Abstract
The phenotypic switch underlying the differentiation of cardiac fibroblasts into hypersecretory myofibroblasts is critical for cardiac remodeling following myocardial infarction. Myofibroblasts facilitate wound repair in the myocardium by secreting and organizing extracellular matrix (ECM) during the wound healing process. However, the molecular mechanisms involved in myofibroblast differentiation are not well known. TGF-β has been shown to promote differentiation and this, combined with the robust mechanical environment in the heart, lead us to hypothesize that the mechanotransduction and TGF-β signaling pathways play active roles in the differentiation of cardiac fibroblasts to myofibroblasts. Here, we show that the mechanosensitve ion channel TRPV4 is required for TGF-β1-induced differentiation of cardiac fibroblasts into myofibroblasts. We found that the TRPV4-specific antagonist AB159908 and siRNA knockdown of TRPV4 significantly inhibited TGFβ1-induced differentiation as measured by incorporation of α-SMA into stress fibers. Further, we found that TGF-β1-induced myofibroblast differentiation was dependent on ECM stiffness, a response that was attenuated by TRPV4 blockade. Finally, TGF-β1 treated fibroblasts exhibited enhanced TRPV4 expression and TRPV4-mediated calcium influx compared to untreated controls. Taken together these results suggest for the first time that the mechanosensitive ion channel, TRPV4, regulates cardiac fibroblast differentiation to myofibroblasts by integrating signals from TGF-β1 and mechanical factors.
Keywords: calcium, cardiac remodeling, myocardial infarction, extracellular matrix, TRPV4
1. Introduction
Cardiac fibroblasts (CF) are one of the populous cell types reside in the interstitial space of the heart, known to secrete extracellular matrix proteins which help to maintain the structural integrity of the myocardium. CF mediated ECM deposition is essential for cardiac remodeling and in response to diseases such as myocardial infarction, hypertrophy and heart failure. The differentiation of CF to myofibroblasts is critical for remodeling of myocardium after myocardial infarction[1, 2]. Myofibroblasts are hypersecretory, highly contractile and facilitate wound healing by forming scar tissue. Moreover, disproportionate production and prolonged survival of myofibroblasts can generate excessive formation of ECM proteins which can lead to pathological fibrosis[1–3]. To date, the molecular mechanisms underlying the differentiation of fibroblasts to myofibroblasts are not well known. Although TGFβ1 and mechanical stress (generated by ECM stiffness) are recognized as major mediators of myofibroblast differentiation[3], the molecular signals that coordinate these soluble and mechanical signals are still elusive[3]. Interestingly, calcium signaling has recently gained much attention as a regulator of myofibroblast contractile activity[4] but it is not known whether calcium signaling is required for differentiation of fibroblasts to myofibroblasts. Recently, it was shown that a Transient Receptor Potential (TRP) channel, TRPM7 is required for atrial fibroblast differentiation[5].
TRPV4 channels, another class of TRP channels, are widely expressed in epithelial, endothelial, chondroblasts, osteoblasts and fibroblasts and are activated by different mechanical forces such as cyclic strain in endothelial cells, cell swelling in epithelial and endothelial cells, and shear stress in endothelial and renal epithelial cells[6–9]. TRPV4 channels were shown to be expressed in cardiac fibroblasts but their function is not known[10]. Since TRPV4 is implicated as a mechanosensor [6–9] and that cardiac fibroblast differentiation requires changes in ECM mechanics and mechanical stretch[3], we speculated that these channels may play a role in this process. Here, we show that the mechanosensitive ion channel TRPV4 is an important mediator of cardiac fibroblast differentiation to myofibroblasts.
2. Material and Methods
Materials
GSK1016790A, 2-APB, carvacrol, gelatin, EDA-FN antibodies and alphasmooth muscle actin (α-SMA) antibodies were purchased from Sigma; AB159908 was obtained from ABCR GmbH (Germany). Fluo-4, phalloidin and Alexa-conjugated secondary antibodies were from Invitrogen. The polyclonal antibodies against TRPV4 were obtained from Alomone (Israel). TGF-β1 was purchased from PeproTech.
Cardiac Fibroblast isolation and Culture
Cardiac fibroblasts were isolated from adult, male, Sprague-Dawley rats as previously described[11–13]. Euthanization of rats was performed according to guidelines and approval of the Institutional Animal Care and Use Committee (IACUC) of the Northeast Ohio Medical University (NEOMED). Cells were cultured in low glucose DMEM supplemented with 10 % FBS, penn/strep. Cells from passage 2–3 were used for calcium and TGF-β1 induced differentiation experiments. Under our culture conditions[11–14], we consistently observe that rat cardiac fibroblasts spontaneously differentiate into myofibroblasts at passages 4–5. Importantly, we found that these cells retain their fibroblast phenotype up to 3 passages [11–14] with few cells showing α-SMA incorporation in to stress fibers (< 20%). The CFs used in the study are negative for smooth muscle cell marker, desmin [11–14] and show very little EDA-FN indicating that these cells are largely undifferentiated CFs (Suppl. Fig.4). However, other studies have shown that fibroblasts isolated from tissues and cultured on tissue culture plates can acquire protomyofibroblastic phenotype [45, 46]. Importantly, the fibroblasts used in this study expressed basal α-SMA expression that was not organized in to stress fibers. Hence, we cannot rule out the possibility that these cells are protomyofibroblasts, however, we have referred to them as fibroblasts throughout the study.
Calcium Imaging
Cells cultured on MatTek glass bottomed dishes were loaded with Fluo-4/AM (4 µM) for 20 min, washed 3 times in calcium medium (136 mM NaCl, 4.7 mM KCl,1.2 mM MgSO4, 1.1 mM CaCl2, 1.2 mM KH2PO4, 5 mM NaHCO3, 5.5 mM glucose, and 20 mM Hepes. pH 7.4) and kept in this medium on an inverted Olympus IX 70 confocal microscope (FV300). Cells were stimulated with GSK1016790A (10–300 nM) and images were acquired every 3 seconds and analyzed using Olympus Fluoview software and Microsoft Excel as previously described[9, 15, 16]. In the indicated experiments, cells were pre-treated with TRPV4 antagonist 10 µM AB159908 for 20 min[16].
Immunofluorescence of alpha-SMA and EDA-FN
Cardiac fibroblasts were serum starved for 24 h and incubated with TGF-β1 (10 ng/ml) for 24 h. For the inhibitor studies, serum starved CFs were pre-treated with TRPV4 antagonist AB159908 (10 µM ) for 30 min and then incubated with TGF-β1 in the continuous presence of antagonist. Cells were then rinsed with phosphate-buffered saline and fixed for 20 min at room temperature in PBS containing 4% paraformaldehyde. After washing with PBS, cells were permeabilized with 0.25% Triton-X100/PBS, washed and blocked with serum containing media for 20 min. Cells were then incubated with the primary antibodies (α- SMA or EDA-FN from Sigma; 1: 1000 ) followed by Alexa Fluor-conjugated secondary antibodies (1:500). Cells were mounted on glass slides using fluoromount containing DAPI (Vector labs). Images were obtained using an Olympus fluorescence microscope with a 20× objective and processed using Image J (NIH) software. Cells with α-SMA positive stress fibers that extend greater than 3/4 of the length of the cell with a 3–5 fold increased in staining intensity (measured by drawing a line across the cell and analyzed using plot profile in Image J) than control cells are considered as myofibroblasts.
To independently confirm myofibroblast content as a change in the total α-SMA levels between treatment groups, we measured the total α-SMA fluorescence of each cell (75–100 cells in each group) using Image J and the results are presented as relative α-SMA levels.
SDS-PAGE and Western blot analysis
Cells were lysed in modified RIPA buffer (50mM Tris-HCl at pH 7.4, 150mM sodium chloride, 1% Triton-X100, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS) with protease and phosphatase inhibitor cocktail (Boston Bioproducts). Cell lysates were separated by electrophoresis on 4–20% gradient SDS- polyacrylamide gels and transferred to Immobilon ® polyvinylidene difluoride membrane. Immunoblotting was performed with primary antibodies as follows: Anti-TRPV4 (Alomone), Anti-tubulin (Abcam) and Anti-α-GAPDH (Cell signaling). The ECL (Pierce West Pico) method was used with anti-rabbit or mouse IgG-conjugated horseradish peroxidase (Jackson Laboratories) at a dilution of 1:10,000 and developed using Protein Simple. Results were quantified using Image J software.
RT-PCR Analysis
RT-PCR analysis of cardiac fibroblasts was performed using Qiagen® One step RT-PCR Kit and specific primers for rat TRPV4, TRPM7 and GAPDH as described previously[16].
siRNA knockdown of TRPV4
CFs were transfected with 10 nM rat TRPV4 specific smartpool siRNAs (Dharmacon) using siLentFect reagent (Biorad). Two days later, TRPV4 knockdown was assessed by Western blot analysis.
Statistical analysis
Statistical analysis was performed using one way ANOVA followed by Tukey post hoc analysis or Student’s t-test and the significance was set at p≤0.05.
3. Results
3.1. TRPV4-dependent calcium influx is required for cardiac fibroblast differentiation
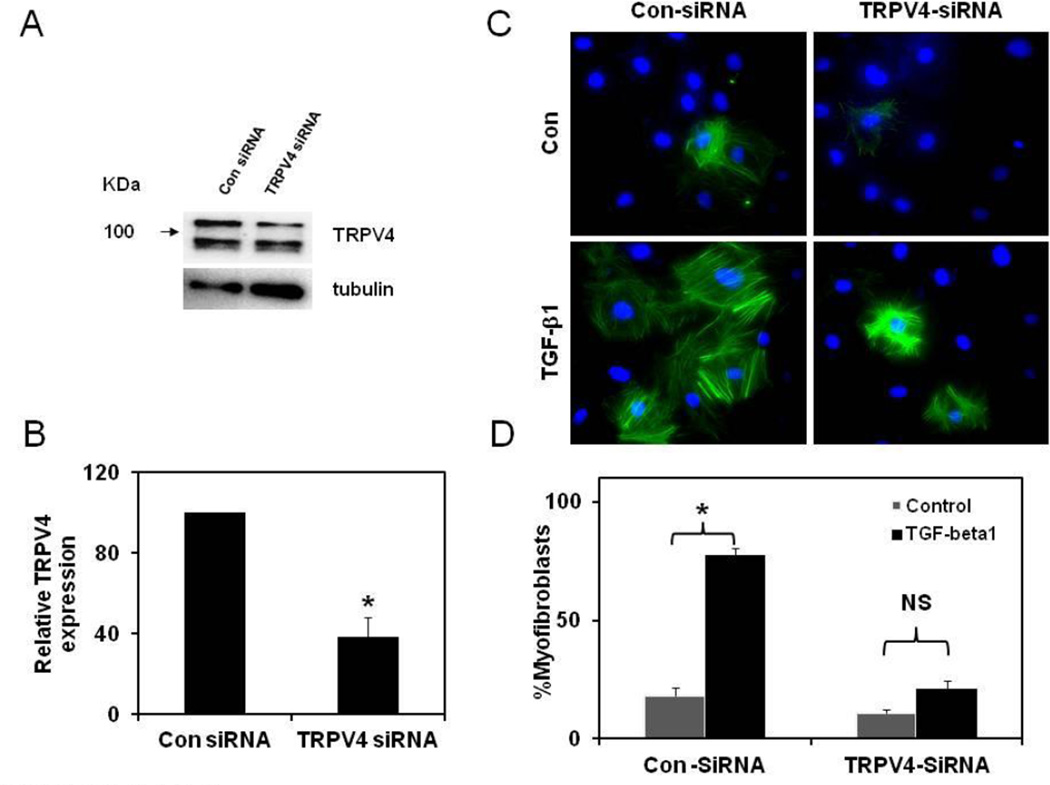
We have previously shown that mechanical stretch activates TRPV4 dependent calcium influx that is required for changes in endothelial cell phenotype [9]. In order to determine if TRPV4 is required for cardiac fibroblast differentiation, we first asked if TRPV4 is expressed in rat CFs. RT-PCR analysis revealed that TRPV4 mRNA is expressed in these cells, and Western blot analysis further confirmed that TRPV4 protein was present in CFs with two bands of molecular weights above and below 100 KDa (Fig.1 A). To find out if these channels are functionally active, we loaded CFs with Fluo-4 and measured calcium influx in response to a specific TRPV4 agonist, GSK1016790A. As shown in Fig.1 B and C, GSK1016790A induced robust concentration-dependent (10–300 nM) calcium influx confirming that TRPV4 channels are functionally active in rat CF. Vehicle(DMSO) or AB159908 alone were used as controls, which were shown to have no effect on basal calcium levels (Suppl. Fig.1). Next, we asked if TRPV4 channels are required for differentiation of CFs to myofibroblasts. Cardiac fibroblasts cultured in cell culture plates were serum starved and stimulated with 10 ng/ml of TGF-β1 for 24 h in the presence or absence of a TRPV4 antagonist, AB159908. TGF-β1 treatment induced robust differentiation of CFs to myofibroblasts as evidenced by increased α-smooth muscle actin (α-SMA) positive stress fibers (Fig.1D, Suppl. Fig.2). Importantly, pre-treatment of cells with TRPV4 antagonist significantly inhibited TGF-β1-induced differentiation of cardiac fibroblasts. Quantitative analysis revealed that TGF-β1 treatment induced significant increase (65%) in the percentage of myofibroblasts which was significantly inhibited by TRPV4 antagonist (35% reduction; Fig.1E, TGF-β1 vs TGF-β1+ AB1; 80.0 ± 2.1 vs 46.0 ± 4.4). Quantitative analysis of α-SMA fluorescence of each cells also revealed that TGF-β1 significantly increased the α-SMA levels which is inhibited by pre-treatment of CFs with TRPV4 antagonist, AB159908 (Suppl. Fig.3). Western blot analysis further confirmed that TGF-β1-induced α-SMA expression is inhibited by TRPV4 antagonist, AB159908 (SuppFig.4A, B). Furthermore, we found that TGF-β1 treatment increased the levels of another marker of myofibroblasts, extra domain A fibronectin (EDA-FN) which was significantly attenuated in the presence of TRPV4 antagonists (Suppl. Fig.5). These findings unequivocally confirm that TRPV4 channels are required for TGF-β1- induced differentiation of CFs. To further confirm the role of TRPV4, we knocked down TRPV4 expression in rat CF using specific TRPV4 siRNAs. We found that transfection of TRPV4 specific siRNA resulted in 60% knockdown of TRPV4 protein (Fig.2A, B). Importantly, we demonstrated that knockdown of TRPV4 significantly inhibited TGF-β1-induced cardiac fibroblast differentiation (Fig.2C, D and Suppl.Fig.6). In contrast, control siRNA had no effect on TGF-β1-induced cardiac fibroblast differentiation. Taken together, these results clearly demonstrate that TRPV4 channels are required for TGF- β1-induced cardiac fibroblast differentiation
Figure 1. TRPV4 channels are required for TGF-β1-induced differentiation of cardiac fibroblasts.
A) RT-PCR and Western blot analysis showing the expression of TRPV4 mRNA and protein in rat left ventricular fibroblasts. B) Calcium transients showing that a specific activator of TRPV4, GSK1016790A (10, 100 and 300 nM), induced calcium influx in rat cardiac fibroblasts. Arrow indicates addition of the stimulator. C) Quantitative analysis of relative changes (Δ F/F0) in calcium influx in CFs loaded with Fluo-4 and stimulated with TRPV4 specific activator, GSK1016790A. D) Immunofluorescence images of fibroblast showing the differentiation as evidenced by the incorporation of α-SMA (green) in to stress fibers. Nuclei were stained with DAPI (blue). CFs were serum starved and stimulated with TGF-β1 for 24 h in the presence or absence of TRPV4 antagonist AB159908 (AB1). E) Quantitative analysis of the fibroblast differentiation. Cells (n=300) were counted from independent images and the percentage of α-SMA positive cells was presented as % myofibroblasts. The results shown are mean ± SEM from 3 independent experiments (* p < 0.05).
Figure 2. siRNA knockdown of TRPV4 channels inhibits TGF-β1-induced differentiation of cardiac fibroblasts.
A) Western blot analysis showing the TRPV4 protein expression in control siRNA and TRPV4 siRNA treated CFs. Tubulin served as a loading control. B) Quantitative analysis showing the specific knockdown of the expression of TRPV4 in rat CFs. C) Immunofluorescence images showing the significant inhibition of TGF-β1-induced CF differentiation in TRPV4 knockdowned cells as evidenced by the incorporation of α-SMA (green) in to stress fibers. Nuclei were stained with DAPI (blue). CFs were transfected with control or TRPV4 specific siRNAs and, serum starved and stimulated with TGF-β1 for 24 h. E) Quantitative analysis of the fibroblast differentiation in control and TRPV4 knockdown cells. Cells (n=300) were counted from independent images and the percentage of α-SMA positive cells was presented as % myofibroblasts. The results shown are mean ± SEM from 3 independent experiments (* p < 0.05). NS=Non-significant).
3.2. TGFβ1-induced cardiac fibroblast differentiation is regulated by matrix stiffness via TRPV4
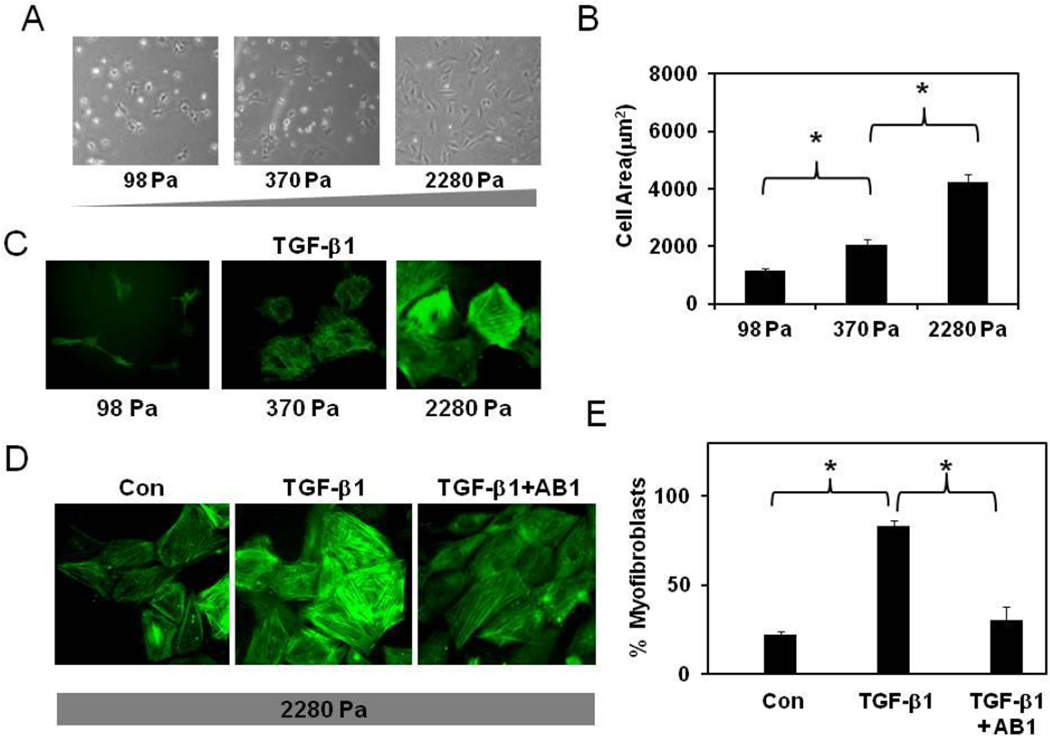
Since ECM stiffness influences the differentiation of fibroblasts[3], we next investigated if cardiac fibroblast differentiation is sensitive to matrix stiffness and requires functional TRPV4 channels. To achieve this, we prepared gelatin hydrogels of varying stiffness (98–2280 Pa) using transglutaminase cross-linking as described previously[17]. Cell spreading is dependent on the force balance between the contractile forces applied by the cell on the ECM and ECM resistance (stiffness) to these forces[17]. First, we measured cardiac fibroblast attachment and spreading on these ECM gels. As shown in Fig.3 A, CFs attached to ECM gels of each stiffness but exhibited little spreading on low stiffness gels and greater spreading on high stiffness gels. Quantification of projected cell areas revealed that CF spreading was increased 3–4 fold on high stiffness gels compared to low stiffness gels (1140 ± 90 versus 4237± 256 on 98 and 2280 Pa gels, respectively; Fig.3B). Since cell spreading is a prerequisite for differentiation, we initially measured TGF-β1-induced differentiation of CFs cultured on all the three ECM gels and found that only the cells on high stiffness gels( that showed maximal spreading) increased α-SMA incorporation into stress fibers (Fig.3C). Next, we asked if TRPV4 is required for differentiation of CFs on high stiffness gels. We found that TGF-β1-treatment increased CF differentiation to myofibroblasts (75%) which was attenuated in CFs pre-treated with TRPV4 antagonist, AB159908 (Fig.3D,E and Suppl.Fig.7). These results indicate that TRPV4 channels are critical for mechanosensing of ECM stiffness by cardiac fibroblasts during their differentiation to myofibroblasts.
Figure 3. TRPV4 channels mediate TGF-β1-ECM stiffness-induced differentiation of cardiac fibroblasts.
A) Photomicrographs of cardiac fibroblasts cultured on transglutaminase-linked gelatin hydrogels of varying stiffness (98, 370 and 2280 Pa). B) The histogram shows the average projected cell areas of cells (n=200) calculated using Metamorph and Image J software. C) Immunofluorescence images of fibroblast differentiation (the incorporation of α-SMA in to stress fibers (green) on gelatin hydrogels of varying stiffness (98–2280 Pa). Note: CFs were differentiated only on high stiffness gels. D) Immunofluorescence images showing TRPV4-dependent differentiation of CFs on high stiffness gels. CFs were serum starved and stimulated with TGF-β1 for 24 ( C, D ) in the presence or absence of TRPV4 antagonist AB159908 (AB1, D). Cells (n=300) were counted from independent images and the percentage of α-SMA positive cells was presented as % myofibroblasts. The results shown are mean ± SEM from 3 independent experiments (* p < 0.05).
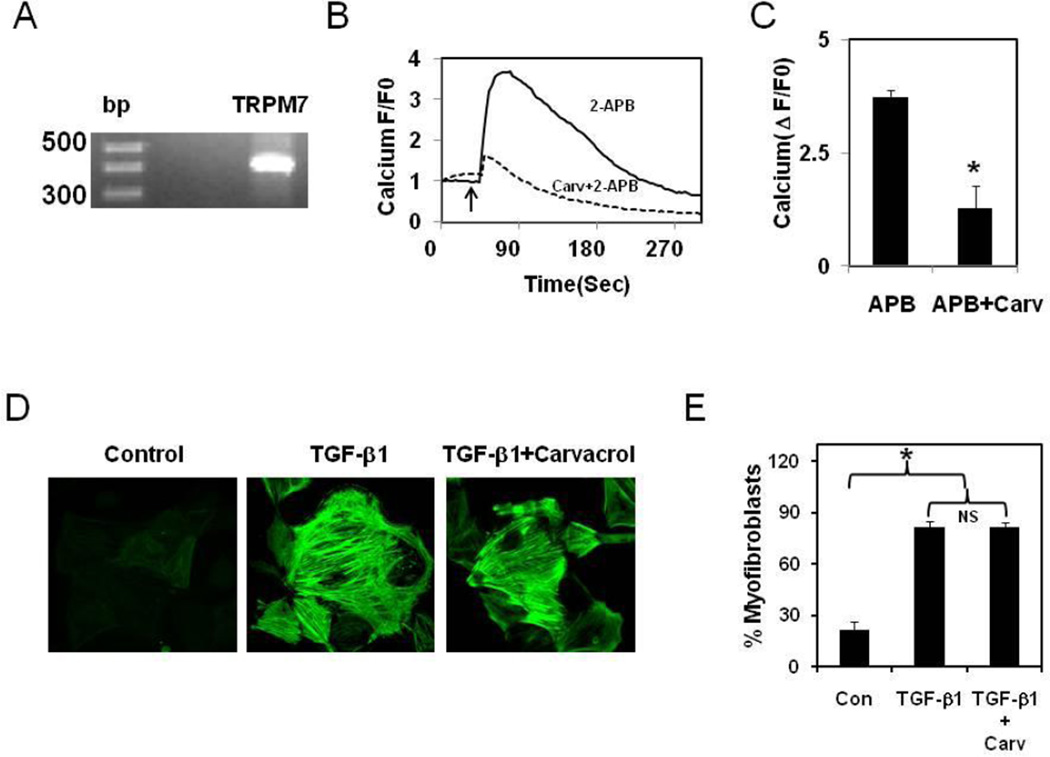
Recently, calcium influx through TRPM7 has been shown to be important for TFG-β1-induced differentiation of atrial fibroblasts[5]. To find out if TRPM7 plays a role in ventricular fibroblast differentiation we investigated its functional expression in rat ventricular fibroblasts. RT-PCR analysis revealed expression of TRPM7 (Fig.4A) and a TRPM7 activator, 2-APB induced robust calcium influx (Fig.4 B,C) which is inhibited by the TRPM7 inhibitor, carvacrol [18]. Importantly, carvacrol failed to inhibit TGF-β1-induced differentiation (Fig.4 D, E and Suppl.Fig.8). These results, thus indicate that TRPV4, but not TRPM7, plays an important role in ventricular fibroblast differentiation and suggest that different regions of heart use distinct signaling mechanisms to control cardiac fibroblast fate.
Figure 4. TRPM7 channels are not required for TGF-β1-induced ECM stiffness dependant differentiation of cardiac fibroblasts.
A) RT-PCR analysis showing the expression of TRPM7 mRNA B) Calcium transients showing that an activator of TRPM7 2-APB (5 mM), induced calcium influx in rat cardiac fibroblasts which is inhibited by TRPM7 blocker, Carvacrol (500 µM). Arrow indicates addition of the stimulator. C) Quantitative analysis of relative changes in calcium influx (Δ F/F0) in CFs loaded with Fluo-4 and stimulated with TRPM7 activator, 2-APB. D) Immunofluorescence images of fibroblast showing the differentiation as evidenced by the incorporation of α-SMA (green) in to stress fibers. CFs cultured on high stiffness gelatin hydrogel (2280 Pa) were serum starved and stimulated with TGF-β1 for 24 h in the presence or absence of TRPM7 antagonist Carvacrol (500 µM) E) Quantitative analysis of the fibroblast differentiation. Cells (n=300) were counted from independent images and the percentage of α-SMA positive cells was presented as % myofibroblasts. The results shown are mean ± SEM from 3 independent experiments (* p < 0.05). NS=Non-significant).
3.3. TGF-β1 modulates TRPV4 expression and activity during cardiac fibroblast differentiation
In order to gain insight into the molecular mechanism through which TGF-β1 mediates TRPV4 dependent differentiation of cardiac fibroblasts, we asked if TGF-β1 regulates TRPV4 expression or function in cardiac fibroblasts. First, we measured TRPV4 mediated calcium influx in cells treated or untreated with TGF-β1 in the presence or absence of TRPV4 antagonist. We found that the TRPV4 specific agonist, GSK1016790A (100 nM) induced a two-fold increase in calcium influx in TGF-β1-treated cardiac fibroblasts compared to the untreated control cells (Fig.5 A, B). Further, we found that pre-treatment of CFs with TRPV4 antagonist abolished the TGF-β1-induced increase in the calcium influx (Fig.5 A, B). Next, we asked if this increase in calcium was due to increased expression of TRPV4. RT-PCR and Western blot analysis showed that TGF-β1 treatment significantly increased the levels of TRPV4 mRNA and protein in differentiated CFs compared to untreated controls (Fig.5 C, D).
Figure 5. TGF-β1 induces increased TRPV4 expression and activity during cardiac fibroblast differentiation.
A) Calcium transients showing that an increase in GSK1016790A (100 nM), induced calcium influx in TGF-β1 treated rat cardiac fibroblasts compared to controls. Arrow indicates addition of the stimulator. B) Quantitative analysis of relative changes (Δ F/F0) in calcium influx in CFs (n=300) pre-treated with TGF-β1 and stimulated with TRPV4 specific activator, GSK1016790A in the presence or absence of TRPV4 antagonist, AB159908 (AB1). RT-PCR (C) and Western blot (D) analysis of TRPV4 mRNA and protein levels in control and TGF-β1 treated CFs. The results shown are mean ± SEM from 3 independent experiments (*p < 0.05).
4. Discussion
ECM stiffening during cardiac remodeling in normal and disease states is often thought to occur as the consequence of fibrosis[19]. However, recent studies have indicated that ECM stiffening also influences fibroblast differentiation to myofibroblasts resulting in further increases in ECM deposition leading to the progression of disease [3, 19]. On the other hand, TGF-β1 has been universally recognized as a potent inducer of fibroblast differentiation to myofibroblasts [3, 19–23]. Nevertheless, the molecular mechanism by which mechanical signals from the ECM (stiffness) and soluble signals (TGF-β1), regulate fibroblast differentiation is not known, specifically in physiological or pathological cardiac remodeling. Our study provides the initial evidence for the role of TRPV4 in the differentiation of CFs and demonstrates that TRPV4 channels could be a convergence point in integrating signals from TGF-β1 and mechanical stimuli in the differentiation of cardiac fibroblasts.
Cells sense mechanical forces generated by external forces or ECM stiffness through the integrins, which facilitate cell binding to the ECM. Integrins are bidirectional mechanical force transducers as they not only transfer mechanical force applied on ECM in to the cells but also transfer contractile forces on to the ECM [24, 25]. The balance between the contractile forces generated by the cell and the resistance offered by ECM stiffness to these forces, both transferred by integrins, decides the phenotype of the cell [24, 25]. Importantly, myofibroblast differentiation has been shown to be regulated by both integrins and TGF-β1[20]. TGF-β1 treatment has been demonstrated to induce the expression and activity of integrins during the differentiation of fibroblasts to myofibroblasts [26–28]. Further, it was shown that TGF-β requires integrin signaling molecules such as integrin-linked kinase and focal adhesion kinase to induce myofibroblast differentiation [29–33]. Interestingly, integrins β5 and β1 were shown to be critical for TGF-β1 release from the ECM[34, 35]. Although ECM stiffness has been shown to influence myofibroblast contractility as well as TGF-β1 release from the ECM[35–38], the molecular mechanisms by which integrins facilitate TGF-β release from ECM has not been identified until recently. Using single molecule-force spectroscopy, Hinz and coworkers demonstrated that applying forces as small as those generated by the pull of single integrin molecule can induce conformational changes in the latent TGF-β1 complex and release active TGF-β1 [38, 39]. TGF-β1 in turn activates additional integrins which increases Rho-dependent cell contractility required for fibroblasts differentiation to myofibroblasts[35–38]. These findings suggest that there is a cross-talk between TGF-β (soluble) and integrin (mechanical) signaling but neither the molecular mechanism nor the coordinating signaling molecule has been identified to date.
We have previously shown that TRPV4 channels are activated by mechanical forces applied on ECM and mediates integrin to integrin signals required for phenotypic changes in endothelial cells [9]. We have observed that direct application of mechanical forces through the RGD beads attached to the cell activates TRPV4-dependent calcium signals locally at focal adhesion sites[15], and that both integrins and TRPV4 colocalized in focal adhesions. Notably, it was also reported that applying mechanical forces using collagen coated magnetic beads attached to the fibroblast induced α-SMA expression via the recruitment of FAK, to the sites of bead binding[29]. Therefore it is conceivable that TRPV4 channels can be activated by changes in ECM stiffness via force transfer through integrin signaling [3, 9, 15] which transduces these mechanical signals to activate down-stream signaling molecules (such as Rho) required for fibroblast differentiation. In the present study, we found that TRPV4 channels are functionally expressed in cardiac fibroblasts and are required for their differentiation to myofibroblasts. Importantly, inhibition of TRPV4 activity (and/or expression) significantly attenuated TGF-β1-induced fibroblast differentiation on culture plates as well as compliant ECM gels of high stiffness but not on low stiffness. Moreover, TGF-β1 treatment induced increased TRPV4 expression and activity. One potential limitation of the study is regarding the phenotype of the cultured cells. The fibroblasts used in the present study expressed detectable levels of α-SMA, which may represent a partially differentiated protomyofibroblastic phenotype[45, 46]. However, irrespective of their exact phenotype, our findings demonstrate that TRPV4 is a critical mediator of myofibroblast differentiation in response to TGF-β1 and ECM stiffness. The estimated stiffness of the normal adult heart tissue is around 10 kPa and in the disease states, between 20–100 kPa[20, 40], suggesting that the stiffness of the tissue is increased during disease states. Although the gels used in the present study are of lower stiffness range, our findings that CF differentiation is enhanced with increased stiffness clearly provides a proof of principle for the physiological phenomenon i.e. increase in stiffness during disease state increases CF differentiation which will eventually lead to scar formation. Indeed, published reports have shown that fibroblasts can differentiate into myofibroblasts (19, 42) and increase collagen expression at low stiffness matrices (1–3 kPa). These findings coupled with our observation that CFs were differentiated on 2.28 kPa stiffness substrates in response to TGF-β1 suggesting that low substrate stiffness (above 2 kPa) is sufficient to induce the differentiation of cardiac fibroblasts. However, the cells in vivo may behave entirely different as in vivo environment presents 3D matrix rather than 2D substrates of gelatin gels (present study) or widely used PA gels[19, 41, 42].
Interestingly, TRPV4 does not appear to play a role in the differentiation of atrial fibroblasts to myofibroblasts [5]. Instead, another TRP channel, TRPM7 is required for the atrial fibroblast differentiation in response to TGF-β1[5]. In our study, we found TRPM7 did not have any effect on fibroblast differentiation and that TRPV4 is essential for TGF-β1-matrix stiffness-induced differentiation of ventricular fibroblasts to myofibroblasts. It is plausible that this difference could arise due to entirely different mechanical environments presented by atria and ventricles, with the more mechanically active ventricular CFs using TRPV4 as the critical mechanosensor. Also, there are data suggesting the embryological origins of atrial and ventricular tissue differ[43], which also may be the reason for the difference in mechanical signaling.
4.1. Conclusion
In conclusion, our results show that TRPV4 channels mediate cardiac fibroblast differentiation by integrating mechanical and soluble signals that are derived from ECM stiffness and TGF-β1, respectively. These results coupled with a recent finding from global network analysis of ion channels in myocardial infarction identifying TRPV4 as one of the hubs for regulatory proteins [44] suggest a novel important biological role for TRPV4 in the regulation of left ventricle function in post-MI remodeling of the heart.
Supplementary Material
Highlights.
-
➢
TRPV4 channels mediate TGF-β1-induced differentiation of cardiac fibroblasts into myofibroblasts.
-
➢
TRPV4 channels are required for high stiffness, matrix-dependent TGF-β1- induced differentiation.
-
➢
TRPV4 expression and activity is enhanced by TGF-β1 treatment.
-
➢
TRPV4 may play a role in the integration of chemical (TGF-β1) and mechanical signals during cardiac fibroblast differentiation.
Acknowledgements
This work is supported by start-up funds from NEOMED (CKT) and, in part, by NIH- 1R15HL106442-01 (JGM and CKT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None
References
- 1.Flack EC, Lindsey ML, Squires CE, Kaplan BS, Stroud RE, Clark LL, et al. Alterations in cultured myocardial fibroblast function following the development of left ventricular failure. J Mol Cell Cardiol. 2006;40(4):474–483. doi: 10.1016/j.yjmcc.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 2.Squires CE, Escobar GP, Payne JF, Leonardi RA, Goshorn DK, Sheats NJ, et al. Altered fibroblast function following myocardial infarction. J Mol Cell Cardiol. 2005;39(4):699–707. doi: 10.1016/j.yjmcc.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 3.Hinz B, Gabbiani G. Fibrosis: recent advances in myofibroblast biology and new therapeutic perspectives. F1000 Biol Rep. 2010;2:78. doi: 10.3410/B2-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Follonier Castella L, Gabbiani G, McCulloch CA, Hinz B. Regulation of myofibroblast activities: calcium pulls some strings behind the scene. Exp Cell Res. 2010;316(15):2390–23401. doi: 10.1016/j.yexcr.2010.04.033. [DOI] [PubMed] [Google Scholar]
- 5.Du J, Xie J, Zhang Z, Tsujikawa H, Fusco D, Silverman D, et al. TRPM7- mediated Ca2+ signals confer fibrogenesis in human atrial fibrillation. Circ Res. 2010;106(5):992–1003. doi: 10.1161/CIRCRESAHA.109.206771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kohler R, Hoyer J. Role of TRPV4 in the Mechanotransduction of Shear Stress in Endothelial Cells. Boca Raton (FL): CRC Press; 2007. Chapter 27. [PubMed] [Google Scholar]
- 7.Liedtke WB. TRPV Channels' Function in Osmo- and Mechanotransduction. Boca Raton (FL): CRC Press; 2007. Chapter 22. [PubMed] [Google Scholar]
- 8.O'Neil RG, Heller S. The mechanosensitive nature of TRPV channels. Pflugers Arch. 2005;451(1):193–203. doi: 10.1007/s00424-005-1424-4. [DOI] [PubMed] [Google Scholar]
- 9.Thodeti CK, Matthews B, Ravi A, Mammoto A, Ghosh K, Bracha AL, et al. TRPV4 channels mediate cyclic strain-induced endothelial cell reorientation through integrin-to-integrin signaling. Circ Res. 2009;104(9):1123–1130. doi: 10.1161/CIRCRESAHA.108.192930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hatano N, Itoh Y, Muraki K. Cardiac fibroblasts have functional TRPV4 activated by 4alpha-phorbol 12,13-didecanoate. Life Sci. 2009;85(23–26):808–814. doi: 10.1016/j.lfs.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 11.Naugle JE, Olson ER, Zhang X, Mase SE, Pilati CF, Maron MB, et al. Type VI collagen induces cardiac myofibroblast differentiation: implications for postinfarction remodeling. Am J Physiol Heart Circ Physiol. 2006;290(1):H323–H330. doi: 10.1152/ajpheart.00321.2005. [DOI] [PubMed] [Google Scholar]
- 12.Olson ER, Naugle JE, Zhang X, Bomser JA, Meszaros JG. Inhibition of cardiac fibroblast proliferation and myofibroblast differentiation by resveratrol. Am J Physiol Heart Circ Physiol. 2005;288(3):H1131–H1138. doi: 10.1152/ajpheart.00763.2004. [DOI] [PubMed] [Google Scholar]
- 13.Shamhart PE, Luther DJ, Hodson BR, Koshy JC, Ohanyan V, Meszaros JG. Impact of type 1 diabetes on cardiac fibroblast activation: enhanced cell cycle progression and reduced myofibroblast content in diabetic myocardium. Am J Physiol Endocrinol Metab. 2009;297(5):E1147–E1153. doi: 10.1152/ajpendo.00327.2009. [DOI] [PubMed] [Google Scholar]
- 14.Swaney JS, Roth DM, Olson ER, Naugle JE, Meszaros JG, Insel PA. Inhibition of cardiac myofibroblast formation and collagen synthesis by activation and overexpression of adenylyl cyclase. Proc Natl Acad Sci USA. 2005;102(2):437–442. doi: 10.1073/pnas.0408704102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matthews BD, Thodeti CK, Tytell JD, Mammoto A, Overby DR, Ingber DE. Ultra-rapid activation of TRPV4 ion channels by mechanical forces applied to cell surface beta1 integrins. Integr Biol (Camb) 2010;2(9):435–442. doi: 10.1039/c0ib00034e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adapala RK, Talasila PK, Bratz IN, Zhang DX, Suzuki M, Meszaros JG, et al. PKCalpha mediates acetylcholine-induced activation of TRPV4-dependent calcium influx in endothelial cells. Am J Physiol Heart Circ Physiol. 2011;301(3):H757–H765. doi: 10.1152/ajpheart.00142.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghosh K, Thodeti CK, Dudley AC, Mammoto A, Klagsbrun M, Ingber DE. Tumor-derived endothelial cells exhibit aberrant Rho-mediated mechanosensing and abnormal angiogenesis in vitro. Proc Natl Acad Sci USA. 2008;105(32):11305–11310. doi: 10.1073/pnas.0800835105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parnas M, Peters M, Dadon D, Lev S, Vertkin I, Slutsky I, et al. Carvacrol is a novel inhibitor of Drosophila TRPL and mammalian TRPM7 channels. Cell Calcium. 2009;45(3):300–309. doi: 10.1016/j.ceca.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu F, Mih JD, Shea BS, Kho AT, Sharif AS, Tager AM, et al. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J Cell Biol. 2010;190(4):693–706. doi: 10.1083/jcb.201004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hinz B. Tissue stiffness, latent TGF-beta1 activation, and mechanical signal transduction: implications for the pathogenesis and treatment of fibrosis. Curr Rheumatol Rep. 2009;11(2):120–126. doi: 10.1007/s11926-009-0017-1. [DOI] [PubMed] [Google Scholar]
- 21.Romeo S, Eyden B, Prins FA, Briaire-de Bruijn IH, Taminiau AH, Hogendoorn PC. TGF-beta1 drives partial myofibroblastic differentiation in chondromyxoid fibroma of bone. J Pathol. 2006;208(1):26–34. doi: 10.1002/path.1887. [DOI] [PubMed] [Google Scholar]
- 22.Shephard P, Hinz B, Smola-Hess S, Meister JJ, Krieg T, Smola H. Dissecting the roles of endothelin, TGF-beta and GM-CSF on myofibroblast differentiation by keratinocytes. Thromb Haemost. 2004;92(2):262–274. doi: 10.1160/TH03-11-0669. [DOI] [PubMed] [Google Scholar]
- 23.Vaahtomeri K, Ventela E, Laajanen K, Katajisto P, Wipff PJ, Hinz B, et al. Lkb1 is required for TGFbeta-mediated myofibroblast differentiation. J Cell Sci. 2008;121(Pt 21):3531–3540. doi: 10.1242/jcs.032706. [DOI] [PubMed] [Google Scholar]
- 24.Ingber DE. Cellular mechanotransduction: putting all the pieces together again. Faseb J. 2006;20(7):811–827. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- 25.Alenghat FJ, Ingber DE. Mechanotransduction: all signals point to cytoskeleton, matrix, and integrins. Sci STKE. 2002;(119) doi: 10.1126/stke.2002.119.pe6. pe6. [DOI] [PubMed] [Google Scholar]
- 26.Kondo S, Kagami S, Urushihara M, Kitamura A, Shimizu M, Strutz F, et al. Transforming growth factor-beta1 stimulates collagen matrix remodeling through increased adhesive and contractive potential by human renal fibroblasts. Biochim Biophys Acta. 2004;1693(2):91–100. doi: 10.1016/j.bbamcr.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Margadant C, Sonnenberg A. Integrin-TGF-beta crosstalk in fibrosis, cancer and wound healing. EMBO Rep. 2010;11(2):97–105. doi: 10.1038/embor.2009.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thannickal VJ, Lee DY, White ES, Cui Z, Larios JM, Chacon R, et al. Myofibroblast differentiation by transforming growth factor-beta1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J Biol Chem. 2003;278(14):12384–12389. doi: 10.1074/jbc.M208544200. [DOI] [PubMed] [Google Scholar]
- 29.Chan MW, Arora PD, Bozavikov P, McCulloch CA. FAK, PIP5KIgamma and gelsolin cooperatively mediate force-induced expression of alpha-smooth muscle actin. J Cell Sci. 2009;122(Pt 15):2769–27681. doi: 10.1242/jcs.044008. [DOI] [PubMed] [Google Scholar]
- 30.Dalla Costa AP, Clemente CF, Carvalho HF, Carvalheira JB, Nadruz W, Jr, Franchini KG. FAK mediates the activation of cardiac fibroblasts induced by mechanical stress through regulation of the mTOR complex. Cardiovasc Res. 2010;86(3):421–431. doi: 10.1093/cvr/cvp416. [DOI] [PubMed] [Google Scholar]
- 31.Dugina V, Fontao L, Chaponnier C, Vasiliev J, Gabbiani G. Focal adhesion features during myofibroblastic differentiation are controlled by intracellular and extracellular factors. J Cell Sci. 2001;114(Pt 18):3285–3296. doi: 10.1242/jcs.114.18.3285. [DOI] [PubMed] [Google Scholar]
- 32.Greenberg RS, Bernstein AM, Benezra M, Gelman IH, Taliana L, Masur SK. FAK-dependent regulation of myofibroblast differentiation. Faseb J. 2006;20(7):1006–1008. doi: 10.1096/fj.05-4838fje. [DOI] [PubMed] [Google Scholar]
- 33.Vi L, de Lasa C, DiGuglielmo GM, Dagnino L. Integrin-linked kinase is required for TGF-beta1 induction of dermal myofibroblast differentiation. J Invest Dermatol. 2011;131(3):586–593. doi: 10.1038/jid.2010.362. [DOI] [PubMed] [Google Scholar]
- 34.Asano Y, Ihn H, Yamane K, Jinnin M, Tamaki K. Increased expression of integrin alphavbeta5 induces the myofibroblastic differentiation of dermal fibroblasts. Am J Pathol. 2006;168(2):499–510. doi: 10.2353/ajpath.2006.041306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carracedo S, Lu N, Popova SN, Jonsson R, Eckes B, Gullberg D. The fibroblast integrin alpha11beta1 is induced in a mechanosensitive manner involving activin A and regulates myofibroblast differentiation. J Biol Chem. 2010;285(14):10434–10445. doi: 10.1074/jbc.M109.078766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Honda E, Yoshida K, Munakata H. Transforming growth factor-beta upregulates the expression of integrin and related proteins in MRC-5 human myofibroblasts. Tohoku J Exp Med. 2010;220(4):319–327. doi: 10.1620/tjem.220.319. [DOI] [PubMed] [Google Scholar]
- 37.Ding Q, Gladson CL, Wu H, Hayasaka H, Olman MA. Focal adhesion kinase (FAK)-related non-kinase inhibits myofibroblast differentiation through differential MAPK activation in a FAK-dependent manner. J Biol Chem. 2008;283(40):26839–26849. doi: 10.1074/jbc.M803645200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol. 2007;179(6):1311–1323. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buscemi L, Ramonet D, Klingberg F, Formey A, Smith-Clerc J, Meister JJ, et al. The single-molecule mechanics of the latent TGF-beta1 complex. Curr Biol. 2011;21(24):2046–2054. doi: 10.1016/j.cub.2011.11.037. [DOI] [PubMed] [Google Scholar]
- 40.Wells RG, Discher DE. Matrix elasticity, cytoskeletal tension, and TGF-beta: the insoluble and soluble meet. Sci Signal. 2008;1(10) doi: 10.1126/stke.110pe13. pe13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hinz B, Dugina V, Ballestrem C, Wehrle-Haller B, Chaponnier C. Alpha-smooth muscle actin is crucial for focal adhesion maturation in myofibroblasts. Mol Biol Cell. 2003;14(6):2508–2519. doi: 10.1091/mbc.E02-11-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Z, Dranoff JA, Chan EP, Uemura M, Sevigny J, Wells RG. Transforming growth factor-beta and substrate stiffness regulate portal fibroblast activation in culture. Hepatology. 2007;46(4):1246–1256. doi: 10.1002/hep.21792. [DOI] [PubMed] [Google Scholar]
- 43.Mikawa T, Fischman DA. The polyclonal origin of myocyte lineages. Annu Rev Physiol. 1996;58:509–521. doi: 10.1146/annurev.ph.58.030196.002453. [DOI] [PubMed] [Google Scholar]
- 44.Zhou R, Hang P, Zhu W, Su Z, Liang H, Du Z. Whole genome network analysis of ion channels and connexins in myocardial infarction. Cell Physiol Biochem. 2011;27(3–4):299–304. doi: 10.1159/000327956. [DOI] [PubMed] [Google Scholar]
- 45.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3(5):349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 46.Follonier L, Schaub S, Meister JJ, Hinz B. Myofibroblast communication is controlled by intercellular mechanical coupling. J Cell Sci. 2008;121(Pt 20):3305–3316. doi: 10.1242/jcs.024521. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.