Abstract
Despite multiple clinical trials utilizing a spectrum of therapeutic modalities, melanoma remains a disease with dismal outcomes in patients with advanced disease. However, it is now clear that melanoma is not a single entity, but can be molecularly divided into subtypes that generally correspond to the anatomical location of the primary melanoma. Melanomas from acral lentiginous, mucosal, and chronic sun-damaged sites frequently harbor activating mutations and/or increased copy number in the KIT tyrosine kinase receptor gene, which are very rare in the more common cutaneous tumors. Multiple case reports and early observations from clinical trials suggest that targeting mutant KIT with tyrosine kinase inhibitors is efficacious in KIT mutant melanoma. This review recounts what is known about the role of KIT in melanocyte maturation, our current understanding of KIT genetic aberrations in melanoma, and how this knowledge is being translated into clinical oncology.
Keywords: KIT, melanoma, acral lentiginous, mucosal
1. The KIT Receptor Tyrosine Kinase
The KIT receptor tyrosine kinase gene (c-kit) was first identified in 1987 based on sequence similarity to the acute transforming Hardy-Zuckerman 4 feline sarcoma virus (v-kit) [1, 2]. KIT (a.k.a., CD117) is a type III receptor tyrosine kinase characterized by a glycosylated extracellular ligand binding domain containing five immunoglobulin-like repeats, a single hydrophobic transmembrane domain, and an intracellular segment containing a juxtamembrane inhibitory domain, and two tyrosine kinase domains separated by a kinase insert region (Figure 1)[3]. Alternative splicing of KIT can result in the loss of a GNNK amino acid sequence at the 5’ end of the extracellular domain and/or the loss of a serine amino acid residue in the kinase region of the intracellular domain[4, 5]. Stem Cell Factor (SCF, a.k.a., kit ligand, steel factor, or mast cell growth factor), the ligand for KIT, is also a glycosylated transmembrane protein. Alternative splicing results in the presence or absence of a proteolytic cleavage site within the SCF protein[6]. SCF that harbors the cleavage site is released as the soluble form whereas SCF without the cleavage site remains on the cell surface. Either form of SCF is capable of binding to KIT resulting in receptor dimerization, autophosporylation, and activation of the intracellular tyrosine kinase domain, although the ultimate signaling effects generated by the soluble versus membrane-bound SCF differ. Binding of the soluble form of SCF causes KIT activation, internalization, and degradation, whereas binding of the membrane-bound form of SCF results in prolonged KIT activation[7]. Activated KIT has been shown to initiate multiple downstream signaling pathways (e.g., MAPK/MEK, PI3K/AKT, JAK/STAT) that can vary depending on the cellular context in which KIT is activated[3, 8-10].
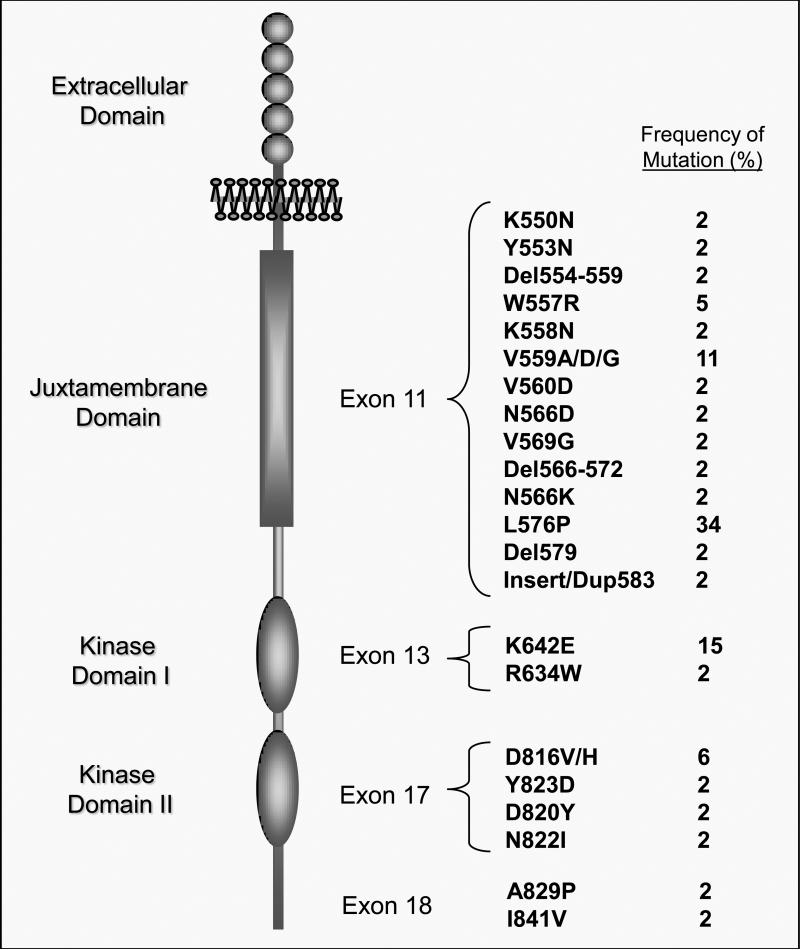
Figure 1.
Schematic Representation of the KIT Tyrosine Kinase Receptor and Mutation Frequency. Five immunoglobulin-like regions (circles) are located in the extracellular domain and serve as the binding site for the KIT ligand, stem cell factor. The juxtamembrane autoinhibitory domain (vertical rectangle) serves to maintain the kinase domains (vertical ovals) in an inhibited state unless the receptor is bound by ligand. KIT mutations occur with highest frequency (~ 70%) in exon 11 of the juxtamembrane domain. Mutations also occur in the kinase domain I, exon 13 (~ 17 %), and kinase domain II, exon 17 (~ 15 %).
The importance of both KIT and SCF for proper melanogenesis, proliferation, migration and survival has been clearly demonstrated by the phenotypes of mice and humans that harbor genetic alterations in these genes. Loss of function mutations in mouse KIT or SCF result in a white color spotting of fur, and loss of function mutations in KIT in humans results in lack of the pigmentation of skin/hair (viz, piebaldism)[11-13]. The pattern of these phenotypes indicates that developing melanocytes with loss of function mutations cannot migrate to distant sites from the neural crest. In addition, other traits associated with proper KIT activity (e.g., gametogenesis, hematopoeisis, mast cell and interstitial cells of Cajal function) are affected by loss of function KIT mutations[10, 14-16]. Despite its essential role in melanogenesis, KIT was not thought to have a major role in promoting melanomas, as KIT protein expression is frequently lost during local melanoma growth/invasion, and overexpression of KIT in metastatic melanoma cells resulted in reduced tumor growth[17, 18].
2. The Identification of KIT Mutations and Amplifications in Melanoma
The first report of a KIT mutation in melanoma came from a 2004 publication by Went et al., who used tissue microarray to screen different tumor types for KIT protein expression level followed by mutation analysis on a small subset of tumors with high KIT protein expression[19]. Fourteen of 39 (36%) primary malignant melanomas showed KIT expression by immunohistochemistry (IHC). Extrapolating from the location of KIT mutations in other tumors, exons 2, 8, 9, 11, 13, and 17 were sequenced (Table 1). Of the two melanoma tumors selected for sequencing analysis, one had a point mutation in KIT. In 2005, Willmore-Payne et al., reported screening 100 melanomas (84 metastatic, 12 primary cutaneous, and 4 in situ, no primary mucosal) for KIT protein expression. Twenty-nine samples (29%) showed KIT expression by IHC, two of which harbored mutations in KIT as analyzed by high resolution amplicon melting[20]. Both of these samples were metastatic samples with high KIT expression and did not have BRAF mutations. In a follow-up paper in 2006 WIllmore-Payne et al. screened an additional 53 cases of melanoma finding KIT protein expression in 6 cases (11%)[21]. Mutation analysis demonstrated one of these samples to harbor a KIT mutation. Flourescent in situ hybridization (FISH) indicated a slight increase in KIT/CEP4 ratio in one of three of the tumors with a KIT mutation.
Table 1.
Frequency of KIT Mutations in Subtypes of Melanoma ¤
| Melanoma Subtype |
||||
|---|---|---|---|---|
| Exons Tested | Not Characterized | Acral | Mucosal | |
| Went 2004+¶ | 2,8,9,11,13,17 | 1/2 (50%) | -- | -- |
| Willmore-Payne 2004+¶ | 9,11,13,17 | 2/29 (7%) | -- | -- |
| Willmore-Payne 2006+¶ | 11 | 1/6 (17%) | -- | -- |
| Curtin 2006¥ | 11, 13, 17, 18 | -- | 3/28 (11%) | 8/38 (21%) |
| Antonescu 2007¥ | 11, 13, 17 | -- | -- | 3/20 (15%) |
| Rivera 2007 | 11, 13 | -- | -- | 4/15 (27%) |
| Beading 2008+# | 11, 13, 17 | -- | 3/13 (23%) | 7/45 (16%) |
| Satzger 2008 | 9, 11, 13, 17, 18 | -- | -- | 6/32 (19%) |
| Ashida 2008 | 11, 13, 17, 18 | -- | 2/16 (13%) | 0/3 (0%) |
| Torres-Cabala 2009 | 11, 13, 17 | -- | 5/39 (13%) | 9/52 (17%) |
| 13/96 | 37/205 | |||
| 14% | 18% | |||
Unless otherwise noted, only samples in which the KIT gene mutation status was documented were included in this assessment.
A distinction between chronic sun-damaged and non-chronic sun-damaged melanoma was not reported.
Only samples with high KIT protein expression were analyzed for KIT mutation.
§KIT exons 9, 13, and 17 were also tested in the one KIT exon 11 containing tumor.
Not all samples were available or evaluable for assessment
KIT Exon 9 was screened in 6 acral and 27 mucosal, and exon 8 was screened in 3 acral and 25 mucosal samples
In 2005, the group of Dr. Boris Bastian published a seminal paper describing DNA copy number changes in melanomas arising from different anatomic sites[22]. The study included a total of 126 melanomas: 30 from skin with chronic sun-damage, 40 from skin without chronic sun-damage, 36 from acral sites, and 20 from mucosal sites. Comparative genome hybridization (CGH) analysis demonstrated that while certain genetic alterations were shared, there were many marked differences between the tumors originating from these four sites.
In particular, the non-cutaneous tumors had a much higher frequency of amplification events than the cutaneous melanomas, and they involved distinct areas. In addition, analysis of the prevalence of mutations in BRAF and NRAS, the most common activating somatic mutations in melanoma, also showed marked differences between the groups. Mutations in BRAF (59%) and NRAS (22%) were very common in tumors arising from skin without chronic sun-damage. BRAF and NRAS mutations were much less common in melanomas with chronic sun damage (11% and 15%), acral melanomas (23% and 10%), and mucosal melanomas (11% and 5%). Subsequent to these findings, Bastian’s group performed an in-depth analysis of chromosomal region 4q12, which had evidence of frequent copy number gain in the mucosal, acral, and chronic sun-damaged melanomas, but not in the non-sun-damaged cutaneous tumors. In addition to this interesting distribution, the reqion was also of interest as it harbored several genes that could be utilized as therapeutic targets. Immunohistochemical and mutational analysis of the tumors with copy gain in this region found both a correlation with stong expression of the KIT protein, and point mutations in the KIT gene. This prompted dedicated analysis of the KIT gene in the full set of tumors, and identified KIT point mutations in 21% of the mucosal, 11% of the acral, 17% of chronic sun-damaged cutaneous, and 0% of non-chronic sun-damaged melanomas[23]. This was the first study to show that KIT genetic aberrations are enriched in specific subtypes of melanoma and are mostly mutually exclusive with BRAF or NRAS mutations within melanoma tumors.
The observations of Bastian significantly enriched the understanding of different types of melanoma. Traditionally, melanomas have been categorized by anatomical location and histological features -- melanomas localized to the skin being designated as superficial spreading, nodular, acral lentiginous, and lentigo maligna, and non-cutaneous melanomas being comprised of uveal and mucosal subtypes. These categorizations are still of importance for clinical-pathological purposes, as there are important differences among these subtypes (Table 2).
Table 2.
Anatomical and Histological Melanoma Categorization
| Cutaneous |
Mucosal |
Uveal |
|||
|---|---|---|---|---|---|
| Nodular/Superficial Spreading |
Lentigo Maligna |
Acral Lentiginous |
|||
| Anatomic Location | any skin site | head/neck | palms soles subungual | sinus, nasal, oral, anal, vulvar, vaginal, urethral mucosa | iris ciliary body choroid |
| Incidence | increasing | increasing | stable | stable | stable |
| Ethnic Frequency | W > AA = As | W > AAA = As | W = AAA = As | W = AAA = As | W > As > AAA |
| Clinical Features | typically diagnosed at earlier stage (superficial spreading) or later stage (nodular) | typically diagnosed at early stage low metastatic potential | typically diagnosed at later stage | typically diagnosed at later stage | typically diagnosed in the intraocular only stage preferentially metastasize to liver |
AA = African-American, As = Asian, W = White
The KIT mutations identified in these subtypes of melanoma differ from those found in gastrointestinal stromal tumors (GIST). Somatic mutations in KIT have been identified in approximately 80% of GISTs. The majority of these mutations are deletions or insertions in the gene,[9] whereas all the melanoma KIT mutations in this study where substitution mutations. Another significant difference in melanoma was that mutations in KIT were not present in exon 9, which is the location of about 15% of KIT mutations in GIST. In addition, the Bastian study identified KIT gene amplification in 7% of acral lentiginous, 8% of mucosal, and 6% of chronic sun-damaged melanomas (Table 3). A substantial number of acral lentiginous and mucosal melanomas also had a KIT copy number that was increased, but did not meet the definition of amplification. In contrast, KIT amplification is rarely observed in GIST tumors. In reviewing all reports to date, about 30% of melanoma samples with KIT mutations also show increased copy number/amplification of KIT [21, 24-26].
Table 3.
Frequency of Increased Copy Number/Amplification¤
| Melanoma Subtype |
|||
|---|---|---|---|
| Uncharacterized | Acral | Mucosal | |
| Willmore-Payne 2006* | 1/3 (33% ) | -- | -- |
| Curtin 2006 | -- | 7/28 (25%) | 10/38 (26%) |
| Antonescu 2007¶ | -- | -- | 1/5 (20%) |
| Beading 2008 | -- | 3/11 (27%) | 10/38 (26%) |
| Ashida 2008 | -- | 3/16 (19%) | 1/3 (33%) |
| 13/55 | 22/84 | ||
| 24% | 26% | ||
Different techniques were used to determine copy number increase, and if copy number could not be determined in a sample, it was not included in the assessment.
Only melanoma cells with a KIT mutation were screened for increased copy number copy number was not available for all samples
A subset of both KIT mutant and non-mutant samples were tested for copy number increase
Multiple groups have now reported the KIT genetic aberration status in additional cohorts of melanoma tumor samples. Antonescu et al., examined 20 primary anal mucosal melanoma samples and discovered a 15% frequency of KIT point mutations, with 1 of 3 KIT mutant samples also showing increased copy number[24]. Of 15 evaluable primary oral mucosal melanoma tumors examined by Rivera et al, 27% had KIT point mutations[27]. KIT copy number and exon 17 mutation status was not determined in this study. Although the total number of patients assessed to date is modest, the frequency of KIT mutation genitourinaryanorectal melanoma is nearly twice that of head and neck mucosal melanoma (Table 4). In studies that separated the genitourinary from anorectal mucosal melanomas, KIT mutations were more common in the former. Whether these differences in the frequency of KIT mutation in different subtypes of mucosal melanoma will be maintained awaits assessment of greater sample sizes.
Table 4.
KIT Mutation Frequency in Mucosal Melanoma
| Mucosal Melanoma Subtype |
Genitourinary vs. Anorectal |
|||
|---|---|---|---|---|
| Head and Neck | Genitourinary Anorectal | Genitourinary | Anorectal | |
| Antonescu 2007 | -- | 3/20 (15%) | -- | 3/20 (15%) |
| Rivera 2007 | 4/15 (27%) | -- | -- | -- |
| Beading 2008 # | 3/36 (8%) | 4/9 (44%) | -- | -- |
| Satzger 2008 | 2/16 (13%) | 4/16 (25%) | 3/8 (38%) | 1/8 (13%) |
| Torres-Cabala 2009 | 1/14 (7%) | 7/47 (15%) | 4/18 (22%) | 3/29 (10%) |
| 10/81 (12%) | 18/92 (20%) | 7/26 (27%) | 7/57 (12%) | |
Genitourinary and anorectal melanomas were grouped together as mucosal type
Beading et al., screened a large number of melanoma subtypes from mostly primary tumors, including conjunctival and choroidal melanomas, for KIT mutations and amplification[26]. They found one of thirteen conjunctival melanomas to harbor a KIT mutation, but none of the sixty choroidal melanomas tested had a KIT mutation. The study showed 23% of acral lentiginous, 15.6% of mucosal, and 2% of cutaneous (sun-damaged status not provided) melanomas to harbor KIT mutations. This was the first study to report non-point KIT mutations in melanoma. One acral melanoma sample had an in-frame deletion mutation and one rectal mucosal melanoma sample was shown to have an insertion/duplication; both of these gene alterations were in exon 11 of KIT. Quantitative PCR was used to assess KIT copy number in these samples, and showed 27.3% of acral lentiginous, 26.3% of mucosal, and 6.7% cutaneous melanoma samples to have an increased KIT copy number compared to a GAPDH control.
Satzger et al., examined 37 mucosal melanoma samples for which DNA was available for KIT mutations, and found 11% of head and neck, 30% of genital tract, 12.5% of anal/rectal mucosal melanomas to harbor mutations in KIT[28]. Of 26 evaluable acral lentiginous melanoma samples screened, Ashida et al. found two with KIT point mutations, one of which also showed an increase in copy number as determined by quantitative PCR. There were no KIT mutations found in the three mucosal samples they evaluated. Consistent with other studies, BRAF mutations were in low abundance in acral melanomas and mutually exclusive with KIT mutant containing samples.
In a recent report from our group at the M. D. Anderson Cancer Center, Torres-Cabala et., showed 12% of acral lentiginous and 17% of mucosal melanoma samples to have mutations in KIT [29]. Two of these mutations were insertions in exon 11 and one primary vulva sample demonstrated KIT point mutations in both exons 13 and 17. In three cases, both primary and metastatic samples from the same patient were available for mutation analysis and the same KIT mutation was demonstrated in both samples.
3. Melanoma KIT Mutant Cell in vitro Experiments
To date, there are only two reports of cultured cells being generated from patients with either acral lentiginous or mucosal melanoma. Jiang, et al., recently analyzed three low passage primary mucosal cell cultures. One of the three cell cultures demonstrated a highly amplified KIT (exon 11 V559D) mutation without evidence of a wild-type allele by sequencing[30]. The other two cell cultures had wild-type KIT without significant changes in copy number. The mutant/amplified KIT cells showed marked KIT phosphorylation at baseline, consistent with constitutive kinase activity, whereas the wild-type/non-amplified KIT cells did not demonstrate baseline KIT phosphorylation. Imatinib (a.k.a., Gleevec, Novartis Pharma AG) treatment of the mutant/amplified KIT cells resulted in G1 cell cycle arrest, induction of apoptosis and a significant reduction in cell proliferation at nanomolar concentrations. The activity of multiple downstream mediators (p42/44, AKT, MTOR, STAT1, STAT3, P70S6K, and S6K) of KIT was markedly reduced after imatinib treatment. Wold-type/non-amplified KIT cells failed to show any of these changes after imatinib treatment.
Ashida et al, recently published the analysis of six acral lentiginous cell lines. One of these cell lines was shown to harbor a non-amplified KIT (exon 17 D820Y) mutation[25]. The remaining acral lentiginous cell lines had wild-type/non-amplified KIT genes with varying degrees of protein expression. The KIT D820Y cell line was the only one to demonstrate KIT phosphorylation in the absence of the KIT ligand, consistent with constitutive activity. KIT D820Y is an imatinib resistant mutation, usually arising as a secondary mutation in the setting of imatinib therapy. Treatment of the KIT D820Y cell line with sunitinib (a.k.a., Sutent, Pfizer), which has greater binding affinity for KIT exon 17 mutations, resulted in a modest reduction in cell proliferation, not seen in KIT wild-type acral cell lines when treated with 1 uM of sunitinib.
4. The Biology of KIT Mutations in Melanoma
Although there are an abundance of published reports on the biology of GIST, mastocytosis and leukemia cells with different KIT mutations, little is known about the behavior of genetically altered KIT in melanoma cells.
Alexeev et al., genetically engineered immortalized mouse melanocytes to express an endogenous, constitutively active KIT D814Y[31]. These cells migrated at a far greater rate in in vitro dual chamber experiments, and when injected into the hypodermis migrated through the dermis to the epidermis. Cells that did not have the KIT D814Y mutation did not demonstrate the same migratory capacity. Curiously, KIT D814Y melanoma cells had reduced cell cycling times and appeared to be less differentiated. Thus the same KIT mutation that drives proliferation in mastocytosis cells, exhibits a migratory phenotype when expressed in melanocytes.
A recent report by Monsel et al., showed that transfection of immortalized mouse melanocytes with KIT mutants failed to result in transformation of these cells[32]. However, when KIT mutants were expressed in the setting of hypoxia or co-expressed with HIF-1α, the melanocytes were transformed, indicated by colony formation in soft agar. Hypoxia resulted in the marked activation of the MAPK pathway in cells expressing mutant KIT, but not in cells expressing wild-type KIT. Imatinib markedly decreased the MAPK phosphorylation and cell proliferation in cells stably transfected with HIF-1α and KIT K642E or KIT 576del mutants, but not in cells transfected with HIF-1α and wild-type KIT. Also, of note, neither mutant BRAF nor NRAS required hypoxic conditions in order to transform cells and there ability to do so was not enhanced by hypoxia, suggesting very different mechanism of cellular transformation between these known melanoma gene mutations.
Bougherara et al., used green fluorescent protein KIT mutant chimeras to track the cellular localization of mutant KIT in CHO cells[33]. KIT mutant proteins were phosphorylated, but exhibited an immature glycosylation pattern (high mannose type) and were retained intracellularly. Imatinib treatment of cells expressing KIT V560G resulted in the loss of mutant KIT phosphorylation, conversion from the high mannose type to the mature complex glycosylated form and redistribution to the cell membrane.
These studies show that the phenotypic expression of KIT mutations may vary under particular cellular and microenvironmental conditions. Although the ongoing clinical trials will provide the response of KIT mutant tumors to TKI treatment, they will not necessarily provide the explanation for that response. As in GIST, cell line generation has been difficult. There is a need to create in vitro or ex vivo systems that will provide workable models for research. Engineering mouse models of mutant KIT melanoma will also likely be important to advance our understanding of the biology of these tumors and their mechanisms of resistance to treatment.
5. Therapeutic Interventions into KIT Mutant Melanoma
Up to this point, patients with metastatic acral lentiginous and mucosal melanoma have generally been treated with the same regimens used to treat patients with superficial spreading cutaneous melanoma, including high dose bolus interleukin-2 (HD IL-2), chemotherapy, and biochemotherapy[34]. In contrast, small molecule tyrosine kinase inhibitors (TKIs) are the standard of care for GIST, where they produce clinical benefit in the overwhelming majority of patients. Three phase II clinical trials examining the efficacy of imatinib in melanoma were performed in the early 2000s, prior to the identification of KIT mutations in subsets of this disease[35-37]. All three trials failed to show significant responsiveness of metastatic melanomas to imatinib treatment. Of 63 patients treated in these studies, only one was reported to have a clinical response. The responding patient had metastatic acral lentiginous melanoma whose tumor had very high KIT protein expression, but did not demonstrate a KIT mutation in exons 9, 11, 13, 15, or 17. KIT copy number status was not determined. As all these studies enrolled patients without regard to melanoma type, they were highly enriched for patients with the most prevalent form of melanoma, non-chronic sun-damaged, which does not harbor KIT mutations. Even if acral, mucosal, or chronic sun-damaged melanoma patients were enrolled, we now know that only about 15% of these patients would have had KIT mutations. If a KIT genetic aberration is necessary for imatinib response in melanoma, then it is likely that the significant lack of response among the patients treated in these studies is due to their lack of having tumors with KIT genetic aberrations.
As these early clinical studies with imatinib were coming to completion, Curtin et al, published the identification of KIT genetic aberrations (mutations +/− increased copy number) in acral, mucosal, or CSD melanomas. This observation prompted researchers to examine the tumors of patients with these subtypes and in some cases to treat patients with TKIs that target KIT. Lutzky et al., chronicled the dramatic clinical course of a patient with anal mucosal melanoma with positive inguinal lymph nodes bilaterally[38]. The patient's melanoma tumor was demonstrated to harbor an amplified KIT K642E mutation. The patient underwent wide local excision of the tumor and bilateral lymph node dissection, followed by adjuvant radiotherapy. Shortly after adjuvant treatment, the patient developed multiple subcutaneous nodules in the anogenital/inguinal areas. After four weeks of imatinib treatment there was a complete resolution of the subcutaneous melanoma metastasis. A recurrence of subcutaneous nodules emerged about six months later following a dose reduction of imatinib due to neutropenic fever. A complete clinical response was again achieved after the imatinib dose was increased and endured for 8 months and was present at the time of the article's publication. At about the same time, our group at M. D. Anderson Cancer Center reported a patient with KIT V560D anal melanoma with isolated lung metastases who had a complete response to a temozolomide/sorafenib (Nexavar, Bayer) regimen[39].
Cases have also been reported in which dramatic responses were achieved in patients with more extensive disease. Hodi, et al. reported a significant clinical response in a patient with a KIT PYDHKWE duplication rectal melanoma that had metastasized to multiple sites[40]. The size and FDG-avidity of pulmonary, epicardial, suprarenal and pelvic metastases were markedly reduced after only 4 weeks of imatinib treatment. Woodman et al, also reported a dramatic reduction in metabolic activity in a KIT L576P vaginal mucosal melanoma that had extensive metastases in the pelvis and inguinal lymph nodes when treated with dasatinib (a.k.a., Sprycel, Brisyol-Myers Squibb) treatment[41]. These case reports suggest that small molecule KIT inhibition has efficacy when used in melanoma patients with KIT mutations.
There are currently multiple ongoing clinical trials prospectively testing TKIs that target KIT in patients with acral lentiginous, mucosal or chronic sun-damaged skin melanoma. Carvajal, et al., reported interim results at the American Society of Clinical Oncology in October 2009 (abstract ID 9001) from a multi-institutional phase II study of imatinib in stage III or IV patients with somatic alterations in KIT[42]. Of the 12 evaluable patients presented, 2 demonstrated a complete response and 2 showed a partial response. All but two of the remaining patients had stable disease on imatinib. Of note, the two patients who achieved a complete response were the only patients to have both amplification and mutation of KIT, whereas the two patients whose disease progressed despite imatinib treatment had KIT mutations known to be resistant to imatinib in gastrointestinal stromal tumor.
Hodi, et al. presented an update on a multi-institutional phase II clinical trial of imatinib in melanoma patients with mucosal, acral/lentiginous or chronically sun damaged skin at the International Melanoma Congress in November 1-4, 2009[43]. Of 20 evaluable patients presented, 0 of 10 patients who had wild-type/amplified KIT showed a clinical response, although two of these patients had stable disease for 6-7 months. No complete responses were achieved, but five of 10 patients with KIT mutations demonstrated a partial response to imatinib treatment, three of whom also had amplified KIT.
The oncology community awaits the final results of these and other trials to better understand the efficacy of TKIs in melanoma subtypes with KIT genetic aberrations.
6. Conclusion
Recent basic and clinical research has generated great excitement in the melanoma research community. The discovery of oncogenes within subtypes of melanoma has provided promising targets for therapy. Particularly, the identification of KIT genetic aberrations in acral lentiginous, mucosal, and CSD melanoma tumors has allowed for trials to be enriched with patients that have a KIT mutation and/or amplification. The availability of FDA-approved TKIs that inhibit KIT has accelerated the pace with which the prospective trials could be performed. After multiple prior negative clinical trials in unselected melanoma patients, the recent case reports and early results from clinical trials suggest that the currently FDA-approved KIT inhibitors have activity in “KIT-driven” melanoma. While these early observations are very encouraging, definitive answers to many key questions await the maturation of the clinical trials and analysis of additional tumors.
The efficacy of treating KIT mutant tumors is best described in GIST, where at least 80% of the tumors harbor a KIT mutation. As most of the KIT-mutant tumors respond to KIT inhibition in GIST, it is easy to make the inference that KIT inhibition will be equally efficacious in KIT mutant melanoma. However, KIT mutations in melanoma differ from those in GIST in several respects. First, KIT mutations in melanoma are almost exclusively point mutations, whereas KIT mutations in GIST are predominantly deletion or insertion mutations. Although more data needs to accrue, the first reports testing TKIs on primary human melanoma cells with a KIT V559D and D820Y mutations in vitro show these cells to respond similarly to GIST tumors with these mutations. Second, exon 9 KIT mutations account for 15% of KIT mutations in GIST, but are very rare in melanoma. In contrast, mutations in imatinib resistant residues in exons 13, 17 and 18 account for up to 15% of KIT mutations in melanoma versus less than 1% in GIST. This difference may affect the ultimate percentage of KIT mutant melanomas that respond to TKI therapy, as to date the TKIs in clinical use do not inhibit these mutations. Third, approximately 30% of mutant KIT and wild-type KIT genes in melanoma show increased copy number/amplification, which is a very rare event in GIST. The early clinical trial data suggests that tumors with amplified wild-type KIT are not very sensitive to imatinib treatment. Intriguingly, the only three patients with complete responses with imatinib therapy reported to date had a KIT mutation that was amplified. It is tempting to speculate that tumors that have both a mutant and amplified KIT are exquisitely oncogene addicted and may exhibit the best responses to KIT inhibition. However, definitive conclusions will require the accrual of additional patients. Finally, although secondary KIT mutations are a clear and common mechanism of treatment resistance in GIST, and by inference are likely to occur in melanoma, they have not yet been reported in melanoma. It will be very interesting to evaluate pre- and post-treatment tumor specimens to evaluate the mechanisms of resistance in these patients to see if they are akin to those observed in GIST. How the differences in KIT genetic aberration in melanoma versus GIST will ultimately unfold clinically is an empirical question for which the melanoma research community anxiously awaits the answer.
Apart from the differences in the KIT gene itself, there may also be significant differences in the cellular milieus of melanoma tumors that alter the behavior of KIT mutant melanoma cells and their response to KIT inhibition. Multiple studies have shown that KIT mutant proteins have different signaling pathways depending on the cellular context. The report by Alexeev et al., showing a KIT mutation to generate a motogenic phenotype in melanoma versus a mitogenic phenotype in mastocytosis cells suggests that the cellular environment in which the KIT mutation occurs can markedly effects its function. Comparing genomic and proteomic profiles between KIT mediated tumor types may provide for a better understanding of common and disparate signaling networks in different KIT mutant tumor types and the mechanisms by which cells are either sensitive or resistant to KIT inhibition.
KIT mutant tumor progression on imatinib treatment has already been reported and may be inevitable in most patients. To date, studies that have been enriched for KIT mutant melanoma patients are testing TKIs directed against the kinase domain of the molecule. Currently, the TKIs that are FDA-approved target multiple kinases, including KIT, and are approved for Philadelphia positive CML, KIT mutant positive GIST and some types of renal cancers. No TKI is yet FDA-approved for KIT-mutant melanoma patients. It is will be interesting to see if the TKIs that are being tested in KIT-mutant melanomas – imatinib, dasatinib, sunitinib, and nilotinib (a.k.a., Tasigna, Novartis) – will have significant differences in efficacy in this disease, or if the activity of the agents will mostly reflect the domain in which the KIT mutation occurs (like in GIST). Other possible therapies should be explored as well, including KIT antibody directed treatment, KIT RNA interference strategies, and combinatorial therapies which couple targeted therapies to KIT with chemo and/or immuno-therapies.
It is an exciting time to be a clinician and researcher in melanoma. The era of being able to molecularly categorize melanoma patients and align them with therapies that more directly treat the underlying mechanism of their tumor pathology is upon us. Hopefully such personalized approaches will lead to rational and more effective treatments that improve outcomes in this challenging disease.
Acknowledgments
Grant Support: NIH K12 CA088084 (S.E.W), Laura and John Arnold Clinical Research Fellowship (S.E.W); University of Texas M. D. Anderson Cancer Center Melanoma Specialized Programs of Research Excellence and the Melanoma Informatics, Tissue Resource, and Pathology Core grant P50 CA93459 (M.A.D); MDACC Melanoma Spore Development Grant (M.A.D); Carol Cogdell Courtney Fellowship (S.E.W and M.A.D); American Society of Clinical Oncology Young Investigator Award (S.E.W and M.A.D); M.A. Davies, commercial research grant, AstraZeneca.
Abbreviations
- KIT
c-kit gene
- KIT
c-kit protein
- SCF
stem cell factor
- CSD
chronic sun-damaged melanoma
- NCSD
non-chronic sun-damaged melanoma
- IHC
immunohistochemistry
- FISH
fluorescent in situ hybridization
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Besmer P, Murphy JE, George PC, Qiu FH, Bergold PJ, Lederman L, et al. A new acute transforming feline retrovirus and relationship of its oncogene v-kit with the protein kinase gene family. Nature. 1986;320:415–21. doi: 10.1038/320415a0. [DOI] [PubMed] [Google Scholar]
- 2.Yarden Y, Kuang WJ, Yang-Feng T, Coussens L, Munemitsu S, Dull TJ, et al. Human proto-oncogene c-kit: a new cell surface receptor tyrosine kinase for an unidentified ligand. EMBO J. 1987;6:3341–51. doi: 10.1002/j.1460-2075.1987.tb02655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ronnstrand L. Signal transduction via the stem cell factor receptor/c-Kit. Cell Mol Life Sci. 2004;61:2535–48. doi: 10.1007/s00018-004-4189-6. [DOI] [PubMed] [Google Scholar]
- 4.Reith AD, Ellis C, Lyman SD, Anderson DM, Williams DE, Bernstein A, et al. Signal transduction by normal isoforms and W mutant variants of the Kit receptor tyrosine kinase. EMBO J. 1991;10:2451–9. doi: 10.1002/j.1460-2075.1991.tb07784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crosier PS, Ricciardi ST, Hall LR, Vitas MR, Clark SC, Crosier KE. Expression of isoforms of the human receptor tyrosine kinase c-kit in leukemic cell lines and acute myeloid leukemia. Blood. 1993;82:1151–8. [PubMed] [Google Scholar]
- 6.Huang EJ, Nocka KH, Buck J, Besmer P. Differential expression and processing of two cell associated forms of the kit-ligand: KL-1 and KL-2. Mol Biol Cell. 1992;3:349–62. doi: 10.1091/mbc.3.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyazawa K, Williams DA, Gotoh A, Nishimaki J, Broxmeyer HE, Toyama K. Membrane-bound Steel factor induces more persistent tyrosine kinase activation and longer life span of c-kit gene-encoded protein than its soluble form. Blood. 1995;85:641–9. [PubMed] [Google Scholar]
- 8.Lennartsson J, Jelacic T, Linnekin D, Shivakrupa R. Normal and oncogenic forms of the receptor tyrosine kinase kit. Stem Cells. 2005;23:16–43. doi: 10.1634/stemcells.2004-0117. [DOI] [PubMed] [Google Scholar]
- 9.Corless CL, Heinrich MC. Molecular pathobiology of gastrointestinal stromal sarcomas. Annu Rev Pathol. 2008;3:557–86. doi: 10.1146/annurev.pathmechdis.3.121806.151538. [DOI] [PubMed] [Google Scholar]
- 10.Orfao A, Garcia-Montero AC, Sanchez L, Escribano L. Recent advances in the understanding of mastocytosis: the role of KIT mutations. Br J Haematol. 2007;138:12–30. doi: 10.1111/j.1365-2141.2007.06619.x. [DOI] [PubMed] [Google Scholar]
- 11.Chabot B, Stephenson DA, Chapman VM, Besmer P, Bernstein A. The proto oncogene c-kit encoding a transmembrane tyrosine kinase receptor maps to the mouse W locus. Nature. 1988;335:88–9. doi: 10.1038/335088a0. [DOI] [PubMed] [Google Scholar]
- 12.Spritz RA, Giebel LB, Holmes SA. Dominant negative and loss of function mutations of the c-kit (mast/stem cell growth factor receptor) proto-oncogene in human piebaldism. Am J Hum Genet. 1992;50:261–9. [PMC free article] [PubMed] [Google Scholar]
- 13.Geissler EN, Ryan MA, Housman DE. The dominant-white spotting (W) locus of the mouse encodes the c-kit proto-oncogene. Cell. 1988;55:185–92. doi: 10.1016/0092-8674(88)90020-7. [DOI] [PubMed] [Google Scholar]
- 14.Rossi P, Sette C, Dolci S, Geremia R. Role of c-kit in mammalian spermatogenesis. J Endocrinol Invest. 2000;23:609–15. doi: 10.1007/BF03343784. [DOI] [PubMed] [Google Scholar]
- 15.Sanders KM, Ward SM. Kit mutants and gastrointestinal physiology. J Physiol. 2007;578:33–42. doi: 10.1113/jphysiol.2006.122473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleischman RA. From white spots to stem cells: the role of the Kit receptor in mammalian development. Trends Genet. 1993;9:285–90. doi: 10.1016/0168-9525(93)90015-a. [DOI] [PubMed] [Google Scholar]
- 17.Huang S, Luca M, Gutman M, McConkey DJ, Langley KE, Lyman SD, et al. Enforced c-KIT expression renders highly metastatic human melanoma cells susceptible to stem cell factor-induced apoptosis and inhibits their tumorigenic and metastatic potential. Oncogene. 1996;13:2339–47. [PubMed] [Google Scholar]
- 18.Lassam N, Bickford S. Loss of c-kit expression in cultured melanoma cells. Oncogene. 1992;7:51–6. [PubMed] [Google Scholar]
- 19.Went PT, Dirnhofer S, Bundi M, Mirlacher M, Schraml P, Mangialaio S, et al. Prevalence of KIT expression in human tumors. J Clin Oncol. 2004;22:4514–22. doi: 10.1200/JCO.2004.10.125. [DOI] [PubMed] [Google Scholar]
- 20.Willmore-Payne C, Holden JA, Tripp S, Layfield LJ. Human malignant melanoma: detection of BRAF- and c-kit-activating mutations by high-resolution amplicon melting analysis. Hum Pathol. 2005;36:486–93. doi: 10.1016/j.humpath.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 21.Willmore-Payne C, Holden JA, Hirschowitz S, Layfield LJ. BRAF and c-kit gene copy number in mutation-positive malignant melanoma. Hum Pathol. 2006;37:520–7. doi: 10.1016/j.humpath.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135–47. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- 23.Curtin JA, Busam K, Pinkel D, Bastian BC. Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol. 2006;24:4340–6. doi: 10.1200/JCO.2006.06.2984. [DOI] [PubMed] [Google Scholar]
- 24.Antonescu CR, Busam KJ, Francone TD, Wong GC, Guo T, Agaram NP, et al. L576P KIT mutation in anal melanomas correlates with KIT protein expression and is sensitive to specific kinase inhibition. Int J Cancer. 2007;121:257–64. doi: 10.1002/ijc.22681. [DOI] [PubMed] [Google Scholar]
- 25.Ashida A, Takata M, Murata H, Kido K, Saida T. Pathological activation of KIT in metastatic tumors of acral and mucosal melanomas. Int J Cancer. 2009;124:862–8. doi: 10.1002/ijc.24048. [DOI] [PubMed] [Google Scholar]
- 26.Beadling C, Jacobson-Dunlop E, Hodi FS, Le C, Warrick A, Patterson J, et al. KIT gene mutations and copy number in melanoma subtypes. Clin Cancer Res. 2008;14:6821–8. doi: 10.1158/1078-0432.CCR-08-0575. [DOI] [PubMed] [Google Scholar]
- 27.Rivera RS, Nagatsuka H, Gunduz M, Cengiz B, Gunduz E, Siar CH, et al. C-kit protein expression correlated with activating mutations in KIT gene in oral mucosal melanoma. Virchows Arch. 2008;452:27–32. doi: 10.1007/s00428-007-0524-2. [DOI] [PubMed] [Google Scholar]
- 28.Satzger I, Schaefer T, Kuettler U, Broecker V, Voelker B, Ostertag H, et al. Analysis of c-KIT expression and KIT gene mutation in human mucosal melanomas. Br J Cancer. 2008;99:2065–9. doi: 10.1038/sj.bjc.6604791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Torres-Cabala CA, Wang WL, Trent J, Yang D, Chen S, Galbincea J, et al. Correlation between KIT expression and KIT mutation in melanoma: a study of 173 cases with emphasis on the acral-lentiginous/mucosal type. Mod Pathol. 2009;22:1446–56. doi: 10.1038/modpathol.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang X, Zhou J, Yuen NK, Corless CL, Heinrich MC, Fletcher JA, et al. Imatinib targeting of KIT-mutant oncoprotein in melanoma. Clin Cancer Res. 2008;14:7726–32. doi: 10.1158/1078-0432.CCR-08-1144. [DOI] [PubMed] [Google Scholar]
- 31.Alexeev V, Yoon K. Distinctive role of the cKit receptor tyrosine kinase signaling in mammalian melanocytes. J Invest Dermatol. 2006;126:1102–10. doi: 10.1038/sj.jid.5700125. [DOI] [PubMed] [Google Scholar]
- 32.Monsel G, Ortonne N, Bagot M, Bensussan A, Dumaz N. c-Kit mutants require hypoxia-inducible factor 1alpha to transform melanocytes. Oncogene. 29:227–36. doi: 10.1038/onc.2009.320. [DOI] [PubMed] [Google Scholar]
- 33.Bougherara H, Subra F, Crepin R, Tauc P, Auclair C, Poul MA. The aberrant localization of oncogenic kit tyrosine kinase receptor mutants is reversed on specific inhibitory treatment. Mol Cancer Res. 2009;7:1525–33. doi: 10.1158/1541-7786.MCR-09-0138. [DOI] [PubMed] [Google Scholar]
- 34.Tsao H, Atkins MB, Sober AJ. Management of cutaneous melanoma. N Engl J Med. 2004;351:998–1012. doi: 10.1056/NEJMra041245. [DOI] [PubMed] [Google Scholar]
- 35.Kim KB, Eton O, Davis DW, Frazier ML, McConkey DJ, Diwan AH, et al. Phase II trial of imatinib mesylate in patients with metastatic melanoma. Br J Cancer. 2008;99:734–40. doi: 10.1038/sj.bjc.6604482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ugurel S, Hildenbrand R, Zimpfer A, La Rosee P, Paschka P, Sucker A, et al. Lack of clinical efficacy of imatinib in metastatic melanoma. Br J Cancer. 2005;92:1398–405. doi: 10.1038/sj.bjc.6602529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wyman K, Atkins MB, Prieto V, Eton O, McDermott DF, Hubbard F, et al. Multicenter Phase II trial of high-dose imatinib mesylate in metastatic melanoma: significant toxicity with no clinical efficacy. Cancer. 2006;106:2005–11. doi: 10.1002/cncr.21834. [DOI] [PubMed] [Google Scholar]
- 38.Lutzky J, Bauer J, Bastian BC. Dose-dependent, complete response to imatinib of a metastatic mucosal melanoma with a K642E KIT mutation. Pigment Cell Melanoma Res. 2008;21:492–3. doi: 10.1111/j.1755-148X.2008.00475.x. [DOI] [PubMed] [Google Scholar]
- 39.Quintas-Cardama A, Lazar AJ, Woodman SE, Kim K, Ross M, Hwu P. Complete response of stage IV anal mucosal melanoma expressing KIT Val560Asp to the multikinase inhibitor sorafenib. Nat Clin Pract Oncol. 2008;5:737–40. doi: 10.1038/ncponc1251. [DOI] [PubMed] [Google Scholar]
- 40.Hodi FS, Friedlander P, Corless CL, Heinrich MC, Mac Rae S, Kruse A, et al. Major response to imatinib mesylate in KIT-mutated melanoma. J Clin Oncol. 2008;26:2046–51. doi: 10.1200/JCO.2007.14.0707. [DOI] [PubMed] [Google Scholar]
- 41.Woodman SE, Trent JC, Stemke-Hale K, Lazar AJ, Pricl S, Pavan GM, et al. Activity of dasatinib against L576P KIT mutant melanoma: molecular, cellular, and clinical correlates. Mol Cancer Ther. 2009;8:2079–85. doi: 10.1158/1535-7163.MCT-09-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carvajal RDC, Wolchok PB, Cane JD, Teitcher L, Lutzky JB, Pavlick J, A. C., Bastian BC, Antonescu CR, Schwartz GK. A phase II study of imatinib mesylate (IM) for patients with advanced melanoma harboring somatic alterations of KIT. J Clin Oncol. 2009:27. [Google Scholar]
- 43.Fisher DE BR, Hodi FS, Herlyn M, Merlino G, Medrano E, Bastian B, Landi TM, Sosman J. Melanoma from bench to bedside: meeting report from the 6th international melanoma congress. Pigment Cell Melanoma Res. 2010;23:14–26. doi: 10.1111/j.1755-148X.2009.00655.x. [DOI] [PubMed] [Google Scholar]