Abstract
Objective
The patterns and risks of perioperative use of anti-tumor necrosis factor (anti-TNF)medication in patients with rheumatoid arthritis (RA) are not well studied. We examined the patterns of perioperative anti-TNF use and risk of postoperative adverse events (AE) in patients undergoing total knee replacement (TKR).
Method
Retrospective cohort study with followup. RA cases within a TKR registry were identified by ICD-9 code (714.0) or self-report. Mailed questionnaires queried anti-TNF use and duration of RA. AE were determined by chart review and patient self-report, and included surgical site infection, pulmonary embolus, deep venous thrombosis, pneumonia, and any infection o rre-operation within 6 months.
Results
There were 268 TKR cases with RA. The stop time for anti-TNF preoperatively correlated with dosing schedule; restart time was after wound healing. There were 7 surgical site infections (3%), one (0.4%) of which was a deep joint infection in bilateral TKA requiring explant. The anti-TNF group had 3.26% (3/92) local site infection versus 2.10% (3/143) in the group without anti-TNF and this difference was not statistically significant (Fisher exact test, p = 0.68). The one deep joint infection was in the anti-TNF group. Six-month AE rate was 7.61% in the anti-TNF group versus 6.99% in the group without anti-TNF (Fisher exact test, p = 1.0).
Conclusion
There was a low risk of infection and perioperative adverse events in patients with RA receiving anti-TNF therapy who were undergoing TKR. This raises the question whether it is necessary to stop anti-TNF for a long period prior to surgery. Given the possible risks associated with stopping anti-TNF, including worsening of disease, further study is needed to determine optimal perioperative use of anti-TNF among patients with RA undergoing TKR.
Key Indexing Terms: RHEUMATOID ARTHRITIS, SURGERY, TUMOR NECROSIS FACTOR INHIBITORS, INFECTION, ARTHROPLASTY
Rheumatoid arthritis (RA) is an autoimmune inflammatory disorder that when uncontrolled can lead to joint destruction warranting total joint replacement. Anti-tumor necrosis factor (anti-TNF) medications developed in the 1990s are extremely effective in treating RA and have greatly increased the quality of life for patients1. However, the use of these immune-modulating medications around the time of joint replacement surgery is controversial. Given that anti-TNF medications suppress the immune system, it has been hypothesized that their use around time of surgery could increase postoperative infection rates, although stopping anti-TNF therapy could increase rates of RA flare.
Recent studies have not clarified whether anti-TNF use truly increases the rate of infection leading to hospitalization or other medical care2,3,4,5,6,7,8,9,10,11. Part of the problem in analyzing this question is that deep joint infection and surgical site infection are relatively rare events, making it difficult to make meaningful comparisons between treatment groups. The rate of postoperative infection is estimated at 1%–2% in the general population and this rate is 2–4 times higher in patients with RA without adjustment for concurrent medication use12. We evaluated a composite outcome consisting of multiple adverse events (AE), including pneumonia (PNA), deep venous thrombosis (DVT), and pulmonary embolism (PE), which are posited to be associated with anti-TNF use13,14,15.
Current physician practice is to stop anti-TNF medications prior to joint replacement surgery, as recommended by numerous international guidelines10,16,17,18,19. However, there are no strong clinical data to support this practice.
National rheumatologic societies differ in their guide- lines for perioperative anti-TNF medication management. For example, the American College of Rheumatology (ACR) recommends stopping anti-TNF therapy 1 week prior to surgery and restarting 1 week after surgery, whereas in Britain the recommendation is to stop 3–5 half-lives pre- operatively and to restart after wound healing. In Japan the recommendation is to stop 2–4 weeks prior to surgery and then to restart after wound healing in 10–14 days10,16,17,18,19. These disparate strategies lack evidence-based studies to support them. To date only 12 studies have examined the perioperative safety of anti-TNF medications for orthopedic surgeries. All but 1 of the 12 studies were entirely retrospective10,12,13,20,21,22,23,24,25,26,27.
We examined the perioperative patterns of anti-TNF medication use in patients with RA undergoing total knee replacement (TKR) at a specialty musculoskeletal hospital that performed over 4600 TKR in 2011. We chose TKR to ensure a homogenous cohort undergoing the same surgical procedure. In addition, TKR is the joint most commonly replaced in patients with RA, and at least 1 study found that it had a higher rate of infection than total hip replacement28. The reasoning in using TKR was that the higher rate of infection would increase the ability to ascertain a difference in infection between the groups. Specific goals were to document the timing of use of anti-TNF medication peri- operatively in patients with RA undergoing TKR, and to examine whether the rate of adverse postsurgical events was increased in anti-TNF users versus those not using anti-TNF medication.
MATERIALS AND METHODS
Patient identification
Patients over 18 years of age with RA or juvenile idiopathic arthritis (JIA) enrolled in our institution’s knee replacement registry between May 1, 2007, and February 29, 2011, were eligible for our study. Institutional review board approval was received and informed consent was obtained per protocol.
RA case definition
Perioperative documentation of potential cases was reviewed by a study rheumatologist (BKJ) based on self-report of RA or International Classification of Diseases code 714.0. The diagnosis of RA was validated if a treating rheumatologist documented that the patient had RA or JIA, or if an internist documented RA/JIA and the patient was taking disease-modifying drugs (DMARD). All uncertain diagnoses were reviewed by 2 additional rheumatologists (SMG, LAM) and agreement reached by consensus.
Both primary and revision TKR were included. Bilateral TKR performed on the same day were counted as 1 procedure. Any revision surgery within 6 months after the initial surgery was counted as a complication of the original case, rather than as a separate new case. Baseline data for all patients in the registry include the Western Ontario and McMaster Universities Arthritis Index (WOMAC), Medical Outcomes Study Short Form-36 (SF-36), EuroQol (EQ-5D), and the Elixhauser comorbidity score29,30. The Elixhauser comorbidity index assigns points to comorbid conditions to predict in-hospital mortality. Recent literature has shown the Elixhauser outperforms other indices, and we chose it as our measure of comorbidity31,32,33,34.
Six-month postoperative AE included surgical site infection (SSI) as defined by the US Centers for Disease Control (CDC), PE as evidenced by computed tomography angiogram, DVT by venous Doppler scan or angiogram, PNA by standard chest radiograph, and any revision surgery within 6 months. Specifically, SSI was defined by the CDC 1992 guide-lines: (1) purulent discharge from a surgical site; (2) a positive culture; (3) a surgical site that requires opening, or (4) SSI is present as determined by the treating surgeon31. AE were evaluated together as a composite endpoint. All self-reported AE were verified by chart review or telephone interview.
In addition, cases were sent a specific RA survey for this study, either by postal mail or e-mail. This survey included questions about duration of and severity of RA, as well as patterns of perioperative anti-TNF medication use. Patient charts were reviewed to confirm self-reported use and timing of all anti-TNF medication. Other RA-specific medication use was also recorded including prednisone use, perioperative “stress-dose” steroids (supraphysiologic doses given peri- and postoperatively), other DMARD, and non-TNF biologic medications. While there was no uniform dosing of stress-dose steroids at our institution, the most common dosing is a 3-day course of hydrocortisone: Day 1, 100 mg intravenous (IV) q8h x3; Day 2, 50 mg IV q8h x3; and Day 3, 25 mg IV q8h x3.
Much has been written about the clinical pharmacokinetics of anti-TNF and how the chemical half-lives of anti-TNF do not necessarily reflect the “therapeutic window” during which the drug is effective in treating the disease35,36. For example, infliximab has a half-life of 8 days, but dosing is IV every 4 to 8 weeks, whereas adalimumab has a half-life of 14 days, which does correlate with every other week of subcutaneous (SC) dosing; this has been attributed to the difference in the route of administration for infliximab being IV and adalimumab being SC36. The actual therapeutic dosing regimen was used in the analysis instead of the pharmacological half-lives, as optimal dosing regimens take into account the biologic effectiveness of the drugs and bioavailability in the body and not just the chemical half-lives. The dosing regimen was the supplier’s recommended therapeutic dosing; for etanercept it was 1 week, for adalimumab it was 2 weeks, for golimumab 4 weeks, and for infliximab 6 weeks based on the dosing ranges of 4 to 8 weeks. For this study we defined an anti-TNF user as someone who had used anti-TNF within 6 months of surgery, as this was the timeframe within which we were looking for adverse postsurgical events.
All analyses were conducted using SAS software, version 9.2 (SAS Institute). Demographic data were analyzed using t tests or chi-square statistics, as appropriate. To further evaluate adverse outcomes, sensitivity analysis was performed using Fisher’s exact test to analyze the effect of (1) excluding patients receiving prednisone, (2) excluding patients receiving rituximab and abatacept, and (3) looking at all infections as the primary outcome.
RESULTS
RA case confirmation
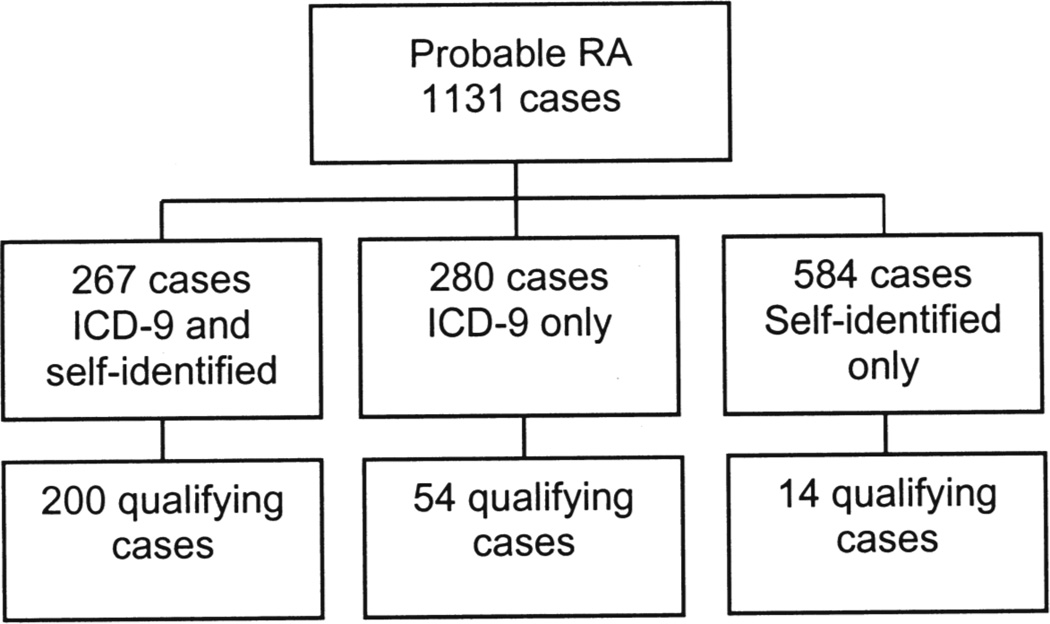
In total, 1131 potential patient cases were identified and reviewed to confirm the diagnosis; 268 cases representing 248 (23.7%) individual patients met RA criteria for this study (Figure 1).
Figure 1.
Case identification; 268 cases representing 248 individual patients met rheumatoid arthritis (RA) criteria for this study. ICD-9:International Classification of Diseases, 9th ed.
Demographic information
Anti-TNF medications were used by 39% of patients; the anti-TNF and non-anti-TNF groups were largely similar. Baseline demographic data were examined to look for potential confounders such as sex, ethnicity, and education level. We also had proxies for RA severity such as measures of functional performance and health-related quality of life including the WOMAC, EuroQol, SF-36, Elixhauser comorbidity scores, duration of RA, and the visual analog scale (VAS) score for pain (Table 1). Only age was statistically significantly different between the groups, the non-anti-TNF group being older (64.4 vs 58.7 yrs; p < 0.0001). However, we did not believe an age difference of about 5 years was clinically significant enough to warrant stratifying the analysis based on age. Overall use of nonbiologic DMARD between the anti-TNF and non-anti-TNF group was not statistically significantly different (p = 0.33). Among specific nonbiologic DMARD only plaquenil use was significantly different between groups, with those receiving anti-TNF medication being less likely to use plaquenil (8.7% vs 20.7%; p = 0.009).
Table 1.
Demographic characteristics and rheumatoid arthritis (RA) history of users of anti-tumor necrosis factor (anti-TNF) medications and nonusers.
| Characteristic | Anti-TNF Use, n = 104 cases |
No Anti-TNF Use, n = 164 cases |
T test or Chi-square p |
|---|---|---|---|
| Age, yrs, mean ± SD | 58.7 ± 12.4 | 64.4 ± 10.5 | < 0.0001 |
| Sex female, % | 84 | 90 | 0.15 |
| Body mass index, mean ± SD | 28.7 ± 6.3 | 28.3 ± 6.3 | 0.64 |
| RA flare rate, % | 26 (n = 77) | 20 (n = 121) | 0.31 |
| VAS pain score 6+ mo postsurgery on survey | 34.8 ± 27.7 | 40.1 ± 27.1 | 0.16 |
| Prednisone use, % | 27 | 37 | 0.10 |
| Prednisone dose at time of surgery, mg, mean ± SD | 7 ± 3.3 | 7.5 ± 4.8 | 0.59 |
| Perioperative stress dose steroid use, % | 26 | 26 | 0.73 |
| Duration of RA reported at time of survey, yrs, mean ± SD | 21.5 ± 16.3 | 19.5 ± 12.4 | 0.32 |
| Elixhauser comorbidity score*, mean ± SD | 2.1 ± 1.3 | 2.2 ± 1.4 | 0.37 |
| Nonbiologic DMARD use, % | 64 (n = 67) | 70 (n = 115) | 0.33 |
| WOMAC function score, range 0–100, mean ± SD, n = 179 | 43.6 ± 20.3 | 43.7 ± 18.61 | 0.96 |
| Euroqol (EQ-5D), range −1 to 1, mean ± SD (n = 214) | 0.58 ± 0.21 | 0.58 ± 0.20 | 0.95 |
| SF-36 function score, mean ± SD (n = 208) | 29.2 ± 7.3 | 29.1 ± 8.2 | 0.92 |
Elixhauser comorbidity score estimates probability of death in hospital based on comorbid diseases; higher scores indicate greater burden of comorbid diseases. VAS: visual analog scale; WOMAC: Western Ontario and McMaster Universities Arthritis Index; SF-36: Medical Outcomes Study Short Form-36; DMARD: disease-modifying antirheumatic drug.
Timing of anti-TNF use
Of the 268 cases, there were 104 cases using anti-TNF for which start and stop times were documented; the other 164 were not using anti-TNF. Data showed that 87% (90/104) of patient charts indicated that anti-TNF medication should be stopped prior to surgery. The remaining 13% (14/104) did not make a recommendation; no chart documented that anti-TNF medication should be continued throughout the surgery. Specific stop times were documented in 71/104 (68%) cases and restart times were listed in 23/104 (22%) cases. Mean stop and restart times of anti-TNF medications are shown in Table 2. Stop times appeared to be based on the drug-specific dosing schedule, although infliximab was stopped a relatively shorter period of time preoperatively than the other anti-TNF medications. The time to restarting medications was uniformly 2–4 weeks, regardless of anti-TNF type, which roughly correlates with the time for wound healing.
Table 2.
Timing of use of anti-tumor necrosis factor (anti-TNF) medication perioperatively.
| Anti-TNF | Stop Time, weeks ± SD, n = 71 | Restart Times, weeks ± SD, n = 23 |
|---|---|---|
| Etanercept (n = 59) Standard dosing: weekly (t1/2= 3–5.5 days) |
2.4 ± 2.4 (n = 39) range 1–14 |
2.1 ± 1.1 (n = 15) range 1–4 |
| Golimumab (n = 2) Standard dosing: weekly (t1/2= 7–20 days) |
8 (n = 1) range NA |
1.5 (n = 1) range NA |
| Adalimumab (n = 25) Standard dosing: every 2 weeks (t1/2 = 10–20 days) |
5 ± 5.6 (n = 20) range 1–24 |
2 ± 1 (n = 3) range 1–3 |
| Infliximab (n = 18) Standard dosing: every 4–8 weeks (t1/2 = 7–12 days) |
4.8 ± 2.2 (n = 11) range 2–9 |
4.4 ± 1.8 (n = 4) Range 2–4 |
t1/2 half-life. NA: not available.
Adverse event data
In total, 233 (87%) cases had 6-month postoperative AE data. The overall AE rate was 7.3% (17/233). Of these, 10 (4.3%) AE met the CDC definition for infection. There were 7 (3%) SSI, only 1 (0.4%) of which was a deep joint infection in bilateral TKA in which both knees required explant. Of note, the deep joint infection was in the anti-TNF group. For local site infections, 3/92 (3.26%) were in the anti-TNF group and 3/143 (2.10%) in the non-anti-TNF group; this difference was not statistically significant (Fisher’s exact test, p = 0.68). Additionally, there was no significant difference between the groups when the data were analyzed for each negative event individually (PNA, PE, DVT, and revision surgery). The AE rate in the non-anti-TNF group, 10/143 (6.99%) versus the anti-TNF group’s 7/92 (7.61%), was not statistically significantly different (Fisher’s exact test, p = 1.0; Table 3). In the sensitivity analysis there was no difference when evaluating only infections as the primary outcome, nor when excluding those using steroids, nor when excluding those using abatacept (n = 12) and rituximab (n = 4). The number of patients using abatacept and rituximab was too small to conduct a separate subgroup analysis.
Table 3.
Adverse outcomes stratified by use of anti-tumor necrosis factor (anti-TNF) medication.
| Anti-TNF Users | Nonusers |
|---|---|
|
|
DISCUSSION
The major finding of our study was that there is a low risk of infection and 6-month AE postoperatively in patients with RA undergoing knee replacement who are receiving anti-TNF medication. This was true for both deep joint infection and surgical site infection. While concerns have been raised that anti-TNF use may increase the rate of prosthetic joint infection, this was not supported by our results. This leads us to question whether use of anti-TNF medication perioperatively needs to be stopped far in advance of surgery or at all; current recommendations vary by country and none are evidence-based10,16,17,18,19. Our infection rate was similar to that reported in other studies examining RA and infection, suggesting that our infection rate results are generalizable to other institutions4,28,37. Considering the low rate of postoperative infection, the question of whether a shorter stop time for anti-TNF or continuation is warranted requires a large randomized clinical trial to achieve statistically significant results.
Our report builds upon 12 clinical studies10,11,12,13,20,21,22,23,24,25,26,27 investigating the patterns of use and AE associated with perioperative anti-TNF therapy. To our knowledge it is the first study to document how anti-TNF is used perioperatively in a large cohort of patients with RA undergoing orthopedic surgery. While there are many recommendations, ours is the first study to examine how physicians are actually practicing in an orthopedic hospital that specializes in joint replacement. This is useful because it might help other clinicians decide how best to manage anti-TNF medications in patients with RA undergoing arthroplasty amid a plethora of conflicting usage recommendations.
The amount of time the medications were stopped prior to surgery appeared to be related to their drug-specific dosing regimen, but was not always consistent. While etanercept and adalimumab were both stopped about 2 doses prior to surgery, infliximab was generally stopped within less than 1 dosing period. We had stop times for only 1 case with golimumab so that was not evaluable. It is unknown whether the decision to stop anti-TNF was influenced by factors other than medication class, such as disease severity.
Our study evaluated anti-TNF use in 1 surgery type, TKR, instead of combining data for surgeries of varying levels of infection risk ranging from abdominal surgery to trauma surgery. In contrast, previous studies grouped many noncomparable surgeries such as general surgical procedures with orthopedic surgeries21,23,24 or several different orthopedic procedures10,11,12,13,20,22,25,26, or focused on different procedures in one anatomic area (e.g., foot and ankle)27. As well, our study focused exclusively on patients with RA. Previous studies grouped together disparate autoimmune diseases that are not necessarily equivalent in terms of infection risk, such as RA, psoriatic arthritis, and spondyloarthropathy22,23,26. Compared to many previous studies we had a large cohort with 268 cases; only 3 of the 12 prior clinical studies had a higher number of cases than ours10,11,24. Other studies had as few as 16 and up to 104 cases12,13,20,21,22,23,25,26,27. Additionally, all RA diagnoses were validated by a rheumatologist instead of relying on ICD-9 coding or self-report alone, as is often the only option in large cohort studies3,7,38.
Our study also stands apart on the matter of data collection; 11 previous studies were retrospective designs with no followup. We had data collected prospectively on a large cohort of RA patients with a survey response rate of 80%. The only previous prospective study was of a small group of 16 patients22. We also had additional demographic data from our hospital database and a response rate of 87% to the 6-month adverse outcomes followup questionnaire. There was a slightly higher response rate for adverse outcomes data, because this information was obtained by both standardized database followup surveys and the additional RA-specific survey for those who did not respond to the database survey.
All incidences of AE including infection were validated in our study. The infection rate was similar to that reported in other studies, suggesting our results are generalizable, although we are a tertiary care specialty hospital4,28,37. Additionally, we used a standardized definition for surgical site infection, from the CDC39. Also, unlike some previous studies13,21,22, we did analyze the therapeutic dosing regimen of the drug. We did this because, after a literature review, we found dosing regimen to be the most robust measure of when the anti-TNF medication was in the system to expose the patient to risk of infection35,36.
Our retrospective study had several limitations, chiefly that the rate of infection was very low, making it difficult to obtain statistically significant results. A prospective randomized controlled trial evaluating the effects of perioperative anti-TNF use would provide the strongest evidence; however, this is unlikely to be feasible given the low rate of infection and other adverse events37,40. Using a Fisher chi-square test for a projected outcome of 1% of non-anti-TNF subjects having 6-month adverse outcomes and 3% of patients using anti-TNF having adverse outcomes, 1644 patients would be needed to achieve 80% power. Based on the actual outcomes of 7.61% in the anti-TNF group and 6.99% in the non-anti-TNF group, 55,890 cases would be needed to achieve 80% power.
As well, RA diagnosis was based on ICD-9 codes and chart review for DMARD use rather than the ACR criteria41. Because our institution is a large referral center, most patients did not have their primary rheumatologist at the hospital and therefore we had access to only perioperative case notes. The literature suggests that using both diagnosis codes for RA and DMARD prescription information increases accuracy in validating RA diagnoses42,43,44. In addition, the fact that RA cases confirmed the diagnosis on our RA-specific self-report questionnaire proves the high positive predictive value of our validation algorithm. RA cases may have been overlooked, but this is unlikely as we reviewed all cases ICD-9 coded as RA. We did not have access in many cases to traditional markers of disease severity such as erythrocyte sedimentation rates or Disease Activity Score results. However, the groups were similar in terms of comorbidities, function, pain, and duration of disease, suggesting the anti-TNF group had a more severe burden of disease.
Overall, we found a very low risk of 6-month infection and adverse events in patients with RA undergoing TKR, regardless of whether they were taking anti-TNF medications perioperatively. Given the lack of evidence behind existing recommendations regarding anti-TNF use at the time of surgery, these data raise the question whether it is actually necessary to stop anti-TNF prior to surgery. Given the possible risks associated with stopping anti-TNF drugs, including worsening of disease, further study is needed to determine optimal perioperative use of anti-TNF therapy.
ACKNOWLEDGMENT
We thank Gina Aharonoff and Huong Do for help in preparation of this report.
Supported by Cornell CTSC Grant UL1-RR024996 (Dr. Mandl), CERT Grant U18-HS016075 (Dr. Mandl), and HSS ARJR Pilot Grant (Dr. Alexiades, Dr. Johnson).
REFERENCES
- 1.Pincus T, Sokka T, Kautiainen H. Patients seen for standard rheumatoid arthritis care have significantly better articular, radiographic, laboratory, and functional status in 2000 than in 1985. Arthritis Rheum. 2005;52:1009–1019. doi: 10.1002/art.20941. [DOI] [PubMed] [Google Scholar]
- 2.Dixon WG, Symmons DP, Lunt M, Watson KD, Hyrich KL, Silman AJ. Serious infection following anti-tumor necrosis factor alpha therapy in patients with rheumatoid arthritis: Lessons from interpreting data from observational studies. Arthritis Rheum. 2007;56:2896–2904. doi: 10.1002/art.22808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curtis JR, Patkar N, Xie A, Martin C, Allison JJ, Saag M, et al. Risk of serious bacterial infections among rheumatoid arthritis patients exposed to tumor necrosis factor alpha antagonists. Arthritis Rheum. 2007;56:1125–1133. doi: 10.1002/art.22504. [DOI] [PubMed] [Google Scholar]
- 4.Bongartz T, Halligan CS, Osmon DR, Reinalda MS, Bamlet WR, Crowson CS, et al. Incidence and risk factors of prosthetic joint infection after total hip or knee replacement in patients with rheumatoid arthritis. Arthritis Rheum. 2008;59:1713–1720. doi: 10.1002/art.24060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson AE, Rieder SW, Pope JE. Tumor necrosis factor therapy and the risk of serious infection and malignancy in patients with early rheumatoid arthritis: A meta-analysis of randomized controlled trials. Arthritis Rheum. 2011;63:1479–1485. doi: 10.1002/art.30310. [DOI] [PubMed] [Google Scholar]
- 6.Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: Systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295:2275–2285. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- 7.Schneeweiss S, Setoguchi S, Weinblatt ME, Katz JN, Avorn J, Sax PE, et al. Anti-tumor necrosis factor alpha therapy and the risk of serious bacterial infections in elderly patients with rheumatoid arthritis. Arthritis Rheum. 2007;56:1754–1764. doi: 10.1002/art.22600. [DOI] [PubMed] [Google Scholar]
- 8.Wolfe F, Caplan L, Michaud K. Treatment for rheumatoid arthritis and the risk of hospitalization for pneumonia: Associations with prednisone, disease-modifying antirheumatic drugs, and anti-tumor necrosis factor therapy. Arthritis Rheum. 2006;54:628–634. doi: 10.1002/art.21568. [DOI] [PubMed] [Google Scholar]
- 9.Listing J, Strangfeld A, Kary S, Rau R, von Hinueber U, Stoyanova-Scholz M, et al. Infections in patients with rheumatoid arthritis treated with biologic agents. Arthritis Rheum. 2005;52:3403–3412. doi: 10.1002/art.21386. [DOI] [PubMed] [Google Scholar]
- 10.Momohara S, Kawakami K, Iwamoto T, Yano K, Sakuma Y, Hiroshima R, et al. Prosthetic joint infection after total hip or knee arthroplasty in rheumatoid arthritis patients treated with nonbiologic and biologic disease-modifying antirheumatic drugs. Mod Rheumatol. 2011;21:469–475. doi: 10.1007/s10165-011-0423-x. [DOI] [PubMed] [Google Scholar]
- 11.Kubota A, Nakamura T, Miyazaki Y, Sekiguchi M, Suguro T. Perioperative complications in elective surgery in patients with rheumatoid arthritis treated with biologics. Mod Rheumatol. 2012;22:844–848. doi: 10.1007/s10165-012-0612-2. [DOI] [PubMed] [Google Scholar]
- 12.Giles JT, Bartlett SJ, Gelber AC, Nanda S, Fontaine K, Ruffing V, et al. Tumor necrosis factor inhibitor therapy and risk of serious postoperative orthopedic infection in rheumatoid arthritis. Arthritis Rheum. 2006;55:333–337. doi: 10.1002/art.21841. [DOI] [PubMed] [Google Scholar]
- 13.Kawakami K, Ikari K, Kawamura K, Tsukahara S, Iwamoto T, Yano K, et al. Complications and features after joint surgery in rheumatoid arthritis patients treated with tumour necrosis factor-alpha blockers: Perioperative interruption of tumour necrosis factor-alpha blockers decreases complications? Rheumatology. 2010;49:341–347. doi: 10.1093/rheumatology/kep376. [DOI] [PubMed] [Google Scholar]
- 14.Ryan BM, Romberg M, Wolters F, Stockbrugger RW. Extensive forearm deep venous thrombosis following a severe infliximab infusion reaction. Eur J Gastroenterol Hepatol. 2004;16:941–942. doi: 10.1097/00042737-200409000-00022. [DOI] [PubMed] [Google Scholar]
- 15.Grange L, Nissen MJ, Garambois K, Dumolard A, Duc C, Gaudin P, et al. Infliximab-induced cerebral thrombophlebitis. Rheumatology. 2005;44:260–261. doi: 10.1093/rheumatology/keh451. [DOI] [PubMed] [Google Scholar]
- 16.Saag KG, Teng GG, Patkar NM, Anuntiyo J, Finney C, Curtis JR, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59:762–784. doi: 10.1002/art.23721. [DOI] [PubMed] [Google Scholar]
- 17.Ding T, Ledingham J, Luqmani R, Westlake S, Hyrich K, Lunt M, et al. BSR and BHPR rheumatoid arthritis guidelines on safety of anti-TNF therapies. Rheumatology. 2010;49:2217–2219. doi: 10.1093/rheumatology/keq249a. [DOI] [PubMed] [Google Scholar]
- 18.Reumatologie NVv. Medications: Use of TNF blockade in the treatment of rheumatoid arthritis. Utrecht: Dutch Society for Rheumatology; 2003. Medicijnen: het toepassen van TNF blockade in de behandeling vav reumatoide arthritis [in Dutch] [Google Scholar]
- 19.Pham T, Claudepierre P, Deprez X, Fautrel B, Goupille P, Hilliquin P, et al. Anti-TNF alpha therapy and safety monitoring. Clinical tool guide elaborated by the Club Rhumatismes et Inflammations (CRI), section of the French Society of Rheumatology (Societe Francaise de Rhumatologie, SFR) . Joint Bone Spine. 2005;72(Suppl 1):S1–S58. doi: 10.1016/s1169-8330(05)80001-8. [DOI] [PubMed] [Google Scholar]
- 20.Hayata K, Kanbe K, Chiba J, Nakamura A, Inoue Y, Hobo K. Clinical factors related to the efficacy and complications of. Rheum Dis. 2011;14:31–36. doi: 10.1111/j.1756-185X.2010.01579.x. [DOI] [PubMed] [Google Scholar]
- 21.Wendling D, Balblanc JC, Brousse A, Lohse A, Lehuede G, Garbuio P, et al. Surgery in patients receiving anti-tumour necrosis factor alpha treatment in rheumatoid arthritis: An observational study on 50 surgical procedures. Ann Rheum Dis. 2005;64:1378–1379. doi: 10.1136/ard.2005.037762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Talwalkar SC, Grennan DM, Gray J, Johnson P, Hayton MJ. Tumour necrosis factor alpha antagonists and early postoperative complications in patients with inflammatory joint disease undergoing elective orthopaedic surgery. Ann Rheum Dis. 2005;64:650–651. doi: 10.1136/ard.2004.028365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruyssen-Witrand A, Gossec L, Salliot C, Luc M, Duclos M, Guignard S, et al. Complication rates of 127 surgical procedures performed in rheumatic patients receiving tumor necrosis factor alpha blockers. Clin Exp Rheumatol. 2007;25:430–436. [PubMed] [Google Scholar]
- 24.den Broeder AA, Creemers MC, Fransen J, de Jong E, de Rooij DJ, Wymenga A, et al. Risk factors for surgical site infections and other complications in elective surgery in patients with rheumatoid arthritis with special attention for anti-tumor necrosis factor: A large retrospective study. J Rheumatol. 2007;34:689–695. [PubMed] [Google Scholar]
- 25.Hirano Y, Kojima T, Kanayama Y, Shioura T, Hayashi M, Kida D, et al. Influences of anti-tumour necrosis factor agents on postoperative recovery in patients with rheumatoid arthritis. Clin Rheumatol. 2010;29:495–500. doi: 10.1007/s10067-009-1346-1. [DOI] [PubMed] [Google Scholar]
- 26.Gilson M, Gossec L, Mariette X, Gherissi D, Guyot MH, Berthelot JM, et al. Risk factors for total joint arthroplasty infection in patients receiving tumor necrosis factor alpha-blockers: A case-control study. Arthritis Res Ther. 2010;12:R145. doi: 10.1186/ar3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bibbo C, Goldberg JW. Infectious and healing complications after elective orthopaedic foot and ankle surgery during tumor necrosis factor-alpha inhibition therapy. Foot Ankle Int. 2004;25:331–335. doi: 10.1177/107110070402500510. [DOI] [PubMed] [Google Scholar]
- 28.Schrama JC, Espehaug B, Hallan G, Engesaeter LB, Furnes O, Havelin LI, et al. Risk of revision for infection in primary total hip and knee arthroplasty in patients with rheumatoid arthritis compared with osteoarthritis: A prospective, population-based study on 108,786 hip and knee joint arthroplasties from the Norwegian Arthroplasty Register. Arthritis Care Res. 2010;62:473–479. doi: 10.1002/acr.20036. [DOI] [PubMed] [Google Scholar]
- 29.Osnes-Ringen H, Kvien TK, Henriksen JE, Mowinckel P, Dagfinrud H. Orthopaedic surgery in 255 patients with inflammatory arthropathies: Longitudinal effects on pain, physical function and health-related quality of life. Ann Rheum Dis. 2009;68:1596–1601. doi: 10.1136/ard.2008.096362. [DOI] [PubMed] [Google Scholar]
- 30.EuroQol — A new facility for the measurement of health-related quality of life. The EuroQol Group. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 31.Southern DA, Quan H, Ghali WA. Comparison of the Elixhauser and Charlson/Deyo methods of comorbidity measurement in administrative data. Med Care. 2004;42:355–360. doi: 10.1097/01.mlr.0000118861.56848.ee. [DOI] [PubMed] [Google Scholar]
- 32.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chron Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 33.Sharabiani MT, Aylin P, Bottle A. Systematic review of comorbidity indices for administrative data. Med Care. 2012;50:1109–1118. doi: 10.1097/MLR.0b013e31825f64d0. [DOI] [PubMed] [Google Scholar]
- 34.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Nestorov I. Clinical pharmacokinetics of tumor necrosis factor antagonists. J Rheumatol Suppl. 2005 Mar;74:13–18. [PubMed] [Google Scholar]
- 36.Nestorov I. Clinical pharmacokinetics of TNF antagonists: How do they differ? Semin Arthritis Rheum. 2005;34(Suppl):12–18. doi: 10.1016/j.semarthrit.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 37.Goh L, Jewell T, Laversuch C, Samanta A. Should anti-TNF therapy be discontinued in rheumatoid arthritis patients undergoing elective orthopaedic surgery? A systematic review of the evidence. Rheumatol Int. 2012;32:5–13. doi: 10.1007/s00296-011-2040-6. [DOI] [PubMed] [Google Scholar]
- 38.Grijalva CG, Chen L, Delzell E, Baddley JW, Beukelman T, Winthrop KL, et al. Initiation of tumor necrosis factor-alpha antagonists and the risk of hospitalization for infection in patients with autoimmune diseases. JAMA. 2011;306:2331–2339. doi: 10.1001/jama.2011.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Consensus paper on the surveillance of surgical wound infections. The Society for Hospital Epidemiology of America; The Association for Practitioners in Infection Control; The Centers for Disease Control; The Surgical Infection Society. Infect Control Hosp Epidemiol. 1992;13:599–605. [PubMed] [Google Scholar]
- 40.Pappas DA, Giles JT. Do antitumor necrosis factor agents increase the risk of postoperative orthopedic infections? Curr Opin Rheumatol. 2008;20:450–456. doi: 10.1097/BOR.0b013e3282fcc345. [DOI] [PubMed] [Google Scholar]
- 41.Singh JA, Furst DE, Bharat A, Curtis JR, Kavanaugh AF, Kremer JM, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res. 2012;64:625–639. doi: 10.1002/acr.21641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim SY, Servi A, Polinski JM, Mogun H, Weinblatt ME, Katz JN, et al. Validation of rheumatoid arthritis diagnoses in health care utilization data. Arthritis Res Ther. 2011;13:R32. doi: 10.1186/ar3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carroll RJ, Thompson WK, Eyler AE, Mandelin AM, Cai T, Zink RM, et al. Portability of an algorithm to identify rheumatoid arthritis in electronic health records. J Am Med Inform Assoc. 2012;19:e162–e169. doi: 10.1136/amiajnl-2011-000583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liao KP, Cai T, Gainer V, Goryachev S, Zeng-Treitler Q, Raychaudhuri S, et al. Electronic medical records for discovery research in rheumatoid arthritis. Arthritis Care Res. 2010;62:1120–1127. doi: 10.1002/acr.20184. [DOI] [PMC free article] [PubMed] [Google Scholar]