Abstract
The accumulation of improperly folded proteins within the endoplasmic reticulum (ER) generates perturbations known as ER stress that engage the unfolded protein response (UPR). ER stress is involved in many inflammatory pathologies that are also associated with the production of the proinflammatory cytokine interleukin-1β (IL-1β). Here we demonstrate that macrophages undergoing ER stress are able to drive the production and processing of pro-IL-1β in response to LPS stimulation in vitro. Interestingly, the classical NLRP3 inflammasome is dispensable, since maturation of pro-IL-1β occurs normally in the absence of the adaptor protein ASC. In contrast, processing of pro-IL-1β is fully dependent on caspase-8. Intriguingly, we found that neither the UPR proteins XBP1 and CHOP or the TLR4 adaptor molecule MyD88 are necessary for caspase-8 activation. Instead, both caspase activation and IL-1β production require the alternative TLR4 adaptor TRIF. This pathway may contribute to IL-1-driven tissue pathology in certain disease settings.
INTRODUCTION
Eukaryotic cells have evolved a complex set of chaperones and quality control mechanisms for ensuring the proper folding of secretory and transmembrane proteins in the endoplasmic reticulum (ER). However, during periods of rapidly increased biosynthetic demand or in response to metabolic, environmental, or infectious insults, these mechanisms can become overwhelmed, leading to accumulation of misfolded proteins in the ER. This state of ER stress activates a cellular pathway known as the unfolded protein response (UPR) (1). In mammalian cells, the UPR pathway involves three primary signaling cascades initiated by the ER-localized transmembrane proteins inositol requiring enzyme 1α (IRE1α), protein kinase RNA-like ER kinase (PERK), and activating transcription factor 6 (ATF6). These signaling cascades act to restore ER homeostasis by increasing the expression of chaperones and other molecules involved in protein folding, inhibiting the translation of most other proteins, and activating ER-associated degradation machinery (1, 2).
In addition to its cell-intrinsic effects on protein translation and folding, ER stress is linked to tissue inflammation. For example, ER stress is thought to contribute to the inflammation observed in chronic metabolic conditions such as obesity and diabetes, in neurodegenerative disorders, and in a variety of infections (1, 3). The mechanistic basis for ER stress-induced inflammation is thought to lie partially downstream of the UPR signaling cascades, which can initiate inflammatory responses through activation of NFκB and the MAP kinase c-Jun N terminal kinase (JNK) (1). Moreover, the major UPR-associated transcription factors X box-binding protein 1 (XBP1) and CCAAT/enhancer-binding protein homologous protein (CHOP) have also been shown to induce expression of the cytokines interferon-β and interleukin-23, respectively, in response to TLR stimulation in the context of ER stress (4, 5). However, ER stress is also thought to have inflammatory effects independent of the UPR by triggering accumulation of reactive oxygen species and Ca2+ flux into the cytosol from the ER (1). Furthermore, ER stress was recently shown to activate the NLRP3 inflammasome, and this was suggested to occur in a manner that did not require the UPR pathway (6).
The inflammasome is a multiprotein complex that proteolytically activates the immature cytokines pro-IL-1β and pro-IL-18 (7). The central component of this complex is traditionally thought to be the enzyme caspase-1, but there has recently been increasing recognition of the role of other proteases. In particular, caspase-8 has been shown to cleave pro-IL-1β at the same site as caspase-1 in response to simultaneous stimulation with TLR3 and TLR4 ligands (8). Interestingly, caspase-8 activation has been found in other systems to be differentially dependent on the adaptor ASC and the receptor-interacting protein 3 kinase (RIPK3), suggesting multiple pathways for triggering caspase-8-mediated IL-1β production (9-11).
In this study, we show that TLR4 stimulation in the context of ER stress triggers caspase-8-mediated IL-1β production. Notably, caspase-8 activation in this model does not require ASC or RIPK3, but is dependent on the signaling adaptor TIR domain-containing adapter-inducing interferon-β (TRIF). Taken together, our data identify a novel pathway for ER stress-induced inflammation involving noncanonical caspase-8 inflammasome activation in response to TLR4 stimulation.
MATERIALS AND METHODS
Mice
C57BL/6 mice were purchased from Taconic Farms and Chop (Ddit3)−/− mice from The Jackson Laboratory. Myd88−/−, Asc−/−, and Casp1−/− Casp11−/− mice were obtained as previously described (12). All animals were maintained in an AALAC-accredited animal facility at the National Institute of Allergy and Infectious Diseases (NIAID). Mice were used according to animal study proposals approved by the NIAID Animal Care and Use Committee. Ripk3+/− Casp8+/−, Ripk3−/− Casp8+/−, and Ripk3−/− Casp8−/− mice (13) were bred at St. Jude Children's Research Hospital. Bone marrow from Xbp1F/F; Mx1-Cre and Xbp1F/F mice that had been treated with Poly(I:C) to delete XBP1 (14) was generously supplied by L. Glimcher (Harvard University). Bone marrow from P2rx7−/− mice was kindly provided by F. Tam (Imperial College).
Reagents
Ultrapure LPS, Pam3CysK4, poly(I:C), CpG, and R848 were purchased from Invivogen. ATP and thapsigargin were from Sigma-Aldrich, and tunicamycin were from Alexis Biochemicals/Enzo Life Sciences. Annexin V and propidium iodide (PI) were from eBioscience and acridine orange from Life Technologies.
Macrophage stimulation and immunoblotting
Bone marrow-derived macrophages (BMDM) prepared as previously described (12) were treated for 4 hours with DMSO (0.1%), tunicamycin (1μg/ml), or thapsigargin (1 μM), washed, and then stimulated for an additional 16-18 hours with LPS (100 ng/ml) or other TLR agonists. Alternatively, cells were primed for 4 hours with LPS (10 ng/ml) and then stimulated for 30-45 min with 5 mM ATP. Supernatants and cell extracts were harvested and processed as previously described (12) and immunoblotted for the following targets with antibodies from Cell Signaling Technology: caspase-8 (4790), cleaved caspase-8 (9429), CHOP (5554 and 2879), BiP (3183), from R&D Systems: IL-1β (AF-401), or from Santa Cruz Biotechnology: caspase-1 (sc-514) and GAPDH (sc-32233). All blots were quantified using ImageJ software (Supplemental Fig. 2F).
Cytokine ELISA
Cytokine levels in macrophage culture supernatants were measured using IL-6, TNF-α, and IL-23 Duoset kits from R&D Systems or the IL-1β Ready-SET-GO! kit from eBioscience.
Epifluorescence Microscopy
After stimulation, cells were washed with annexin V binding buffer (eBioscience) and incubated for 15 minutes with annexin V followed by acridine orange and PI. Fluorescent images were captured using a Leica AF 6000 LX microscope and positive cells enumerated using ImageJ software.
RESULTS AND DISCUSSION
ER stress modulates TLR agonist-induced cytokine production
To investigate how ER stress affects proinflammatory cytokine production, we treated bone marrow-derived macrophages (BMDM) with the ER stress-inducing drugs tunicamycin (TM) or thapsigargin (TG), followed by stimulation with various TLR agonists. We found that ER stress induction enhanced IL-6 and TNF-α production in response to subsequent TLR stimulation, whereas production of IL-12/23p40 was unaffected or reduced (Supplemental Fig. 1A). In agreement with a previous report (4), IL-23 production in response to LPS was also enhanced by ER stress (Supplemental Fig. 1B). Interestingly, we noted that IL-1β, which was not produced in response to treatment with any of the individual TLR agonists in the absence of ER stress, was secreted by TM or TG pre-treated cells stimulated with LPS (Fig. 1A). ER stress induction plus LPS stimulation was associated with some loss of cell viability, but this was also observed with other TLR agonists that did not induce IL-1β production (Supplemental Fig. 1C).
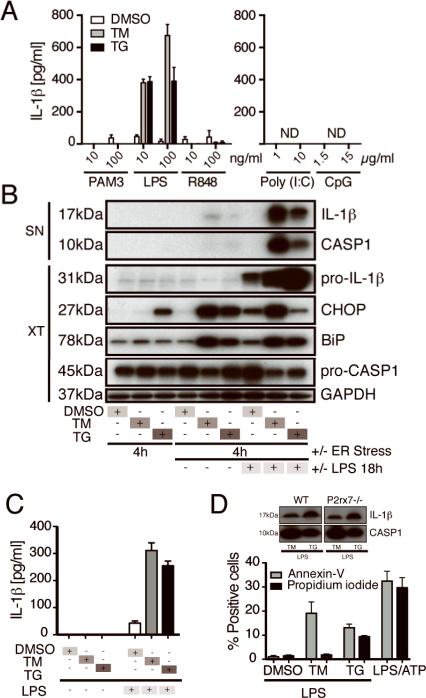
Figure 1.
ER Stress licenses macrophages to produce mature IL-1β in response to LPS stimulation. (A) IL-1β concentration in the supernatants (SN) of BMDMs treated with DMSO, the ER stress inducer tunicamycin (TM), or thapsigargin (TG) followed by the indicated TLR agonists (ND: not detected). (B) Immunoblots of SN and cell extracts (XT) from BMDM treated with ER stress-inducing drugs and/or LPS. (C) IL-1β ELISA of SN from BMDM treated with DMSO, TM, or TG for 4h or further stimulated with LPS or left in medium for 18h. (D) Percentage of cells positive for annexin V or propidium iodide in four random fields counted using ImageJ software after epifluoresence microspcopy (D, insert) WT or P2x7r−/− BMDM were treated with TM or TG and LPS, and the SN were immunoblotted for IL-1β and caspase-1. Data are representative of 2-3 experiments.
Detection of IL-1β in the supernatants of cells treated with ER stress-inducing drugs and LPS was of particular interest because IL-1β is a complexly regulated cytokine that requires two signals for its secretion (7). While LPS can induce pro-IL-1β, it does not usually activate the inflammasome. Therefore, we verified the presence of mature IL-1β and cleaved caspase-1 in the supernatants of BMDM treated with TM or TG and LPS (Fig. 1B). ER stress was confirmed by assessing the induction of ER stress markers BiP and CHOP in the cell extracts (Fig. 1B). Interestingly, the order of ER stress induction and LPS stimulation was critical, since we observed a minimal response to LPS priming and subsequent treatment with ER stress-inducing drugs (Supplemental Fig. 1D). Importantly, treatment of cells with ER stress-inducing drugs alone was not sufficient to induce IL-1β production (Fig. 1B and 1C).
To investigate the role of cell death, we stained TM or TG and LPS-treated BMDM with annexin V and propidium iodide (PI). We then performed epifluorescence microscopy and quantified the percentage of cells that incorporated each stain. We found that TM appeared to induce mild apoptotic-type cell death (annexin V positive cells) but very little necrotic-type cell death (PI positive cells), whereas TG induced mild apoptosis and necrosis (Fig. 1D). Necrotic cell death has been shown to activate the inflammasome through the P2X7 purinergic receptor (15). However, we did not observe any defect in IL-1β production or caspase-1 cleavage in response to ER stress and LPS in P2rx7−/− BMDM (Fig. 1D, insert).
We next asked whether the caspase-1 inflammasome is necessary for IL-1β maturation in this setting. To address this question, we treated WT, Asc−/−, or Casp1−/− Casp11−/− BMDM with ER stress-inducing drugs and LPS (Fig. 2A). In the absence of caspase-1/11, we noted a sizable defect in mature IL-1β secretion in response to TM, while the response to TG was reduced but not abrogated. Importantly, we found that ASC is dispensable for IL-1β secretion in response to both drugs although it is partially required for the maturation of caspase-1. This led us to hypothesize that the canonical inflammasome is not essential for IL-1β processing in response to ER stress and to investigate the role of other potential pathways.
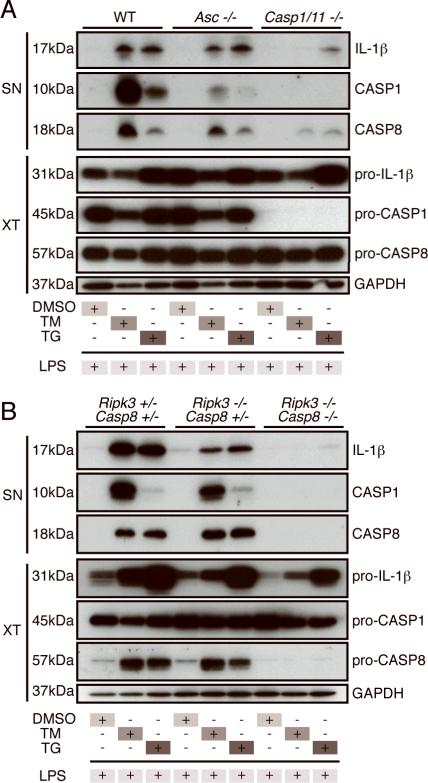
Figure 2.
IL-1β maturation is independent of ASC but dependent on caspase-1/caspase-11 and caspase-8. Immunoblots of SN and XT from WT, Asc−/−, and Casp1/11−/− (A) or from Ripk3+/− Casp8+/−, Ripk3−/− Casp8+/− and Ripk3−/− Casp8−/− (B) BMDM treated with DMSO, TM, or TG for 4h and then stimulated with LPS for 18h. Data are representative of at least 3 independent experiments.
ER stress and LPS-induced IL-1β production requires caspase-8
Recent studies have demonstrated that caspase-8 can proteolytically activate pro-IL-1β (8). Therefore, we asked whether this enzyme accounts for the caspase-1-independent IL-1β maturation triggered by ER stress plus LPS stimulation. Supporting this hypothesis, we found that TM or TG and LPS induced caspase-8 cleavage much more effectively than LPS and ATP (Fig. 2A, Supplemental Fig. 1E and 1F). Caspase-8 deficiency is embryonically lethal, but this lethality can be rescued by concomitant deletion of RIPK3. Therefore, to investigate the role of caspase-8 in IL-1β maturation in response to ER stress and LPS, we used BMDM from Ripk3+/− Casp8+/−, Ripk3−/− Casp8+/− and Ripk3−/− Casp8−/− mice (13). We found that IL-1β production in response to TM or TG and LPS was slightly reduced in the absence of RIPK3, but was ablated in the Ripk3−/− Casp8−/− BMDM (Fig. 2B). Importantly, Ripk3+/− Casp8+/−, Ripk3−/− Casp8+/−, and Ripk3−/− Casp8−/− BMDM were comparable in their production of IL-1β and cleaved caspase-1 in response to LPS and ATP stimulation, demonstrating that caspase-8 is not universally required for IL-1β or inflammasome activation (Supplemental Fig. 1F).
Interestingly, in response to ER stress and LPS stimulation, the Ripk3−/− Casp8−/− BMDM were also unable to produce cleaved caspase-1, suggesting that caspase-8 is acting upstream of caspase-1 inflammasome activation in this system. Furthermore, levels of pro-IL-1β were reduced in caspase-8-deficient macrophages. Using densitometric analysis to measure the relative defects in pro-IL-1β and mature IL-1β production, we found that the reduction in pro-IL-1β levels in caspase-8 deficient macrophages is not sufficient to explain their lack of mature IL-1β (Supplemental Fig. 1G and 1H). Therefore, these data suggest that caspase-8 plays a role in both pro-IL-1β production and IL-1β processing in response to ER stress and TLR4 stimulation.
Caspase-8 activation and IL-1β production in response to ER stress and LPS stimulation requires TRIF but not MyD88, XBP1, or CHOP
Having shown that IL-1β production in response to ER stress and subsequent TLR4 stimulation requires caspase-8, we then asked how this enzyme is activated. First, we considered that ER stress might sensitize cells to produce TNF-α or Fas/FasL, either of which could induce caspase-8 activation (10). However, Tnf−/− and Fas−/− BMDM were comparable to WT BMDM in their activation of caspase-8 and secretion of mature IL-1β in response to TM or TG and LPS (Supplemental Fig. 2A). We next tested whether caspase-8 might be activated downstream of the UPR. One of the three arms of the mammalian UPR is initiated by the ER transmembrane protein IRE1α, which splices the mRNA encoding the transcription factor XBP1 (1). Using BMDM from Xbp1F/F and Xbp1F/F; Mx1-Cre mice (14) we found that XBP1 is not necessary for caspase-8 activation or IL-1β secretion in response to ER stress and LPS (Supplemental Fig. 2B). Similarly, using Chop (Ddit3) −/− BMDM, we found that CHOP, another transcription factor involved in the UPR, was also dispensable for the response to TM or TG and LPS (Supplemental Fig. 2C). While it is surprising that neither of these UPR-associated transcription factors play a role in ER stress and LPS-induced IL-1β production, it is possible that other factors downstream of the PERK- or ATF6-dependent arms of the UPR mediate this response or that there is redundancy in UPR signaling.
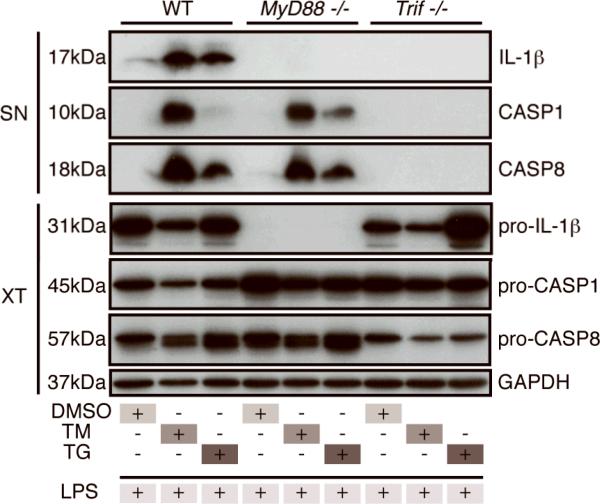
Since ER stress itself is not sufficient to activate caspase-8 and LPS stimulation is also required, we asked whether TLR4 signaling is necessary for caspase-8 activation and IL-1β secretion. Although a TLR4-independent pathway for LPS sensing has recently been described (16), TLR4 was found to be critical in our model (Supplemental Fig. 2D). Interestingly, while both the MyD88 and TRIF-dependent arms of TLR4 signaling are important for mature IL-1β secretion, we found that only TRIF is necessary for caspase-8 cleavage (Fig. 3A). The defect in Myd88−/− BMDM could be explained by the lack of pro-IL-1β production, whereas Trif−/− BMDM displayed normal pro-IL-1β levels (Fig. 3A). These data suggest that, in response to ER stress and LPS stimulation, MyD88-dependent signaling downstream of TLR4 provides “signal one” for pro-IL-1β production, whereas TRIF-dependent signaling provides a second signal for caspase-8 activation and IL-1β maturation.
Figure 3.
The TLR adaptor protein TRIF is critical for ER stress mediated IL-1β processing. Immunoblots of SN and XT from WT, MyD88−/−, and Trif−/− BMDM treated with DMSO, TM, or TG for 4h and then stimulated with LPS for 18h. The difference in pro-caspase-8 levels seen in Trif−/−cell extracts was not observed in subsequent experiments. Data are representative of 2-3 experiments.
In summary, we have identified a novel pathway linking ER stress to inflammation through non-canonical caspase-8 inflammasome activation. Interestingly, both caspase-8 and caspase-1/caspase-11 play a role in IL-1β maturation in response to ER stress and TLR4 stimulation, and there is cross-talk between these enzymes, as caspase-1 cleavage in response to these stimuli is ablated in caspase-8-deficient mice, and caspase-8 cleavage is reduced in caspase-1/11-deficient mice (Supplemental Fig. 2E).
In contrast to a previous report suggesting that ER stress induction after LPS priming triggers caspase-1 inflammasome activation (6), we observed caspase-1 cleavage and mature IL-1β secretion only when first treating with ER stress-inducing drugs and then stimulating with LPS. Moreover, whereas these authors observed a requirement for the NLRP3 inflammasome, IL-1β production in our studies was ASC-independent and partially required caspase-1/caspase-11, while being almost completely caspase-8-dependent. The reason for the discrepancy is unclear, but may stem from the use of the THP1 cell line or their longer (6 hr) treatment with TM and TG, which may have triggered cell death leading to canonical inflammasome activation.
We were surprised to note that caspase-8 plays a role in pro-IL-1β production as well as IL-1β maturation in response to ER stress induction and TLR4 stimulation. It is unclear how caspase-8 is linked to pro-IL-1β production and whether this occurs at the transcriptional or translational level. Pro-IL-1β production is highly NFκB-dependent, and caspase-8 has previously been shown to interact with NFκB (17), so the most likely possibility is that NFκB activation is altered in ER stress drug and LPS-treated cells, leading to reduced Il1b gene induction.
Many viruses are known to induce ER stress when they hijack the cell's translational machinery to produce enormous amounts of viral proteins (18). Moreover, stressed or dying cells are known to release endogenous TLR4 agonists such as HMGB1 (19). Therefore, ER stress may condition cells to produce proinflammatory cytokines in response to endogenous TLR agonists, so that virally-infected cells are exquisitely sensitive to cellular stress or death in their neighbors, leading to enhanced anti-viral responses. Moreover, metabolic conditions such as obesity and diabetes are known to lead to ER stress and have been linked to chronic inflammation (1), so it is possible that a similar phenomenon of endogenous TLR4 agonists acting on cells undergoing ER stress contributes to the inflammation observed during these diseases.
Supplementary Material
Acknowledgements
This project was supported by the Intramural Research Program of the National Institutes of Allergy and Infectious Diseases, NIH and by grants from the Medical Research Council, Cancer Research UK (Programme Grant # C399/A2291 to VC) and the Wellcome Trust. KS was supported by a scholarship from the Rhodes Trust and by MSTP funding from the Johns Hopkins University School of Medicine. We are grateful to Dr. Mahtab Moayeri and Eduardo Pinheiro Amaral for scientific discussion.
REFERENCES
- 1.Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martinon F. The endoplasmic reticulum: a sensor of cellular stress that modulates immune responses. Microbes and infection / Institut Pasteur. 2012;14:1293–1300. doi: 10.1016/j.micinf.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garg AD, Kaczmarek A, Krysko O, Vandenabeele P, Krysko DV, Agostinis P. ER stress-induced inflammation: does it aid or impede disease progression? Trends in molecular medicine. 2012;18:589–598. doi: 10.1016/j.molmed.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Goodall JC, Wu C, Zhang Y, McNeill L, Ellis L, Saudek V, Gaston JS. Endoplasmic reticulum stress-induced transcription factor, CHOP, is crucial for dendritic cell IL-23 expression. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:17698–17703. doi: 10.1073/pnas.1011736107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith JA, Turner MJ, DeLay ML, Klenk EI, Sowders DP, Colbert RA. Endoplasmic reticulum stress and the unfolded protein response are linked to synergistic IFN-beta induction via X-box binding protein 1. European journal of immunology. 2008;38:1194–1203. doi: 10.1002/eji.200737882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menu P, Mayor A, Zhou R, Tardivel A, Ichijo H, Mori K, Tschopp J. ER stress activates the NLRP3 inflammasome via an UPR-independent pathway. Cell death & disease. 2012;3:e261. doi: 10.1038/cddis.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rathinam VA, Vanaja SK, Fitzgerald KA. Regulation of inflammasome signaling. Nature immunology. 2012;13:333–332. doi: 10.1038/ni.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maelfait J, Vercammen E, Janssens S, Schotte P, Haegman M, Magez S, Beyaert R. Stimulation of Toll-like receptor 3 and 4 induces interleukin-1beta maturation by caspase-8. The Journal of experimental medicine. 2008;205:1967–1973. doi: 10.1084/jem.20071632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gringhuis SI, Kaptein TM, Wevers BA, Theelen B, van der Vlist M, Boekhout T, Geijtenbeek TB. Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1beta via a noncanonical caspase-8 inflammasome. Nature immunology. 2012;13:246–254. doi: 10.1038/ni.2222. [DOI] [PubMed] [Google Scholar]
- 10.Bossaller L, Chiang PI, Schmidt-Lauber C, Ganesan S, Kaiser WJ, Rathinam VA, Mocarski ES, Subramanian D, Green DR, Silverman N, Fitzgerald KA, Marshak-Rothstein A, Latz E. Cutting edge: FAS (CD95) mediates noncanonical IL-1beta and IL-18 maturation via caspase-8 in an RIP3-independent manner. Journal of immunology. 2012;189:5508–5512. doi: 10.4049/jimmunol.1202121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vince JE, Wong WW, Gentle I, Lawlor KE, Allam R, O'Reilly L, Mason K, Gross O, Ma S, Guarda G, Anderton H, Castillo R, Hacker G, Silke J, Tschopp J. Inhibitor of apoptosis proteins limit RIP3 kinase-dependent interleukin-1 activation. Immunity. 2012;36:215–227. doi: 10.1016/j.immuni.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Shenderov K, Barber DL, Mayer-Barber KD, Gurcha SS, Jankovic D, Feng CG, Oland S, Hieny S, Caspar P, Yamasaki S, Lin X, Ting JP, Trinchieri G, Besra GS, Cerundolo V, Sher A. Cord factor and peptidoglycan recapitulate the Th17-promoting adjuvant activity of mycobacteria through mincle/CARD9 signaling and the inflammasome. Journal of immunology. 2013;190:5722–5730. doi: 10.4049/jimmunol.1203343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, Hakem R, Salvesen GS, Green DR. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011;471:363–367. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee AH, Scapa EF, Cohen DE, Glimcher LH. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 2008;320:1492–1496. doi: 10.1126/science.1158042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iyer SS, Pulskens WP, Sadler JJ, Butter LM, Teske GJ, Ulland TK, Eisenbarth SC, Florquin S, Flavell RA, Leemans JC, Sutterwala FS. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:20388–20393. doi: 10.1073/pnas.0908698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kayagaki N, Wong MT, Stowe IB, Ramani SR, Gonzalez LC, Akashi-Takamura S, Miyake K, Zhang J, Lee WP, Muszynski A, Forsberg LS, Carlson RW, Dixit VM. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science. 2013;341:1246–1249. doi: 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- 17.Lemmers B, Salmena L, Bidere N, Su H, Matysiak-Zablocki E, Murakami K, Ohashi PS, Jurisicova A, Lenardo M, Hakem R, Hakem A. Essential role for caspase-8 in Toll-like receptors and NFkappaB signaling. The Journal of biological chemistry. 2007;282:7416–7423. doi: 10.1074/jbc.M606721200. [DOI] [PubMed] [Google Scholar]
- 18.He B. Viruses, endoplasmic reticulum stress, and interferon responses. Cell death and differentiation. 2006;13:393–403. doi: 10.1038/sj.cdd.4401833. [DOI] [PubMed] [Google Scholar]
- 19.Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annual review of immunology. 2011;29:139–162. doi: 10.1146/annurev-immunol-030409-101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.