Abstract
Diarrheal diseases constitute a significant global health burden and are a major cause of childhood mortality and morbidity. Treatment of diarrheal disease has centered on the replacement of fluid and electrolyte losses using oral rehydration solutions (ORS). Although ORS has been highly successful, significant mortality and morbidity due to diarrheal disease remains. Secretory diarrheas, such as those caused by bacterial and viral enterotoxins, result from activation of cyclic nucleotide and/or Ca2+ signaling pathways in intestinal epithelial cells, enterocytes, which increase the permeability of Cl− channels at the lumen-facing membrane. Additionally, there is often a parallel reduction in intestinal Na+ absorption. Inhibition of enterocyte Cl− channels, including the cystic fibrosis transmembrane conductance regulator (CFTR) and Ca2+-activated Cl− channels, represents an attractive strategy for antisecretory drug therapy. High-throughput screening of synthetic small molecule collections has identified several classes of Cl− channel inhibitors that show efficacy in animal models of diarrhea but remain to be tested clinically. In addition, several natural-product extracts with Cl− channel inhibition activity have shown efficacy in diarrhea models. However, a number of challenges remain to translate the promising bench science into clinically useful therapeutics, including efficiently targeting orally administered drugs to enterocytes during diarrhea, funding development costs, and carrying out informative clinical trials. Nonetheless, Cl− channel inhibitors may prove to be effective adjunctive therapy in a broad spectrum of clinical diarrheas, including acute infectious and drug-related diarrheas, short-bowel syndrome, and congenital enteropathies.
Keywords: diarrhea, small molecules, chloride channels, CFTR, CaCC, rotavirus
Diarrheal disease – a global health burden
Diarrheal disease is a major health burden worldwide and still represents a leading cause of mortality and morbidity. This burden, as with many major diseases, falls disproportionately on the very young and the elderly. At present, diarrheal disease is the second leading cause of mortality globally in children under age five, and repeated episodes of dehydration from diarrhea are associated with impaired physical and mental development 1, 2. In developing countries, the major causes of diarrheal diseases are primarily infectious, including enterotoxin-producing bacteria such as Vibrio cholerae and enterotoxigenic E. coli, viruses such as rotavirus, enteroinvasive bacteria such as Shigella and Salmonella, and parasites such as Entamoeba histolytica and Cryptosporidium parvum 1. Major non-infectious causes of diarrhea include drug side-effects, particularly with certain cancer and HIV therapeutics, and diarrhea secondary to intestinal inflammatory/autoimmune diseases such as ulcerative colitis, Crohn’s disease and celiac disease 3, 4. Diarrhea is also a major problem in patients with short-bowel syndrome, in rare congenital disorders such as microvillus inclusion disease and tufting enteropathy, and with peptide-secreting neuroendocrine tumors 5. Oral rehydration solution (ORS) to replace fluid losses and promote intestinal fluid absorption has been the primary therapy for infectious diarrheas, reducing mortality four-fold over the last 30 years 6. However, there remains an unmet need for alternative and adjunctive antidiarrheal therapeutics to complement ORS, in part because of limitations in ORS availability, acceptability and adequate administration, as well as for symptom relief.
Technological primer: Transporter drug targets for diarrheal disease
Diarrhea results from excessive secretion and/or impaired absorption of fluid and electrolytes across the intestinal epithelium. Movement of fluid across the intestinal epithelium is driven by active transport of ions, mainly Na+, Cl+ and K+, and solutes, mainly glucose. Fluid absorption in the small intestine involves several luminal transporters, including a Na+/H+ exchanger (NHE3), Na+-glucose cotransporter (SGLT1), and Cl−/HCO3− exchanger (DRA) 7,8. As in epithelia in general, the electrochemical driving force is established by a basolateral Na+K+-ATPase pump. In the colon, fluid absorption is also facilitated by the epithelial Na+ channel (ENaC) and short-chain fatty acid transporters (SMCT1) 9. Targeting of fluid absorptive pathways for diarrheal therapy is the subject of a companion review.
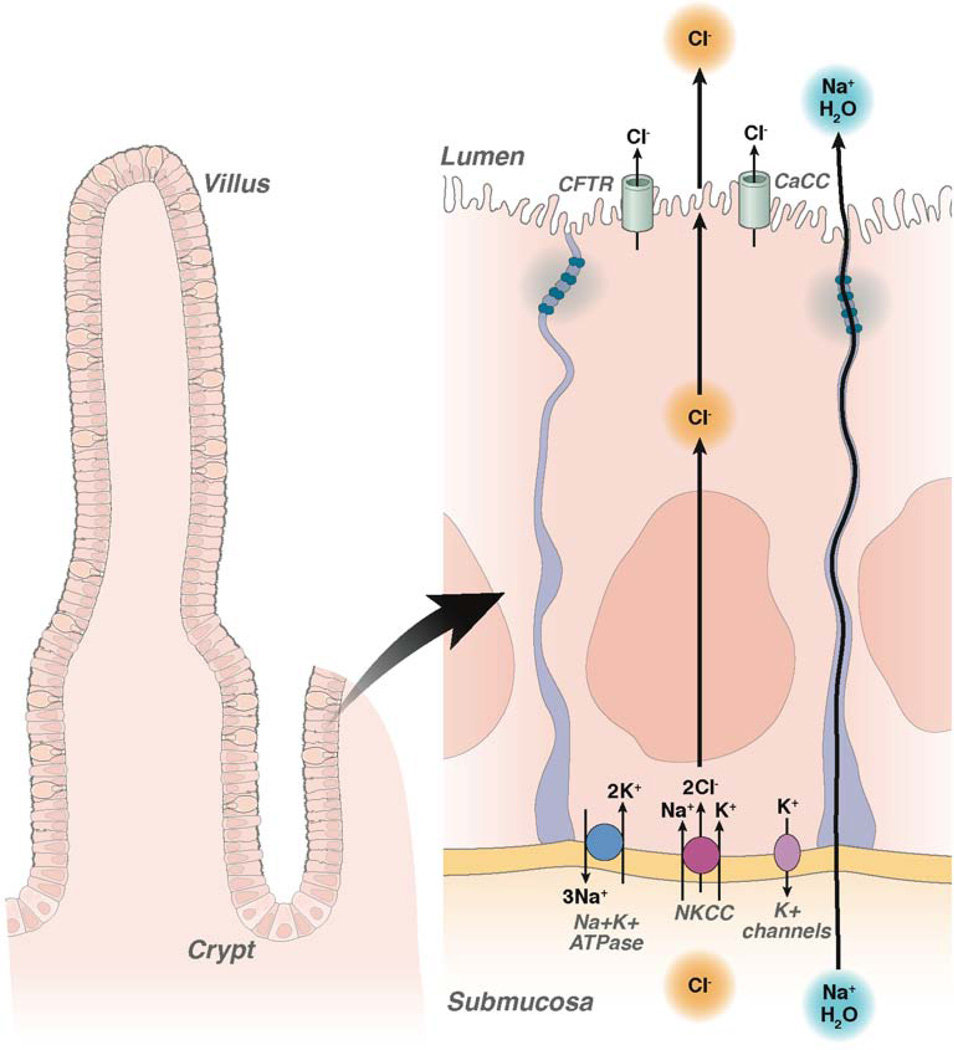
Intestinal fluid secretion is driven by active transepithelial Cl− secretion, creating the electrochemical force for paracellular Na+ secretion and the osmotic driving force for transcellular water secretion (Figure 1). Cl− is transported into the cell at the basolateral membrane by a Na+/K+/2Cl− cotransporter (NKCC1), which is driven by Na+ and Cl− concentration gradients produced by the Na+K+-ATPase and basolateral K+ channels. The electrochemical gradient drives Cl− secretion across the luminal membrane Cl− channels, primarily the cAMP-activated channel CFTR and Ca2+-activated Cl− channels (CaCC) 12. Enterotoxin-producing bacteria such as Vibrio cholerae and enterotoxigenic E. coli produce secretory diarrhea primarily by activation of CFTR-mediated Cl− secretion 13. Viral diarrheas such as caused by rotavirus are thought to result in secretion by causing elevation in cytoplasmic Ca2+ and consequent activation of luminal CaCCs 14. Drug-related diarrhea caused by HIV protease inhibitors is also thought to involve CaCCs 15. However, the contribution of Cl− secretion in the pathogenesis of most drug-related diarrheas, congenital pediatric enteropathies, and many bacterial, viral and parasitic infections remains untested. Despite these limitations in our current knowledge, inhibition of luminal CFTR and CaCC Cl− channels represent an attractive target for potential antidiarrheal therapeutics.
Figure 1. Cl− channels as targets for therapy of secretory diarrheas.
Diagram of fluid secretory mechanism in enterocytes lining intestinal crypts and villi, showing active Cl− transport from the blood/sub-mucosa to the intestinal lumen facilitated by luminal membrane CFTR and CaCC channels. Top inset. CFTR channel pore showing proposed site of action of CFTRinh-172 (arginine 347) and external pore blocking action of GlyH-101. N- NBD binding domain, r- regulatory domain.
Findings: Discovery and development of chloride channel inhibitors
High-throughput screening for discovery of small-molecule CFTR and CaCC inhibitors
Our lab developed and carried out cell-based high-throughput screens to identify Cl− channel modulators using genetically encoded, cytoplasmic fluorescent halide sensors, including the yellow fluorescent protein YFP-H148Q/I152L, whose fluorescence is strongly reduced by I− 17. Target-based assays utilized epithelial cells expressing YFP-H148Q/I152L and CFTR 17 or the CaCC TMEM16A 18. The high-throughput screens involved addition of test compound and Cl− channel activation (by cAMP agonists for CFTR, Ca2+ agonists for TMEM16A), followed by extracellular I− addition to drive cellular I− influx. Potential inhibitors were identified as compounds reducing I− influx as monitored by the kinetics of YFP-H148Q/I152L fluorescence decrease. Because the identity of the major enterocyte CaCC is not clear, phenotype-based screening was done to identify intestinal CaCC inhibitors, utilizing a human intestinal epithelial cell line (HT-29) stably expressing YFP-H148Q/I152L by lentiviral transfection 19.
Small-molecule CFTR inhibitors
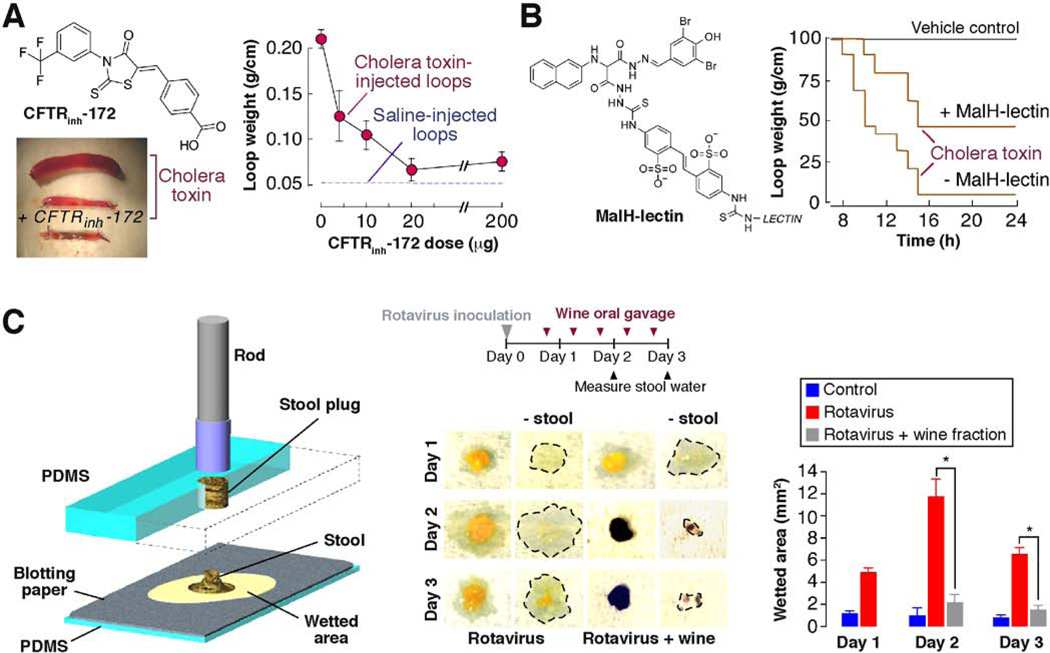
Three chemical classes of nanomolar-potency small-molecule CFTR inhibitors have been identified from screening of synthetic small molecule collections. The thiazolidinone CFTRinh-172 (Fig. 2A) inhibits CFTR Cl− conductance by binding near arginine-347 on the cytoplasmic side of CFTR and stabilizing the channel closed-state 20. Studies on CFTRinh-172 analogs have identified the chemical structural determinants of CFTR inhibition and have provided analogs with a range of activities and aqueous solubilities 21. CFTRinh-172 has shown antisecretory efficacy in rodent diarrhea models, including a closed-intestinal loop model in which fluid accumulation is measured in response to luminal cholera toxin (Fig. 2A). A more recently identified class of CFTR inhibitors targeting the cytoplasmic surface of CFTR are the PPQ/BPO compounds, with the best compound (R-BPO-27) having IC50 ~ 4 nM 22. The PPQ/BPO compounds have shown efficacy in models of polycystic kidney disease in which cyst expansion involves CFTR Cl− secretion, but have not been tested in diarrhea models 23.
Figure 2. Efficacy of Cl− channel inhibitors in animal models of secretory diarrheas.
A. CFTR inhibition prevents cholera toxin-induced fluid secretion. CFTRinh-172 structure (left, top) and photographs of intestinal loops at 6 hours after injection with saline or cholera toxin (left, bottom). Dose-response for inhibition of loop fluid accumulation (right). Mice were given single dose of CFTRinh-172 by intraperitoneal injection and loop weight measured at 6 hours. From ref. 13. B. Improved survival of suckling mice following gavage with cholera toxin without vs. with the lectin conjugate MalH-ConA showing chemical structure (left) and survival plot (right). From ref. 27. C. CaCC inhibition by a red wine extract prevents rotavirus-induced fluid secretion in neonatal mice. Schematic showing expulsion of a 1.9 mm-diameter, 1.5 mm-thick cylindrical volume of stool onto absorbent tissue paper in which stool water content is quantified by wetted area (left). (center) Mice were inoculated with rotavirus at day 0 and gavaged with 1-kDa wine extract twice a day. Photographs of absorbent tissue at 1 min after contacting stool specimen, just before (photos at left) and after (photos at right) removal of stool mass. Wetted area demarcated by thin line. (right) Wetted area at indicated days. From ref. 28.
A third chemical class of small-molecule CFTR inhibitors, the glycine hydrazides, target the extracellular CFTR surface in the channel pore itself 24. These compounds offer the unique opportunity for development as externally acting, non-absorbable antisecretory agents; however, by targeting an external site on the intestinal lumen a potential barrier is accessing CFTR in the deep intestinal crypts against a strong convective washout force during secretory diarrheas. Small-molecule glycine hydrazides, such as the original compound GlyH-101 25 and an analog being studied clinically (iOWH032 26) are unlikely to be effective in significant diarrheas as they are washed off within seconds and have poor IC50 (> 10 µM) at the interior-negative enterocyte membrane potential. Following structure-activity analysis, we synthesized ‘sticky’ glycine hydrazide analogs with improved potency down to 50 nM that resist intestinal washout 27. A macromolecular conjugate containing a CFTR inhibitor moiety linked by a polar spacer to a lectin, which bound strongly to the enterocyte surface glycocalyx, produced improved survival in a suckling mouse model of cholera (Fig. 2B).
Small-molecule CaCC inhibitors
The initial phenotype-based screen in HT-29 cells yielded several small-molecule CaCC inhibitors, the most potent being the 3-acyl-2-aminothiophene CaCCinh-A01, which fully inhibited CaCC-dependent halide flux in different intestinal cell lines with IC50 ~ 1 µM. CaCCinh-A01 likely targets the CaCC directly based on patch-clamp studies and its lack of effect on cytoplasmic Ca2+ signaling. CaCCinh-A01 was shown recently to prevent watery diarrhea in a neonatal mouse model of rotavirus 28. Smallmolecule inhibitors of the CaCC TMEM16A were identified in a target-based screen 18. However, while TMEM16A is the major CaCC in salivary gland and in interstitial cells of Cajal in the intestine, it probably represents a minor contributor to Cl− conductance in enterocytes.
Natural-product Cl− channel inhibitors
Natural products represent a potentially attractive source of antidiarrheal therapeutics, as they are generally inexpensive and have the potential for rapid translation to the clinic. In addition, there is a long history of anecdotal evidence of efficacy of various antidiarrheal remedies in many parts of the world. A drug / natural-product screen done in our lab for CaCC inhibitors revealed, unexpectedly, tannic acid as a general CaCC inhibitor. Subsequent studies showed strong CaCC inhibition by red wines that contain chemically related polyphenolic gallotannins29. A wide range of CaCC inhibition activities was found in different red wines, though white wines and various grape extracts showed no inhibition. Motivated by the likely involvement of CaCC activation in rotaviral diarrhea, we recently showed that an alcohol-free >1 kdalton red wine extract prevented rotaviral diarrhea in neonatal mice, without effect on the rotaviral infection 28. As shown in Fig. 2C, oral administration of the red wine extract stained the stool red but prevented watery diarrhea. In control studies, a red wine extract with minimal CaCC activity in vitro did not prevent rotaviral diarrhea, and the red wine extract used in Fig. 2C did not inhibit CFTR or prevent cholera toxin-induced diarrhea. The use of red wine extracts for CaCC-dependent diarrheas thus warrants potential clinical testing.
In a separate study, we recently screened various diarrhea remedies from sources around the world for those showing Cl− channel inhibition. A commonly used Thai herbal remedy was identified that fully inhibited both CFTR and CaCC Cl− conductance in vitro, and was efficacious in mouse models of cholera and rotaviral diarrhea 30. The remedy also inhibited intestinal smooth muscle contraction and motility. Natural products thus represent a potentially inexpensive and readily available therapy for secretory diarrheas with a defined mechanism of action.
A natural product extract, Crofelemer, was recently approved for treatment of diarrhea associated with HIV drug therapy 31. Crofelemer is a heterogeneous proanthocyanidin oligomer extracted from the bark latex of South American tree Croton lechleri. In vitro studies showed crofelemer as a weak and partial (~60 %) antagonist of CFTR, though a relatively strong inhibitor of CaCC with IC50 < 10 µM 32. It is not clear at this time whether the reported antidiarrheal efficacy of crofelemer in HIV drug-associated diarrhea is related to CaCC inhibition or unrelated actions.
Translation and roadblocks
A number of hurdles remain in the translation of anti-diarrheal drug candidates to widely used therapy. Although a number of compounds have been advanced through pre-clinical testing in murine models, new high-throughput model systems of enterocyte fluid secretion, such as human intestinal enteroids or genetically tractable systems such as zebrafish, warrant development to identify novel compounds and antidiarrheal drug targets. A major translational roadblock, however, is the difficulty in designing and funding informative clinical trials. Field trials in developing countries are logistically difficult to implement and require considerable funding from non-governmental agencies, but have large patient populations with a relatively small number of specific infectious pathogens. In developed countries challenges includes enrollment of adequate and specific patient cohorts that are not confounded by multiple diarrheal etiologies or existing medications. Nevertheless, a number of specific patient populations, such as norovirus diarrheal infections and congential pediatric enteropathies, may allow testing of new antisecretory drugs.
Barriers to diarrheal drug development in developing countries include the need for very low manufacture cost, high stability in hot / humid environments, as well as obtaining funding to support commercial development of new chemical entities with relatively low profit potential.
For drugs targeting the enterocyte extracellular surface, an additional challenge, as mentioned above, is convective washout in which secreted fluid in intestinal crypts washes away inhibitor drugs. A mathematical model of intestinal convection-diffusion concluded that in severe secretory diarrheas such as cholera the antisecretory efficacy of an orally administered, surface-targeted inhibitor requires: (i) high inhibitor affinity to its target (low nanomolar Kd) in order to obtain sufficiently high luminal inhibitor concentration (> 100-fold Kd); and (ii) sustained high luminal inhibitor concentration or slow inhibitor dissociation 33. Washout is a significant concern for small-molecule CFTR glycine hydrazides such as iOWH032 and potentially for several of the natural-product agents.
Conclusions
Antisecretory drug therapy has considerable potential in reducing morbidity and mortality associated with infectious, drug-induced and other diarrheas. The identification of synthetic small molecules and potent natural products, as well as the repurposing of existing medications, present a promising pre-clinical pipeline of drug candidates. The overall human and economic cost of diarrheal disease globally demands a comprehensive approach that includes pharmacological therapies in addition to existing public health priorities such as improvements in access to ORS, vaccination and sanitation. Although multiple challenges remain in the development of antisecretory drugs, including the funding of informative clinical trials, the next decade may bring the exciting prospect of new drugs to combat diarrheal disease.
Acknowledgments
Grant support: Grants DK72517, DK35124, EY13574 and EB00415 from the National Institutes of Health, and a Research Development Program grant from the Cystic Fibrosis Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: No relevant conflicts of interest exist
References
- 1.Walker CL, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA, O'Brien KL, Campbell H, Black RE. Global burden of childhood pneumonia and diarrhoea. Lancet. 2013;381:1405–1416. doi: 10.1016/S0140-6736(13)60222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore SR, Lima NL, Soares AM, Oriá RB, Pinkerton RC, Barrett LJ, Guerrant RL, Lima AA. Prolonged episodes of acute diarrhea reduce growth and increase risk of persistent diarrhea in children. Gastroenterology. 2010;139:1156–1164. doi: 10.1053/j.gastro.2010.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Binder HJ. Mechanisms of diarrhea in inflammatory bowel diseases. Ann NY Acad Sci. 2009;1165:285–293. doi: 10.1111/j.1749-6632.2009.04039.x. [DOI] [PubMed] [Google Scholar]
- 4.Green PH, Jabri B. Celiac disease. Annu Rev Med. 2006;57:207–221. doi: 10.1146/annurev.med.57.051804.122404. [DOI] [PubMed] [Google Scholar]
- 5.Canani RB, Terrin G. Recent progress in congenital diarrheal disorders. Curr Gastroenterol Rep. 2011;13:257–264. doi: 10.1007/s11894-011-0188-6. [DOI] [PubMed] [Google Scholar]
- 6.Munos MK, Walker CL, Black RE, Lipecka J, Bali M, Thomas A, Fanen P, Edelman A. The effect of oral rehydration solution and recommended home fluids on diarrhoea mortality. Int J Epidemiol. 2010;39:i75–i87. doi: 10.1093/ije/dyq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin R, Murtazina R, Cha B, Chakraborty M, Sarker R, Chen TE, Lin Z, Hogema BM, de Jonge HR, Seidler U, Turner JR, Li X, Kovbasnjuk O, Donowitz M. D-glucose acts via sodium/glucose cotransporter 1 to increase NHE3 in mouse jejunal brush border by a Na+/H+ exchange regulatory factor 2-dependent process. Gastroenterology. 2011;140:560–571. doi: 10.1053/j.gastro.2010.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker NM, Simpson JE, Brazill JM, Gill RK, Dudeja PK, Schweinfest CW, Clarke LL. Role of down-regulated in adenoma anion exchanger in HCO3 - secretion across murine duodenum. Gastroenterology. 2009;136:893–901. doi: 10.1053/j.gastro.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gawenis LR, Franklin CL, Simpson JE, Palmer BA, Walker NM, Wiggins TM, Clarke LL. cAMP inhibition of murine intestinal Na/H exchange requires CFTR-mediated cell shrinkage of villus epithelium. Gastroenterology. 2003;125:1148–1163. doi: 10.1016/s0016-5085(03)01212-5. [DOI] [PubMed] [Google Scholar]
- 10.Murtazina R, Kovbasnjuk O, Chen TE, Zachos NC, Chen Y, Kocinsky HS, Hogema BM, Seidler U, de Jonge HR, Donowitz M. NHERF2 is necessary for basal activity, second messenger inhibition, and LPA stimulation of NHE3 in mouse distal ileum. Am J Physiol Cell Physiol. 2011;301:C126–C136. doi: 10.1152/ajpcell.00311.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Binder HJ. Role of colonic short-chain fatty acid transport in diarrhea. Annu Rev Physiol. 2010;72:297–313. doi: 10.1146/annurev-physiol-021909-135817. [DOI] [PubMed] [Google Scholar]
- 12.Barrett KE, Keely SJ. Chloride secretion by the intestinal epithelium: molecular basis and regulatory aspects. Annu Rev Physiol. 2000;62:535–572. doi: 10.1146/annurev.physiol.62.1.535. [DOI] [PubMed] [Google Scholar]
- 13.Thiagarajah JR, Broadbent T, Hsieh E, Verkman AS. Prevention of toxin-induced intestinal ion and fluid secretion by a small-molecule CFTR inhibitor. Gastroenterology. 2004;126:511–519. doi: 10.1053/j.gastro.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Morris AP, Scott JK, Ball JM, Zeng CQ, O'Neal WK, Estes MK. NSP4 elicits age-dependent diarrhea and Ca2+ mediated I− influx into intestinal crypts of CF mice. Am J Physiol. 1999;277:G431–G444. doi: 10.1152/ajpgi.1999.277.2.G431. [DOI] [PubMed] [Google Scholar]
- 15.Rufo PA, Lin PW, Andrade A, Jiang L, Rameh L, Flexner C, Alper SL, Lencer WI. Diarrhea-associated HIV-1 APIs potentiate muscarinic activation of Cl− secretion by T84 cells via prolongation of cytosolic Ca2+ signaling. Am J Physiol Cell Physiol. 2004;286:C998–C1008. doi: 10.1152/ajpcell.00357.2003. [DOI] [PubMed] [Google Scholar]
- 16.Jayaraman S, Haggie P, Wachter R, Remington SJ, Verkman AS. Mechanism and cellular applications of a green fluorescent protein-based halide sensor. J. Biol. Chem. 2000;275:6047–6050. doi: 10.1074/jbc.275.9.6047. [DOI] [PubMed] [Google Scholar]
- 17.Ma T, Thiagarajah JR, Yang H, Sonawane ND, Folli C, Galietta LJ, Verkman AS. Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion. J Clin Invest. 2002;110:1651–1658. doi: 10.1172/JCI16112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Namkung W, Phuan P, Verkman AS. TMEM16A inhibitors reveal TMEM16A as a minor component of CaCC conductance in airway and intestinal epithelial cells. J Biol Chem. 2011;286:2365–2374. doi: 10.1074/jbc.M110.175109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De La Fuente R, Namkung W, Mills S, Verkman AS. Small-molecule screen identifies inhibitors of a human intestinal calcium-activated chloride channel. Mol Pharmacol. 2008;73:758–768. doi: 10.1124/mol.107.043208. [DOI] [PubMed] [Google Scholar]
- 20.Caci E, Caputo A, Hinzpeter A, Arous N, Fanen P, Sonawane ND, Verkman AS, Ravazzolo R, Zegarra-Moran O, Galietta LJ. Evidence for direct CFTR inhibition by CFTRinh-172 based on arginine 347 mutagenesis. Biochem J/ 2008;413:135–142. doi: 10.1042/BJ20080029. [DOI] [PubMed] [Google Scholar]
- 21.Sonawane ND, Verkman AS. Thiazolidinone CFTR inhibitors with improved water solubility identified by structure-activity analysis. Bioorg Med Chem. 2008;16:8187–8195. doi: 10.1016/j.bmc.2008.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snyder DS, Tradtrantip L, Yao C, Kurth MJ, Verkman AS. Potent, metabolically stable benzopyrimido-pyrrolo-oxazine-dione (BPO) CFTR inhibitors for polycystic kidney disease. J Med Chem. 2011;54:5468–5477. doi: 10.1021/jm200505e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tradtrantip L, Sonawane ND, Namkung W, Verkman AS. Nanomolar potency pyrimido-pyrrolo-quinoxalinedione CFTR inhibitor reduces cyst size in a polycystic kidney disease model. J Med Chem. 2009;52:6447–6455. doi: 10.1021/jm9009873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norimatsu Y, Ivetac A, Alexander C, O'Donnell N, Frye L, Sansom MS, Dawson DC. Locating a plausible binding site for an open channel blocker, GlyH-101, in the pore of the cystic fibrosis transmembrane conductance regulator. Mol Pharmacol. 2012;82:1042–1055. doi: 10.1124/mol.112.080267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muanprasat C, Sonawane ND, Salinas D, Taddei A, Galietta LJ, Verkman AS. Discovery of glycine hydrazide pore-occluding CFTR inhibitors: mechanism, structure-activity analysis, and in vivo efficacy. J Gen Physiol. 2004;124:125–137. doi: 10.1085/jgp.200409059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Hostos EL, Choy RK, Nguyen T. Developing novel antisecretory drugs to treat infectious diarrhea. Future Med Chem. 2011;3:1317–1325. doi: 10.4155/fmc.11.87. [DOI] [PubMed] [Google Scholar]
- 27.Sonawane ND, Zhao D, Zegarra-Mora O, Galietta LJ, Verkman AS. Lectin conjugates as potent, nonabsorbable CFTR inhibitors for reducing intestinal fluid secretion in cholera. Gastroenterology. 2007;132:1234–1244. doi: 10.1053/j.gastro.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 28.Ko EA, Jin BJ, Namkung W, Ma T, Thiagarajah JR, Verkman AS. Chloride channel inhibition by a red wine extract prevents rotaviral secretory diarrhea in neonatal mice. Gut. 2013 doi: 10.1136/gutjnl-2013-305663. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Namkung W, Thiagarajah JR, Phuan PW, Verkman AS. Inhibition of Ca2+-activated Cl− channels by gallotannins as a possible molecular basis of health benefits of green tea and red wine. Faseb J. 2010;24:4178–4186. doi: 10.1096/fj.10-160648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tradtrantip L, Ko EA, Verkman AS. Antidiarrheal efficacy and cellular mechanisms of a Thai herbal remedy. doi: 10.1371/journal.pntd.0002674. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeo QM, Crutchley R, Cottreau J, Tucker A, Garey KW. Crofelemer, a novel antisecretory agent approved for the treatment of HIV-associated diarrhea. Drugs Today (Barc) 2013;49:239–252. doi: 10.1358/dot.2013.49.4.1947253. [DOI] [PubMed] [Google Scholar]
- 32.Tradtrantip L, Namkung W, Verkman AS. Crofelemer, an antidiarrheal proanthocyanidin oligomer extracted from Croton lechleri, targets two distinct intestinal chloride channels. Mol Pharmacol. 2010;77:69–78. doi: 10.1124/mol.109.061051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin BJ, Thiagarajah JR, Verkman AS. Convective washout reduces the antidiarrheal efficacy of enterocyte surface-targeted antisecretory drugs. J Gen Physiol. 2013;141:261–272. doi: 10.1085/jgp.201210885. [DOI] [PMC free article] [PubMed] [Google Scholar]