Abstract
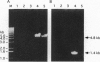
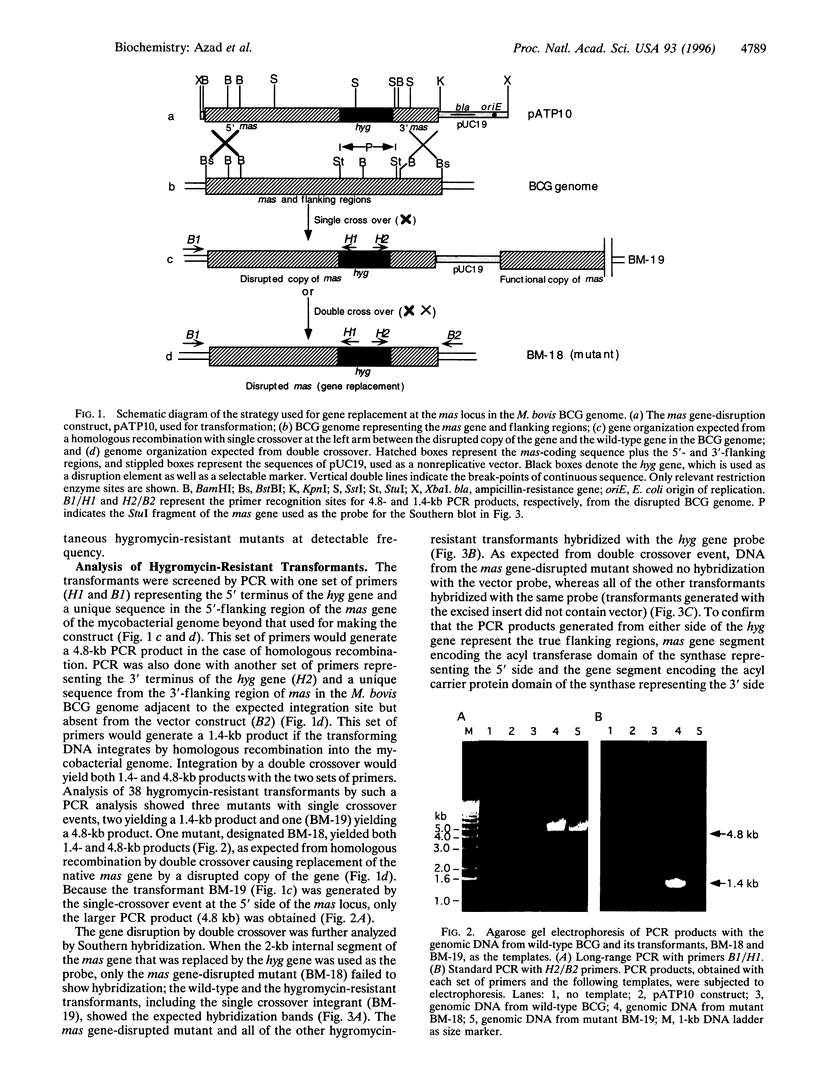
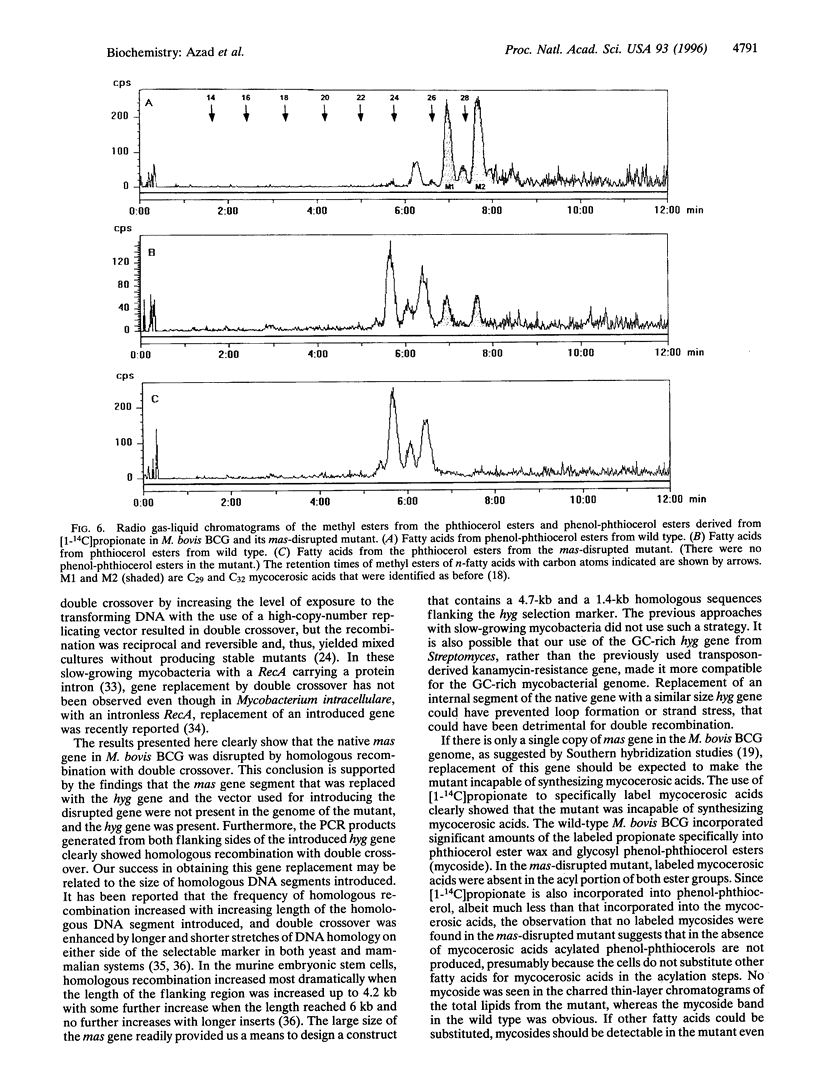
A single gene (mas) encodes the multifunctional enzyme that catalyzes the synthesis of very long chain multiple methyl branched fatty acids called mycocerosic acids that are present only in slow-growing pathogenic mycobacteria and are thought to be important for pathogenesis. To achieve a targeted disruption of mas, an internal 2-kb segment of this gene was replaced with approximately the same size hygromycin-resistance gene (hyg), such that hyg was flanked by 4.7- and 1.4-kb segments of mas. Transformation of Mycobacterium bovis BCG with this construct in a plasmid that cannot replicate in mycobacteria yielded hygromycin-resistant transformants. Screening of 38 such transformants by PCR revealed several transformants representing homologous recombination with single crossover and one with double crossover. With primers representing the hyg termini and those representing the mycobacterial genome segments outside that used to make the transformation construct, the double-crossover mutant yielded PCR products expected from either side of hyg. Gene replacement was further confirmed by the absence of the vector and the 2-kb segment of mas replaced by hyg from the genome of the mutant. Thin-layer and radio-gas chromatographic analyses of the lipids derived from [1-14C]propionate showed that the mutant was incapable of synthesizing mycocerosic acids and mycosides. Thus, homologous recombination with double crossover was achieved in a slow-growing mycobacterium with an intron-containing RecA. The resulting mas-disrupted mutant should allow testing of the postulated roles of mycosides in pathogenesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldovini A., Husson R. N., Young R. A. The uraA locus and homologous recombination in Mycobacterium bovis BCG. J Bacteriol. 1993 Nov;175(22):7282–7289. doi: 10.1128/jb.175.22.7282-7289.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian V., Pavelka M. S., Jr, Bardarov S. S., Martin J., Weisbrod T. R., McAdam R. A., Bloom B. R., Jacobs W. R., Jr Allelic exchange in Mycobacterium tuberculosis with long linear recombination substrates. J Bacteriol. 1996 Jan;178(1):273–279. doi: 10.1128/jb.178.1.273-279.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A., Dubnau E., Quemard A., Balasubramanian V., Um K. S., Wilson T., Collins D., de Lisle G., Jacobs W. R., Jr inhA, a gene encoding a target for isoniazid and ethionamide in Mycobacterium tuberculosis. Science. 1994 Jan 14;263(5144):227–230. doi: 10.1126/science.8284673. [DOI] [PubMed] [Google Scholar]
- Bloom B. R., Murray C. J. Tuberculosis: commentary on a reemergent killer. Science. 1992 Aug 21;257(5073):1055–1064. doi: 10.1126/science.257.5073.1055. [DOI] [PubMed] [Google Scholar]
- Brennan P. J., Nikaido H. The envelope of mycobacteria. Annu Rev Biochem. 1995;64:29–63. doi: 10.1146/annurev.bi.64.070195.000333. [DOI] [PubMed] [Google Scholar]
- Chan J., Fujiwara T., Brennan P., McNeil M., Turco S. J., Sibille J. C., Snapper M., Aisen P., Bloom B. R. Microbial glycolipids: possible virulence factors that scavenge oxygen radicals. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2453–2457. doi: 10.1073/pnas.86.7.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daffé M., Lanéelle M. A., Asselineau C., Lévy-Frébault V., David H. Intérêt taxonomique des acides gras des mycobactéries: proposition d'une méthode d'analyse. Ann Microbiol (Paris) 1983 Sep-Oct;134B(2):241–256. [PubMed] [Google Scholar]
- Daffé M., Lanéelle M. A., Lacave C., Lanéelle G. Monoglycosyldiacylphenol-phthiocerol of Mycobacterium tuberculosis and Mycobacterium bovis. Biochim Biophys Acta. 1988 Feb 19;958(3):443–449. doi: 10.1016/0005-2760(88)90230-5. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Frieden T. R., Sterling T., Pablos-Mendez A., Kilburn J. O., Cauthen G. M., Dooley S. W. The emergence of drug-resistant tuberculosis in New York City. N Engl J Med. 1993 Feb 25;328(8):521–526. doi: 10.1056/NEJM199302253280801. [DOI] [PubMed] [Google Scholar]
- Garbe T. R., Barathi J., Barnini S., Zhang Y., Abou-Zeid C., Tang D., Mukherjee R., Young D. B. Transformation of mycobacterial species using hygromycin resistance as selectable marker. Microbiology. 1994 Jan;140(Pt 1):133–138. doi: 10.1099/13500872-140-1-133. [DOI] [PubMed] [Google Scholar]
- Hasty P., Rivera-Pérez J., Bradley A. The length of homology required for gene targeting in embryonic stem cells. Mol Cell Biol. 1991 Nov;11(11):5586–5591. doi: 10.1128/mcb.11.11.5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husson R. N., James B. E., Young R. A. Gene replacement and expression of foreign DNA in mycobacteria. J Bacteriol. 1990 Feb;172(2):519–524. doi: 10.1128/jb.172.2.519-524.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs W. R., Jr, Kalpana G. V., Cirillo J. D., Pascopella L., Snapper S. B., Udani R. A., Jones W., Barletta R. G., Bloom B. R. Genetic systems for mycobacteria. Methods Enzymol. 1991;204:537–555. doi: 10.1016/0076-6879(91)04027-l. [DOI] [PubMed] [Google Scholar]
- Kalpana G. V., Bloom B. R., Jacobs W. R., Jr Insertional mutagenesis and illegitimate recombination in mycobacteria. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5433–5437. doi: 10.1073/pnas.88.12.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund B. I., Speert D. P., Stokes R. W. Gene replacement through homologous recombination in Mycobacterium intracellulare. J Bacteriol. 1995 Nov;177(21):6100–6105. doi: 10.1128/jb.177.21.6100-6105.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur M., Kolattukudy P. E. Molecular cloning and sequencing of the gene for mycocerosic acid synthase, a novel fatty acid elongating multifunctional enzyme, from Mycobacterium tuberculosis var. bovis Bacillus Calmette-Guerin. J Biol Chem. 1992 Sep 25;267(27):19388–19395. [PubMed] [Google Scholar]
- Mehra V., Brennan P. J., Rada E., Convit J., Bloom B. R. Lymphocyte suppression in leprosy induced by unique M. leprae glycolipid. Nature. 1984 Mar 8;308(5955):194–196. doi: 10.1038/308194a0. [DOI] [PubMed] [Google Scholar]
- Norman E., Dellagostin O. A., McFadden J., Dale J. W. Gene replacement by homologous recombination in Mycobacterium bovis BCG. Mol Microbiol. 1995 May;16(4):755–760. doi: 10.1111/j.1365-2958.1995.tb02436.x. [DOI] [PubMed] [Google Scholar]
- Perriëns J. H., Colebunders R. L., Karahunga C., Willame J. C., Jeugmans J., Kaboto M., Mukadi Y., Pauwels P., Ryder R. W., Prignot J. Increased mortality and tuberculosis treatment failure rate among human immunodeficiency virus (HIV) seropositive compared with HIV seronegative patients with pulmonary tuberculosis treated with "standard" chemotherapy in Kinshasa, Zaire. Am Rev Respir Dis. 1991 Oct;144(4):750–755. doi: 10.1164/ajrccm/144.4.750. [DOI] [PubMed] [Google Scholar]
- Rainwater D. L., Kolattukudy P. E. Fatty acid biosynthesis in Mycobacterium tuberculosis var. bovis Bacillus Calmette-Guérin. Purification and characterization of a novel fatty acid synthase, mycocerosic acid synthase, which elongates n-fatty acyl-CoA with methylmalonyl-CoA. J Biol Chem. 1985 Jan 10;260(1):616–623. [PubMed] [Google Scholar]
- Reyrat J. M., Berthet F. X., Gicquel B. The urease locus of Mycobacterium tuberculosis and its utilization for the demonstration of allelic exchange in Mycobacterium bovis bacillus Calmette-Guérin. Proc Natl Acad Sci U S A. 1995 Sep 12;92(19):8768–8772. doi: 10.1073/pnas.92.19.8768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein R. Targeting, disruption, replacement, and allele rescue: integrative DNA transformation in yeast. Methods Enzymol. 1991;194:281–301. doi: 10.1016/0076-6879(91)94022-5. [DOI] [PubMed] [Google Scholar]
- Sander P., Meier A., Böttger E. C. rpsL+: a dominant selectable marker for gene replacement in mycobacteria. Mol Microbiol. 1995 Jun;16(5):991–1000. doi: 10.1111/j.1365-2958.1995.tb02324.x. [DOI] [PubMed] [Google Scholar]
- Schlesinger L. S., Horwitz M. A. Phenolic glycolipid-1 of Mycobacterium leprae binds complement component C3 in serum and mediates phagocytosis by human monocytes. J Exp Med. 1991 Nov 1;174(5):1031–1038. doi: 10.1084/jem.174.5.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider D. E., Jr, Roper W. L. The new tuberculosis. N Engl J Med. 1992 Mar 5;326(10):703–705. doi: 10.1056/NEJM199203053261011. [DOI] [PubMed] [Google Scholar]
- Takayama K., Wang L., David H. L. Effect of isoniazid on the in vivo mycolic acid synthesis, cell growth, and viability of Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1972 Jul;2(1):29–35. doi: 10.1128/aac.2.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman P. F., Chai W., Rosankiewicz J. R., Rogers H. J., Lawson A. M., Draper P. Possible intermediates in the biosynthesis of mycoside B by Mycobacterium microti. Eur J Biochem. 1993 Mar 15;212(3):705–711. doi: 10.1111/j.1432-1033.1993.tb17708.x. [DOI] [PubMed] [Google Scholar]
- Thurman P., Draper P. Biosynthesis of phenolic glycolipids in M. microti. Acta Leprol. 1989;7 (Suppl 1):74–74. [PubMed] [Google Scholar]
- Vachula M., Holzer T. J., Andersen B. R. Suppression of monocyte oxidative response by phenolic glycolipid I of Mycobacterium leprae. J Immunol. 1989 Mar 1;142(5):1696–1701. [PubMed] [Google Scholar]