Abstract
Aims
To assess the relationship between pain and HbA1c levels in a predominantly black population with diabetes, and to determine whether self-management behaviours (exercise and diet) and symptoms of depression mediate this relationship.
Methods
We analysed cross-sectional data from 417 community-dwelling individuals with diabetes in rural Alabama, USA. Binary logistic regression was used to analyse the relationship between pain and HbA1c levels, defined as relatively good [≤ 64 mmol/mol (≤ 8.0%)] and relatively poor [> 64 mmol/mol (> 8.0%)], after adjusting for sociodemographics, insulin use, medication count, cigarette smoking history and body mass index (BMI). We examined the mediating roles of exercise, diet, and symptoms of depression using bootstrapping.
Results
Participants were primarily black (86.6%), female (76.1%) and reported an annual income of ≤$20,000 (52.7%). Their mean (SD) age was 59.6 (12.8) years. The majority of the participants reported moderate to extreme pain (71.5%). Participants reporting pain were more than twice as likely to have HbA1c levels > 64 mmol/mol (8.0%) in the fully adjusted model (odds ratio 2.33 [95% CI 1.28–4.24]; P < 0.05). Diet significantly mediated the relationship between pain and HbA1c control (β = 0.06; 95% CI: 0.01–0.17), but only in the unadjusted model. Exercise and symptoms of depression were not significant mediators.
Conclusions
A significant independent relationship between pain and HbA1c control was found in this mainly black population, which was not explained by self-management behaviours or symptoms of depression. Future research is needed to delineate the mechanism by which pain influences HbA1c control, especially among black people with diabetes on low incomes.
Introduction
Compared with the white US population, the black US population is more likely to be diagnosed with diabetes, suffer from diabetes-related complications and have worse glycaemic control [1,2]. Although factors such as genetics and healthcare utilization have been proposed as explanations for this discrepancy [1], the underlying mechanisms are probably multifactorial. One variable that may contribute to the poor outcomes associated with black people with diabetes is pain.
Recurrent or persistent pain affects one-half or more of individuals with Type 2 diabetes [3,4]. Despite the frequent occurrence of pain in diabetes, very few studies have examined the relationship between pain and glycaemic control. Bair et al. [5] reported a positive association between pain and HbA1c levels in a national sample, but this association was no longer significant after controlling for patient characteristics such as demographics, smoking history, and BMI. It should be noted, however, that only 16.5% of their study population comprised black people; thus, the strength of the relationship between pain and HbA1c levels in black people has received little attention. The aim of the present study was to assess the relationship between pain and HbA1c levels in a predominantly black population, and to determine the role of self-management behaviours and symptoms of depression in this relationship.
Compared to white people, healthy black people have a greater pain sensitivity to standard noxious stimuli in the laboratory [6], and black people with chronic pain conditions reliably report greater clinical pain intensity than their white counterparts [7]. This ethnic difference in clinical pain reports is also found among people with osteoarthritis, a condition that affects as many as 40% of individuals with Type 2 diabetes [8]. In addition, black people report greater pain-related interference with daily activities (e.g. recreation activities, household chores) and higher levels of a concept termed ‘pain catastrophizing’, which is associated with pain-related disability [9]. Because black people experience more pain and are more negatively affected by pain than white people, any relationship between pain and HbA1c control may be more pronounced in black people.
Individuals with diabetes who experience pain are more likely to report poorer self-management behaviours. For example, Krein et al. [4] found that US veterans reporting pain were less likely to follow a recommended eating and exercise plan than those without pain. In the Translating Research into Action for Diabetes study, reports of new or ongoing pain at baseline were associated with decreases in sustained walking at follow-up [10]; thus, pain may be a barrier to self-management behaviours, which may in turn worsen glycaemic control.
An important aspect of any study on the relationship between pain and HbA1c control is depression. Symptoms of depression are present in one third or more of individuals with Type 2 diabetes [11], and are associated with lower diet and medication adherence, worse glycaemic control and greater functional impairment [12]. In addition, there is a strong relationship between pain and depression, and depression in patients with pain is associated with greater functional and social impairments [13]. For these reasons, depression might play an important role in the relationship between pain and HbA1c control.
The present study analysed cross-sectional baseline data from the Evaluating Community Peer Advisors and Diabetes Outcomes in Rural Alabama study (Encourage), a community-based group-randomized controlled trial testing the effectiveness of volunteer peer support plus diabetes education on diabetes outcomes. A unique aspect of our study was that the majority of participants were black people and of low socio-economic status. We hypothesized that participants who reported having moderate to extreme pain would have higher HbA1c levels than those without pain after taking into account confounding variables. In addition, we hypothesized that self-management behaviours (reports of diet and exercise), and/or symptoms of depression would partially mediate the relationship between pain and HbA1c control.
Patients and methods
Details of the Encourage study are provided elsewhere [14]. Participants in Encourage were 424 individuals ≥ 19 years of age residing in one of nine rural Alabama counties. This region is known for its high proportion of black residents, and also for its high poverty level and high prevalence of diabetes and obesity. Enrollment and baseline data collection took place between February 2010 and August 2010. Participants were excluded if they did not have a primary care provider, and had not been diagnosed with diabetes by a doctor. Important requirements for enrolment were that participants wanted to learn self-management skills and were willing to work with a peer coach. The Encourage study team obtained informed consent and collected all baseline data during enrolment. The institutional review board at the University of Alabama at Birmingham reviewed and approved the study.
The outcome variable in this study was HbA1c level, which was measured with a capillary blood sample and analysed using an in2it device (Bio-Rad, Hercules, CA, USA) during baseline biometrics assessment. This machine has been waived by the Clinical Laboratory Improvement Amendments and certified by the National Glycohemoglobin Standardization Program to have variation of no more than 10% [15]. HbA1c was dichotomized as ≤ 64 mmol/mol (≤ 8.0%) vs > 64 mmol/mol (> 8.0%), indicating relatively good and relatively poor glycaemic control, respectively.
Pain was assessed by a single item on the EuroQuol Quality-of-Life scale (EQ-5D). The EQ-5D has been validated in patients with Type 2 diabetes [16], and is strongly correlated (0.68) with pain on the Medical Outcome Study 36-item Short Form [17]. Participants indicated the degree of pain ‘today’ by selecting one of three responses: 1) I have no pain or discomfort; 2) I have moderate pain or discomfort; or 3) I have extreme pain or discomfort. This variable was then dichotomized as no pain or discomfort vs moderate/extreme pain or discomfort.
Self-management behaviours included diet and exercise. To assess diet, participants were asked, ‘How many days during the past week did you eat high-fat foods?’ In addition, participants were presented with the following list of items that were considered to be high fat: fried foods; snack foods such as pork skins and chips; fatty meats, such as bologna, sausage, ribs, hot dogs, burgers; breads, such as biscuits and cornbread; dairy foods, such as whole milk and regular cheese; and desserts, such as pie, ice cream, snack cakes, puddings. Based on the distribution of responses, this variable was dichotomized as 0–2 days vs 3–7 days. Exercise was measured by asking participants ‘Other than your regular job or what you do around the house, on how many of the last 7 days did you participate in a specific exercise session (such as swimming, walking, running, biking)?’ Based on the distribution of responses, this variable was similarly dichotomized as 0–2 days vs 3–7 days. Symptoms of depression were determined by the eight-item Patient Health Questionnaire (PHQ-8). The PHQ-8 contains Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) criteria for major depression and has been validated as a measure of current depression [18].
Covariates included sociodemographic data, illness burden, smoking history and BMI. Sociodemographics included age, gender, race, income and education. Race was self-reported and classified as white or black race. Income was assessed by asking participants what their household income was for the past year. Education was assessed as the highest level of school completed or the highest educational degree received. Illness burden was measured using a simple sum of prescription medications taken and, separately, insulin use. For medication count, participants were asked to bring all of their medications to the baseline assessment and the number was recorded by Encourage staff. For anyone who did not bring their medications, a telephone appointment was made and medication count was recorded after baseline assessment. For insulin use, participants indicated if they were or were not using insulin. Cigarette smoking history was assessed by asking if participants had smoked 100 cigarettes in their life.
Statistical Analysis
Chi-squared analyses and t-tests were performed to assess group differences on the study variables. We used logistic regression to test the relationship between pain and HbA1c levels. Unadjusted models were analysed first, followed by models adjusted for age, gender, race, education, income, insulin use, medication count, cigarette smoking history and BMI.
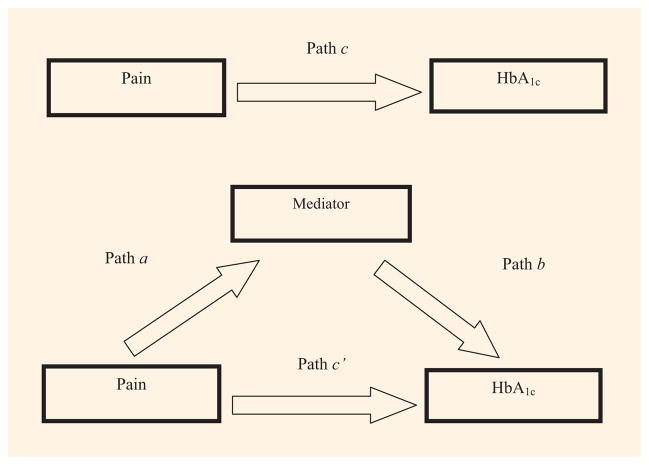
Mediation was assessed by the bootstrapping technique and macro put forth by Preacher and Hayes [19]. Specifically, a 95% bias-corrected CI was calculated to determine if the proposed mediating variables (diet, exercise or symptoms of depression) help explain the relationship between pain and HbA1c levels. The bootstrapped mediation analysis indicates whether the total effect (path c) of pain on HbA1c comprised a significant direct effect (path c′) and a significant indirect effect (path a x b) through one of the proposed mediators [diet, exercise or symptoms of depression (Fig 1)]. In the present study, path a refers to the effect of pain on a proposed mediator, whereas path b refers to the effect of a proposed mediator on HbA1c. A significant indirect effect between pain and HbA1c is determined if the 95% CI does not contain zero. A total of six separate mediational analyses were performed, one for each proposed mediator without statistical adjustment for covariates (i.e. unadjusted analyses), followed by full adjustment for covariates.
Fig. 1.
Conceptual study model of mediation. Mediators assessed included eating high-fat foods at least 2 days per week, exercising fewer than 3 days per week, and symptoms of depression as reflected in the eight-item Patient Health Questionnaire score.
The present study included 417 individuals with baseline HbA1c data available. There were no missing data for the EQ-5D, diet, exercise or PHQ-8 variables. Missing data for covariates were < 5%, thus no imputation was performed (i.e. missing cases were deleted listwise). All analyses were carried out using SPSS, version 19.
Results
Table 1 shows characteristics of the sample on study variables. Participants were primarily female (76.1%), black (86.6%), and reported an annual income ≤$20,000 (52.7%) with a high-school education or lower (58.2%). The mean (SD) age was 59.6 (12.8). The majority of the sample reported moderate to extreme pain (71.5%). As shown in Table 1, participants reporting moderate to extreme pain were more likely to use more medications, have a history of cigarette smoking, have a greater BMI, eat high-fat foods more than twice per week, participate in < 3 days of exercise per week, have a higher number of symptoms of depression, and have HbA1c > 64 mmol/mol (8.0%) compared with those reporting no pain (P < 0.05). In addition, there was a tendency for those who reported pain to report insulin use (P = 0.08).
Table 1.
Characteristics of the participants in the Evaluating Community Peer Advisors and Diabetes Outcomes in Rural Alabama (Encourage) study
| Total sample | No pain | Pain* | P | |
|---|---|---|---|---|
| No. of participants | 417 | 119 | 298 | |
| Mean (SD) age, years | 59.6 (12.8) | 60.6 (13.0) | 59.1 (12.7) | 0.29 |
| Female,% | 76.1 | 71.4 | 77.9 | 0.17 |
| Black,% | 86.6 | 89.1 | 85.9 | 0.60 |
| Education High school or lower,% | 58.2 | 64.7 | 55.9 | 0.10 |
| Annual income,% | ||||
| ≤ $20,000 | 52.7 | 53.5 | 52.2 | 0.45 |
| > $20,000 and ≤ $40,000 | 27.5 | 21.9 | 29.8 | 0.61 |
| > $40,000 | 19.8 | 24.6 | 18.0 | 0.37 |
| Using insulin,% | 39.1 | 32.8 | 41.9 | 0.08 |
| Mean (SD) medication count | 7.7 (4.0) | 6.8 (3.3) | 8.0 (4.3) | <0.01 |
| History of cigarette use,% | 34.1 | 26.1 | 36.9 | 0.03 |
| Mean (SD) BMI, kg/m2 | 36.3 (8.69) | 34.9 (8.36) | 36.8 (8.77) | 0.05 |
| Eat high fat food > 2 days/week,% | 48.7 | 39.5 | 52.7 | 0.02 |
| Exercise < 3 days/week,% | 47.3 | 36.1 | 52.0 | <0.01 |
| Mean (SD) PHQ-8 score | 6.58 (5.71) | 4.06 (4.69) | 7.61 (5.80) | <0.001 |
| HbA1c > 64 mmol/mol (8.0%),% | 35.6 | 26.9 | 39.3 | 0.02 |
Participants reporting moderate or extreme pain on the EuroQuol Quality-of-Life scale.
PHQ-8, eight-item Patient Health Questionnaire.
Pain was independently associated with HbA1c > 64 mmol/mol (8.0%) in both unadjusted (odds ratio 1.76 [95% CI 1.10–2.80]; P < 0.05) and fully adjusted analyses (odds ratio 2.33 [95% CI 1.28–4.24]; P < 0.05). Specifically, the odds of having HbA1c levels > 64 mmol/mol (8.0%) were more than twice as high in people with moderate to extreme pain after accounting for age, gender, race, income, education, insulin use, cigarette smoking history, medication count and BMI.
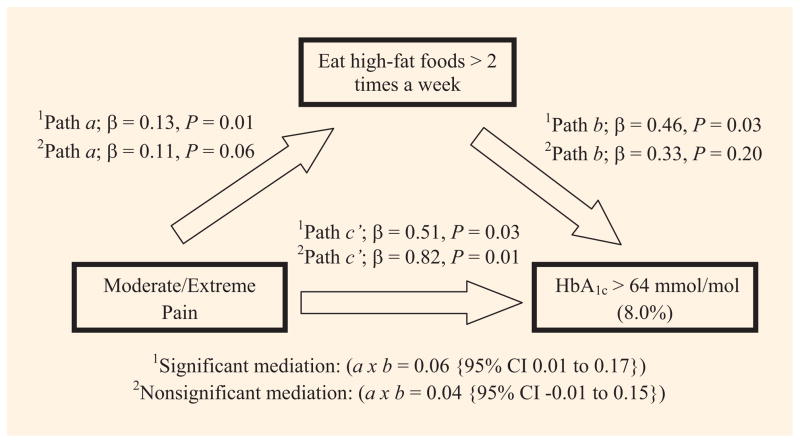
Diet mediated the association between pain and HbA1c when the model was unadjusted for covariates. Specifically, the indirect effect (path a x b) of pain on HbA1c through diet had a point estimate of 0.06 and a 95% CI of 0.01–0.17 (Fig. 2), but in the fully adjusted analysis, diet was no longer a significant mediator (Fig. 2). Neither exercise nor symptoms of depression were significant mediators in unadjusted or fully adjusted analyses.
Fig. 2.
Mediation analysis of pain, diet and HbA1c levels.
Discussion
The present study showed that participants who self-reported moderate to extreme levels of pain were more likely to have relatively poor HbA1c levels compared with those without pain, even after adjusting for confounding variables. Furthermore, when compared to participants without pain, those with pain were more likely to frequently eat high-fat foods, exercise less, and have a greater number of symptoms of depression; however, none of these variables mediated the relationship between pain and HbA1c levels. These findings suggest that moderate to extreme pain may have effects on HbA1c control unrelated to the self-management of at least two health behaviours or the presence of symptoms of depression.
Pain is a very common comorbidity in patients with diabetes and can stem from multiple sources. A common type of pain is painful peripheral neuropathy, affecting ~20% of individuals with Type 2 diabetes and 5% with Type 1 diabetes [20]. Musculoskeletal pain is also common; recent data suggest that patients with diabetes are 1.7 to 2.1 times more likely to have chronic musculoskeletal pain than the population without diabetes [21]. In addition, many individuals with diabetes have osteoarthritis-related pain [8]. Indeed, nearly 75% of our sample reported moderate to extreme levels of pain, although we do not know the exact sources of pain in each participant.
Unlike the recent study by Bair et al. [5], the current study revealed a significant relationship between pain and HbA1c, even after adjusting for a large number of potentially confounding variables. A fundamental difference between the studies is the percentage of black participants (86.6 vs 16.5%). Black people report a greater prevalence and severity of pain in a number of chronic pain conditions [7], and have higher levels of pain-related physical and psychosocial disability than their white counterparts [22]. In addition, black people are more likely to use passive pain-coping strategies (e.g. hoping), which have been associated with greater pain-related disability [23]; thus, pain may be particularly burdensome among black people with diabetes.
The socio-economic status of our study sample may also have contributed to the present results. All participants in Encourage resided in the Alabama counties collectively referred to as the ‘Black Belt,’ a region with limited healthcare resources and high rates of unemployment and poverty [24]. In the study by Bair et al. [5], 39.2% of the sample reported annual household income > $40,000, compared with 19.8% in our sample. This may be important, because people with low socio-economic status are more likely to report chronic pain symptoms and pain disability than people with higher socio-economic status [25]. Differences in racial composition and socio-economic status, therefore, may be two important factors underlying the differing results between the current study and that of Bair et al. It would be useful to determine the relationship between pain and HbA1c levels in black people with higher socio-economic status in future research.
It has been previously shown that pain is associated with poorer self-management behaviours [4]. In addition, the presence of depression and poor self-management behaviours are associated with worse glycaemic control [12,26]; however, in the present study, neither self-management behaviours nor symptoms of depression mediated the relationship between pain and HbA1c control. These findings suggest that the relationship between pain and glycaemic control may be influenced by pathways unrelated to diet, exercise or symptoms of depression. It is possible that the relationship between pain and higher HbA1c levels is partially mediated by neurohormones. Pain triggers the release of a number of neurohormonal substances including cortisol [27], which have been associated with higher serum glucose values in patients with diabetes [28].
The present study has some limitations. Firstly, the chronicity or type of pain was not determined so it was not known which pain condition(s) were most highly related to worse glycaemic control. Nevertheless, there is a growing body of literature that acknowledges the importance of studying pain regardless of the etiology. For example, the reporting of non-specific pain is related to greater mortality [29] and lower quality of life in patients with diabetes [30]. Despite this, future research is needed to determine whether the associations among pain, glycaemic control, and other diabetes complications vary according to the chronicity and/or type of pain.
Secondly, our study had a relatively modest sample size, which may have masked subtle mediation effects. Post hoc power analyses demonstrated that our mediational analyses had low statistical power, ranging from 0.15 to 0.27. Thirdly, the question used to assess high-fat food consumption did not include details such as the amount of saturated fats consumed. In addition, self-reported dietary data have well--known limitations, including the under-reporting of deleterious dietary behaviours [31]. Fourthly, we assessed on how many days during the past week exercise was undertaken, but did not include information such as intensity or duration, which have been shown to result in greater reductions in HbA1c levels [26]. Furthermore, self-reported exercise is prone to over-reporting compared with objective measures such as accelerometer data [32]. Thus, more specific information about diet and exercise may yield a more robust analysis of the mediational relationship between pain and HbA1c control, as the measurement of diet and exercise in the current study may have been affected by recall bias.
The present study also has numerous noteworthy strengths, including the inclusion of individuals at very high risk for uncontrolled diabetes and poor outcomes that were recruited from community-based settings. In addition, we were able to control for a large number of confounding variables.
Unlike previous studies, we found a strong and significant independent relationship between pain and HbA1c control after adjusting for important covariates in this predominantly black population. We did not observe a significant mediating role for health behaviours including frequent ingestion of high-fat foods and less exercise frequency, nor for symptoms of depression. Future research is needed to delineate the mechanisms by which pain may influence glycaemic control, especially among black people with diabetes.
What’s new?
Participants in the study were predominantly black people with diabetes, living in under-served, rural areas of the USA; a population that has received limited research attention.
Results showed a significant, independent relationship between pain and HbA1c levels > 64 mmol/mol (8.0%), suggesting that greater pain is associated with poorer HbA1c control, particularly among the black population of the USA, who are at high risk for uncontrolled diabetes.
We extend the work of previous investigations by examining the mediating roles of self-management behaviours (exercise and diet) and depression symptoms in the relationship between pain and HbA1c control.
Addressing and treating pain in health-discrepant areas may improve diabetes outcomes.
Acknowledgments
Funding sources
This study was funded by Peers for Progress, a collaboration between the American Academy of Family Physicians Foundation and the Eli Lily and Company Foundation.
Significant contributions were made by all authors. We acknowledge the work of the Encourage staff and thank all of the participants.
Footnotes
Competing interests
None declared.
References
- 1.CDC, editor. National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States. Atlanta, GA: Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 2.McWilliams JM, Meara E, Zaslavsky AM, Ayanian JZ. Differences in control of cardiovascular disease and diabetes by race, ethnicity, and education: U.S. trends from 1999 to 2006 and effects of medicare coverage. Ann Intern Med. 2009;150:505–515. doi: 10.7326/0003-4819-150-8-200904210-00005. [DOI] [PubMed] [Google Scholar]
- 3.Davies M, Brophy S, Williams R, Taylor A. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care. 2006;29:1518–1522. doi: 10.2337/dc05-2228. [DOI] [PubMed] [Google Scholar]
- 4.Krein SL, Heisler M, Piette JD, Makki F, Kerr EA. The effect of chronic pain on diabetes patients’ self-management. Diabetes Care. 2005;28:65–70. doi: 10.2337/diacare.28.1.65. [DOI] [PubMed] [Google Scholar]
- 5.Bair MJ, Brizendine EJ, Ackermann RT, Shen C, Kroenke K, Marrero DG. Prevalence of pain and association with quality of life, depression and glycaemic control in patients with diabetes. Diabet Med. 2010;27:578–584. doi: 10.1111/j.1464-5491.2010.02971.x. [DOI] [PubMed] [Google Scholar]
- 6.Rahim-Williams B, Riley JL, Williams AK, Fillingim RB. A quantitative review of ethnic group differences in experimental pain response: do biology, psychology, and culture matter? Pain Med. 2012;13:522–540. doi: 10.1111/j.1526-4637.2012.01336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allen KD, Chen JC, Callahan LF, Golightly YM, Helmick CG, Renner JB, et al. Racial differences in knee osteoarthritis pain: potential contribution of occupational and household tasks. J Rheumatol. 2012;39:337–344. doi: 10.3899/jrheum.110040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Douloumpakas I, Pyrpasopoulou A, Triantafyllou A, Sampanis Ch, Aslanidis S. Prevalence of musculoskeletal disorders in patients with type 2 diabetes mellitus: a pilot study. Hippokratia. 2007;11:216–218. [PMC free article] [PubMed] [Google Scholar]
- 9.Arnow BA, Blasey CM, Constantino MJ, Robinson R, Hunkeler E, Lee J, et al. Catastrophizing, depression and pain-related disability. Gen Hosp Psychiatry. 2011;33:150–156. doi: 10.1016/j.genhosppsych.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Duru OK, Gerzoff RB, Brown AF, Karter AJ, Kim C, Kountz D, et al. Predictors of sustained walking among diabetes patients in managed care: the Translating Research into Action for Diabetes (TRIAD) study. J Gen Intern Med. 2008;23:1194–1199. doi: 10.1007/s11606-008-0629-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ali S, Stone MA, Peters JL, Davies MJ, Khunti K. The prevalence of co-morbid depression in adults with type 2 diabetes: a systematic review and meta-analysis. Diabet Med. 2006;23:1165–1173. doi: 10.1111/j.1464-5491.2006.01943.x. [DOI] [PubMed] [Google Scholar]
- 12.Ciechanowski PS, Katon WJ, Russo JE. Depression and diabetes: impact of depressive symptoms on adherence, function, and costs. Arch Intern Med. 2000;160:3278–3285. doi: 10.1001/archinte.160.21.3278. [DOI] [PubMed] [Google Scholar]
- 13.Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Inter Med. 2003;163:2433–2445. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- 14.Andreae SJ, Halanych JH, Cherrington A, Safford MM. Recruitment of a rural, southern, predominantly African-American population into a diabetes self-management trial. Contemp Clin Trials. 2012;33:499–506. doi: 10.1016/j.cct.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 15. [Last accessed 19 December 2012];in2it (II) Analyzer [article online] 2012 Available from http://www.bio-rad.com/prd/en/US/CDG/PDP/16c8f689-4b6a-4952-967f-1802779c2c58/in2it-%28II%29-Analyzer.
- 16.Matza LS, Boye KS, Yurgin N. Validation of two generic patient-reported outcome measures in patients with type 2 diabetes. Health Qual Life Outcomes. 2007;5:47. doi: 10.1186/1477-7525-5-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nowels D, McGloin J, Westfall JM, Holcomb S. Validation of the EQ-5D quality of life instrument in patients after myocardial infarction. Qual Life Res. 2005;14:95–105. doi: 10.1007/s11136-004-0614-4. [DOI] [PubMed] [Google Scholar]
- 18.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114:163–173. doi: 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 19.Preacher K, Hayes A. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004;36:717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- 20.Hartemann A, Attal N, Bouhassira D, Dumont I, Gin H, Jeanne S, et al. Painful diabetic neuropathy: diagnosis and management. Diabetes Metab. 2011;37:377–388. doi: 10.1016/j.diabet.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Molsted S, Tribler J, Snorgaard O. Musculoskeletal pain in patients with type 2 diabetes. Diabetes Res Clin Pract. 2012;96:135–140. doi: 10.1016/j.diabres.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 22.Cano A, Mayo A, Ventimiglia M. Coping, pain severity, interference, and disability: the potential mediating and moderating roles of race and education. J Pain. 2006;7:459–468. doi: 10.1016/j.jpain.2006.01.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edwards RR, Moric M, Husfeldt B, Buvanendran A, Ivankovich O. Ethnic similarities and differences in the chronic pain experience: a comparison of african american, Hispanic, and white patients. Pain Med. 2005;6:88–89. doi: 10.1111/j.1526-4637.2005.05007.x. [DOI] [PubMed] [Google Scholar]
- 24.Savage RM, Dillon JM, Hammel JC, Lewis TC, Johnson NC, Barlow LM, et al. The Alabama coalition for a healthier black belt: a proof of concept project. Community Ment Health J. 2012;49:79–85. doi: 10.1007/s10597-012-9488-z. [DOI] [PubMed] [Google Scholar]
- 25.Dorner TE, Muckenhuber J, Stronegger WJ, Rasky E, Gustorff B, Freidl W. The impact of socio-economic status on pain and the perception of disability due to pain. Euro J Pain. 2011;15:103–109. doi: 10.1016/j.ejpain.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 26.Zanuso S, Jimenez A, Pugliese G, Corigliano G, Balducci S. Exercise for the management of type 2 diabetes: a review of the evidence. Acta Diabetol. 2010;47:15–22. doi: 10.1007/s00592-009-0126-3. [DOI] [PubMed] [Google Scholar]
- 27.Van Uum SH, Sauve B, Fraser LA, Morley-Forster P, Paul TL, Koren G. Elevated content of cortisol in hair of patients with severe chronic pain: a novel biomarker for stress. Stress. 2008;11:483–488. doi: 10.1080/10253890801887388. [DOI] [PubMed] [Google Scholar]
- 28.Chiodini I, Adda G, Scillitani A, Coletti F, Morelli V, Di Lembo S, et al. Cortisol secretion in patients with type 2 diabetes: relationship with chronic complications. Diabetes Care. 2007;30:83–88. doi: 10.2337/dc06-1267. [DOI] [PubMed] [Google Scholar]
- 29.Landman GW, van Hateren KJ, Kleefstra N, Groenier KH, Gans RO, Bilo HJ. Health-related quality of life and mortality in a general and elderly population of patients with type 2 diabeties (ZODIAC-18) Diabetes Care. 2010;33:2378–2382. doi: 10.2337/dc10-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Redekop WK, Koopmanschap MA, Stolk RP, Rutten GE, Wolf-fenbuttel BH, Niessen LW. Health-related quality of life and treatment satisfaction in Dutch patients with type 2 diabetes. Diabetes Care. 2002;25:458–463. doi: 10.2337/diacare.25.3.458. [DOI] [PubMed] [Google Scholar]
- 31.Livingstone MB, Black AE. Markers of the validity of reported energy intake. J Nutr. 2003;133:895S–920S. doi: 10.1093/jn/133.3.895S. [DOI] [PubMed] [Google Scholar]
- 32.Tucker JM, Welk GJ, Beyler NK. Physical activity in U.S: adults compliance with the Physical Activity Guidelines for Americans. Am J Prev Med. 2011;40:454–461. doi: 10.1016/j.amepre.2010.12.016. [DOI] [PubMed] [Google Scholar]