Abstract
Iron has emerged as a significant cause of neurotoxicity in several neurodegenerative conditions, including Alzheimer's disease (AD), Parkinson's disease (PD), sporadic Creutzfeldt-Jakob disease (sCJD), and others. In some cases, the underlying cause of iron mis-metabolism is known, while in others, our understanding is, at best, incomplete. Recent evidence implicating key proteins involved in the pathogenesis of AD, PD, and sCJD in cellular iron metabolism suggests that imbalance of brain iron homeostasis associated with these disorders is a direct consequence of disease pathogenesis. A complete understanding of the molecular events leading to this phenotype is lacking partly because of the complex regulation of iron homeostasis within the brain. Since systemic organs and the brain share several iron regulatory mechanisms and iron-modulating proteins, dysfunction of a specific pathway or selective absence of iron-modulating protein(s) in systemic organs has provided important insights into the maintenance of iron homeostasis within the brain. Here, we review recent information on the regulation of iron uptake and utilization in systemic organs and within the complex environment of the brain, with particular emphasis on the underlying mechanisms leading to brain iron mis-metabolism in specific neurodegenerative conditions. Mouse models that have been instrumental in understanding systemic and brain disorders associated with iron mis-metabolism are also described, followed by current therapeutic strategies which are aimed at restoring brain iron homeostasis in different neurodegenerative conditions. We conclude by highlighting important gaps in our understanding of brain iron metabolism and mis-metabolism, particularly in the context of neurodegenerative disorders. Antioxid. Redox Signal. 20, 1324–1363.
I. Overview of Brain Iron Homeostasis and Scope of the Review
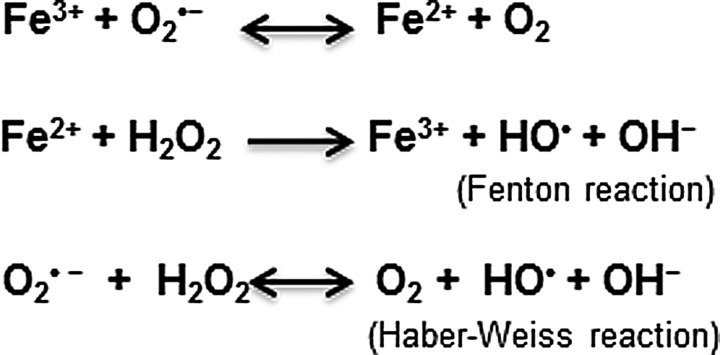
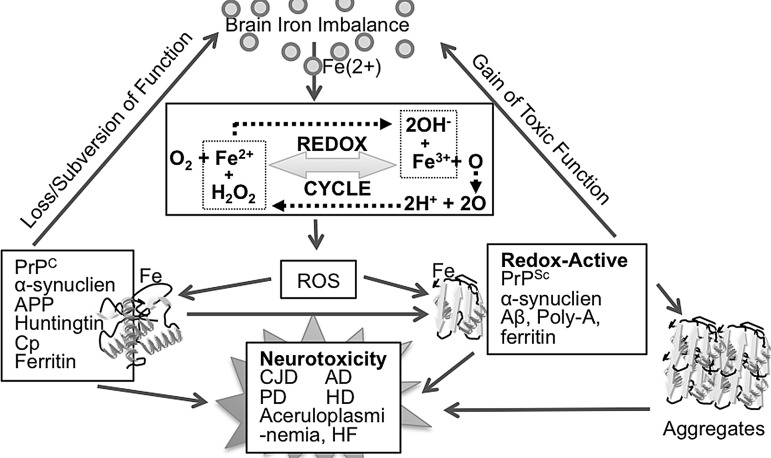
Redox-active metals such as iron and copper are necessary for normal cellular function because of their ability to participate in electron-transfer reactions. Iron, in particular, participates in several vital biochemical reactions, including synthesis of DNA, RNA, and proteins; serves as a co-factor for several enzymatic reactions, including heme synthesis; and aids in the synthesis of myelin that is necessary for brain function (197, 451). At the same time, free iron is cytotoxic due to its ability to react with molecular oxygen that is usually abundant in metabolically active cells to generate hydroxyl radicals and hydroxyl anions by the Fenton chemistry (186) (Fig. 1). These cause lipid peroxidation, DNA strand breaks, and protein modifications that ultimately lead to cell death (251). Metabolism of iron is, therefore, tightly regulated by a set of iron uptake, transport, and storage proteins that are themselves regulated by iron at the cellular and systemic levels.
FIG. 1.
Fenton and Haber Weiss reactions resulting in the generation of hydroxyl radicals in the presence of iron.
Regulation of iron homeostasis begins at the gastro-intestinal tract where duodenal cells are primed to absorb iron based on systemic iron needs. Once in circulation, most of the iron is bound tightly to serum transferrin (Tf). Small amounts associate with albumin, citrate, amino acids, and other unidentified negatively charged ligands. Most parenchymal cells take up iron from Tf for their metabolic needs by the Tf-transferrin receptor 1 (Tf-TfR) pathway. Non-transferrin bound iron (NTBI) is taken up by the divalent metal transporter 1 (DMT1) as well as by Zip 14/Slc39a14 (197, 275). Within the cell, iron is used for essential metabolic processes, and any excess is stored in a relatively inert form in cytosolic ferritin (451). The brain acquires iron from the systemic circulation through a tightly controlled mechanism at the blood brain barrier (BBB). Within the brain, iron serves essential functions that are best exemplified by brain disorders which arise from iron deficiency, excess, or mis-metabolism (97, 224, 312, 413). Table 1 provides a partial list of human disorders that result from dysfunction or absence of one or more proteins involved in brain iron metabolism, and representative animal models.
Table 1.
Mouse Models and Corresponding Human Disorders of Iron Metabolism
| Gene | Mouse model | Iron phenotype | Human disease parallel | Reference |
|---|---|---|---|---|
| Abcb7 | Abcb7−/− | Siderocytosis | X-linked sideroblastic anemia | (153) |
| Abcb7E433K | ||||
| Aco1 | Irp1−/− | NA | Cardiac problems | (163) |
| Alas2 | Alas2−/− | Increased iron in primitive erythroid cells | X-linked sideroblastic anemia | (153) |
| Beta-2 microglobulin | B2m−/− | Parenchymal iron deposition; decreased hepcidin | ND | (382) |
| Bmp6 | Bmp6−/− | Increased body iron; decreased hepcidin | ND | (16, 304) |
| Ceruloplasmin | Cp−/− | Iron accumulation in hepatocytes and macrophages | Aceruloplasminemia | (189, 211, 341) |
| DMT1 (G185R) | mk (missense) | Systemic iron deficiency; impaired iron uptake from the duodenum and by erythroid precursors | ND | (52, 151, 320) |
| Belgrade rat | ||||
| Duodenal cytochrome B | Cybrd1−/− | Little impact | ND | (153) |
| Feline leukemia virus receptor | Flvcr−/− | Macrocytic anemia | ND | (153) |
| Ferrochelatase | FECHm1Pas | Microcytic hypochromic anemia & severe porphyria | ND | (279) |
| FECH−/− | Homozygous state is embryonic lethal; Heterozygotes have decreased FECH activity and mild porphyria | ND | (284) | |
| Ferroportin | Slc40a1 (missense) | Iron loading of Kupffer cells, high serum ferritin, low transferrin saturation | ND | (153) |
| Fpn−/− | Increased iron absorption and overload | Hfe4 hemochromatosis | (124) | |
| Frataxin | Fxn−/− with human mutant Fxn YAC constructs | Mitochondrial iron deposits; neurodegeneration; cardiomyopathy; embryonic lethal | Friedreich's ataxia | (10, 153) |
| Fxn−/− | Embryonic lethal | NA | (153) | |
| H-ferritina | Fth+/− | Elevated tissue and serum L-ferritin | ND | (147, 428) |
| Haptoglobin | Hp−/− | Increased export of iron from duodenum to plasma | ND | (270, 293) |
| Heme oxygenase 1 | Hmox1−/− | Anemia; low serum iron; tissue iron deposition | Hmox 1 deficiency | (354) |
| Hemojuvelin | Hjv−/− | Increased body iron, decreased hepcidin | ND | (153) |
| Hemopxin | Hx−/− | Increased CNS iron | ND | (153) |
| Hepcidin | HAMP (transgene) | Iron deficiency; Anemia | NA | (261) |
| Hepcidinb | Usf2−/− | Hepatocellular iron accumulation; decreased macrophage iron; increased Tf saturation | Juvenile hemochromatosis | (321) |
| Hepcidin/HFE | Hfe−/− | Amelioration of hepatic iron loading relative to Hfe−/− mice; increased body iron; decreased hepcidin | NA | (321) |
| HfeC282Y/C282 | ||||
| HFEH63D/H63D | ||||
| Hephaestin | sla (deletion) | Microcytic hypochromic anemia; impaired iron transfer from the duodenum | ND | (447) |
| Hfe | Hfe−/− | Hepatocellular iron accumulation; decreased macrophage iron; increased Tf saturation | Hfe hemochromatosis | (484) |
| HfeC282Y/C282Yc | ||||
| Irp2 | Irp2−/− | Microcytic anemia; duodenal and hepatic iron loading | Pulmonary disease | (89, 163) |
| Mitoferrin 1 | Mfrn1−/− | Severe anemia | ND | (153) |
| Sec1511 | Hbd | Deficiency in Tf cycling | ND | (464) |
| Slc25a37 | Mfrn1−/− | Embryonic lethal with profound anemia | Tumors or cancer of the prostate | (81) |
| Smad4 | Smad4−/− (liver specific) | Increased body iron; decreased hepcidin | ND | (452) |
| Steap3 | Steap3−/− | Congenital, hypochromic, microcytic anemia; elevated red cell zinc protoporphyrin; functional erythroid iron deficiency; Impaired ferriereductase activity | ND | (153, 279, 327) |
| Nm1054 (deletion) | ||||
| Tmprss6 (matriptase-2) | Tmprss6−/− | Microcytic anemia; low iron stores; low serum iron; increased hepcidin | ND | (128) |
| Tmprss6msk/msk | ||||
| Transferrin | Hpx | Microcytic hypochromic anemia; tissue iron deposition | Atransferrinemia | (434) |
| Transferrin receptor-1d | TfR+/− | Microcytic hypochromic erythrocytes; decreased iron stores; embryonic death | ND | (264) |
| Transferrin receptor-2 | TfRr2246x/246 | Hepatocellular iron accumulation; decreased macrophage iron; increased Tf saturation | TfR2 hemochromatosis | (125, 152, 222) |
| TfR2−/− | ||||
| Transferrin | Tfhpx/hpx | Severe anemia, iron overload | Congenital hypotransferrinemia | (153) |
Fth−/− mice: early embryonic lethality.
Includes deletion of gene for upstream stimulatory factor-2.
Mutation is in mouse codon 294.
Tf−/− mice: embryonic lethal by E 12.5.
ND, not described; NA, not applicable; BMP, bone morphogenetic protein; Cp, ceruloplasmin; DMT1, divalent metal transporter 1; FECH, ferrochelatase; Fxn, frataxin; HFE, hereditary hemochromatosis gene product; Hp, hephaestin; Hpx, hemopexin; Irp1/Irp2, iron regulatory proteins 1 and 2; Tf, transferrin; TfR, transferrin receptor.
Next, we discuss recent information on the regulation of iron in the systemic circulation, across the BBB, within the complex milieu of the brain, and consequences of brain iron mis-metabolism and associated brain disorders. The main emphasis is on brain disorders associated with dysfunction of iron-modulating proteins ferritin and ceruloplasmin (Cp) that result in hereditary ferritinopathies and aceruloplasminemia, respectively, and neurodegenerative conditions which are associated with brain iron mis-metabolism such as Alzheimer's disease (AD), Parkinson's disease (PD), and sporadic Creutzfeldt-Jakob disease (sCJD). Recent information on the regulation of iron transport to the mitochondria is discussed in the context of Friedreich's ataxia (FA) and sideroblastic anemias to highlight the importance of iron mis-metabolism at this site on brain function. Current information on the yeast Saccharomyces cerevisiae (S. cerevisiae) as models of PD, FA, and Huntington's disease (HD) is discussed to emphasize its usefulness in understanding the complexity underlying these conditions. Finally, therapeutic strategies aimed at reducing iron-induced oxidative stress, including chelation, are discussed.
II. Molecular Regulation of Iron Metabolism
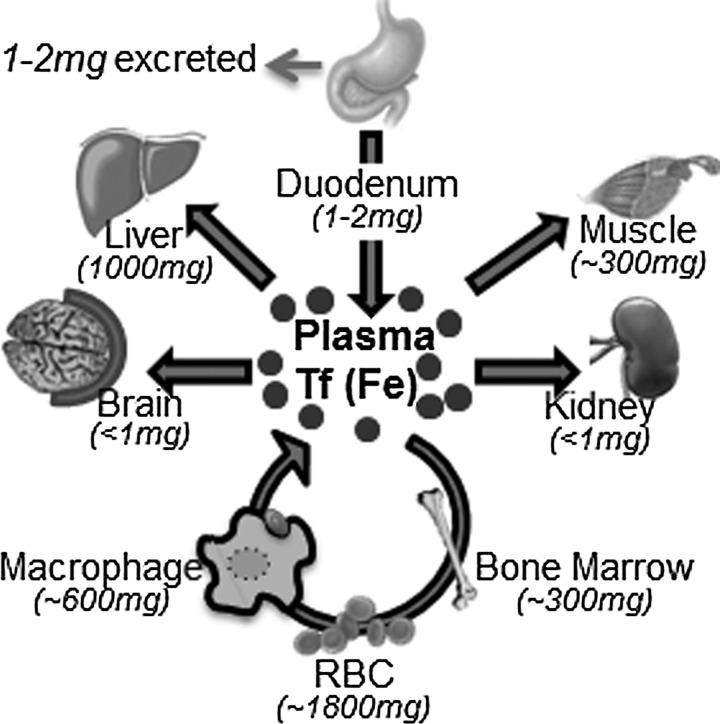
The only route of iron acquisition by the body is through duodenal epithelial cells that absorb heme and non-heme iron from ingested food in response to body iron stores. Absorbed iron is loaded to serum Tf for utilization by systemic organs and for hematopoiesis. Daily absorption of iron in a healthy individual rarely exceeds 1–2 mg, and a similar amount is lost through intestinal epithelial shedding, desquamation, or menstruation. Most of the body iron (80%) is in hemoglobin in the erythroid compartment, and 10%–15% is in myoglobin in muscle fibers. Other tissues contain variable amounts of iron that is used for metabolic purposes. The liver is the main site of iron storage where excess iron is stored in ferritin within the parenchymal cells and reticuloendothelial macrophages (Fig. 2). Iron from senescent red blood cells is recycled by the spleen and bone marrow macrophages that degrade hemoglobin and release associated iron for reloading to serum Tf for re-utilization (Fig. 3) (197, 451).
FIG. 2.
Systemic iron cycle. Duodenal enterocytes absorb 1–2 mg of dietary iron daily and transport to plasma Tf. Most of the absorbed iron is utilized by precursor red blood cells in the bone marrow for Hb synthesis. Iron from senescent red blood cells is recovered by macrophages and released to the plasma for re-utilization. Excess iron is stored in the liver and macrophages. Other tissues take up various amounts of iron from plasma Tf. Only 1–2 mg of iron is excreted daily. Numbers in parentheses indicate the amount of iron in each tissue. Tf, transferrin.
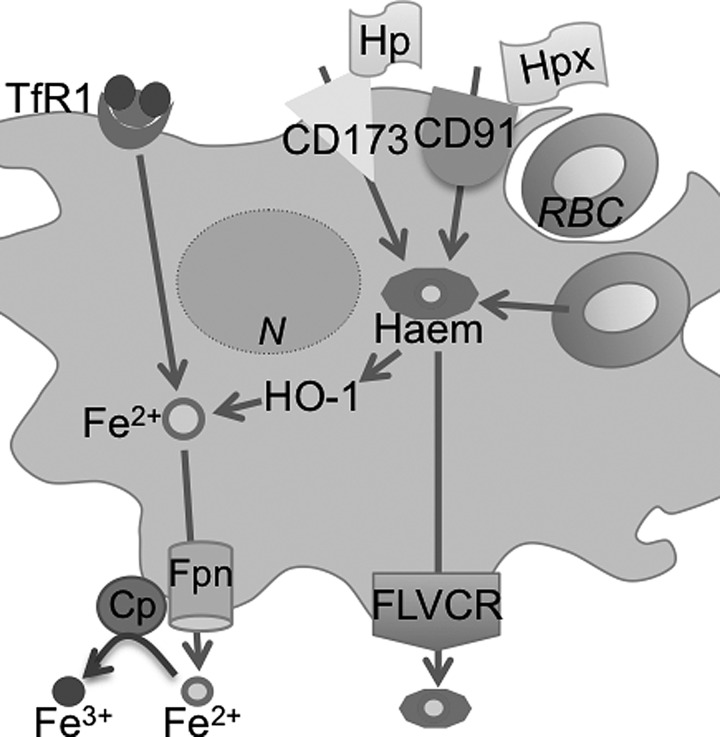
FIG. 3.
Iron recycling by macrophages. Almost 20–30 mg of Iron is recycled by the macrophages and reused for erythropoiesis daily. Macrophages phagocytize senescent red blood cells, and the heme-associated iron released by HO-1 activity is exported out by Fpn for loading to Tf. Macrophages also acquire heme by endocytosis of haptoglobin and hemopexin. In addition, these cells possess the capability of acquiring iron by the Tf/TfR pathway and exporting heme through the FLVCR receptor. CD173 (marker of early hematopoiesis) and CD91 (low density lipoprotein receptor 1 or α-2-macroglobulin receptor). Cp, ceruloplasmin; Fe2+, ferrous iron; Fe3+, ferric iron; FLVCR, feline leukemia virus subgroup C receptor; Fpn, ferroportin; HO-1, heme-oxygenase-1; Hp, hephaestin; TfR, transferrin receptor.
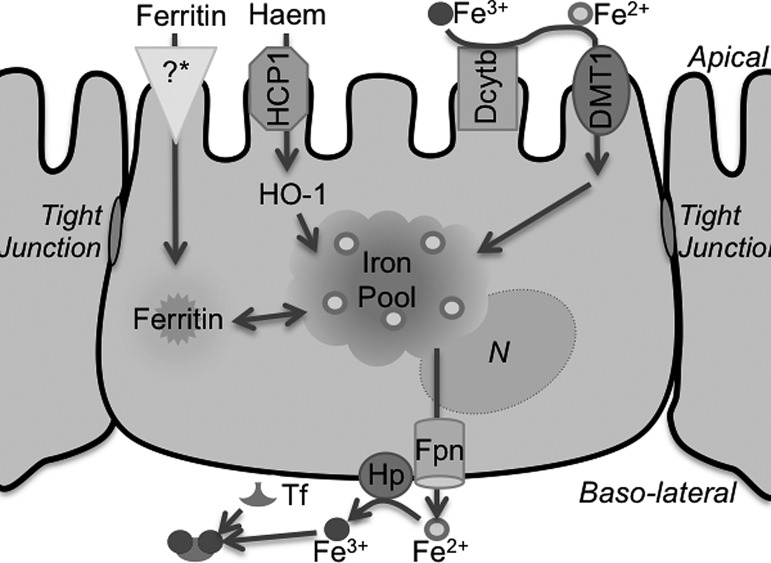
Duodenal epithelial cells form a tight monolayer that separates intestinal luminal contents from the blood stream while allowing access to selected molecules. Heme and elemental iron, the main sources of iron in ingested food, are absorbed through distinct proteins on the apical membrane of epithelial cells. Heme is taken up by the heme carrier protein and broken down by heme-oxygenase-1 (HO-1) to release the associated iron moiety. Elemental or non-heme iron in ingested food is mainly in the relatively stable ferric (Fe3+) form, and, unless associated with chelatable compounds, is reduced to ferrous (Fe2+) iron by the ferrireductase (FR) duodenal cytochrome b (Dcytb) on the apical plasma membrane before uptake by DMT1. In the cytosol, iron contributes to the labile iron pool and is utilized for metabolic reactions, or is transported to the basolateral membrane for efflux to the blood stream by the coupled action of the metal transporter ferroportin (Fpn) and the ferroxidase hephaestin (Hp) that oxidizes released Fe2+ to the Fe3+ form for loading to serum Tf (Fig. 4). Excess Fe2+ is converted to Fe3+ by the ferroxidase activity of ferritin H-chain (Ft-H) and stored in cytosolic ferritin (451, 462).
FIG. 4.
Iron transport across the enterocyte. Oxidized Fe3+ iron in the food is reduced by the ferrireductase Dcytb on the AP plasma membrane to the Fe2+ form for transport by DMT1 to the cytosol. Heme is taken up via heme carrier protein 1 and is degraded by HO-1 to release Fe2+ iron. A receptor for ferritin has also been described on the AP plasma membrane. Iron from these sources enters the common labile iron pool, and is either stored in ferritin or exported from the baso-lateral membrane by the Fpn/Hp complex that oxidizes exported Fe2+ iron to the Fe3+ form for loading to plasma Tf. Dcytb, duodenal cytochrome b; DMT1, divalent metal transporter 1.
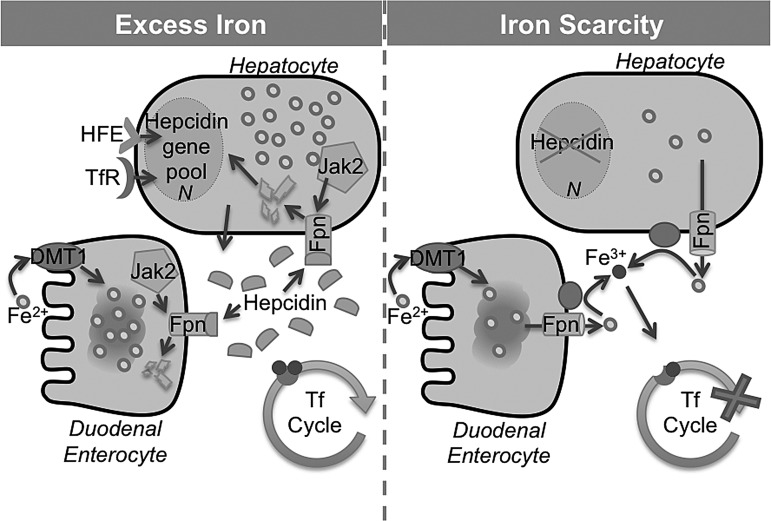
The uptake and release of iron from the duodenal epithelial cells to the blood stream is regulated by hepcidin, a peptide hormone that is released by hepatocytes in response to iron saturation of Tf. When serum iron is adequate or high, hepcidin binds to the extracellular region of Fpn between transmembrane domains 7 and 8. Binding causes Jak-2 mediated tyrosine phosphorylation at residues 302 and 303 in the cytosolic loop of Fpn, which is internalized, dephosphorylated, ubiquitinated, and, ultimately, degraded in the late endosome/lysosome compartment, thus blocking transport of iron from the basolateral membrane to the blood stream. When iron saturation of Tf is low, expression of hepcidin is down-regulated, sparing Fpn and allowing iron transport (313) (Fig. 5). Non-transported iron is lost by shedding of epithelial cells into the intestinal lumen.
FIG. 5.
Role of hepcidin in systemic iron homeostasis. Systemic iron homeostasis is regulated by hepcidin, a peptide hormone that is released by hepatocytes in response to iron saturation of serum Tf. Excess iron (left) triggers the release of hepcidin that binds to Fpn. This initiates the binding of Jak2 to the cytosolic portion of Fpn. Subsequent autophosphorylation of Jak2 leads to phosphorylation of Fpn, followed by internalization and degradation of the Fpn-hepcidin complex. In an iron deficiency (right), hepcidin expression is inhibited, sparing Fpn that restores normal iron transport. Figure adapted form Hentze et al. (197). HFE, hereditary hemochromatosis gene product.
Most cells are equipped for taking up Tf-bound and NTBI from the serum, but with varying efficiency. Cells that express TfR1 use the Tf-TfR pathway, a process modulated by the hemochromatosis gene product (HFE) (164). The Tf-TfR complex is internalized in clathrin-coated pits, and Tf-bound Fe3+ is released in the acidic environment of the endosomes while the Tf-TfR complex is recycled back to the plasma membrane. Fe3+ released in endosomes is reduced to Fe2+ by membrane-bound FR proteins such as the Steap family of proteins (Steap 2–4, depending on the organ), and transported to the cytosol through DMT1 where it contributes to the labile iron pool. Excess Fe2+ is oxidized and stored in the ferritin shell in the cytosol. Uptake of NTBI is mediated by DMT1 after reduction to Fe2+ by FR proteins at the plasma membrane, and joins the cellular labile iron pool or is stored within ferritin. Iron homeostasis within the cells is maintained by the coordinated regulation of Tf, TfR1, and ferritin at the transcriptional and translational level by iron regulatory proteins 1 and 2 (IRP1/IRP2). Based on cellular iron levels, IRPs interact with iron-responsive elements (IREs) in the mRNA of specific proteins, modulating their stability (and translation) or degradation to meet cellular iron demands (196, 197, 218) (Fig. 6). Specific regulation of these proteins is discussed next with relevance to brain disorders that are associated with iron mis-metabolism.
FIG. 6.
Iron regulatory proteins. IRP-IRE system regulates iron uptake and storage by modulating the expression of mRNAs coding for iron uptake, storage, and export proteins. When iron levels are low (top), IRP binds to 3′ IREs of target mRNAs (TfR1, DMT1), stabilizing the transcript to enable translation and increase uptake of iron. Concomitant binding to 5′ IRE of target mRNAs (ferritin, Fpn, ALAS2, HIF-2α, APP, and, possibly, α-synuclein) prevents binding of the 43S pre-initiation complex, thus inhibiting translation and reducing iron storage and efflux. In the presence of excess iron (bottom), IRP1 incorporates ISC to acquire aconitase activity while IRP2 is degraded. IRPs, therefore, lose their affinity for IREs, resulting in degradation of mRNAs with 3′ IRE sequences coding for iron uptake proteins and translation of mRNAs with 5′ IREs encoding iron storage and efflux proteins. Figure adapted from Crichton et al. (97). ALAS2, δ-aminolevulinate synthase 2; APP, amyloid precursor protein; HIF-2α, hypoxia-inducible factor-2α; ISC, iron-sulfur cluster; IREs, iron-responsive elements; IRP, iron regulatory protein.
III. Iron Homeostasis Within the Brain and Regulation at the BBB
Brain is rich in non-heme iron, reflecting its high metabolic rate. Most of the iron is concentrated in the substantia nigra pars compacta (SN) and basal ganglia, the latter reaching levels equivalent to the liver (126, 177, 183). The BBB protects the brain from fluctuations in systemic iron levels, and disturbances of iron homeostasis in peripheral organs have a minimal effect on brain iron metabolism. Thus, levels of iron and iron-modulating proteins in the serum and cerebrospinal fluid (CSF) that bathe the brain differ significantly (311).
The BBB is formed by a monolayer of polarized capillary endothelial cells with tight junctions that regulate the transport of cargo from blood at the luminal or apical surface to the abluminal or basolateral surface bathed by the CSF and brain interstitial fluid (Fig. 7) (110, 376). Transport of iron across this barrier is mediated by the Tf-TfR and DMT1-Fpn pathways as in systemic organs. Fe2+ released by Fpn at the basolateral surface of endothelial cells is oxidized to Fe3+ by Cp expressed on astrocytic foot processes lining the endothelial cells (210), and captured by circulating Tf in the brain interstitial fluid and CSF. A significant amount of transported iron circulates in association with citrate, ascorbate, or ATP (224, 286). Brain Tf is secreted by cells of the choroid plexus and oligodendrocytes, though participation of the latter is controversial. Serum Tf does not gain access to the brain, and brain Tf does not leak into the serum except in certain pathological conditions (376). Other pathways of iron transport across the BBB involve uptake of ferritin by a specific though unidentified receptor on the apical surface of endothelial cells (Fig. 8) (53, 150, 429).
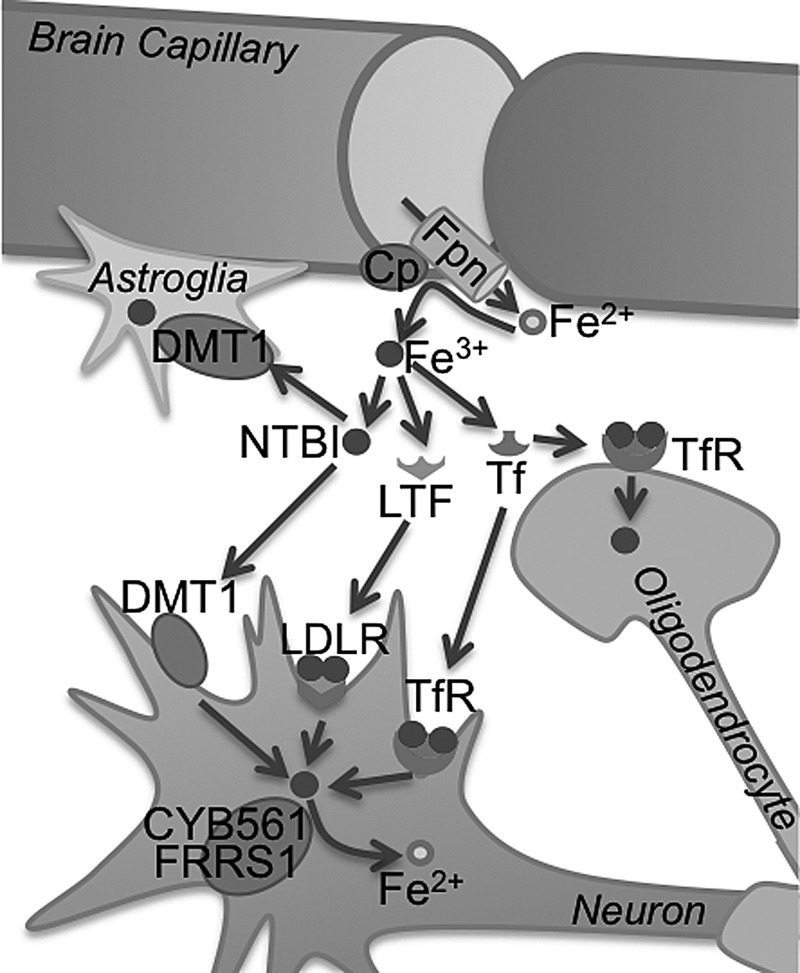
FIG. 7.
Transport of iron to the brain. Iron is exported from endothelial cells of the BBB to brain interstitial fluid through Fpn, and is oxidized to Fe3+ by Cp. Within the brain, iron is bound to Tf or exists as NTBI. Tf bound iron is taken up by TfRs on the neuronal and oligodendroglial plasma membrane. NTBI enters the neuron and astroglia by the DMT1 pathway. Figure adapted from Ke and Qian (224). BBB, blood brain barrier; LDLR, low density lipoprotein receptor; LTF, lactotransferrin; NTBI, non-transferrin bound iron.
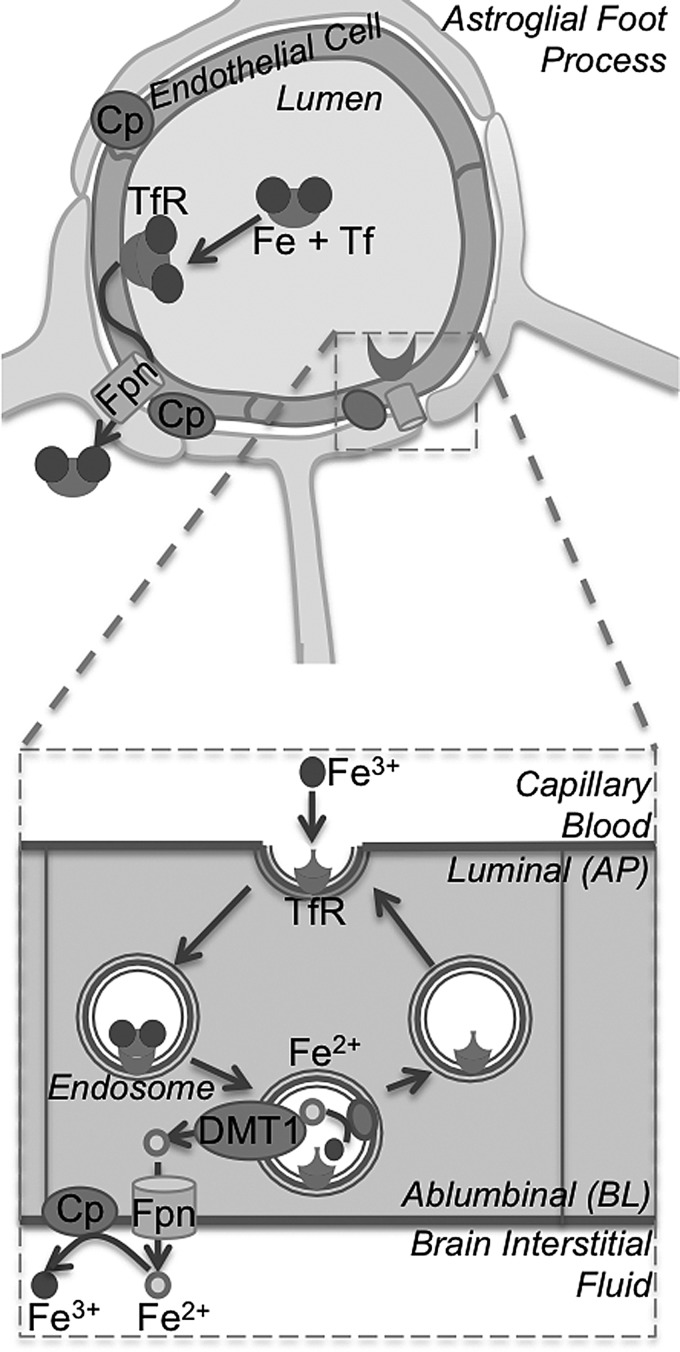
FIG. 8.
Iron transport across the BBB. The BBB (top) is formed by a tight monolayer of endothelial cells surrounded by astroglial foot processes on the baso-lateral surface. Tf-bound Fe3+ iron is captured by TfRs on the luminal or apical membrane of endothelial cells and released in the acidic environment of the endosomes (below). Released Fe3+ iron is reduced to the Fe2+ form before transport across DMT1 to the cytosol. Subsequently, iron is transported across the abluminal or basolateral membrane through Fpn to the brain interstitial fluid where it is oxidized back to Fe3+ by Cp or Hp for loading to Tf. The TfR is recycled back to the apical membrane. Figure adapted from Crichton et al. (97) and Ke and Qian (224).
Different cell types in the brain acquire iron by distinct pathways, and iron homeostasis within the organ is maintained by a complex set of interacting mechanisms. Neurons express abundant TfR and acquire most of their iron from Tf. Astrocytes express DMT1 and reduce Fe3+ in the interstitial fluid to the Fe2+ form before uptake (253). Oligodendrocyte progenitors acquire most of their iron from Tf by the TfR1 pathway, and from ferritin through the Tim-2 receptor as they mature (429). Most brain cells express cytosolic ferritin for storing excess iron, but the abundance of ferritin varies based on functional requirements for iron in different cell types. Neurons contain the least, and microglia contain the most amount of cytosolic ferritin. In addition, the abundance of ferritin subunits varies between cell types. Neuronal ferritin has a higher proportion of Ft-H, oligodendrocyte ferritin has equivalent amounts of Ft-H and ferritin light chain (Ft-L), and microglial ferritin is comprised mostly of Ft-L chains. However, the relative abundance of Ft-H is higher than Ft-L in total brain homogenates compared with liver and spleen tissue. Excess iron is exported from cells by the combined action of Fpn and Hp, similar to other cell types (281, 312).
It is notable that the iron saturation of CSF Tf is ∼100%, while that of serum Tf is ∼30% (53, 312). Several factors are likely to contribute to this observation, including low concentrations of Tf in the CSF relative to serum, high concentrations of ascorbic acid, and low concentrations of Cp that are necessary for oxidizing available Fe2+ to the Fe3+ form for binding to Tf. Serum Tf, therefore, has a much higher buffering capacity under conditions of iron overload relative to CSF Tf, increasing the vulnerability of neuronal cells to iron-induced toxicity. Thus, redundant pathways of iron import, export, and storage exist to protect the neurons from iron imbalance. This is exemplified by mouse models of hypo-transferrinemia and loss of function mutations of Fpn that show minimal change in brain iron levels (38, 124). These observations also indicate that mechanisms regulating brain iron homeostasis are not fully known. Although mouse models carrying a deletion of one or more genes involved in iron regulation or representing specific brain disorders have been helpful (Table 1), continued research in this area is necessary to understand the pathogenesis of brain disorders that are associated with iron metabolism.
IV. Brain Iron Mis-Metabolism and Associated Disorders
Functional mutation(s) in iron-modulating proteins disrupt iron homeostasis in systemic organs and the brain to a varying extent, and, in some cases, result in specific human disorders (61, 226, 283). Ferritinopathies and aceruloplasminemia are clear examples where dysfunction of ferritin or absence of Cp alter brain iron homeostasis and induce neurotoxicity. The correlation between brain iron metabolism and neurotoxicity associated with neurodegenerative conditions such as AD, PD, HD, and sCJD remains controversial (13). Some studies suggest non-specific co-precipitation of iron and other metals with aggregated proteins, while others associate brain iron directly with disease pathogenesis (129, 372). Current information on the role of iron in the pathogenesis of some of these disorders is reviewed next.
A. Ferritin and ferritinopathies
Ferritin performs the essential function of storing excess iron within its shell, and is considered a pro- as well as an anti-oxidant. In addition to its function in iron storage, ferritin is involved in several physiological and pathological processes. We will focus on the role of ferritin in brain iron metabolism and disorders that result from its dysfunction (20, 281, 451).
Ferritin is a ubiquitous, mainly cytosolic, globular protein of ∼450 kDa comprising 24 subunits of Ft-H and Ft-L chains (20, 21, 87, 216). Ft-H possesses an active ferroxidase center that catalyzes the oxidation of Fe2+ to the Fe3+ form, while Ft-L mediates its nucleation within the protein shell for storage. Together, these chains form a nano-cage that can store approximately 4500 Fe3+ ions in a non-toxic, bioavailable form as the mineral ferrihydrite (258, 306, 425). High Ft-H:Ft-L ratios are found in tissues with high rates of oxidative respiration to maximize iron turnover, while high Ft-L:Ft-H ratios are observed in tissues specialized for iron storage, such as the liver (20, 21, 64). Ferritin also circulates in the CSF, although at 10-fold lower concentrations than in the plasma. Hemosiderin is a degradation product of ferritin and is often observed in areas of hemorrhage and massive cell death. Iron within hemosiderin is insoluble, bio-unavailable, and unlikely to react with free radicals.
The precise mechanism of binding, storage, and release of iron from ferritin requires more clarity. Available information suggests that a cytosolic iron chaperone Poly (rC)-binding protein 1 binds cytosolic iron for delivery to ferritin (398). Poorly characterized ferritin-binding proteins, amino acids, and small molecules regulate the release of iron from ferritin, supporting the gated pore model (273, 274, 427). Stored iron is released in response to low intracellular iron, thus maintaining equilibrium between ferritin iron and the labile iron pool. Expression of ferritin is regulated post-transcriptionally by IRPs, and degradation is induced by certain iron chelators and lysosomal activity (108, 227). Free radicals, high levels of ascorbic acid, and acidic milieu also trigger release of iron from ferritin, leading to oxidative burst and cytotoxicity. The cell protects itself against such events by up-regulating ferritin approximately 80-fold through the antioxidant-response element, in part by binding to the transcription factor NF-E2-related factor 2 (200, 348). The resultant increase in ferritin sequesters iron and minimizes the generation of reactive oxygen species (ROS) (200, 346, 431). Other regulators of ferritin expression include inflammatory cytokines, tumor necrosis factor alpha, and interleukins, IL-1 and IL-6 (431). Inflammation up-regulates the expression of Ft-H, which sequesters iron as a protection against oxidative stress (139, 432, 457), resulting in the anemia of chronic inflammation (461). This is compounded further by the release of hepcidin that blocks export of iron from the macrophage/reticuloendothelial system, resulting in intracellular iron overload (313, 460). Thus, the role of ferritin extends beyond that of iron storage.
Hereditary ferritinopathy or neurodegeneration with brain iron accumulation (NBIA) type 3 is an autosomal dominant condition that affects motor and cognitive function (94, 316). The underlying cause is duplication of nucleotides in exon 4 of Ft-L gene on chromosome 19 that alters the C-terminus of the translated product (28). Mutant Ft-L (Phe167SerfsX26) accumulates intracellularly in inclusion bodies that include wild-type Ft-L and Ft-H polypeptides (28, 59). Crystal structure of mutant Ft-L at 2.85 Å resolution shows insertion of structures that are similar to wild-type Ft-L between residues Ile-5 and Arg-154, resulting in multiple polypeptide conformations instead of the normal E-helices, and disruption of the fourfold (∼2 Å) or threefold (3.4 Å) axis pores in the ferritin nano-cage (27). This structural disruption manifests as loss of iron storage ability and accumulation of ferritin aggregates in association with iron (25, 27). The accompanying increase in brain iron levels triggers translation of additional ferritin by the IRE/IRP system, accentuating ferritin aggregation and toxicity (28, 29, 442, 444). The inclusions stain positive for iron, ferritin, and ubiquitin (101), and are prominent in the nuclei of neurons, oligodendrocytes, and microglial cells as well as the extracellular space, increasing brain iron levels. Similar inclusions are also noted in muscles, peripheral nerves, and the skin of affected individuals (389, 442, 444). So far, six such insertions, 442InsC (292), 442Ins 4nt (246), 458InsA (116), 460InsA (101), 469–484dup16nt (329), and 498InsTC (443), have been reported that cause frame shifts (59, 101). In addition, a missense mutation A96T that results in a similar disease phenotype has been identified (280). The neurological symptoms manifest in the fourth to sixth decade, and include choreoathetosis, dystonia, spasticity, and rigidity. Cognitive decline is rare or subtle in the early stages, but deteriorates with disease progression (59, 98, 471).
More than 30 additional mutations have been reported in the Ft-L chain, mostly in the regulatory 5′ IRE stem-loop region, that cause hereditary hyperferritinemia cataract syndrome with increased serum ferritin, and early-onset bilateral cataracts. Serum iron and Tf saturation are normal to low in most cases (168, 303). Mutations in this region reduce the binding affinity of IRE to the IRPs, up-regulating Ft-L expression and altering the ratio of Ft-H to Ft-L. This affects the ability of lens ferritin to store iron, and micro-crystalline aggregates of Ft-L result in cataracts. The degree of hyperferritinemia and severity of cataracts are characteristic of the individual mutation. Affected individuals do not show any change in systemic or brain iron homeostasis (171, 317).
Mutations in the coding region of Ft-L are rare. The heterozygous missense mutation Thr30Ile in the N-terminus of Ft-L causes genetic hyperferritinemia without iron overload or clinical symptoms. The unusually high glycosylation of mutant Ft-L is probably due to increased hydrophobicity of the N-terminal α-helix (217). In addition, a His133Pro mutation with low Ft-L levels and mild chronic anemia in a case of PD (154) and a heterozygous mutation of the first nucleotide of Ft-L start codon have been reported. The latter mutation did not alter synthesis of the functional protein (95). Mutations in the Ft-H chain are infrequent. Of the three reported mutations in the 5′ IRE of Ft-H chain mRNA, only A49U identified in a Japanese subject is associated with iron overload; the others (C20G and G34T) are silent (96, 219). It is likely that genetic alterations in Ft-H are lethal, and, hence, detected infrequently.
Pathologically, the brain tissue of hereditary ferritinopathy cases shows over-expression of neuroglobin, deposits of cytoglobin, and p53-mediated apoptosis of neurons and glia in specific regions (355). The presence of lipid peroxidation and abnormal nitration of proteins suggests contribution of Fe2+-mediated oxidative stress in the pathophysiology of these disorders (292). Imbalance of iron metabolism in affected regions also causes mitochondrial abnormalities as evidenced by changes in the biochemical and histochemical characteristics and accumulation of oxidatively damaged DNA in the mitochondria of a mouse model of hereditary ferritinopathy (114). Interestingly, mutant Ft-L itself is targeted by ROS, resulting in its cleavage and disruption of the ferritin shell. The free radical trap 5,5-dimethyl-1-pyrroline N-oxide effectively rescues mutant Ft-L from cleavage, confirming the role of free radicals in the process (26). It is likely that co-aggregation of wild-type Ft chains is initiated by the free radicals generated by mutant Ft-L, creating iron imbalance in the affected brains (25). Accumulation of iron-rich, aggregated ferritin has also been observed in sCJD brains, suggesting the presence of common pathogenic events initiated by brain iron imbalance and free radicals (407, 410).
B. Cp and aceruloplasminemia
Cp is a copper-containing, acute-phase α2-glycoprotein that functions as a multi-copper ferroxidase to regulate body iron homeostasis (331). The holoprotein contains six copper atoms that confer ferroxidase activity which is responsible for the oxidation of Fe2+ iron released from the intestinal epithelial, capillary endothelial, and reticuloendothelial cells to Fe3+, thereby modulating iron transport at multiple sites. Cp exists as two isoforms; a soluble form in the plasma synthesized mainly by hepatocytes, and a glycosylphosphatidyl inositol-(GPI)-linked form synthesized by astrocytes. The GPI-linked isoform is generated by alternate splicing of exons 19 and 20 and replacement of the C-terminal 5 amino acids of the secretory form by a 30-amino acid signal peptide for GPI anchor addition (340). Within the brain, Cp is expressed mainly on glial cells in the cerebellum, SN, microvasculature, (235), and the inner nuclear layer of the retina (236).
Although Cp requires copper for its function, absence of Cp causes imbalance of iron metabolism rather than disturbance of copper homeostasis (308). This is exemplified by the human disease aceruloplasminemia, a rare autosomal recessive disorder resulting from mutations in the Cp gene, and characterized by progressive accumulation of iron in the liver, pancreas, basal ganglia, SN, and the retina. Typical clinical presentation includes ataxia, involuntary movements, parkinsonism, cognitive dysfunction, retinal degeneration, and diabetes mellitus (283, 309). More than 30 mutations in the Cp gene segregate with aceruloplasminemia. Most are truncation mutations due to a premature stop-codon, resulting in the generation of a protein product that is either not transported to the plasma membrane or lacks one or more copper binding sites, rendering it non-functional (199, 283). Absence of Cp causes iron overload mainly by down-regulating Fpn, the iron export protein that is stabilized by the ferroxidase activity of Cp (107). Hp, another copper containing ferroxidase, is unable to substitute for the complete lack of Cp, highlighting important differences between the two ferroxidases (78, 79, 189).
Aceruloplasminemia is best exemplified by transgenic mouse models with a targeted deletion of the Cp gene. These mice exhibit age-dependent accumulation of non-heme iron in the brain, especially in the brainstem and cerebellar regions. The spinal cord and retina also show a significant increase in iron (341). Most of the accumulated iron is within astrocytes; oligodendrocytes, Purkinje neurons, and large neurons of the deep nuclei do not accumulate iron. Instead, Purkinje neurons up-regulate DMT1 expression and show evidence of iron deprivation, probably because of sequestration in astrocytes (Fig. 9). Iron-loaded astrocytes are a source of ROS, and neuronal death ensues through free radical injury and lack of trophic support by astrocytes that eventually succumb to iron-mediated injury (211). It is interesting to note that ROS down-regulates Cp through an mRNA decay mechanism, creating a destructive positive-feedback loop (423). Since ROS is implicated in the neurotoxicity associated with several neurodegenerative conditions, it is likely that ROS-mediated down-regulation of Cp and perhaps Fpn contributes to the neurotoxicity associated with diverse neurodegenerative conditions.
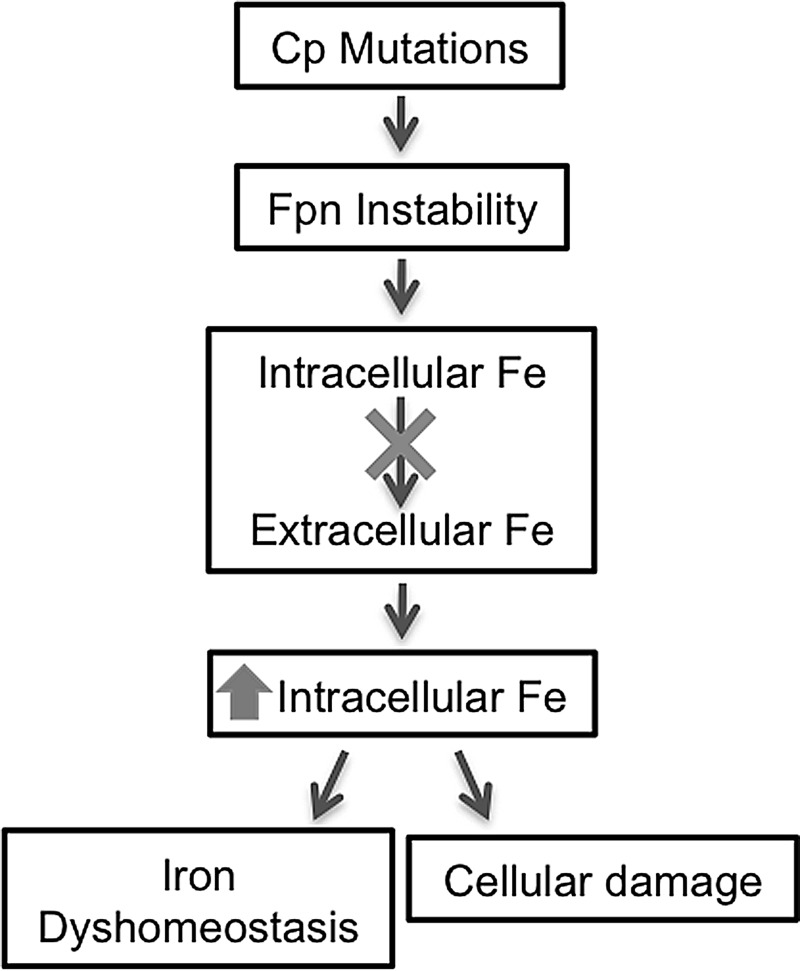
FIG. 9.
Aceruloplasminemia: absence or dysfunction of Cp destabilizes Fpn, resulting in intracellular accumulation of iron.
C. Alzheimer's disease
AD is the most common dementia of individuals older than the age of 65, accounting for 50%–80% of all dementias. An estimated 5 million Americans currently suffer from AD, and the number is likely to reach 11–16 million by the year 2050. The majority of AD cases are sporadic in origin and do not show symptoms till advanced age. A small number (5%–10%) are inherited as an autosomal dominant trait and develop the disease at a much younger age. Affected individuals present with short-term memory deficits that gradually progress to loss of cognition, aphasia, apraxia, agnosia, and general loss of executive functions. The hallmark lesion of AD brains is the extracellular presence of amyloid plaques comprising mainly of amyloid β (Aβ), and intra-neuronal accumulation of neurofibrillary tangles (NFT) composed of hyperphosphorylated tau. Aβ arises from sequential proteolytic processing of amyloid precursor protein (APP), a transmembrane protein that is expressed abundantly on the neuronal plasma membrane. Mutations in APP and presenilin genes 1 and 2 increase Aβ generation and are associated with the dominantly inherited form of AD, implicating Aβ as the principal cause of neurotoxicity (184). However, Aβ plaques and NFT do not account for all aspects of AD, which is now believed to involve additional pathways that converge to produce the typical pathology and clinical symptoms. Prominent among these are brain metal dyshomeostasis, mitochondrial dysfunction, impaired glial cell function, inflammation, and oxidative stress (61, 446). These mechanisms are not mutually exclusive, and are likely to work through intersecting biochemical pathways.
Although brain iron increases with aging, this process is enhanced and localized to certain regions in AD such as the parietal cortex, motor cortex, and hippocampus (45, 480). Most of the iron is associated with Aβ plaques or sequestered within ferritin in the surrounding glial cells (60, 277, 414). It is believed that iron accumulation precedes aggregation of tau and formation of NFTs, and is a major cause of protein and DNA oxidation, lipid peroxidation, and accumulation of advanced glycation end products, carbonyls, malondialdehyde, and peroxynitrite in AD brains. A concomitant decrease in anti-oxidant enzymes is also noted, potentiating the oxidative stress. Neurons are particularly vulnerable to this change because of their high metabolic rate and dependence on oxidative metabolism (415).
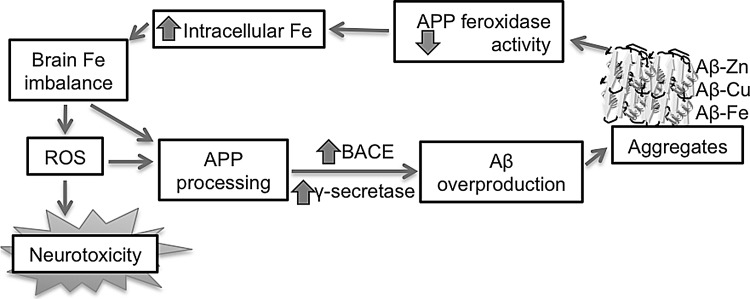
Accumulation of iron in AD brains is attributed to two main causes: (i) relatively high affinity of Aβ for metals such as iron, copper, and zinc (203), and (ii) impaired ferroxidase activity of APP at the neuronal plasma membrane (129). Although Aβ is generated under normal physiological conditions and is neurotrophic, increased production and aggregation into insoluble fibrils renders it pathogenic. A possible cause of Aβ toxicity is the binding of iron and copper to histidine residues 6, 13, and 14 in its hydrophilic N-terminal domain, and the reduction of these metals by methionine at residue 35 to release hydrogen peroxide and hydroxyl radicals through Fenton chemistry. This causes oxidation, cross-linking, and aggregation of Aβ, sequestering associated iron and copper that confer pro-oxidant activity to Aβ. Aggregation of additional Aβ peptides then proceeds exponentially (63). Insoluble Aβ aggregates activate surrounding microglia, contributing to oxidative stress (Fig. 10). NFTs also bind iron and copper, complementing the actions of Aβ to create an environment of metal imbalance and oxidative stress. Zinc, on the other hand, allosterically inhibits the iron and copper-binding sites on Aβ, and, therefore, may play a protective role. However, the role of zinc in AD pathogenesis is complex, and it is premature to assign a clear protective or toxic role to this metal (Fig. 10) (366).
FIG. 10.
Over-production of Aβ by ROS. Amyloidogenic processing of APP to generate Aβ is increased by ROS. Accumulated Aβ binds redox-active metals, acquires pro-oxidant characteristics, and creates a vicious feed-back loop that generates additional ROS. Aβ, amyloid β; ROS, reactive oxygen species.
An important consideration in discussing the role of iron in AD pathogenesis is that APP functions as a major ferroxidase on the neuronal plasma membrane, and is regulated by cellular iron levels (129). A functional “type-II” IRE has been identified in the 5′ UTR of APP mRNA, including it in the family of proteins that are regulated by IRPs (373). An increase in intracellular iron increases APP expression, while exposure to iron chelators down-regulates its expression in neuroblastoma cells. In neuronal cells, APP functions as a ferroxidase partner for Fpn-coupled export of iron from cells, an activity that is inhibited by zinc released from Aβ plaques (129, 366). Chelation of zinc restores the ferroxidase activity of APP to control levels, implicating APP and zinc as pro-oxidants in AD pathogenesis (129). Although Cp is an equally effective ferroxidase and facilitates the export of iron from cells, it is mainly expressed on astrocytes. Thus, the absence of APP increases intracellular iron in neocortical and hippocampal neurons of APP−/− mice, while deletion of Cp causes iron accumulation in the astrocytes of Cp−/− mice (129, 211). APP is also involved in the efflux of copper from cells by functioning as a copper chaperone, though the details of this pathway are not clear (31). A recent study explains neuronal iron accumulation in taupathies, AD, and PD due to a common defect in APP trafficking, a process that is usually facilitated by tau. Consequent loss of ferroxidase activity of APP links the three neurodegenerative disorders to a common pathway of intracellular iron accumulation and oxidative stress (259).
Neurons and glial cells protect themselves against oxidative stress with the help of inducible and constitutive forms of heme-oxygenase enzymes HO-1 and HO-2, respectively, and biliverdin reductase (BVR). However, both isoforms of heme-oxygenase are induced by oxidative stress and free heme (66, 134) and catalyze the degradation of heme to generate equimolar amounts of Fe2+, carbon monoxide, and biliverdin (229, 470). Fe2+ participates in the generation of ROS, while biliverdin is further reduced by BVR to the lipophilic antioxidant bilirubin (285, 420). Although increased activity of these enzymes in AD brains is believed to be neuroprotective (290), recent studies suggest that Fe2+ released by this reaction causes aggregation and phosphorylation of tau that aggravates the toxicity by interfering with APP transport and other cellular processes (205, 259). Other studies suggest that heme-oxygenase and biliverdin-reductase undergo post-translational modifications in subjects with mild cognitive impairment and AD, reducing their ability to generate antioxidant molecules including bilirubin (33–35), thereby potentiating the oxidative damage by Fe2+ (35, 121). These findings provide a new perspective on the imbalance between oxidant species such as iron and anti-oxidant enzymes in AD pathogenesis.
D. Parkinson's disease
PD is the second most common age-related neurodegenerative disorder affecting 1%–2% of the population older than the age of 65 (254, 295, 323). Clinically, patients present with motor dysfunction due to loss of dopamine-producing neurons in the SN, the primary neurotransmitter in the extrapyramidal system. Typical symptoms of PD include dyskinesia, rigidity, and tremor, eventually followed by cognitive decline and depression (204, 310). Pathologically, PD is characterized by loss of neuromelanin in the SN and the appearance of proteinaceous inclusions or Lewy bodies in the surviving neurons (142, 276).
Most cases of PD (∼90%) are sporadic and attributed to diverse causes, including exposure to certain toxins (106, 206, 384). A small percentage (∼10%) are familial in nature, and segregate with mutations in α-synuclein, parkin, PTEN-induced putative kinase 1 (pink1), DJ-1, leucine rich repeat kinase-2 (LRRK2), ubiquitin carboxy-terminal hydrolase L1, and ATP13A2 (49, 140, 233, 352, 438). The majority of familial cases are associated with mutations in α-synuclein, a protein involved in synaptic vesicle formation in addition to other partially characterized roles (1, 479). The normal function of other proteins is still emerging. It is believed that Parkin and ubiquitin carboxy-terminal hydrolase L1 play a role in ubiquitination and de-ubiquitination of proteins targeted for proteasomal degradation (482). DJ-1 is an antioxidant and a redox-sensitive chaperone (49), and PINK-1 is a mitochondrial protein kinase (438). LRRK2 is associated with mitochondria, binds to parkin, and is involved in membrane and protein trafficking (140).
Mutations in α-synuclein and LRRK2 are autosomal dominant, while those in DJ-1, parkin, pink1, and ATP13A2 segregate with autosomal recessive forms of PD. Notably, over-expression of α-synuclein is sufficient to cause PD in humans and animal models (111, 188, 193, 324, 365), suggesting a central role in PD pathogenesis. Pathogenic events common to most sporadic and familial forms of PD include accumulation of misfolded α-synuclein, mitochondrial dysfunction, oxidative stress, impaired autophagy, and neuronal iron accumulation (137, 296, 403, 433). Some of these defects are partially reversed by over-expression of parkin, pink1, DJ1, or the mitochondrial chaperone TRAP1 that requires phosphorylation by pink1 for functional activity (62, 349), suggesting a prominent role of autophagy in this process (445). Impaired autophagy due to mutations in parkin or pink1 impacts mitochondrial function by inhibiting mitophagy (257), and mitochondrial dysfunction directly inhibits autophagy by interfering with microtubule-dependent transport of autophagosomes, ultimately increasing intracellular levels of ROS and associated lysosomal leakage (17, 112). Lysosomal deficiency and α-synuclein rich Lewy bodies reactive for the autophagosomal marker LC3 have been reported in the SN of PD brains and mouse models of PD, supporting this sequence of events (445). Conditional inhibition of autophagy and mitochondrial complex 1 activity in experimental mouse models causes age-dependent loss of dopaminergic (DA) neurons (9, 86), further supporting the observations cited earlier.
Accumulation of iron in the SN of PD cases has been described by several groups, and is attributed to the chain of events triggered by mitochondrial dysfunction (301) and death of the relatively iron-rich DA neurons. However, the presence of iron in PD brains is not simply an outcome of α-synuclein aggregation, mitochondrial dysfunction, and DA cell death. An active process of iron import is suggested by the up-regulation of DMT1 (+IRE), TfR1, and transferrin receptor 2 (TfR2) (278, 298, 380), and down-regulation of Fpn (6) in DA neurons of PD cases and mouse models of PD, while loss-of-function mutations in DMT1 abolish this effect (380). Mutations in parkin increase DMT1 levels by inhibiting its degradation by the proteasomal pathway (374), providing the principal pathway of iron accumulation in DA neurons of the SN (380). Other brain regions such as the cerebellum, caudate nucleus, putamen, and cerebral cortex show minimal change (117, 118). Exposure of a DA cell line MES23.5 to the active metabolite of PD-inducing toxin MPTP (1-methyl-4-phenylpyridinium or MPP+) induces up-regulation of DMT1 and increased uptake of Fe2+ from the extracellular milieu, supporting these observations (481). The presence of a mitochondrial-targeting signal in TfR2 and alteration of its function by rotenone, another toxin used for inducing experimental PD, further suggests that alteration of iron metabolism in the SN is an active process (298, 325).
Accumulation of iron in DA neurons through DMT1 is further compounded by impaired activity of Cp, APP, and Hp, principal brain ferroxidases that are responsible for the export of excess iron from cells (30, 201, 259, 330, 367). Thus, mutations in Cp co-segregate with PD, and treatment of mouse models of PD exposed to 6 hydroxydopamine (6-OHDA) causes down-regulation of Fpn and Hp (271, 418, 450). Likewise, mouse models with deletion of tau develop PD due to impaired transport of APP to the plasma membrane, suggesting that a combination of increased iron uptake by DMT1 and decreased efflux due to compromised activity of APP-Fpn complex is responsible for the accumulation of iron in DA neurons of the SN (259). On similar lines, aggregates of α-synuclein cross-seed intracellular aggregation of tau in cell lines, inhibiting the transport of APP and accumulation of intracellular iron. This scenario provides a plausible explanation for the co-existence of AD and PD pathology in certain cases (455).
Similar to APP, α-synuclein has an IRE element in the 5′ UTR of its mRNA at exon 1–2 splice junction. The functionality of this element, however, has not been demonstrated unequivocally (159). Apart from this, α-synuclein functions as a FR when expressed in neuroblastoma cells, suggesting a role in cellular iron uptake and transport (104). When exposed to free radicals or excess iron, α-synuclein aggregates and induces the aggregation of additional molecules due to the redox-active nature of these aggregates, creating an environment of ongoing oxidative stress (149, 332). In transfected cell lines, co-expression of DMT1 with wild-type or mutant α-synuclein enhances toxicity of the latter (84), and oral administration of excess iron to neonatal or early post-natal mice predisposes these animals to MPTP and paraquat induced PD (220, 343, 367). Iron-fed mice show significantly more DA toxicity relative to controls, indicating that excess iron increases the vulnerability of DA neurons to toxic insults (220). Furthermore, mutations in genes involved in cellular iron homeostasis increase susceptibility to PD (50, 178) while chelation of iron is protective (221), underscoring the role of iron and brain iron mis-metabolism in PD pathogenesis.
Paradoxically, L-DOPA, the mainstay of PD treatment, triggers oxidative stress and contributes to the already existing redox imbalance in PD cases. Catabolism of dopamine by monoamine oxidase results in the generation of free radicals under physiological conditions, and rats treated with L-DOPA show biomarkers of oxidative and nitrosative stress and activation of the heat shock pathway in the SN and striatum (65). Additional factors that contribute to an environment of oxidative stress in DA neurons include (i) pro-oxidant characteristics of iron-rich neuromelanin that releases Fe2+ because of weak affinity; (ii) release of Fe3+ from ferritin in the presence of superoxide; (iii) inefficient activity of superoxide dismutase (SOD) that prevents the conversion of superoxide radical to hydrogen peroxide; (iv) decrease in astrocytes expressing glutathione peroxidase which reduces hydrogen peroxide to water and prevents the generation of hydroxyl radicals; and (v) dysfunction of the ubiquitin-proteasome system (9, 41, 118, 154, 172, 310, 417, 482). Together, the observations cited earlier suggest a significant role of iron-induced oxidative stress in the pathogenesis of PD.
E. Sporadic Creutzfeldt-Jakob disease
Prion diseases are a group of neurodegenerative conditions of humans and animals that are sporadic, familial, and infectious in origin. Common disorders include sCJD in humans, scrapie in sheep and goats, and chronic wasting disease in the deer and elk population (359, 360). Approximately 80% of CJD cases are sporadic in origin with no known underlying cause, and the remaining cases segregate with point mutations in the prion protein gene. Only 1%–2% are acquired from an external source through contaminated meat or iatrogenically following a medical procedure (8). Although relatively rare in comparison to the aforementioned neurodegenerative conditions, the infectious nature of these disorders has prompted intense investigations in this area. It is now clear that the main event underlying sCJD and other prion disorders is a conformational change of the prion protein (PrPC), a ubiquitously expressed glycoprotein that is most abundant on neuronal cells, from α-helical to a β-sheet rich isoform termed PrP-scrapie (PrPSc) (8, 359). Deposits of PrPSc in the brain parenchyma are the principal cause of infectivity and neurotoxicity in all prion disorders, though the mechanisms underlying these processes are not clear (32). Amplification of PrPSc from recombinant PrPC in vitro by several laboratories leaves little doubt that PrPSc arises from PrPC, and is sufficient to initiate infection and cause disease when inoculated intracerebrally into recipient animals (113, 449). However, the mechanisms by which PrPSc induces neurotoxicity are not as clear, and search for the toxic molecule continues. It is clear that expression of host-encoded PrPC on the neuronal plasma membrane is necessary for transmitting the toxic signal of PrPSc, but the nature of this signal and the pathways involved remain ambiguous (83, 288, 362).
As noted for AD and PD, markers of oxidative stress are prominent in prion disease affected brains, and have been attributed to the loss of anti-oxidant activity of PrPC combined with gain of pro-oxidant activity of PrPSc in diseased brains. Though plausible, direct evidence for either of these processes is lacking. Regarding the loss of normal function of PrPC, several studies have demonstrated a protective role of PrPC in the face of oxidative stress and other insults, and possible mechanisms for this activity have been proposed (85). In addition, controlled alteration of certain parameters in PrP−/− mice and cell lines lacking PrPC expression has revealed several putative functions of PrPC (7). However, lack of an overt phenotype in transgenic mice carrying a deletion of the PrP gene (PrP−/−) suggests that either the normal function of PrPC is not significant, or is essential for life and is compensated for by other proteins in its absence. Further studies are necessary to resolve this question. In this review, we will focus on the antioxidant function of PrPC and its role in iron uptake and transport, and possible mechanisms leading to the pro-oxidant nature of PrPSc-aggregates.
Several studies have indicated that absence of PrPC increases the vulnerability of PrP−/− mice and neuronal cell lines to superoxide, hydrogen peroxide, and copper ions, an observation attributed to the function of PrPC as an SOD (234, 474). A similar susceptibility to oxidative stress is noted in cell lines propagating PrPSc in culture (463), though it is unclear whether the decrease in PrPC levels due to conversion to PrPSc or the pro-oxidant activity of PrPSc aggregates is responsible for this phenotype. Although both processes could be involved to varying degrees, it is likely that PrPSc aggregates play a dominant role, as cells propagating PrPSc in culture show alteration of iron-modulating proteins, cellular iron mis-metabolism, and markers of oxidative stress (144, 145). Likewise, scrapie-infected mouse brains show increased amounts of Fe2+ and Fe3+ in the cerebral cortex, striatum, and brain stem. Deposition of iron is also noted around amyloid plaques in certain human prion disorders. (207, 230, 345).
It is unclear whether the pro-oxidant characteristics of PrPSc are due to non-specific co-precipitation with iron or other metals as observed for Aβ, or results from a specific physiological or pathological process. Evidence from cell and animal models suggests that PrPC binds copper in the micromolar range in a pH-sensitive manner. The C-terminal residues 90–126 and N-terminal histidine-rich octa-peptide repeat region serve as copper-binding sites (19, 57, 214, 215). Externally added copper stimulates the endocytosis of PrPC in cell models, suggesting that PrPC captures copper ions from the extracellular milieu for delivery to intracellular compartments (342). In addition, PrPC reduces Cu2+ to Cu1+ prior to transport via copper-specific intracellular trafficking proteins, suggesting a dual function in copper uptake and reduction to facilitate transport across the endosomal membrane (307). The interaction of PrPC with other metals such as manganese, iron, zinc, and nickel, and the physiological implications of this interaction are poorly understood (85, 198). The majority of these metals induce aggregation of purified or recombinant PrPC to a form resembling PrPSc under certain conditions, but the contribution of these metals to prion disease pathogenesis in vivo is unclear.
Although PrPC does not appear to bind iron under in vivo conditions (412), recent studies on cell and mouse models indicate that PrPC promotes iron uptake and transport in neuroblastoma cells in culture, and uptake by hematopoietic precursors and parenchymal cells of major organs in mouse models (408, 409). When over-expressed in neuroblastoma cells, PrPC increases the labile iron pool and iron saturation of ferritin. Deletion of the octa-peptide repeat region in the N-terminus of PrPC abolishes iron uptake, implicating this region in iron uptake in addition to its role in copper uptake (409). The differential iron content of cells expressing PrPC and the mutant PrP isoform lacking the octa-peptide repeat region is maintained in the presence of excess extracellular iron, suggesting a dominant role of PrPC in iron uptake and transport (409). Consistent with these observations, PrP−/− mice display a phenotype of systemic iron deficiency relative to matched wild-type controls (408). Absence of PrPC in PrP−/− mice affects iron transport at several sites. When introduced orally, radioactive iron is transferred to peripheral red blood cells of PrP−/− mice much before wild-type controls, but unlike wild-type mice in which the mature red cells continue to accumulate radioactive iron from recycled hemoglobin for several days, the amount of radioactive iron in red cells of PrP−/− mice does not increase significantly with time (408). These results are explained by a recent report in which bone marrow macrophages of PrP−/− mice show less stainable iron and do not incorporate radioactive iron from the medium effectively when cultured in vitro. These findings have been attributed to the FR function of PrPC, thereby facilitating the transport of NTBI through DMT1 (406). The iron-deficient phenotype of PrP−/− mice is reversed by expressing wild-type PrPC on the PrP−/− background, reinforcing the functional role of PrPC in iron transport (408).
Consistent with the role of PrPC in iron uptake, conversion to the PrPSc form induces a phenotype of apparent iron deficiency in sCJD-affected human and scrapie infected mouse brains (409). However, this phenotype is not merely due to loss of function of PrPC in iron uptake, as diseased brains show minimal change in total iron and a significant increase in Fe2+ iron. Evaluation of scrapie-infected animal brains shows progressive increase in iron deficiency that correlates directly with PrPSc levels, implicating sequestration of iron in PrPSc-protein complexes in a biologically unavailable form (407). Isolation of PrPSc using harsh conditions co-purifies iron rich ferritin, suggesting co-aggregation of PrPSc with ferritin in a detergent insoluble complex. Likewise, ferritin isolated from sCJD brain homogenates shows significant alterations in its biochemical characteristics. Unlike normal brain ferritin, ferritin isolated from prion disease-affected brains is insoluble, partitions with denatured ferritin when purified using conventional methods, and retains associated iron even after boiling in the presence of SDS (410). The PrPSc-ferritin aggregates are rich in iron and induce the aggregation of additional PrPC to the PrPSc form due to their pro-oxidant characteristics (36). It is likely that PrPSc and ferritin co-aggregate in lysosomal structures where the two proteins turnover and are likely to encounter low pH conditions which favor denaturation. Co-localization of PrPSc and ferritin has been observed in scrapie infected cell lines, supporting this hypothesis (36, 408). Notably, PrPSc and ferritin resist harsh biochemical procedures and co-purify from sCJD and scrapie-infected brain homogenates, suggesting the formation of these complexes during disease progression (408). Sequestration of iron in such PrPSc-ferritin complexes in vivo is likely to induce an iron-deficient phenotype in diseased brains and confer pro-oxidant characteristics to PrPSc. Analysis of CSF from sCJD cases indicates that iron deficiency occurs relatively early in the disease process and is reflected in the CSF as a significant increase in ferroxidase activity and decrease in Tf levels (185). When used in combination, these biomarkers detect CJD with an accuracy of 88.9%, providing a disease specific pre-mortem diagnostic test (185, 405). These observations also suggest mis-regulation of signaling between brain parenchymal cells and the blood-brain and brain-CSF barriers that are responsible for regulating iron uptake into the brain. Some of these observations have been reproduced using a systems biology approach, highlighting the significance of brain iron imbalance in prion disease pathogenesis (185, 208, 225).
F. Neurodegeneration with brain iron accumulation
NBIA is a heterogeneous group of inherited neurologic disorders that is characterized by excessive deposition of iron in the basal ganglia, globus pallidus, SN, striatum, and the cerebellar dentate nuclei. Age of onset is variable, and most cases present with a broad spectrum of overlapping clinical presentations, including progressive extrapyramidal signs with various combinations of movement disorders, seizures, visual disturbances, followed ultimately by cognitive decline (175, 248, 388).
NBIA disorders comprise two main syndromes: pantothenate kinase-associated neurodegeneration (PKAN) or NBIA type 1 comprising ∼50% of all reported cases, and phospholipase A2-associated neurodegeneration (PLAN) or NBIA-type 2 (101, 190, 194). Other relatively rare forms of NBIA include fatty acid hydroxylase-associated neurodegeneration (FAHN), mitochondrial membrane protein associated neurodegeneration (MPAN) (192), Kufor–Rakeb disease associated with a mutation in ATP13A2, static encephalopathy of childhood with neurodegeneration in adulthood syndrome (SENDA) (388), and aceruloplasminemia and neuroferritinopathy described above.
Multiple metabolic pathways are believed to contribute to the pathogenesis of NBIA, in particular the genes involved in iron and phospholipid metabolism (133). PKAN is associated with mutations in the PANK2 gene located on chromosome 20p, and expressed mainly in neurons of the cortex, globus pallidus, nucleus basalis of Meynert, and the pontine nuclei. Several mutations in the PANK2 gene segregate with PKAN, including missense mutations, deletions, duplications, and splice-site mutations (191). The two most common mutations G to A in codon 1231, and C to T in codon 1253 are detected in about a third of all cases. Several other mutations have also been detected in individual cases, resulting in unique phenotypes (174). Although the pathophysiology of PKAN is not entirely clear, PANK2 protein is believed to play a regulatory role in coenzyme A synthesis by catalyzing the phosphorylation of pantothenate (239). Since PANK2 is mainly targeted to the mitochondria, it is believed that dysfunction caused by mutations interferes with vital metabolic processes, compromising neuronal viability (239). A role for PANK2 in lipid metabolism has also been suggested, though the details are not clear. The correlation between PANK2 mutations and accumulation of iron in certain brain regions, especially the globus pallidus interna, remains unclear, though some studies suggest dys-regulation of Fpn as the underlying cause (350).
NBIA type 2, or the PLAN group of disorders are associated with missense, insertion, deletion, and splice site mutations in PLA2G6 (175). This gene is located on chromosome 22q and encodes iPLA2 β, a calcium-independent phospholipase A2 that hydrolyzes phospholipids to free fatty acids and lysophospholipids. Consequently, iPLA2 β plays a significant role in membrane remodeling, and is, therefore, implicated in vital cellular processes such as signal transduction, cell proliferation, and apoptosis. Dysfunction of iPLA2β alters membrane lipid composition, thus compromising the functional activity of proteins regulating vesicle transport within axons and dendrites. This causes accumulation of membranes in distal axons, resulting in neuronal dysfunction (287). Interestingly, certain human cases of NBIA type 2 show evidence of α-synuclein-positive Lewy bodies and hyperphosphorylated tau and NFT, suggesting an overlap with pathogenic mechanisms underlying PD (335).
Several other rare forms of NBIA have been recently recognized, and they include FA2H-associated neurodegeneration (FAHN) (244), Kufor–Rakeb Disease (PARK9) (365), MPAN, and SENDA (243). FA2H plays a critical role in the maintenance of normal myelin integrity, and mutations in this gene segregate with childhood NBIA characterized by altered brain iron homeostasis, lipid signal transduction, cyclin-dependent kinase inhibitor expression, and intracellular ceramide pool composition (244, 248). Kufor–Rakeb disease is a rare type of NBIA caused by mutations in ATP13A2 (245), and MPAN segregates with mutations in the open reading frame 12 (c19orf12) of chromosome 19 that encodes an orphan mitochondrial protein (119, 192). SENDA has also been associated with mutations in WD-repeat domain 45 (WDR45), which might lead to a defect in autophagy (377).
Current diagnosis of NBIA disorders is limited to clinical assessment, neuroimaging, and, in some cases, molecular genetic testing. Treatment options are limited at this time, and focus mainly on palliation therapy. A better understanding of the pathogenic mechanisms underlying these disorders is essential for developing viable therapeutic strategies.
V. Mitochondrial Iron Homeostasis and Associated Disorders
The brain consumes ∼20% of resting total body oxygen despite comprising only 2% of total body weight, and utilizes a substantial amount of glucose to meet its high metabolic demands. Both oxygen and glucose metabolism occur in the mitochondria and are highly dependent on iron, which serves as an essential cofactor (326). Mitochondria are the site of critical reactions in the pathway, leading to heme synthesis that uses iron as a prosthetic group. Heme is required for the functioning of hemoglobin, is a component of cytochromes abundant in neurons, and paradoxically, can also function as a pro-oxidant (22). Mitochondria are also major generators of iron-sulfur clusters (ISCs) that are essential for the electron transport ability of various proteins (368). Thus, maintenance of iron homeostasis in the mitochondrion is essential for its function and overall cell viability.
A. Iron uptake and utilization in the mitochondria
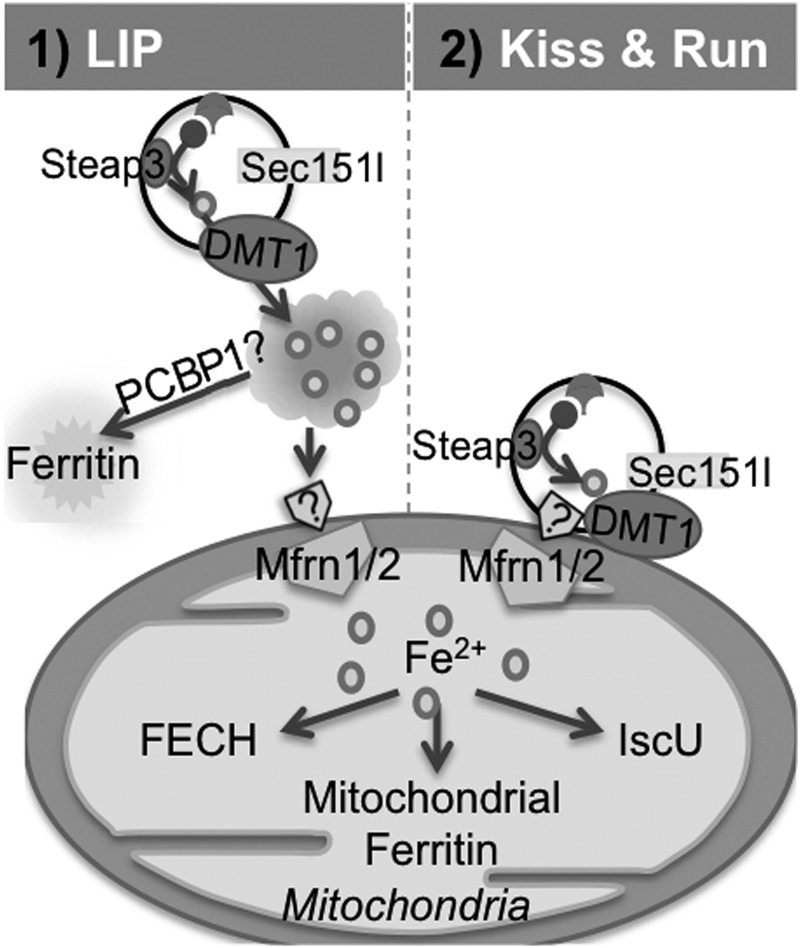
Iron uptake and utilization by the mitochondria is a tightly regulated because of the ready availability of oxygen that can react with Fe2+ to generate hydroxyl radicals (135, 182, 272). Several different, though not mutually exclusive, hypotheses of iron transport to the mitochondrion have been proposed. The prevailing hypothesis suggests that the labile iron pool in the cytosol contributes to the mitochondrial iron content (357, 368). Other studies suggest that Fe2+, once transported out of the endosome, is bound by hydrophobic pockets of chaperone proteins for transport to the mitochondria (369). One such protein is Poly (rC)-binding protein 1 (PCBP1) that transports iron to cytosolic ferritin (398). Additional cytosolic protein chaperones are believed to transport iron to mitochondria in a chelator-impermeable, endosome-independent manner (402). Depending on the cell lineage, however, the specific transport mechanism may vary (396). A third hypothesis proposes the “kiss and run” mechanism of iron delivery to the mitochondria, especially in developing reticulocytes (397). According to this model, endosomes form a close contact with mitochondria to accomplish the transport of iron, bypassing the cytosol. It is likely that Sec15l1, a component of the mammalian exocyst complex involved in TfR-endosome recycling modulates the docking of endosomes to the mitochondria for the transfer of iron (Fig. 11) (269). It is unclear whether this mechanism is limited to cells of the erythroid lineage or operates in other cells. As mentioned earlier, iron transport to DA neurons of the SN is mediated by an additional mechanism involving TfR2 (298) that shares 45% homology with the extracellular domain of TfR1, but unlike TfR1, does not contain an IRE (223, 436). TfR2 contains an N-terminal mitochondrial targeting sequence and facilitates the delivery of Tf bound iron to this organelle (298).
FIG. 11.
Transport of iron for mitochondrial uptake. Transport of iron to mitochondria occurs by two potential mechanisms: [1] Fe2+ from the cytosolic labile iron pool is transported by low-molecular-weight chaperones for delivery to mitochondria, and [2] Tf-iron containing endosomes transiently fuse with the mitochondria for iron delivery. Within the mitochondria, iron is used for heme or ISC synthesis or stored in mitochondrial ferritin. FECH, ferrochelatase; IscU, ISC assembly protein U.
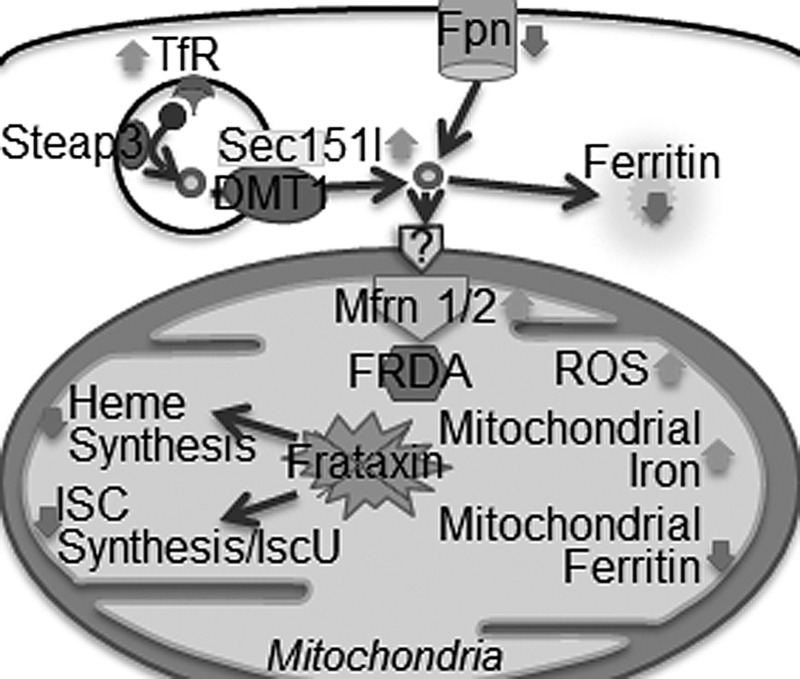
To access the respiratory chain in the mitochondrial matrix, iron needs to be ferried across the outer and inner mitochondrial membranes (368, 395). The mechanisms underlying iron transport across the outer mitochondrial membrane are not well understood. Transport across the inner membrane is mediated by mitoferrin 1 and mitoferrin 2, the conserved vertebrate homologs of yeast mitochondrial carrier genes Mrs3 and Mrs4 (158, 160, 315). Silencing of mitoferrin 1 and mitoferrin 2 in mammalian cells reduces mitochondrial iron transport and decreases heme and ISC synthesis (338). Mitoferrin 1 functions in erythroid tissues with high iron demands, while mitoferrin 2 is expressed ubiquitously in tissues with lower iron demands (395). Abcb10 is a mitochondrial inner membrane ATP-binding cassette transporter that stabilizes mitoferrin 1 and enhances iron transport to the mitochondria (81). The Abcb10 protein physically interacts with mitoferrin 1 but not with mitoferrin 2 (80, 81). It was additionally shown that ferrochelatase (FECH), the final enzyme in the heme synthesis pathway, can form an oligomeric complex with mitoferrin 1 and Abcb10, thereby bypassing the release of iron in the mitochondrial matrix in favor of a highly efficient direct delivery to heme synthesis enzymes (Fig. 12) (80).
FIG. 12.
Uptake of iron by mitochondria. Mfrn1 and Mfrn2 are located on the inner mitochondrial membrane transport Fe2+ from the inter-membrane space to the matrix, where it is used for heme or ISC synthesis, or stored in mitochondrial ferritin. Fxn participates in heme and ISC-synthesis pathways. Fxn knock-out mice show up-regulation of cellular iron uptake proteins, down-regulation of storage and export proteins, and increased uptake of iron by mitochondria. Heme and ISC synthesis are decreased, as in FA patients. FA, Friedreich's ataxia; Fxn, frataxin.
Within the mitochondrial matrix, iron is used as a catalyst for oxidative metabolism and for the synthesis of heme and ISC proteins (263). Since free iron in the mitochondrial matrix produces ROS, mechanisms are in place to limit the exposure of mitochondrial matrix and inter-membrane space to iron (272). These include targeted delivery of iron to the heme and ISC synthesis complexes by protein chaperones, and storage of excess iron in mitochondrial ferritin (262, 387, 478). Human mitochondrial ferritin is a H-type ferritin encoded by an intron-less gene on chromosome 5q23.1, and translates a 30 kDa precursor protein that matures to a 22 kDa product after cleavage of the N-terminal mitochondrial targeting signal which is rich in positively charged arginine residues (90, 127, 262). Unlike cytosolic ferritin that is rich in organs with iron storage functions such as the liver and spleen, mitochondrial ferritin is highly expressed in the brain, spinal cord, and certain other organs that have high metabolic demands (381). Mitochondrial ferritin forms a homopolymer of 24 subunits that shares a high degree (79%) of sequence homology with cytoplasmic H-ferritin, assembles into shells that bind iron, and demonstrates ferroxidase activity, though the activity is lower than cytosolic H-ferritin chain (51, 90, 262). Over-expression of mitochondrial ferritin results in cytosolic iron deficiency, up-regulation of iron uptake proteins, increased iron import into the cell, and sequestration of iron within mitochondrial ferritin (322). High levels of mitochondrial ferritin in the SN have been reported in cases of Restless-leg syndrome, and could account for the brain iron deficiency reported in these cases (416). Thus, levels of mitochondrial ferritin influence iron metabolism throughout the cell. Unlike cytosolic ferritin, no IRE has been identified in mitochondrial ferritin, and little is known about its regulation (21, 262).
Mitochondria are the key site for the biogenesis of ISCs that function as cofactors for proteins of cellular respiration, DNA replication and transcription, FECH, and regulatory proteins such as IRP1(476). Mitochondrial ISC assembly is a complex pathway in which more than 20 components participate. In brief, ISCs are composed of iron and sulfide ions that form either 2Fe-2S or 4Fe-4S clusters. The human cysteine desulfurase nitrogen fixation 1 homologue (Nfs1) along with an accessory protein Isd11 serves as sulfur donors for the assembly of ISCs (44, 252, 336). The frataxin (Fxn) protein has been implicated in ISC synthesis in addition to other proposed functions (383, 421). ISCs are assembled on the human scaffold protein ISC assembly protein U (IscU), and interactions between IscU, Nfs1, and Isd11 form the core ISC complex, which then interacts with Fxn (387).
The precise mechanism of ISC export from the mitochondria to the cytoplasm has not been established, except that the ATP-binding cassette (ABC) protein ABCB7 is involved (40). ABCB7 shares a high degree of sequence identity with the yeast inner mitochondrial membrane protein Atm1p (400). Deletion of Atm1p in yeast has been shown to result in mitochondrial iron overload (393). In humans, mutations in ABCB7 cause mitochondrial iron overload and X-linked sideroblastic anemia with ataxia (XLSA/A) (11). ABCB7 and Atm1p are also required for the biogenesis of mature cytosolic ISC proteins in humans and yeast, respectively (40, 232, 353). Interestingly, ABCB7 has also been shown to interact with FECH (422). Further research is needed to fully elucidate the details of this interaction.
B. Regulation of mitochondrial iron homeostasis
Irp1 is an ISC-containing protein that functions as a cytosolic aconitase in the presence of an intact ISC (131), and it undergoes a conformational shift which facilitates IRE binding in its absence (448). When iron levels and ISC synthesis are low, transcripts with 5′ IREs such as δ-aminolevulinate synthase 2 (ALAS2), Fpn, and ferritin are translationally repressed; while those with 3′ IREs such as TfR1 and DMT1 are stabilized, allowing for the import of iron to the cytosol and mitochondria (72, 92, 180, 299) (Fig. 6). Irp2 is regulated by the F-box and leucine-rich repeat protein 5 (FBXL5), an oxygen and iron-sensing protein that is a component of the E3 ubiquitin ligase complex. When iron and oxygen levels are low, FBXL5 is targeted for degradation, blocking the formation of E3 ubiquitin ligase complex and thereby causing Irp2 levels to remain high and allow binding to IREs (379, 439). Ablation of Irp2 function in mice causes severe iron dysregulation and a progressive neurodegenerative disease with an average onset of 6 months (255). Mitochondrial dysfunction in these mice is mediated by decreased levels of ISC-containing proteins, which, in turn, results in decreased respiratory chain complex I and II activity (209). Studies have shown that Irp1 function may partially compensate for the lack of Irp2 activity, as mice with a homozygous ablation of Irp2 and heterozygous for Irp1 show a more severe phenotype of neurodegeneration and axonopathy, and activation of Irp1 IRE-binding activity in the Irp2 homozygous mutant mice restores activity of the respiratory chain complex 1 (209).
Export of iron from mitochondria is another important aspect of mitochondrial iron homeostasis. Decreased export of iron as heme or ISCs contributes to mitochondrial iron overload as noted in the Fxn-knockout mouse model (202). Only a small portion of heme and ISCs are used for mitochondrial metabolism. The rest are exported by unknown transporters for use as cofactors in numerous proteins important for cell physiology. ABC transporters Abcb7 and Abcb10 are expressed on the inner mitochondrial membrane in higher eukaryotes and are essential for iron transfer and homeostasis as well as export of mitochondrial ISC intermediates (40, 319, 353, 401). Targeted knockout of Abcb7 is lethal, implying its essential role in various tissues (353). Deletions of ATM1, the yeast ABCB7 homolog causes a thirty-fold increase in mitochondrial iron accumulation along with reduced activity of cytosolic but not mitochondrial ISC-containing enzymes (231). ATM1 is also required for maturation of cytosolic ISC-containing proteins that could be complemented with Abcb7 in ATM-deficient cells (40, 100, 353). These findings strongly suggest that Abcb7 is a mitochondrial transporter for efflux of ISC intermediates that are important for use in the cytosol (40). Abcb10 participates in heme synthesis, but its precise role in this process is not clear.
Absence or dysfunction of one or more of the proteins involved in mitochondrial iron homeostasis causes systemic and brain disorders, some with serious consequences. Recent advances in our understanding of two such disorders, FA and sideroblastic anemias, are reviewed next.
C. Brain disorders of mitochondrial iron dyshomeostasis
1. Fxn and Friedriech's ataxia
Mutations in Fxn result in FA, a disorder that is characterized by severe neurodegeneration and cardiac disease accompanied by iron-sulfur protein deficiency and altered mitochondrial iron metabolism (54, 337, 361). It is a progressive disease with mean age of loss of ambulation at 25 years, causing 95% of patients to be wheelchair bound by the fourth decade (187). Symptoms of FA typically begin to manifest in puberty, but onset of the disease can range from infancy to as late as the fifth decade in atypical presentations (42). Consistent diagnostic findings are limb and truncal ataxia, absent tendon reflexes in the legs, pyramidal tract dysfunction, loss of joint proprioception and vibration sense, and eventual dysarthria (187). Other findings include impaired glucose tolerance or overt diabetes mellitus, as well as cardiac hypertrophy followed by cardiomyopathy, which is a leading cause of death in FA patients (132, 370). In 96% of patients, the underlying cause is expansion of GAA repeats in the first intron of the Fxn gene, resulting in markedly reduced levels of Fxn mRNA (70, 148). This reduction is caused by an unusual structure termed sticky DNA, which sequesters RNA polymerases (43, 70, 378). In addition, gene silencing due to repeat expansions has also been implicated in FA (328). These patients have an average of between 200 to more than 1000 intronic GAA repeat units compared with the normal number of 7–22 units (70, 148). Other cases are heterozygous with GAA expansions in one allele and point mutations in the other allele that result in weak binding to the Nfs1, Isd11, and IscU complex and loss of ISC assembly activities (56, 91, 138).
The human Fxn gene is encoded on chromosome 9q13, and translates a 210 amino acid protein that is targeted to the mitochondria where its transit sequence is cleaved to yield a 17 kDa mature form (69, 240). Fxn is highly conserved from bacteria to humans (166). Crystal structure-based studies of bacterial, yeast, and human Fxn reveal a conserved, negatively charged patch of glutamic and aspartic acid residues that are thought to be involved in cation binding (3, 120). The conserved C-terminal beta sheet structure is important for mediating partner interactions (120, 260, 318). Fxn lacks any canonical structural motif that would provide a membrane anchor, and is associated with the membrane by forming a complex with mitochondrial proteins like FECH (478).
The function of Fxn and consequences of decreased Fxn levels on the pathophysiology of FA have been well studied in animal and cell models. Of these, the function of Fxn as an iron chaperone for ISC and heme synthesis is well supported. Initial evidence for this hypothesis came from findings of decreased ISC-dependent protein activities in FA patients and in yeast and animal models of the disease (54, 314, 361, 375). Subsequent studies have demonstrated interactions between Fxn and the ISC assembly complex (421). Results with the bacterial Fxn ortholog CyaY demonstrate interactions between CyaY and IscS, the bacterial cysteine desulfurase involved in ISC assembly (256, 358, 399). Yeast Fxn homologue Yfh1 is stable as an iron-loaded monomer under physiologic conditions and binds iron at micromolar binding affinity, consistent with the proposed function of Yfh1 as an iron donor (88). Yfh1 also interacts with the mitochondrial scaffold protein Isu1, and can form iron-dependent complexes with Nfs1, and Isu1, a finding reproduced with human Fxn (165, 364, 387). In addition, holo-Fxn but not apo-Fxn forms complexes with IscU, and it stimulates ISC assembly (477). Fxn also interacts with heme-synthesis pathways (195, 478). In the presence of iron, holo-Fxn binds with a higher affinity to FECH than to IscU, indicating a possible regulatory role in allocating iron toward heme or ISC synthesis. This is supported by studies showing down-regulation of Fxn in erythroid cells (39, 478). Yfh1p appears to bind, retain, and transfer Fe2+ iron to FECH through protein–protein interactions (339). Fxn has also been shown to transfer Fe2+ iron to mitochondrial aconitase, but whether it functions more ubiquitously as an iron donor remains to be characterized (58).
Diminished levels of Fxn lead to impaired ISC biogenesis, decreased respiratory chain function, altered cellular iron homeostasis, and increased oxidative stress (54, 202, 361, 375, 391). At the cellular level, degeneration is detected in the dorsal root ganglia with large sensory neurons (238). Eventual degeneration of sensory axons is seen in peripheral nerves, posterior columns of the spinal cord, corticospinal tracts, and dentate nucleus of the cerebellum ensues (237, 337). Immuno-cytochemical studies show dysregulation of ferritin, suggesting that depletion of Fpn occurs in the neuropil of the dentate nucleus (237).
The precise mechanism and role of iron in cell dysfunction and death in FA is unknown. Mouse models with complete loss of Fxn expression are embryonic lethal and exhibit no mitochondrial iron accumulation (91). In a conditional FA mouse model, deficits in respiratory chain complexes I–III precede intra-mitochondrial iron deposition in the cardiac tissue (91, 361). In yeast models carrying a deletion of Yfh1, mitochondria show iron accumulation and impaired mitochondrial function (24, 157, 363). Expression of mitochondrial ferritin in models of FA attenuates the pathological phenotype, providing evidence for the causative role of iron in FA pathology (67, 68). In addition, iron chelation therapy shows improvement of neurologic symptoms in some cases of FA, limits cardiac hypertrophy in mouse models, and rescues human FA fibroblasts from oxidant stress, further suggesting the involvement of iron in the pathogenic process (48, 467, 473). It is believed that accumulation of iron in the mitochondria is due to the deficiency of ISCs rather than absence of Frx per se. Sequestration of iron in the mitochondria results in cytosolic iron depletion, further decreasing the transcription of Fxn and creating a negative feed-back loop (266). Thus, therapeutic strategies for FA need to be directed at increasing transport of accumulated iron from the mitochondria to the cytosol and restore iron homeostasis in affected neurons.
2. Sideroblastic anemias
Inherited sideroblastic anemias are a heterogeneous group of diseases that are characterized by iron-laden mitochondria clustered around the nucleus of red cell precursors. These disorders have provided valuable insights into the regulation of iron metabolism in the mitochondria and interactions between the heme and ISC synthesis pathways. A number of mutations responsible for congenital sideroblastic anemias have been characterized, including mutations in Abcb7, ALAS2, the proposed glycine/5-ALA exchanger SCL25A38, and GLRX5, a mitochondrial thiol-disulfide oxidoreductase involved in ISC biogenesis (213).
Mutations in ABCB7 have been described in patients with XLSA/A, a condition of mild sideroblastic anemia with early onset cerebellar ataxia (11, 40, 353). Deletion of ATM1, the yeast ABCB7 homolog, results in reduced activity of cytosolic ISC-containing proteins and increased mitochondrial iron. The activity of mitochondrial ISC-containing proteins, however, is not affected (231). Decreased levels of cytosolic ISC may affect the Irp system, explaining the anemia exhibited in XLSA/A patients. Anemia may also be caused by dysfunction of FECH (102, 334), as evidenced by elevated levels of free protoporphyrin in XLSA/A patients, or by the proposed interactions between ABCB7 and FECH in erythroid cells (422). The pathogenesis of cerebellar ataxia has not been well characterized for this disease, but the ubiquitous role for ABCB7 in iron metabolism may provide an important link to this question.
X-linked sideroblastic anemia (XLSA) is due to a mutation in the heme synthesis mitochondrial enzyme ALAS2 (46, 93). The phenotype of anemia in XSLA is likely due to the restricted expression of ALAS2 in erythroid cells (241). Studies have revealed increased expression of mitochondrial ferritin in the erythroblasts of patients with XLSA (74, 262), which may be either the cause or consequence of cellular iron overloading. Previous experiments have shown that elevated levels of mitochondrial ferritin sequester iron away from cytosolic ferritin and result in up-regulation of TfR levels (90). Expression of mitochondrial ferritin in yeast models of FA, and in fibroblasts from FA patients attenuates the pathological phenotype, supporting this hypothesis (67, 68). Mitochondrial ferritin is not expressed at high levels in normal erythroid cells, and its regulation is not well understood (262). Future studies of XLSA and associated changes in mitochondrial ferritin will elucidate the mechanisms underlying the pathobiology of sideroblastic anemias.
VI. Yeast as Models of Neurodegenerative Conditions
Many cellular and biochemical processes are highly conserved from unicellular yeast to humans. The ease of genetic manipulations in the budding yeast S. cerevisiae provide a simple and experimentally manipulable model for understanding complex cellular and biochemical processes that are difficult to model in higher eukaryotes (179, 250, 472). The model yeast S. cerevisiae has provided important information on the biochemical and genetic basis of several human disorders, including AD, PD, prion disorders, and HD (297, 305, 435, 468, 469). Several factors make yeast a convenient model for investigations pertaining to brain iron homeostasis and dyshomeostasis: (i) pathways regulating iron metabolism are highly conserved between yeast and humans; (ii) iron transport pathways are well characterized in S. cerevisiae, and the ease of genetic manipulation provides an opportunity for identifying downstream targets that are unapproachable in higher eukaryotes; and (iii) yeast growth conditions are well defined and can be altered to monitor the effect of metal ion concentrations. However, several limitations need to be considered while interpreting data from yeast models. S. cerevisiae is a unicellular organism and is, therefore, unable to recapitulate the cross-talk among cells as in multicellular organisms. Proteins involved in many of the mammalian disorders such as prion and Alzheimers' disorders are membrane associated, and unlike neurons, the plasma membrane S. cerevisiae is surrounded by a cell wall. Thus, biology at membranes could differ in S. cerevisiae and neuronal cells. Unlike neurons, yeast replicate and it is believed that asymmetric distribution during cytokinesis sequesters potentially toxic protein species in mother cells, generating healthier daughter cells. Thus, the protective mechanisms in yeast could differ from neuronal cells (5). In addition, yeast lack many of the components of protein quality control machinery present in mammalian cells such as the Hsc70 partner Carboxyl terminus of Hsp70-interacting protein (CHIP) that bridges Hsc70 to the proteasomal machinery. Thus, results obtained using yeast models need to be validated in higher eukaryotic models such as neuronal cells and animal models. Despite these limitations, the fundamental biological processes are highly conserved between yeast and mammalian cells, providing a convenient tool to rapidly screen components of the cellular machinery as well as chemical compounds to identify targets against several human disorders, including AD, PD, HD, and CJD (Fig. 13).
FIG. 13.
Schematic describing yeast as model organisms for the study of metal dyshomeostasis in human neurodegenerative disorders. (A) To construct a yeast model of a specific neurodegenerative disease, the disease-associated human gene is overexpressed in yeast and examined for its pathological effects. If expression is toxic to yeast growth, a suppressor screen using chemically synthesized compounds is carried out. Obtained suppressors are further analyzed to understand the underlying mechanism. (B) The yeast orthologue of disease-associated protein is knocked out, and the growth medium is supplemented in various metal ions to assess the effect on growth. Normal growth is restored on incubation with specific rescuing compounds or by restoration of the native protein expression in the knockout strain. PARK9 metal specificity was explored using a similar strategy. Figure adapted from Froschauer et al. (160).
A. Iron metabolism in S. cerevisiae
As for most organisms, iron is an essential element for yeast growth, and exists primarily in the Fe3+ form to prevent interaction with available oxygen. For cellular utilization, the more soluble Fe2+ iron is preferred. Thus, yeast cells have two distinct reductive and non-reductive pathways for iron transport. The reductive pathway is facilitated by transcriptionally regulated FRs Fre1p and Fre2p present on cellular membranes that reduce Fe3+ to Fe2+ for further uptake by a low or high affinity pathway (15, 103). The low-affinity pathway is utilized when iron is abundant in the growth media. Due to its poor specificity, the low-affinity pathway also mediates the transport of other metals such as copper. The low-affinity pathway for direct uptake of Fe2+ is mediated by FET4 or SMF1 gene products (82, 123, 454). Smf1 mediated iron uptake is proton dependent. The high-affinity pathway is negatively regulated by iron abundance and is specific for iron uptake. The high-affinity iron uptake is induced on iron deprivation in growth media and is mediated by the ferroxidase Fet3p and permease Ftr1p (109, 419). The ferroxidase Fet3p catalyzes the oxidation of Fe2+ to Fe3+, which is then further transported into the cell by Ftr1p permease. Both Fet3p and Ftr1 are transcriptionally regulated by the transcription factor Aft1p and localize to cellular membranes only when both proteins are present in the cell (71).
The non-reductive pathway involves internalization of the Fe3+-siderophore complex. Siderophores are low-molecular-weight organic compounds with high affinity for iron and are secreted by many microorganisms. Due to their ability to chelate iron, siderophores solubilize otherwise insoluble Fe3+, facilitating transport across the plasma membrane. S. cerevisiae itself does not synthesize siderophores, but has the ability to use siderophores secreted by other microbes. Plasma membrane permeases belonging to the major facilitator superfamily mediate iron-siderophore uptake. The four distinct facilitators in S. cerevisiae are Arn1p, Arn2p (Taf1p), Arn3p (Sit1p), and Arn4p (Enb1p) (47).
B. S. cerevisiae as a model of brain disorders of iron dyshomeostasis
1. Parkinson's disease
Yeast models of PD-associated α-synuclein toxicity are among the most established models of neurodegenerative disease. Though a clear homolog of α-synuclein has not been identified in yeast, heterologous overexpression of human α-synuclein causes its aggregation in S. cerevisiae and suppresses growth, recapitulating familial forms of PD associated with duplication and sometimes triplication of the gene encoding wild-type α-synuclein (333). Likewise, mutant forms of α-synuclein (E46K and A53T) cause increased toxicity in yeast as in cell models, reproducing certain aspects of familial PD. Important neurotoxic processes of PD are recapitulated by yeast models such as defects in mitochondrial biogenesis, protein homeostasis, vesicular trafficking, and increased sensitivity to oxidative stress (333, 394). Though limited in scope at the present time, these models have helped in the identification of genetic suppressors of α-synuclein toxicity, belonging mainly to the ER-to-Golgi transport proteins.
Yeast models have also been successful in understanding the correlation between environmental risk factors and PD pathogenesis. Regulated expression of α-synuclein-GFP chimeras under the control of galactose-inducible promoter has been instrumental in identifying modifiers of α-synuclein toxicity (333). Thus, metals such as iron, zinc, and manganese induce aggregation of α-synuclein and cytotoxicity, presumably due to oxidative stress (176). Yeast cells grown in the presence of excess Fe3+ induce aggregation and aggravate the toxicity of wild-type and pathogenic mutations of α-synuclein, while iron chelators and ROS-quenching agents are protective, providing a model that mimics mammalian cell culture models, and yet is manipulable at the genetic level to enable identification of gene products involved in toxicity and the rescue of this phenotype. A suppressor screen of ∼10,000 compounds yielded two compounds that reduced FeCl3-mediated toxicity. Interestingly, these compounds were flavonoids that are known to possess anti-oxidant and metal-chelating properties (176).
A clear example of the versatility of this model system is the functional characterization of PARK9 as a manganese transporter. The human gene PARK9 encoding a P-type transmembrane cation-transporting ATPase (ATP13A2) is linked to early onset-Parkinsonism. This hereditary form of PD is caused by homozygous mutations in PARK9 (122, 365). The role of wild-type ATP13A2 and its disease causing mutants has primarily been deduced from yeast models using the PARK9 ortholog YPK9, a transmembrane cationic metal transporter in S. cerevisiae (169). The substrate specificity of Ypk9 was explored by growing wild-type and ypk9Δ yeast strains in the presence of a wide range of metal ions, including cadmium, calcium, cobalt, Fe3+, zinc, and copper, and metal chelators EDTA, EGTA. The ypk9Δ strain was more sensitive to manganese, and the sensitivity was reversed on overexpression of wild-type Ypk9 but not mutant Ypk9 carrying the disease-associated mutation. It is likely that mis-metabolism of manganese leads to downstream events leading to early onset PD.
2. Prion disorders and HD
As discussed earlier, mammalian prion disorders result from the conformational transition of PrPC to PrPSc. Since S. cerevisiae does not encode a PrP orthologue, attempts have been made to establish yeast models by expressing mammalian PrP in yeast. However, until now, there are no well-established yeast models of PrP that recapitulate PrP associated toxicity as seen in mammalian cells. Similarly, yeast models expressing PrP have been unable to recapitulate the role of PrP in iron or copper metabolism as seen in higher eukaryotic models (265), and iron metabolism has not been thoroughly investigated.
Yeast models of HDs have been constructed by expressing huntingtin protein fragment containing polyQ stretch of varying lengths (130, 302). As observed in neurons, length of the glutamine expansion determines toxicity in yeast. Significant toxicity is generally seen when the polyQ stretch exceeds 72 glutamines. Though not extensively studied, compounds analogous to clioquinol (CQ), a weak metal chelator of the hydroxyquinoline family, show protection against polyglutamine toxicity, suggesting a role of metals, primarily Fe3+, Cu2+, and Zn2+ in HD pathogenesis (424).
3. Fxn toxicity
S. cerevisiae encodes the Fxn homolog Yfh1p, which, similar to Fxn, is involved in ISC biosynthesis (12, 58, 339, 392). Deletion of Yfh1 causes iron accumulation in mitochondria, reduced cell growth, and increased sensitivity to H2O2. Reconstitution of Yfh1 expression restores cell growth on non-fermentable carbon source, clarifying the role of Yfh1 in iron metabolism (73). Moreover, wild-type Fxn complements yfh1 deletion while FA-associated mutations fail to do so, suggesting that Fxn is involved in mitochondrial iron homeostasis, and disease-associated mutations cause accumulation of iron in mitochondria and oxidative stress. Observations on Fxn mouse models support these findings (466).
VII. Therapeutic Options
The potential for iron to cause oxidative stress and protein aggregation is well established, and appropriate chelation provides an opportunity to alleviate this component of the pathogenic process (162). When considering chelation for clinical application, factors such as efficacy versus toxicity, route of administration, and transport across the BBB need to be considered. An ideal iron chelator, for instance, should bind iron in a nontoxic metal complex, have appreciable lipid solubility to allow for penetration of the BBB and inner mitochondrial membrane, and be uncharged and of small molecular size (<500 KDa) to facilitate membrane diffusion (161). Some studies suggest that an iron chelator should have good oral bioavailability and have even smaller size (<300 Da); however, the main delivery methods with proven effectiveness so far have been through the intra-venous route (483). It would also be preferable for iron chelators to selectively bind Fe3+, as chelators that prefer Fe2+ can also bind other divalent metals such as copper and zinc and inhibit important metalloenzyme activity (465).
Generally, iron chelators can be classified into four categories: desferrioxamine (DFO), 8-hydroxyquinolone (8HQ) analogs, prochelators, and aroylhydrazones. DFO is a hexadentate ligand with a higher binding affinity for Fe3+ than Fe2+. It has been traditionally used to treat β-thalassemia to minimize iron overload from chronic blood transfusions. Some studies have shown that DFO also prolongs life in AD mouse models (105). In AD, DFO is believed to reduce Aβ levels by disrupting the iron-mediated up-regulation of APP through its 5′- UTR, and by stabilizing the transcriptional activator hypoxia-inducible factor-1α (HIF-1α) (458). The stability of HIF-1α is under the control of a class of iron-dependent and oxygen-sensing enzymes, HIF prolyl-4-hydroxylases that target it for degradation. Prolyl-4-hydroxylases contain non-heme Fe2+ in their catalytic center, and are able to extract iron from the intracellular chelatable labile iron pool as a cofactor (458). Iron chelation can inhibit the action of prolyl-4-hydroxylases and stabilize HIF-1α, increasing expression of target genes that include Tf, TfR1, and other survival genes (23). Iron chelation may also benefit PD patients (344). Thus, DFO pretreatment in PD mouse models of 6-OHDA, MPTP, or proteasome inhibitor show beneficial results (458). However, DFO is highly hydrophilic, making its oral bioavailability and BBB penetration extremely low and necessitating parenteral administration. Due to its half life of 12 min, patients on DFO therapy should undergo subcutaneous infusions from 8–12 h approximately 7 times a week (351, 430, 437). A recent study, however, highlights the possibility of intranasal DFO administration, which may improve patient compliance (181). Deferiprone and deferasirox are two drugs with iron-chelation activity that are designed to overcome the hydrophilic and poor BBB penetration of DFO. They have been approved by the FDA for treatment of secondary iron overload and have been demonstrated to reduce ataxia and neuropathy in FA, but are difficult to manage in FA because some of the patients develop anemia (48). However, current clinical trials and studies show promising results for AD and PD (115, 156, 440).
CQ is an analog of 8HQ, and confers metal chelation properties by forming stable 5-membered chelate rings with Fe3+ (347). CQ is small, lipophilic, and can freely traverse the BBB. However, large quantities can induce neurotoxicity as evidenced by the development of sub-acute myelo-optico-neuropathy in a group of Japanese patients in the 1960s (136). It is also able to form complexes with copper and zinc that play a significant role in plaque and amyloid formation in AD (453, 465). Recent studies have demonstrated reduction in the number and size of zinc-containing plaques in the brains of AD mouse models (APP/presenilin 1 double transgenic mice), as well as decreased expression of APP, BACE1, and presenilin-1 after CQ treatment (453). Thus, CQ has the potential to reduce neuritic plaque formation and oxidative stress in AD and other neurodegenerative disorders of metal imbalance. The drawback of possible CQ-induced neurotoxicity has led to the development of other CQ analogs that include VK28, HLA-20, and M30. VK28 is an antioxidant-iron chelating agent with the 8HQ moiety that protects against MPTP- and 6-OHDA-induced lesions in rat brains (14, 267). HLA-20 and M30 are hybrid drugs that combine the N-propargyl moiety found in rasagiline and selegiline (MAO-B inhibitors used to treat PD) with an 8HQ moiety (458). It is of note that MAO inhibitors inhibit oxidation of DA by MAO-A and MAO-B, thus preventing the formation of hydrogen peroxide generation and hydroxyl radicals and associated neuronal damage. Compared with CQ, HLA-20 and M30 have a higher binding affinity for iron than copper and may have less cross-metalloenzyme effects (344). The multimodal functions of HLA-20 and M30 make them more effective than previous “silver-bullet” iron chelators. M30, for instance, has been shown to up-regulate HIF-1α expression, attenuate tau phosphorylation, protect cultured cortical neurons from Aβ toxicity, and prevent lipid peroxidation (23, 247) (Table 2).
Table 2.
Major Classes of Chelators
| Chelator | Properties | Mechanism of action | Clinical studies |
|---|---|---|---|
| DFO and analogs | |||
| DFO | Hexadentate chelator hydrophilic, poor BBB penetration, short half-life (8–12 min) | Up-regulates HIF-1α by inhibiting PHDs (458), down-regulates APP via 5′-UTR (373, 441, 458), down-regulates α-synuclein via 5′-UTR (143) | DFO treatment may slow the rate of decline in AD patients by as much as twofold (300). |
| DFP | Bidentate chelator, lipophilic, high BBB penetration, iron-DFP complex is hydrophilic | Reduces Aβ40, Aβ42, and BACE1 levels in the brain (356), decreases phosphorylation of tau but not ROS formation or brain iron levels (356). | Approved by FDA for treatment of secondary iron overload, phase II trials for treatment of PD undergoing (115, 156), open-label single-arm study for treatment of FA using combination therapy of idebenone and DFP (440).Treatment of FA adolescents with DFP reduced neuropathy and ataxic gait (48). |
| DFX | Tridentate chelator, high bioavailability, long half-life (8–16 h) | Chelates iron in 2:1 DFX:iron ratio | Approved by FDA for treatment of secondary iron overload |
| 8-HQ analogs | |||
| CQ | Lipophilic, high BBB penetration, may induce SMON (136) | 8HQ moiety forms 5-member chelate rings with Fe3+, forms complexes with copper and zinc: reduces plaque formation in AD (75, 453, 474), decreases levels of interstitial Aβ (4), down-regulates APP, BACE1, and presenilin 1 (453, 474) | Pilot phase II trial shows significant decrease in AD plasma Aβ42 after CQ treatment (371). |
| PBT2 | Lipophilic, high BBB penetration | Inhibits redox activity via metal chelation (146), neutralizes Aβ toxicity by promoting clearance from existing deposits (99) | Phase IIa trial shows significant decrease in AD CSF Aβ42 following PBT2 treatment (141) |
| VK28 | Contains 8HQ moiety | Protects against MPTP and 6-OHDA induced lesions in rat brains (14) | |
| HLA-20 and M30 | Contains N-propargyl moiety, contains 8HQ moiety | Prevents ROS generation by inhibiting MAO (458), up-regulates HIF-1α (23), and attenuates tau phosphorylation (23, 247). | |
| Prochelators | |||
| SIH-B BSIH |
Parent molecule SIH, lipophilic, tridentate chelator | SIH-B is converted to SIH after exposure to hydrogen peroxide, which can then bind iron and copper (77, 282), BSIH is converted to SIH after removal of boron ester by hydrogen peroxide (76, 77, 282) | |
| BHAPI | Isonicotynoyl backbone, binds trivalent and divalent metal ions | BHAPI is converted to HAPI after reaction with hydrogen peroxide or paraquat (228). | |
| SWH | Lipophilic, contains metal chelating and amyloid binding moieties | SWH is converted to copper chelator CP after enzyme modification by β-secretase (155). Inhibits Aβ aggregation (155). | |
| Aroylhydrazones | |||
| PCIH | PIH analog, lipophilic, forms more stable complex with copper than iron (18, 268) | Induces iron mobilization and prevents iron uptake from Tf (268, 475), permeates mitochondrion to induce iron release (475) | |
| PCTH | PCIH analog, high oral bioavailability | Faster membrane penetration than DFO or PCIH (268), able to penetrate inner and outer membrane of mitochondria, and prevents hydrogen peroxide-induced cytotoxicity (268) | |
6-OHDA, 6 hydroxydopamine; 8HQ, 8-hydroxyquinolone; Aβ, amyloid β; AD, Alzheimer's disease; APP, amyloid precursor protein; BBB, blood brain barrier; CSF, cerebrospinal fluid; CQ, clioquinol; DFO, desferrioxamine; DFP, deferiprone; DFX, deferasirox; FA, Friedreich's ataxia; Fe3+, ferric iron; HIF-1α, hypoxia-inducible factor-1α; PCTH, 2-pyridylcarboxaldehyde 2-thiophenecarboxyl hydrazone; PCIH, 2-pyridylcarboxaldehyde isonicotinoyl hydrazine; PD, Parkinson's disease; PHD, prolyl-4-hydroxylases; ROS, reactive oxygen species; SIH, salicylaldehyde isonicotynoyl hydrazine.
Prochelators are molecules that can be converted into chelators under certain circumstances such as oxidative stress. The advantage of this model compared with other iron chelators is the ability to concentrate its activity at sites of damage (77). Two prominent prochelators, SIH-B and BSIH, as well as a few other derivatives have recently come to light. The parent molecule, salicylaldehyde isonicotynoyl hydrazine (SIH), is a lipophilic tridentate chelator (282). SIH-B does not bind iron or copper strongly, but is converted to SIH after exposure to hydrogen peroxide, which can then bind iron and copper. Similarly, BSIH interacts weakly with iron unless hydrogen peroxide removes the boron-ester-protecting group to reveal SIH (282). Thus, SIH-B and BSIH prevent the generation of harmful hydroxyl radicals from hydrogen peroxide and the formation of ROS from iron (76). Another derivative BHAPI (based on metal chelator HAPI, which possesses an isonicotynoyl backbone) has been shown to bind divalent and trivalent metal ions when converted to its chelator form (228). A prochelator termed SWH is enzymatically activated by β-secretase, which is up-regulated in AD, to produce a high affinity copper chelator that sequesters copper from Aβ, preventing copper-induced Aβ aggregation, and decreasing copper-promoted ROS formation (155). Thus, prochelators can be targeted to multiple pathways of disease pathogenesis.
The first aroylhydrazones were derived from pyridoxal isonicotinoyl hydrazone, highly effective and lipophilic molecules that prevent iron uptake while mobilizing intracellular iron (268). Subsequently, new iron chelators known as the 2-pyridylcarboxaldehyde isonicotinoyl hydrazone analogs were developed. Initial studies revealed that isonicotinoyl hydrazone was not a selective iron chelator and instead, formed a more stable complex with copper (268, 344). This led to subsequent derivatives, of which 2-pyridylcarboxaldehyde 2-thiophenecarboxyl hydrazone (PCTH) was the most effective (465). Experiments conducted on FA fibroblasts treated with PCTH revealed significant improvement in cell viability (70%) when compared with cells treated with DFO (1%–5%) (268). The effectiveness of PCTH in rescuing cells from hydrogen peroxide-induced cytotoxicity could be attributed to its more efficient rate of penetration. In FA, iron accumulation occurs in the mitochondria, and chelating agents should cross both the outer and inner mitochondrial membrane to have noticeable effects. With its highly lipophilic nature and rapid penetration ability, PCTH is a promising candidate for FA treatment (Table 2).
Plant polyphenols are a class of naturally occurring, non-toxic compounds that exhibit metal chelating, anti-inflammatory, radical scavenging, and neuroprotective effects. Green tea is high in catechin, a subclass of polyphenols, with epigallocatechin gallate (EGCG) comprising more than two-thirds of the catechin content (459). EGCG has been shown to protect against hydrogen-peroxide-induced toxicity in astroglial cells and to alleviate mitochondrial oxidative stress (2, 386, 390). There are conflicting opinions on the effect of polyphenols on transcription factor NF-E2-related factor 2 activation that up-regulates ferritin (386, 390). High consumption of green tea, as shown by a cross-sectional Japanese study, has a neuroprotective function and slows the progression of AD (249, 459). Several epidemiological studies have confirmed the moderate risk reduction in tea drinkers compared with non-tea drinkers (411, 459). The neuroprotective effects of tea are believed to stem from the flavanol class-related catechins and their derivatives, which possess antioxidant, radical scavenging, and transcription activating capabilities. EGCG has been shown to rescue DA depletion and SN neuronal loss in MPTP-induced PD mouse brains, possibly through its catechol-like structure that can act as a potent anti-oxidant and iron chelator (411). Its effect in AD is still unclear, but in vitro studies have demonstrated that EGCG destabilizes Aβ aggregates, prevents elongation of fibrils, and prevents Aβ-induced neurotoxicity. In addition, EGCG promotes the non-amyloidogenic pathway through up-regulation of α-secretase, suggesting a promising therapeutic option for AD (459). Interestingly, however, some studies have found that tea catechins function as pro-oxidants at high concentrations or in the presence of copper ions, necessitating further research to fully characterize this compound. While EGCG is the main focus of plant polyphenol research, other compounds such as ethyl ferulate have been shown to induce heme oxygenase-1 and antioxidant activity (385).
Other naturally occurring compounds include curcumin, rosmarinic acid, and the wine polyphenols resveratrol, myricetin, quercetin, and kaemferol. Curcumin is a major compound found in the Indian spice turmeric and has been shown to decrease the concentration of free Fe3+ in thalassemia plasma. The beta-diketo moiety of curcumin is likely its iron-binding motif. Studies in pheochromocytoma PC12 cells have shown that curcumin increases cell viability against MPTP induction (212). Similarly, rats injected with 6-OHDA benefit from curcumin treatment. However, other reports disagree with this conclusion, as 1–4 g/day of curcumin for 6 months was unable to improve cognitive performance or reduce biomarkers of inflammation in AD patients (37). In addition, since curcumin interferes with drug metabolism, it should be used with caution (289, 291). Wine polyphenols, especially resveratrol, have come under scrutiny of late due to its proposed neuroprotective effects. Resveratrol is a natural polyphenol found in many plants and, in particular, the skin of red grapes. While several studies have highlighted the role of resveratrol as anti-amyloidogenic and fibril-destabilizing, there is still dispute on whether these properties can fully account for its neuroprotective effects (173). There is wide consensus, however, that resveratrol possesses antioxidant activity, scavenges free radicals, and prevents toxicity-mediated through Aβ and Aβ-metal complexes (173). Diketopiperazine is another compound that is believed to be neuroprotective, and has shown beneficial effects in animal models of PD (242).
The development of therapeutic options for the treatment of CJD and other prion disorders has been limited by the lack of knowledge concerning PrPC and its role in metal homeostasis, and the contribution of PrPSc in disturbing brain iron metabolism. In the absence of a clear understanding of the mechanism of neurotoxicity by PrPSc, the current target of therapy involves blocking the conversion of PrPC to PrPSc. Toward this end, PrP autoantibodies have been shown to block PrPSc formation, possibly by down-regulating PrP expression (456). It is also suggested that manganese plays a role in disease pathogenesis by facilitating the refolding of normal PrPC to PrPSc and promoting subsequent aggregation; this is supported by higher levels of manganese in prion-infected brains as well as evidence of PrPSc reduction in mice treated with the manganese chelator Na2CaCDTA (55). Likewise, a modest delay in disease onset has been observed in scrapie-inoculated mice treated with the copper chelator D-penicillamine (404). Although metal imbalance appears to trigger the conversion of normal prion protein to its scrapie isoform, it is unclear whether chelation can modify its metal ion occupancy and revert PrPSc to its α-helical rich form.
Lipid peroxidation and decreased levels of SOD in prion-infected animals and cell cultures implicates oxidative stress in disease pathogenesis (167). Contributing factors are increased concentration of total iron in the brains of scrapie-infected mice, and the ability of an internal fragment of PrP (PrP106–126) to promote hydroxyl-radical formation through Fenton chemistry in the presence of copper (162). Loss of the antioxidant SOD-like activity of PrPC due to aggregation to the PrPSc form may also contribute to the overall state of oxidative stress in diseased brains. This is likely to be compounded by reduced iron export due to reduction of microtubule affinity regulating kinase-4 that decreases the phosphorylation of tau (170), thereby destabilizing tubulin and impairing the transport of APP to the plasma membrane (259). Thus, therapies targeted at restoring brain iron homeostasis and reducing oxidative stress in prion-infected brains may prove useful.
VIII. Conclusions
Iron is a well-known initiator of toxic hydroxyl-radicals, necessitating intricate mechanisms of uptake, utilization, and storage in systemic organs and the brain. Regulation of iron homeostasis in systemic organs has become increasingly clear over the past few years, and has provided important information on the transport of iron across the BBB and regulation within the brain. Although most iron-modulating proteins are synthesized locally in the brain, their functional similarity has helped in understanding their role in the complex environment of the brain. Unlike most systemic organs, the brain is unique in its region- and cell-specific distribution of iron. Moreover, the mechanism of iron uptake, storage, utilization, and vulnerability to changes in the intra- and extracellular environment is specific to each cell type within the brain. For example, astrocytes are more resistant to toxicity by iron compared with neurons and brain vascular endothelial cells, and may even provide transient protection to the neurons. Cell-specific expression of certain iron-modulating proteins has clarified these observations and the role of different cell types in maintaining brain iron homeostasis. Absence of certain proteins, such as Cp, results in iron accumulation and associated pathology in systemic organs as well as the brain. In others, such as hereditary ferritinopathy, the disease is limited to the brain; while in still others such as hemochromatosis, iron accumulation is mainly in systemic organs. The cause of organ- and cell-specific accumulation of iron and associated pathology is clear for some of these disorders; while for others, additional information on functional interaction among different iron-modulating proteins and overall maintenance of iron homeostasis within the brain is necessary. Nevertheless, these disorders provide proof of concept that excess iron in the brain is neurotoxic, especially as unlike serum Tf that is ∼30% saturated, brain Tf is ∼100% saturated with iron, and has minimal buffering capacity when faced with excess iron.
For neurodegenerative conditions such as AD, PD, sCJD, and HD, the underlying cause of brain iron dyshomeostasis is not entirely clear. The debate over whether iron is the cause or consequence of disease pathogenesis, therefore, continues. However, cumulative evidence implicating key proteins involved in the pathogenesis of these disorders in iron metabolism favors the former possibility. The presence of 5′ UTR sequences in the APP and α-synuclein transcripts, the activity of APP as a ferroxidase, α-synuclein and PrPC as a FR, the functional role of PrPC in iron transport, and sequestration of iron in PrPSc-protein complexes leaves little doubt that iron plays a significant role in the neurotoxicity associated with AD, PD, and sCJD. The accuracy of CSF ferroxidase activity and Tf in distinguishing sCJD from AD and other dementias suggests that the underlying cause of iron mis-metabolism is distinct in these disorders. Accumulated iron may induce direct neurotoxicity, or increase the vulnerability of affected neurons to toxic insults. The phenotype of iron deficiency in the SN of PD brains and neurons of sCJD cases despite accumulation of iron in these areas and consequent up-regulation of iron import proteins DMT1 and TfR1, respectively, suggests mis-regulation of the iron homeostatic machinery, emphasizing the underlying complexity (Fig. 14). Thus, chelation of iron alone is unlikely to ameliorate the neurotoxicity associated with these disorders. It is, therefore, imperative to understand the cause of iron mis-metabolism in these conditions, and device therapeutic strategies to restore brain iron homeostasis and halt the ongoing state of oxidative stress.
FIG. 14.
Brain iron dyshomeostasis in neurodegenerative conditions. Excess Fe2+ iron in the brain leads to the generation of ROS, resulting in the aggregation of certain proteins. Some of these proteins acquire pro-oxidant characteristics. Neuronal death ensues due to the combined effect of loss of function of these proteins in iron metabolism and gain of toxic function by redox-active protein aggregates. AD, Alzheimer's disease; CJD, Creutzfeldt-Jakob disease; HD, Huntington's disease; PD, Parkinson's disease; PrPC, prion protein; PrPSc, PrP-scrapie.
Several gaps in our understanding of brain iron metabolism have hampered progress toward this goal. For example, it is unclear how the iron transport machinery at the BBB senses brain iron levels and regulates import of iron from systemic circulation to the brain. The cross-talk between different cell types and pathways regulating brain iron levels at the inter-cellular level are still unclear. Thus, the neurotoxic signals generated by the absence of an astrocyte specific ferroxidase Cp are difficult to understand except for the general hypothesis of oxidative stress and lack of trophic support due to degenerating astrocytes. Likewise, the functional role of ferritin as an iron transport protein is unclear. Receptors for ferritin have been identified in endothelial cells lining the BBB and in oligodendrocytes, and radiolabeled ferritin is absorbed by rat intestinal segments (294, 426). However, further studies are necessary to elucidate the functional role of ferritin in brain iron regulation. Since ferritin is rich in iron, is fairly resistant to degradation by proteases, and co-aggregates with denatured proteins such as PrPSc, it is likely to influence brain iron levels significantly. The role of other iron-modulating proteins such as Fpn, Hp, and hepcidin or a hepcidin-like molecule in maintaining brain iron homeostasis is still emerging.
A complete understanding of these pathways and their disruption by pathogenic processes involved in various brain disorders is necessary to understand the cause of iron mis-metabolism in familial and sporadic neurodegenerative disorders associated with iron imbalance. The initial trigger of iron accumulation or deficiency is likely to vary with each condition. The subsequent cascade of events resulting in the generation of ROS and the ensuing self-enhancing vicious cycle is likely to be similar among the various brain disorders. Differences among the brain regions and cell types involved in each disorder probably reflect the proteins and biochemical pathways involved in a specific disorder. Thus, a common strategy of iron chelation is unlikely to succeed as a therapeutic strategy for all disorders associated with brain iron imbalance, though it may thwart the neurotoxicity caused by redox-active iron to some extent. However, iron chelators have provided neuroprotection for some brain disorders. Continued efforts at understanding the cause of iron imbalance and search for drugs that restore brain iron homeostasis are necessary to identify therapeutic drugs which can benefit the increasing population suffering from neurodegenerative and other disorders associated with iron imbalance.
Abbreviations Used
- 6-OHDA
6 hydroxydopamine
- 8HQ
8-hydroxyquinolone
- Aβ
amyloid β
- ABCB7
ATP-binding cassette (ABC) protein
- AD
Alzheimer's disease
- ALAS2
δ-aminolevulinate synthase 2
- APP
amyloid precursor protein
- ATP13A2
ATPase
- BBB
blood brain barrier
- CJD
Creutzfeldt-Jakob disease
- Cp
ceruloplasmin
- CSF
cerebrospinal fluid
- CQ
clioquinol
- DA
dopaminergic
- Dcytb
duodenal cytochrome b
- DFO
desferrioxamine
- DMT1
divalent metal transporter 1
- EGCG
epigallocatechin gallate
- FA
Friedreich's ataxia
- FAHN
fatty acid hydroxylase-associated neurodegeneration
- Fe2+
ferrous iron
- Fe3+
ferric iron
- FECH
ferrochelatase
- FLVCR
feline leukemia virus subgroup C. receptor
- Fpn
ferroportin
- FR
ferrireductase
- Ft-H
ferritin H-chain
- Ft-L
ferritin light chain
- Fxn
frataxin
- HD
Huntington's disease
- HFE
hereditary hemochromatosis gene product
- HIF-1α
hypoxia-inducible factor
- HO-1
heme-oxygenase-1
- Hp
hephaestin
- IREs
iron-responsive elements
- Irp1/Irp2
iron regulatory proteins 1 and 2
- ISCs
iron-sulfur clusters
- IscU
ISC assembly protein U
- LDLR
low density lipoprotein receptor
- LRRK2
leucine rich repeat kinase-2
- LTF
lactotransferrin
- MPAN
mitochondrial membrane protein associated neurodegeneration
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- NBIA
neurodegeneration with brain iron accumulation
- Nfs1
nitrogen fixation 1 homologue
- NFT
neurofibrillary tangles
- NTBI
non-transferrin bound iron
- PCIH
2-pyridylcarboxaldehyde isonicotinoyl hydrazine
- PCTH
2-pyridylcarboxaldehyde 2-thiophenecarboxyl hydrazone
- PD
Parkinson's disease
- PHD
prolyl-4-hydroxylases
- PKAN
pantothenate kinase-associated neurodegeneration
- PLAN
phospholipase A2-associated neurodegeneration
- PrPC
prion protein
- PrPSc
PrP-scrapie
- PINK1
PTEN-induced putative kinase 1
- ROS
reactive oxygen species
- S. cerevisiae
Saccharomyces cerevisiae
- sCJD
sporadic Creutzfeldt-Jakob disease
- SENDA
static encephalopathy of childhood with neurodegeneration in adulthood syndrome
- SIH
salicylaldehyde isonicotynoyl hydrazine
- SN
substantia nigra pars compacta
- SOD
superoxide dismutase
- Tf
transferrin
- TfR1
transferrin receptor 1
- TfR2
transferrin receptor 2
- XLSA/A
X-linked sideroblastic anemia with ataxia
- Yfh1
yeast frataxin homologue
References
- 1.Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JMG, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, and Rosenthal A. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25: 239–252, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Abib RT, Quincozes-Santos A, Zanotto C, Zeidan-Chulia F, Lunardi PS, Goncalves CA, and Gottfried C. Genoprotective effects of the green tea-derived polyphenol/epicatechin gallate in C6 astroglial cells. J Med Food 13: 1111–1115, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Adinolfi S, Trifuoggi M, Politou AS, Martin S, and Pastore A. A structural approach to understanding the iron-binding properties of phylogenetically different frataxins. Hum Mol Genet 11: 1865–1877, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Adlard PA, Cherny RA, Finkelstein DI, Gautier E, Robb E, Cortes M, Volitakis I, Liu X, Smith JP, Perez K, Laughton K, Li QX, Charman SA, Nicolazzo JA, Wilkins S, Deleva K, Lynch T, Kok G, Ritchie CW, Tanzi RE, Cappai R, Masters CL, Barnham KJ, and Bush AI. Rapid restoration of cognition in Alzheimer's transgenic mice with 8-hydroxy quinoline analogs is associated with decreased interstitial A beta. Neuron 59: 43–55, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Aguilaniu H, Gustafsson L, Rigoulet M, and Nystrom T. Asymmetric inheritance of oxidatively damaged proteins during cytokinesis. Science 299: 1751–1753, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Aguirre P, Urrutia P, Tapia V, Villa M, Paris I, Segura-Aguilar J, and Nunez MT. The dopamine metabolite aminochrome inhibits mitochondrial complex I and modifies the expression of iron transporters DMT1 and FPN1. Biometals 25: 795–803, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Aguzzi A, Baumann F, and Bremer J. The prion's elusive reason for being. Annu Rev Neurosci 31: 439–477, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Aguzzi A. and Falsig J. Prion propagation, toxicity and degradation. Nat Neurosci 15: 936–939, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Ahmed I, Liang Y, Schools S, Dawson VL, Dawson TM, and Savitt JM. Development and characterization of a new Parkinson's disease model resulting from impaired autophagy. J Neurosci 32: 16503–16509, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Mahdawi S, Pinto RM, Varshney D, Lawrence L, Lowrie MB, Hughes S, Webster Z, Blake J, Cooper JM, King R, and Pook MA. GAA repeat expansion mutation mouse models of Friedreich ataxia exhibit oxidative stress leading to progressive neuronal and cardiac pathology. Genomics 88: 580–590, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allikmets R, Raskind WH, Hutchinson A, Schueck ND, Dean M, and Koeller DM. Mutation of a putative mitochondrial iron transporter gene (ABC7) in X-linked sideroblastic anemia and ataxia (XLSA/A). Hum Mol Genet 8: 743–749, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Aloria K, Schilke B, Andrew A, and Craig EA. Iron-induced oligomerization of yeast frataxin homologue Yfh1 is dispensable in vivo. EMBO Rep 5: 1096–1101, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altamura S. and Muckenthaler MU. Iron toxicity in diseases of aging: Alzheimer's disease, Parkinson's disease and atherosclerosis. J Alzheimers Dis 16: 879–895, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Amit T, Avramovich-Tirosh Y, Youdim MB, and Mandel S. Targeting multiple Alzheimer's disease etiologies with multimodal neuroprotective and neurorestorative iron chelators. FASEB J 22: 1296–1305, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Anderson GJ, Lesuisse E, Dancis A, Roman DG, Labbe P, and Klausner RD. Ferric iron reduction and iron assimilation in Saccharomyces cerevisiae. J Inorg Biochem 47: 249–255, 1992 [DOI] [PubMed] [Google Scholar]
- 16.Andriopoulos B, Corradini E, Xia Y, Faasse SA, Chen SZ, Grgurevic L, Knutson MD, Pietrangelo A, Vukicevic S, Lin HY, and Babitt JL. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat Genet 41: 482–487, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arduino DM, Esteves AR, Cortes L, Silva DF, Patel B, Grazina M, Swerdlow RH, Oliveira CR, and Cardoso SM. Mitochondrial metabolism in Parkinson's disease impairs quality control autophagy by hampering microtubule-dependent traffic. Hum Mol Genet 21: 4680–4702, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armstrong CM, Bernhardt PV, Chin P, and Richardson DR. Structural Variations and Formation Constants of First-Row Tr ansition Metal Complexes of Biologically Active Aroylhydrazones. Eur J Inorg Chem 6: 1145–1156, 2003 [Google Scholar]
- 19.Aronoff-Spencer E, Burns CS, Avdievich NI, Gerfen GJ, Peisach J, Antholine WE, Ball HL, Cohen FE, Prusiner SB, and Millhauser GL. Identification of the Cu2+ binding sites in the N-terminal domain of the prion protein by EPR and CD spectroscopy. Biochemistry 39: 13760–13771, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arosio P, Ingrassia R, and Cavadini P. Ferritins: a family of molecules for iron storage, antioxidation and more. Biochim Biophys Acta 1790: 589–599, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Arosio P. and Levi S. Cytosolic and mitochondrial ferritins in the regulation of cellular iron homeostasis and oxidative damage. Biochim Biophys Acta 1800: 783–792, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Atamna H. Heme, iron, and the mitochondrial decay of ageing. Ageing Res Rev 3: 303–318, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Avramovich-Tirosh Y, Bar-Am O, Amit T, Youdim MB, and Weinreb O. Up-regulation of hypoxia-inducible factor (HIF)-1alpha and HIF-target genes in cortical neurons by the novel multifunctional iron chelator anti-Alzheimer drug, M30. Curr Alzheimer Res 7: 300–306, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Babcock M, de Silva D, Oaks R, Davis-Kaplan S, Jiralerspong S, Montermini L, Pandolfo M, and Kaplan J. Regulation of mitochondrial iron accumulation by Yfh1p, a putative homolog of frataxin. Science 276: 1709–1712, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Baraibar MA, Barbeito AG, Muhoberac BB, and Vidal R. Iron-mediated aggregation and a localized structural change characterize ferritin from a mutant light chain polypeptide that causes neurodegeneration. J Biol Chem 283: 31679–31689, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baraibar MA, Barbeito AG, Muhoberac BB, and Vidal R. A mutant light-chain ferritin that causes neurodegeneration has enhanced propensity toward oxidative damage. Free Radic Bio Med 52: 1692–1697, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baraibar MA, Muhoberac BB, Garringer HJ, Hurley TD, and Vidal R. Unraveling of the E-helices and disruption of 4-fold pores are associated with iron mishandling in a mutant ferritin causing neurodegeneration. J Biol Chem 285: 1950–1956, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barbeito AG, Garringer HJ, Baraibar MA, Gao X, Arredondo M, Nunez MT, Smith MA, Ghetti B, and Vidal R. Abnormal iron metabolism and oxidative stress in mice expressing a mutant form of the ferritin light polypeptide gene. J Neurochem 109: 1067–1078, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barbeito AG, Levade T, Delisle MB, Ghetti B, and Vidal R. Abnormal iron metabolism in fibroblasts from a patient with the neurodegenerative disease hereditary ferritinopathy. Mol Neurodegener 5: 50, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barnham KJ. and Bush AI. Metals in Alzheimer's and Parkinson's diseases. Curr Opin Chem Biol 12: 222–228, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Barnham KJ, McKinstry WJ, Multhaup G, Galatis D, Morton CJ, Curtain CC, Williamson NA, White AR, Hinds MG, Norton RS, Beyreuther K, Masters CL, Parker MW, and Cappai R. Structure of the Alzheimer's disease amyloid precursor protein copper binding domain. A regulator of neuronal copper homeostasis. J Biol Chem 278: 17401–17407, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Baron GS, Magalhaes AC, Prado MA, and Caughey B. Mouse-adapted scrapie infection of SN56 cells: greater efficiency with microsome-associated versus purified PrP-res. J Virol 80: 2106–2117, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barone E, Di Domenico F, Cenini G, Sultana R, Cini C, Preziosi P, Perluigi M, Mancuso C, and Butterfield DA. Biliverdin reductase—a protein levels and activity in the brains of subjects with Alzheimer disease and mild cognitive impairment. Biochim Biophys Acta 1812: 480–487, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barone E, Di Domenico F, Cenini G, Sultana R, Coccia R, Preziosi P, Perluigi M, Mancuso C, and Butterfield DA. Oxidative and nitrosative modifications of biliverdin reductase-A in the brain of subjects with Alzheimer's disease and amnestic mild cognitive impairment. J Alzheimers Dis 25: 623–633, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Barone E, Di Domenico F, Sultana R, Coccia R, Mancuso C, Perluigi M, and Butterfield DA. Heme oxygenase-1 posttranslational modifications in the brain of subjects with Alzheimer disease and mild cognitive impairment. Free Radic Biol Med 52: 2292–2301, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Basu S, Mohan ML, Luo X, Kundu B, Kong Q, and Singh N. Modulation of proteinase K-resistant prion protein in cells and infectious brain homogenate by redox iron: implications for prion replication and disease pathogenesis. Mol Biol Cell 18: 3302–3312, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baum L, Lam CW, Cheung SK, Kwok T, Lui V, Tsoh J, Lam L, Leung V, Hui E, Ng C, Woo J, Chiu HF, Goggins WB, Zee BC, Cheng KF, Fong CY, Wong A, Mok H, Chow MS, Ho PC, Ip SP, Ho CS, Yu XW, Lai CY, Chan MH, Szeto S, Chan IH, and Mok V. Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. J Clin Psychopharmacol 28: 110–113, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Beard JL, Wiesinger JA, Li N, and Connor JR. Brain iron uptake in hypotransferrinemic mice: influence of systemic iron status. J Neurosci Res 79: 254–261, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Becker EM, Greer JM, Ponka P, and Richardson DR. Erythroid differentiation and protoporphyrin IX down-regulate frataxin expression in Friend cells: characterization of frataxin expression compared to molecules involved in iron metabolism and hemoglobinization. Blood 99: 3813–3822, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Bekri S, Kispal G, Lange H, Fitzsimons E, Tolmie J, Lill R, and Bishop DF. Human ABC7 transporter: gene structure and mutation causing X-linked sideroblastic anemia with ataxia with disruption of cytosolic iron-sulfur protein maturation. Blood 96: 3256–3264, 2000 [PubMed] [Google Scholar]
- 41.Ben-Shachar D, Riederer P, and Youdim MB. Iron-melanin interaction and lipid peroxidation: implications for Parkinson's disease. J Neurochem 57: 1609–1614, 1991 [DOI] [PubMed] [Google Scholar]
- 42.Berciano J, Infante J, Garcia A, Polo JM, Volpini V, and Combarros O. Very late-onset Friedreich's ataxia with minimal GAA1 expansion mimicking multiple system atrophy of cerebellar type. Mov Disord 20: 1643–1645, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Bidichandani SI, Ashizawa T, and Patel PI. The GAA triplet-repeat expansion in Friedreich ataxia interferes with transcription and may be associated with an unusual DNA structure. Am J Hum Genet 62: 111–121, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Biederbick A, Stehling O, Rosser R, Niggemeyer B, Nakai Y, Elsasser HP, and Lill R. Role of human mitochondrial Nfs1 in cytosolic iron-sulfur protein biogenesis and iron regulation. Mol Cell Biol 26: 5675–5687, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bilgic B, Pfefferbaum A, Rohlfing T, Sullivan EV, and Adalsteinsson E. MRI estimates of brain iron concentration in normal aging using quantitative susceptibility mapping. Neuroimage 59: 2625–2635, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bishop DF, Tchaikovskii V, Hoffbrand AV, Fraser ME, and Margolis S. X-linked sideroblastic anemia due to carboxyl-terminal ALAS2 mutations that cause loss of binding to the beta-subunit of succinyl-CoA synthetase (SUCLA2). J Biol Chem 287: 28943–28955, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bleackley MR. and Macgillivray RT. Transition metal homeostasis: from yeast to human disease. Biometals 24: 785–809, 2011 [DOI] [PubMed] [Google Scholar]
- 48.Boddaert N, Le Quan Sang KH, Rotig A, Leroy-Willig A, Gallet S, Brunelle F, Sidi D, Thalabard JC, Munnich A, and Cabantchik ZI. Selective iron chelation in Friedreich ataxia: biologic and clinical implications. Blood 110: 401–408, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P, Joosse M, van Dongen JW, Vanacore N, van Swieten JC, Brice A, Meco G, van Duijn CM, Oostra BA, and Heutink P. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 299: 256–259, 2003 [DOI] [PubMed] [Google Scholar]
- 50.Borie C, Gasparini F, Verpillat P, Bonnet AM, Agid Y, Hetet G, Brice A, Durr A, and Grandchamp B. Association study between iron-related genes polymorphisms and Parkinson's disease. J Neurol 249: 801–804, 2002 [DOI] [PubMed] [Google Scholar]
- 51.Bou-Abdallah F, Santambrogio P, Levi S, Arosio P, and Chasteen ND. Unique iron binding and oxidation properties of human mitochondrial ferritin: a comparative analysis with human H-chain ferritin. J Mol Biol 347: 543–554, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Bowen BJ. and Morgan EH. Anemia of the Belgrade rat: evidence for defective membrane transport of iron. Blood 70: 38–44, 1987 [PubMed] [Google Scholar]
- 53.Bradbury MW. Transport of iron in the blood-brain-cerebrospinal fluid system. J Neurochem 69: 443–454, 1997 [DOI] [PubMed] [Google Scholar]
- 54.Bradley JL, Blake JC, Chamberlain S, Thomas PK, Cooper JM, and Schapira AH. Clinical, biochemical and molecular genetic correlations in Friedreich's ataxia. Hum Mol Genet 9: 275–282, 2000 [DOI] [PubMed] [Google Scholar]
- 55.Brazier MW, Volitakis I, Kvasnicka M, White AR, Underwood JR, Green JE, Han S, Hill AF, Masters CL, and Collins SJ. Manganese chelation therapy extends survival in a mouse model of M1000 prion disease. J Neurochem 114: 440–451, 2010 [DOI] [PubMed] [Google Scholar]
- 56.Bridwell-Rabb J, Winn AM, and Barondeau DP. Structure-function analysis of Friedreich's ataxia mutants reveals determinants of frataxin binding and activation of the Fe-S assembly complex. Biochemistry 50: 7265–7274, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brown DR, Qin K, Herms JW, Madlung A, Manson J, Strome R, Fraser PE, Kruck T, von Bohlen A, Schulz-Schaeffer W, Giese A, Westaway D, and Kretzschmar H. The cellular prion protein binds copper in vivo. Nature 390: 684–687, 1997 [DOI] [PubMed] [Google Scholar]
- 58.Bulteau AL, O'Neill HA, Kennedy MC, Ikeda-Saito M, Isaya G, and Szweda LI. Frataxin acts as an iron chaperone protein to modulate mitochondrial aconitase activity. Science 305: 242–245, 2004 [DOI] [PubMed] [Google Scholar]
- 59.Burn J. and Chinnery PF. Neuroferritinopathy. Semin Pediatr Neurol 13: 176–181, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Bush AI. The metallobiology of Alzheimer's disease. Trends Neurosci 26: 207–214, 2003 [DOI] [PubMed] [Google Scholar]
- 61.Bush AI. and Curtain CC. Twenty years of metallo-neurobiology: where to now? Eur Biophys J 37: 241–245, 2008 [DOI] [PubMed] [Google Scholar]
- 62.Butler EK, Voigt A, Lutz AK, Toegel JP, Gerhardt E, Karsten P, Falkenburger B, Reinartz A, Winklhofer KF, and Schulz JB. The mitochondrial chaperone protein TRAP1 mitigates alpha-Synuclein toxicity. PLoS Genet 8: e1002488, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Butterfield DA. and Kanski J. Brain protein oxidation in age-related neurodegenerative disorders that are associated with aggregated proteins. Mech Ageing Dev 122: 945–962, 2001 [DOI] [PubMed] [Google Scholar]
- 64.Cairo G, Rappocciolo E, Tacchini L, and Schiaffonati L. Expression of the genes for the ferritin H and L subunits in rat liver and heart. Evidence for tissue-specific regulations at pre- and post-translational levels. Biochem J 275 (Pt 3): 813–816, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Calabrese V, Mancuso C, Ravagna A, Perluigi M, Cini C, De Marco C, Butterfield DA, and Stella AM. In vivo induction of heat shock proteins in the substantia nigra following L-DOPA administration is associated with increased activity of mitochondrial complex I and nitrosative stress in rats: regulation by glutathione redox state. J Neurochem 101: 709–717, 2007 [DOI] [PubMed] [Google Scholar]
- 66.Calabrese V, Sultana R, Scapagnini G, Guagliano E, Sapienza M, Bella R, Kanski J, Pennisi G, Mancuso C, Stella AM, and Butterfield DA. Nitrosative stress, cellular stress response, and thiol homeostasis in patients with Alzheimer's disease. Antioxid Redox Signal 8: 1975–1986, 2006 [DOI] [PubMed] [Google Scholar]
- 67.Campanella A, Isaya G, O'Neill HA, Santambrogio P, Cozzi A, Arosio P, and Levi S. The expression of human mitochondrial ferritin rescues respiratory function in frataxin-deficient yeast. Hum Mol Genet 13: 2279–2288, 2004 [DOI] [PubMed] [Google Scholar]
- 68.Campanella A, Rovelli E, Santambrogio P, Cozzi A, Taroni F, and Levi S. Mitochondrial ferritin limits oxidative damage regulating mitochondrial iron availability: hypothesis for a protective role in Friedreich ataxia. Hum Mol Genet 18: 1–11, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Campuzano V, Montermini L, Lutz Y, Cova L, Hindelang C, Jiralerspong S, Trottier Y, Kish SJ, Faucheux B, Trouillas P, Authier FJ, Durr A, Mandel JL, Vescovi A, Pandolfo M, and Koenig M. Frataxin is reduced in Friedreich ataxia patients and is associated with mitochondrial membranes. Hum Mol Genet 6: 1771–1780, 1997 [DOI] [PubMed] [Google Scholar]
- 70.Campuzano V, Montermini L, Molto MD, Pianese L, Cossee M, Cavalcanti F, Monros E, Rodius F, Duclos F, Monticelli A, Zara F, Canizares J, Koutnikova H, Bidichandani SI, Gellera C, Brice A, Trouillas P, De Michele G, Filla A, De Frutos R, Palau F, Patel PI, Di Donato S, Mandel JL, Cocozza S, Koenig M, and Pandolfo M. Friedreich's ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science 271: 1423–1427, 1996 [DOI] [PubMed] [Google Scholar]
- 71.Casas C, Aldea M, Espinet C, Gallego C, Gil R, and Herrero E. The AFT1 transcriptional factor is differentially required for expression of high-affinity iron uptake genes in Saccharomyces cerevisiae. Yeast 13: 621–637, 1997 [DOI] [PubMed] [Google Scholar]
- 72.Casey JL, Hentze MW, Koeller DM, Caughman SW, Rouault TA, Klausner RD, and Harford JB. Iron-responsive elements: regulatory RNA sequences that control mRNA levels and translation. Science 240: 924–928, 1988 [DOI] [PubMed] [Google Scholar]
- 73.Cavadini P, Gellera C, Patel PI, and Isaya G. Human frataxin maintains mitochondrial iron homeostasis in Saccharomyces cerevisiae. Hum Mol Genet 9: 2523–2530, 2000 [DOI] [PubMed] [Google Scholar]
- 74.Cazzola M, Invernizzi R, Bergamaschi G, Levi S, Corsi B, Travaglino E, Rolandi V, Biasiotto G, Drysdale J, and Arosio P. Mitochondrial ferritin expression in erythroid cells from patients with sideroblastic anemia. Blood 101: 1996–2000, 2003 [DOI] [PubMed] [Google Scholar]
- 75.Ceccom J, Cosledan F, Halley H, Frances B, Lassalle JM, and Meunier B. Copper chelator induced efficient episodic memory recovery in a non-transgenic Alzheimer's mouse model. PLoS One 7: e43105, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Charkoudian LK, Dentchev T, Lukinova N, Wolkow N, Dunaief JL, and Franz KJ. Iron prochelator BSIH protects retinal pigment epithelial cells against cell death induced by hydrogen peroxide. J Inorg Biochem 102: 2130–2135, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Charkoudian LK, Pham DM, and Franz KJ. A pro-chelator triggered by hydrogen peroxide inhibits iron-promoted hydroxyl radical formation. J Am Chem Soc 128: 12424–12425, 2006 [DOI] [PubMed] [Google Scholar]
- 78.Chen H, Attieh ZK, Su T, Syed BA, Gao H, Alaeddine RM, Fox TC, Usta J, Naylor CE, Evans RW, McKie AT, Anderson GJ, and Vulpe CD. Hephaestin is a ferroxidase that maintains partial activity in sex-linked anemia mice. Blood 103: 3933–3939, 2004 [DOI] [PubMed] [Google Scholar]
- 79.Chen H, Huang G, Su T, Gao H, Attieh ZK, McKie AT, Anderson GJ, and Vulpe CD. Decreased hephaestin activity in the intestine of copper-deficient mice causes systemic iron deficiency. J Nutr 136: 1236–1241, 2006 [DOI] [PubMed] [Google Scholar]
- 80.Chen W, Dailey HA, and Paw BH. Ferrochelatase forms an oligomeric complex with mitoferrin-1 and Abcb10 for erythroid heme biosynthesis. Blood 116: 628–630, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen W, Paradkar PN, Li L, Pierce EL, Langer NB, Takahashi-Makise N, Hyde BB, Shirihai OS, Ward DM, Kaplan J, and Paw BH. Abcb10 physically interacts with mitoferrin-1 (Slc25a37) to enhance its stability and function in the erythroid mitochondria. Proc Natl Acad Sci U S A 106: 16263–16268, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen XZ, Peng JB, Cohen A, Nelson H, Nelson N, and Hediger MA. Yeast SMF1 mediates H(+)-coupled iron uptake with concomitant uncoupled cation currents. J Biol Chem 274: 35089–35094, 1999 [DOI] [PubMed] [Google Scholar]
- 83.Chesebro B, Race R, and Kercher L. Scrapie pathogenesis in brain and retina: effects of prion protein expression in neurons and astrocytes. J Neurovirol 11: 476–480, 2005 [DOI] [PubMed] [Google Scholar]
- 84.Chew KC, Ang ET, Tai YK, Tsang F, Lo SQ, Ong E, Ong WY, Shen HM, Lim KL, Dawson VL, Dawson TM, and Soong TW. Enhanced autophagy from chronic toxicity of iron and mutant A53T alpha-synuclein: implications for neuronal cell death in Parkinson disease. J Biol Chem 286: 33380–33389, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Choi CJ, Anantharam V, Saetveit NJ, Houk RS, Kanthasamy A, and Kanthasamy AG. Normal cellular prion protein protects against manganese-induced oxidative stress and apoptotic cell death. Toxicol Sci 98: 495–509, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chou AP, Li S, Fitzmaurice AG, and Bronstein JM. Mechanisms of rotenone-induced proteasome inhibition. Neurotoxicology 31: 367–372, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Connor JR, Boeshore KL, Benkovic SA, and Menzies SL. Isoforms of Ferritin Have a Specific Cellular-Distribution in the Brain. J Neurosci Res 37: 461–465, 1994 [DOI] [PubMed] [Google Scholar]
- 88.Cook JD, Bencze KZ, Jankovic AD, Crater AK, Busch CN, Bradley PB, Stemmler AJ, Spaller MR, and Stemmler TL. Monomeric yeast frataxin is an iron-binding protein. Biochemistry 45: 7767–7777, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cooperman SS, Meyron-Holtz EG, Olivierre-Wilson H, Ghosh MC, McConnell JP, and Rouault TA. Microcytic anemia, erythropoietic protoporphyria, and neurodegeneration in mice with targeted deletion of iron-regulatory protein 2. Blood 106: 1084–1091, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Corsi B, Cozzi A, Arosio P, Drysdale J, Santambrogio P, Campanella A, Biasiotto G, Albertini A, and Levi S. Human mitochondrial ferritin expressed in HeLa cells incorporates iron and affects cellular iron metabolism. J Biol Chem 277: 22430–22437, 2002 [DOI] [PubMed] [Google Scholar]
- 91.Cossee M, Puccio H, Gansmuller A, Koutnikova H, Dierich A, LeMeur M, Fischbeck K, Dolle P, and Koenig M. Inactivation of the Friedreich ataxia mouse gene leads to early embryonic lethality without iron accumulation. Hum Mol Genet 9: 1219–1226, 2000 [DOI] [PubMed] [Google Scholar]
- 92.Cox TC, Bawden MJ, Martin A, and May BK. Human erythroid 5-aminolevulinate synthase: promoter analysis and identification of an iron-responsive element in the mRNA. EMBO J 10: 1891–1902, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cox TC, Bottomley SS, Wiley JS, Bawden MJ, Matthews CS, and May BK. X-linked pyridoxine-responsive sideroblastic anemia due to a Thr388-to-Ser substitution in erythroid 5-aminolevulinate synthase. N Engl J Med 330: 675–679, 1994 [DOI] [PubMed] [Google Scholar]
- 94.Cozzi A, Santambrogio P, Corsi B, Campanella A, Arosio P, and Levi S. Characterization of the L-ferritin variant 460InsA responsible of a hereditary ferritinopathy disorder. Neurobiol Dis 23: 644–652, 2006 [DOI] [PubMed] [Google Scholar]
- 95.Cremonesi L, Cozzi A, Girelli D, Ferrari F, Fermo I, Foglieni B, Levi S, Bozzini C, Camparini M, Ferrari M, and Arosio P. Case report: a subject with a mutation in the ATG start codon of L-ferritin has no haematological or neurological symptoms. J Med Genet 41: e81, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cremonesi L, Foglieni B, Fermo I, Cozzi A, Paroni R, Ruggeri G, Belloli S, Levi S, Fargion S, Ferrari M, and Arosio P. Identification of two novel mutations in the 5′ untranslated region of H-ferritin using denaturing high performance liquid chromatography scanning. Haematologica 88: 1110–1116, 2003 [PubMed] [Google Scholar]
- 97.Crichton RR, Dexter DT, and Ward RJ. Brain iron metabolism and its perturbation in neurological diseases. J Neural Transm 118: 301–314, 2011 [DOI] [PubMed] [Google Scholar]
- 98.Crompton DE, Chinnery PF, Bates D, Walls TJ, Jackson MJ, Curtis AJ, and Burn J. Spectrum of movement disorders in neuroferritinopathy. Mov Disord 20: 95–99, 2005 [DOI] [PubMed] [Google Scholar]
- 99.Crouch PJ, Savva MS, Hung LW, Donnelly PS, Mot AI, Parker SJ, Greenough MA, Volitakis I, Adlard PA, Cherny RA, Masters CL, Bush AI, Barnham KJ, and White AR. The Alzheimer's therapeutic PBT2 promotes amyloid-beta degradation and GSK3 phosphorylation via a metal chaperone activity. J Neurochem 119: 220–230, 2011 [DOI] [PubMed] [Google Scholar]
- 100.Csere P, Lill R, and Kispal G. Identification of a human mitochondrial ABC transporter, the functional orthologue of yeast Atm1p. FEBS Lett 441: 266–270, 1998 [DOI] [PubMed] [Google Scholar]
- 101.Curtis AR, Fey C, Morris CM, Bindoff LA, Ince PG, Chinnery PF, Coulthard A, Jackson MJ, Jackson AP, McHale DP, Hay D, Barker WA, Markham AF, Bates D, Curtis A, and Burn J. Mutation in the gene encoding ferritin light polypeptide causes dominant adult-onset basal ganglia disease. Nat Genet 28: 350–354, 2001 [DOI] [PubMed] [Google Scholar]
- 102.Dailey HA, Sellers VM, and Dailey TA. Mammalian Ferrochelatase—Expression and Characterization of Normal and two Human Protoporhyric Ferrochelatases. J Biol Chem 269: 390–395, 1994 [PubMed] [Google Scholar]
- 103.Dancis A, Klausner RD, Hinnebusch AG, and Barriocanal JG. Genetic evidence that ferric reductase is required for iron uptake in Saccharomyces cerevisiae. Mol Cell Biol 10: 2294–2301, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Davies P, Moualla D, and Brown DR. Alpha-Synuclein Is a Cellular Ferrireductase. PLoS One 6: e15814, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Davis BA, O'Sullivan C, Jarritt PH, and Porter JB. Value of sequential monitoring of left ventricular ejection fraction in the management of thalassemia major. Blood 104: 263–269, 2004 [DOI] [PubMed] [Google Scholar]
- 106.Dawson TM. and Dawson VL. Molecular pathways of neurodegeneration in Parkinson's disease. Science 302: 819–822, 2003 [DOI] [PubMed] [Google Scholar]
- 107.De Domenico I, Ward DM, Di Patti MCB, Jeong SY, David S, Musci G, and Kaplan J. Ferroxidase activity is required for the stability of cell surface ferroportin in cells expressing GPI-ceruloplasmin. EMBO J 26: 2823–2831, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.De Domenico I, Ward DM, and Kaplan J. Specific iron chelators determine the route of ferritin degradation. Blood 114: 4546–4551, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.De Silva DM, Askwith CC, Eide D, and Kaplan J. The FET3 gene product required for high affinity iron transport in yeast is a cell surface ferroxidase. J Biol Chem 270: 1098–1101, 1995 [DOI] [PubMed] [Google Scholar]
- 110.Deane R, Zheng W, and Zlokovic BV. Brain capillary endothelium and choroid plexus epithelium regulate transport of transferrin-bound and free iron into the rat brain. J Neurochem 88: 813–820, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Decressac M, Mattsson B, Lundblad M, Weikop P, and Bjorklund A. Progressive neurodegenerative and behavioural changes induced by AAV-mediated overexpression of alpha-synuclein in midbrain dopamine neurons. Neurobiol Dis 45: 939–953, 2012 [DOI] [PubMed] [Google Scholar]
- 112.Dehay B, Bove J, Rodriguez-Muela N, Perier C, Recasens A, Boya P, and Vila M. Pathogenic lysosomal depletion in Parkinson's disease. J Neurosci 30: 12535–12544, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Deleault NR, Harris BT, Rees JR, and Supattapone S. Formation of native prions from minimal components in vitro. Proc Natl Acad Sci U S A 104: 9741–9746, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Deng XL, Vidal R, and Englander EW. Accumulation of oxidative DNA damage in brain mitochondria in mouse model of hereditary ferritinopathy. Neurosci Lett 479: 44–48, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Devos D. 2012. Efficacy and safety of the iron chelator deferiprone in Parkinson's disease (FAIR-PARK-I). http://clinicaltrials.gov/ct2/show/NCT00943748?term=iron+chelator+neurodegeneration&rank=1
- 116.Devos D, Tchofo PJ, Vuillaume I, Destee A, Batey S, Burn J, and Chinnery PF. Clinical features and natural history of neuroferritinopathy caused by the 458dupA FTL mutation. Brain 132: e109, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dexter DT, Carayon A, Javoy-Agid F, Agid Y, Wells FR, Daniel SE, Lees AJ, Jenner P, and Marsden CD. Alterations in the levels of iron, ferritin and other trace metals in Parkinson's disease and other neurodegenerative diseases affecting the basal ganglia. Brain 114 (Pt 4): 1953–1975, 1991 [DOI] [PubMed] [Google Scholar]
- 118.Dexter DT, Wells FR, Lees AJ, Agid F, Agid Y, Jenner P, and Marsden CD. Increased nigral iron content and alterations in other metal ions occurring in brain in Parkinson's disease. J Neurochem 52: 1830–1836, 1989 [DOI] [PubMed] [Google Scholar]
- 119.Dezfouli MA, Alavi A, Rohani M, Rezvani M, Nekuie T, Klotzle B, Tonekaboni SH, Shahidi GA, and Elahi E. PANK2 and C19orf12 mutations are common causes of neurodegeneration with brain iron accumulation. Mov Disord 28: 228–232, 2013 [DOI] [PubMed] [Google Scholar]
- 120.Dhe-Paganon S, Shigeta R, Chi YI, Ristow M, and Shoelson SE. Crystal structure of human frataxin. J Biol Chem 275: 30753–30756, 2000 [DOI] [PubMed] [Google Scholar]
- 121.Di Domenico F, Barone E, Mancuso C, Perluigi M, Cocciolo A, Mecocci P, Butterfield DA, and Coccia R. HO-1/BVR-A system analysis in plasma from probable Alzheimer's disease and mild cognitive impairment subjects: a potential biochemical marker for the prediction of the disease. J Alzheimers Dis 32: 277–289, 2012 [DOI] [PubMed] [Google Scholar]
- 122.Di Fonzo A, Chien HF, Socal M, Giraudo S, Tassorelli C, Iliceto G, Fabbrini G, Marconi R, Fincati E, Abbruzzese G, Marini P, Squitieri F, Horstink MW, Montagna P, Libera AD, Stocchi F, Goldwurm S, Ferreira JJ, Meco G, Martignoni E, Lopiano L, Jardim LB, Oostra BA, Barbosa ER, and Bonifati V. ATP13A2 missense mutations in juvenile parkinsonism and young onset Parkinson disease. Neurology 68: 1557–1562, 2007 [DOI] [PubMed] [Google Scholar]
- 123.Dix DR, Bridgham JT, Broderius MA, Byersdorfer CA, and Eide DJ. The FET4 gene encodes the low affinity Fe(II) transport protein of Saccharomyces cerevisiae. J Biol Chem 269: 26092–26099, 1994 [PubMed] [Google Scholar]
- 124.Donovan A, Lima CA, Pinkus JL, Pinkus GS, Zon LI, Robine S, and Andrews NC. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab 1: 191–200, 2005 [DOI] [PubMed] [Google Scholar]
- 125.Drake SF, Morgan EH, Herbison CE, Delima R, Graham RM, Chua AC, Leedman PJ, Fleming RE, Bacon BR, Olynyk JK, and Trinder D. Iron absorption and hepatic iron uptake are increased in a transferrin receptor 2 (Y245×) mutant mouse model of hemochromatosis type 3. Am J Physiol Gastrointest Liver Physiol 292: G323–G328, 2007 [DOI] [PubMed] [Google Scholar]
- 126.Drayer B, Burger P, Darwin R, Riederer S, Herfkens R, and Johnson GA. MRI of brain iron. AJR Am J Roentgenol 147: 103–110, 1986 [DOI] [PubMed] [Google Scholar]
- 127.Drysdale J, Arosio P, Invernizzi R, Cazzola M, Volz A, Corsi B, Biasiotto G, and Levi S. Mitochondrial ferritin: A new player in iron metabolism. Blood Cells Mol Dis 29: 376–383, 2002 [DOI] [PubMed] [Google Scholar]
- 128.Du X, She E, Gelbart T, Truksa J, Lee P, Xia Y, Khovananth K, Mudd S, Mann N, Moresco EMY, Beutler E, and Beutler B. The serine protease TMPRSS6 is required to sense iron deficiency. Science 320: 1088–1092, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Duce JA, Tsatsanis A, Cater MA, James SA, Robb E, Wikhe K, Leong SL, Perez K, Johanssen T, Greenough MA, Cho HH, Galatis D, Moir RD, Masters CL, McLean C, Tanzi RE, Cappai R, Barnham KJ, Ciccotosto GD, Rogers JT, and Bush AI. Iron-export ferroxidase activity of beta-amyloid precursor protein is inhibited by zinc in Alzheimer's disease. Cell 142: 857–867, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Duennwald ML, Jagadish S, Muchowski PJ, and Lindquist S. Flanking sequences profoundly alter polyglutamine toxicity in yeast. Proc Natl Acad Sci U S A 103: 11045–11050, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dupuy J, Volbeda A, Carpentier P, Darnault C, Moulis JM, and Fontecilla-Camps JC. Crystal structure of human iron regulatory protein 1 as cytosolic aconitase. Structure 14: 129–139, 2006 [DOI] [PubMed] [Google Scholar]
- 132.Durr A, Cossee M, Agid Y, Campuzano V, Mignard C, Penet C, Mandel JL, Brice A, and Koenig M. Clinical and genetic abnormalities in patients with Friedreich's ataxia. N Engl J Med 335: 1169–1175, 1996 [DOI] [PubMed] [Google Scholar]
- 133.Dusek P. and Schneider SA. Neurodegeneration with brain iron accumulation. Curr Opin Neurol 25: 499–506, 2012 [DOI] [PubMed] [Google Scholar]
- 134.Dwyer BE, Nishimura RN, and Lu SY. Differential expression of heme oxygenase-1 in cultured cortical neurons and astrocytes determined by the aid of a new heme oxygenase antibody. Response to oxidative stress. Brain Res Mol Brain Res 30: 37–47, 1995 [DOI] [PubMed] [Google Scholar]
- 135.Eaton JW. and Qian M. Molecular bases of cellular iron toxicity. Free Radic Biol Med 32: 833–840, 2002 [DOI] [PubMed] [Google Scholar]
- 136.Egashira Y. and Matsuyama H. Subacute myelo-optico-neuropathy (SMON) in Japan. With special reference to the autopsy cases. Acta Pathol Jpn 32Suppl 1: 101–116, 1982 [PubMed] [Google Scholar]
- 137.Esteves AR, Arduino DM, Silva DF, Oliveira CR, and Cardoso SM. Mitochondrial dysfunction: the road to alpha-synuclein oligomerization in PD. Park Dis 2011: 693761, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Evans-Galea MV, Corben LA, Hasell J, Galea CA, Fahey MC, du Sart D, and Delatycki MB. A novel deletion-insertion mutation identified in exon 3 of FXN in two siblings with a severe Friedreich ataxia phenotype. Neurogenetics 12: 307–313, 2011 [DOI] [PubMed] [Google Scholar]
- 139.Fahmy M. and Young SP. Modulation of iron metabolism in monocyte cell line U937 by inflammatory cytokines: changes in transferrin uptake, iron handling and ferritin mRNA. Biochem J 296: 175–181, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Farrer M, Stone J, Mata IF, Lincoln S, Kachergus J, Hulihan M, Strain KJ, and Maraganore DM. LRRK2 mutations in Parkinson disease. Neurology 65: 738–740, 2005 [DOI] [PubMed] [Google Scholar]
- 141.Faux NG, Ritchie CW, Gunn A, Rembach A, Tsatsanis A, Bedo J, Harrison J, Lannfelt L, Blennow K, Zetterberg H, Ingelsson M, Masters CL, Tanzi RE, Cummings JL, Herd CM, and Bush AI. PBT2 rapidly improves cognition in Alzheimer's Disease: additional phase II analyses. J Alzheimers Dis 20: 509–516, 2010 [DOI] [PubMed] [Google Scholar]
- 142.Fearnley JM. and Lees AJ. Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain 114 (Pt 5): 2283–2301, 1991 [DOI] [PubMed] [Google Scholar]
- 143.Febbraro F, Giorgi M, Caldarola S, Loreni F, and Romero-Ramos M. alpha-Synuclein expression is modulated at the translational level by iron. Neuroreport 23: 576–580, 2012 [DOI] [PubMed] [Google Scholar]
- 144.Fernaeus S, Halldin J, Bedecs K, and Land T. Changed iron regulation in scrapie-infected neuroblastoma cells. Brain Res Mol Brain Res 133: 266–273, 2005 [DOI] [PubMed] [Google Scholar]
- 145.Fernaeus S. and Land T. Increased iron-induced oxidative stress and toxicity in scrapie-infected neuroblastoma cells. Neurosci Lett 382: 217–220, 2005 [DOI] [PubMed] [Google Scholar]
- 146.Fernandez-Bachiller MI, Perez C, Gonzalez-Munoz GC, Conde S, Lopez MG, Villarroya M, Garcia AG, and Rodriguez-Franco MI. Novel tacrine-8-hydroxyquinoline hybrids as multifunctional agents for the treatment of Alzheimer's disease, with neuroprotective, cholinergic, antioxidant, and copper-complexing properties. J Med Chem 53: 4927–4937, 2010 [DOI] [PubMed] [Google Scholar]
- 147.Ferreira C, Bucchini D, Martin ME, Levi S, Arosio P, Grandchamp B, and Beaumont C. Early embryonic lethality of H ferritin gene deletion in mice. J Biol Chem 275: 3021–3024, 2000 [DOI] [PubMed] [Google Scholar]
- 148.Filla A, DeMichele G, Cavalcanti F, Pianese L, Monticelli A, Campanella G, and Cocozza S. The relationship between trinucleotide (GAA) repeat length and clinical features in friedreich ataxia. Am J Hum Genet 59: 554–560, 1996 [PMC free article] [PubMed] [Google Scholar]
- 149.Fink AL. The aggregation and fibrillation of alpha-synuclein. Acc Chem Res 39: 628–634, 2006 [DOI] [PubMed] [Google Scholar]
- 150.Fisher J, Devraj K, Ingram J, Slagle-Webb B, Madhankumar AB, Liu X, Klinger M, Simpson IA, and Connor JR. Ferritin: a novel mechanism for delivery of iron to the brain and other organs. Am J Physiol Cell Physiol 293: C641–C649, 2007 [DOI] [PubMed] [Google Scholar]
- 151.Fleming MD, Trenor CC, Su MA, Foernzler D, Beier DR, Dietrich WF, and Andrews NC. Microcytic anaemia mice have a mutation in Nramp2, a candidate iron transporter gene. Nat Genet 16: 383–386, 1997 [DOI] [PubMed] [Google Scholar]
- 152.Fleming RE, Ahmann JR, Migas MC, Waheed A, Koeffler HP, Kawabata H, Britton RS, Bacon BR, and Sly WS. Targeted mutagenesis of the murine transferrin receptor-2 gene produces hemochromatosis. Proc Natl Acad Sci U S A 99: 10653–10658, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fleming RE, Feng Q, and Britton RS. Knockout mouse models of iron homeostasis. Annu Rev Nutr 31: 117–137, 2011 [DOI] [PubMed] [Google Scholar]
- 154.Foglieni B, Ferrari F, Goldwurm S, Santambrogio P, Castiglioni E, Sessa M, Volonte MA, Lalli S, Galli C, Wang XS, Connor J, Sironi F, Canesi M, Biasiotto G, Pezzoli G, Levi S, Ferrari M, Arosio P, and Cremonesi L. Analysis of ferritin genes in Parkinson disease. Clin Chem Lab Med 45: 1450–1456, 2007 [DOI] [PubMed] [Google Scholar]
- 155.Folk DS. and Franz KJ. A prochelator activated by beta-secretase inhibits Abeta aggregation and suppresses copper-induced reactive oxygen species formation. J Am Chem Soc 132: 4994–4995, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Forni GL. 2012. Ferrochelating treatment in patients affected by neurodegeneration with brain iron accumulation. http://clinicaltrials.gov/ct2/show/NCT00907283?term=iron+chelator+neurodegeneration&rank=2
- 157.Foury F. and Cazzalini O. Deletion of the yeast homologue of the human gene associated with Friedreich's ataxia elicits iron accumulation in mitochondria. FEBS Lett 411: 373–377, 1997 [DOI] [PubMed] [Google Scholar]
- 158.Foury F. and Roganti T. Deletion of the mitochondrial carrier genes MRS3 and MRS4 suppresses mitochondrial iron accumulation in a yeast frataxin-deficient strain. J Biol Chem 277: 24475–24483, 2002 [DOI] [PubMed] [Google Scholar]
- 159.Friedlich AL, Tanzi RE, and Rogers JT. The 5′-untranslated region of Parkinson's disease alpha-synuclein messengerRNA contains a predicted iron responsive element. Mol Psychiatry 12: 222–223, 2007 [DOI] [PubMed] [Google Scholar]
- 160.Froschauer EM, Schweyen RJ, and Wiesenberger G. The yeast mitochondrial carrier proteins Mrs3p/Mrs4p mediate iron transport across the inner mitochondrial membrane. Biochim Biophys Acta 1788: 1044–1050, 2009 [DOI] [PubMed] [Google Scholar]
- 161.Gabathuler R. Approaches to transport therapeutic drugs across the blood-brain barrier to treat brain diseases. Neurobiol Dis 37: 48–57, 2010 [DOI] [PubMed] [Google Scholar]
- 162.Gaeta A. and Hider RC. The crucial role of metal ions in neurodegeneration: the basis for a promising therapeutic strategy. Br J Pharmacol 146: 1041–1059, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Galy B, Ferring D, Minana B, Bell O, Janser HG, Muckenthaler M, Schumann K, and Hentze MW. Altered body iron distribution and microcytosis in mice deficient in iron regulatory protein 2 (IRP2). Blood 106: 2580–2589, 2005 [DOI] [PubMed] [Google Scholar]
- 164.Gao JW, Chen JX, Kramer M, Tsukamoto H, Zhang AS, and Enns CA. Interaction of the Hereditary hemochromatosis protein HFE with transferrin receptor 2 is required for transferrin-induced hepcidin expression. Cell Metab 9: 217–227, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gerber J, Muhlenhoff U, and Lill R. An interaction between frataxin and Isu1/Nfs1 that is crucial for Fe/S cluster synthesis on Isu1. EMBO Rep 4: 906–911, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gibson TJ, Koonin EV, Musco G, Pastore A, and Bork P. Friedreich's ataxia protein: phylogenetic evidence for mitochondrial dysfunction. Trends Neurosci 19: 465–468, 1996 [DOI] [PubMed] [Google Scholar]
- 167.Giese A. and Kretzschmar HA. Prion-induced neuronal damage—the mechanisms of neuronal destruction in the subacute spongiform encephalopathies. Curr Top Microbiol Immunol 253: 203–217, 2001 [DOI] [PubMed] [Google Scholar]
- 168.Girelli D, Corrocher R, Bisceglia L, Olivieri O, De Franceschi L, Zelante L, and Gasparini P. Molecular basis for the recently described hereditary hyperferritinemia-cataract syndrome: a mutation in the iron-responsive element of ferritin L-subunit gene (the “Verona mutation”). Blood 86: 4050–4053, 1995 [PubMed] [Google Scholar]
- 169.Gitler AD, Chesi A, Geddie ML, Strathearn KE, Hamamichi S, Hill KJ, Caldwell KA, Caldwell GA, Cooper AA, Rochet JC, and Lindquist S. Alpha-synuclein is part of a diverse and highly conserved interaction network that includes PARK9 and manganese toxicity. Nat Genet 41: 308–315, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Gong HS, Guo Y, Tian C, Xie WL, Shi Q, Zhang J, Xu Y, Wang SB, Zhang BY, Chen C, Liu Y, and Dong XP. Reduction of protein kinase MARK4 in the brains of experimental scrapie rodents and human prion disease correlates with deposits of PrPSc. Int J Mol Med 30: 569–578, 2012 [DOI] [PubMed] [Google Scholar]
- 171.Goralska M, Holley BL, and McGahan MC. Overexpression of H- and L-ferritin subunits in lens epithelial cells: Fe metabolism and cellular response to UVB irradiation. Invest Ophthalmol Vis Sci 42: 1721–1727, 2001 [PubMed] [Google Scholar]
- 172.Gotz ME, Double K, Gerlach M, Youdim MB, and Riederer P. The relevance of iron in the pathogenesis of Parkinson's disease. Ann N Y Acad Sci 1012: 193–208, 2004 [DOI] [PubMed] [Google Scholar]
- 173.Granzotto A. and Zatta P. Resveratrol acts not through anti-aggregative pathways but mainly via its scavenging properties against Abeta and Abeta-metal complexes toxicity. PLoS One 6: e21565, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gregory A, Polster BJ, and Hayflick SJ. Clinical and genetic delineation of neurodegeneration with brain iron accumulation. J Med Genet 46: 73–80, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gregory A, Westaway SK, Holm IE, Kotzbauer PT, Hogarth P, Sonek S, Coryell JC, Nguyen TM, Nardocci N, Zorzi G, Rodriguez D, Desguerre I, Bertini E, Simonati A, Levinson B, Dias C, Barbot C, Carrilho I, Santos M, Malik I, Gitschier J, and Hayflick SJ. Neurodegeneration associated with genetic defects in phospholipase A(2). Neurology 71: 1402–1409, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Griffioen G, Duhamel H, Van Damme N, Pellens K, Zabrocki P, Pannecouque C, van Leuven F, Winderickx J, and Wera S. A yeast-based model of alpha-synucleinopathy identifies compounds with therapeutic potential. Biochim Biophys Acta 1762: 312–318, 2006 [DOI] [PubMed] [Google Scholar]
- 177.Griffiths PD. and Crossman AR. Distribution of iron in the basal ganglia and neocortex in postmortem tissue in Parkinson's disease and Alzheimer's disease. Dementia 4: 61–65, 1993 [DOI] [PubMed] [Google Scholar]
- 178.Guerreiro RJ, Bras JM, Santana I, Januario C, Santiago B, Morgadinho AS, Ribeiro MH, Hardy J, Singleton A, and Oliveira C. Association of HFE common mutations with Parkinson's disease, Alzheimer's disease and mild cognitive impairment in a Portuguese cohort. BMC Neurol 6: 24, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Guldener U, Munsterkotter M, Kastenmuller G, Strack N, van Helden J, Lemer C, Richelles J, Wodak SJ, Garcia-Martinez J, Perez-Ortin JE, Michael H, Kaps A, Talla E, Dujon B, Andre B, Souciet JL, De Montigny J, Bon E, Gaillardin C, and Mewes HW. CYGD: the Comprehensive Yeast Genome Database. Nucleic Acids Res 33: D364–D368, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gunshin H, Allerson CR, Polycarpou-Schwarz M, Rofts A, Rogers JT, Kishi F, Hentze MW, Rouault TA, Andrews NC, and Hediger MA. Iron-dependent regulation of the divalent metal ion transporter. FEBS Lett 509: 309–316, 2001 [DOI] [PubMed] [Google Scholar]
- 181.Guo C, Wang T, Zheng W, Shan ZY, Teng WP, and Wang ZY. Intranasal deferoxamine reverses iron-induced memory deficits and inhibits amyloidogenic APP processing in a transgenic mouse model of Alzheimer's disease. Neurobiol Aging 34: 562–575, 2013 [DOI] [PubMed] [Google Scholar]
- 182.Gutteridge JM, Rowley DA, and Halliwell B. Superoxide-dependent formation of hydroxyl radicals and lipid peroxidation in the presence of iron salts. Detection of ‘catalytic’ iron and anti-oxidant activity in extracellular fluids. Biochem J 206: 605–609, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Haacke EM, Cheng NY, House MJ, Liu Q, Neelavalli J, Ogg RJ, Khan A, Ayaz M, Kirsch W, and Obenaus A. Imaging iron stores in the brain using magnetic resonance imaging. Magn Reson Imaging 23: 1–25, 2005 [DOI] [PubMed] [Google Scholar]
- 184.Haass C. and Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat Rev Mol Cell Biol 8: 101–112, 2007 [DOI] [PubMed] [Google Scholar]
- 185.Haldar S, Beveridge AJ, Wong J, Singh A, Galimberti D, Borroni B, Zhu X, Blevins J, Greenlee JJ, Perry G, Mukhopadhyay C, Schmotzer C, and Singh N. A low-molecular-weight ferroxidase is increased in the CSF of sCJD cases: CSF ferroxidase and transferrin as diagnostic biomarkers for sCJD. Antioxid Redox Signal 19: 1662–1675, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Halliwell B. and Gutteridge JMC. Biologically Relevant Metal Ion-Dependent Hydroxyl Radical Generation—an Update. FEBS Lett 307: 108–112, 1992 [DOI] [PubMed] [Google Scholar]
- 187.Harding AE. “Idiopathic” late onset cerebellar ataxia. A clinical and genetic study of 36 cases. J Neurol Sci 51: 259–271, 1981 [DOI] [PubMed] [Google Scholar]
- 188.Hardy J. Genetic analysis of pathways to Parkinson disease. Neuron 68: 201–206, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Harris ZL, Durley AP, Man TK, and Gitlin JD. Targeted gene disruption reveals an essential role for ceruloplasmin in cellular iron efflux. Proc Natl Acad Sci U S A 96: 10812–10817, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Harris ZL, Takahashi Y, Miyajima H, Serizawa M, MacGillivray RT, and Gitlin JD. Aceruloplasminemia: molecular characterization of this disorder of iron metabolism. Proc Natl Acad Sci U S A 92: 2539–2543, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hartig MB, Hortnagel K, Garavaglia B, Zorzi G, Kmiec T, Klopstock T, Rostasy K, Svetel M, Kostic VS, Schuelke M, Botz E, Weindl A, Novakovic I, Nardocci N, Prokisch H, and Meitinger T. Genotypic and phenotypic spectrum of PANK2 mutations in patients with neurodegeneration with brain iron accumulation. Ann Neurol 59: 248–256, 2006 [DOI] [PubMed] [Google Scholar]
- 192.Hartig MB, Iuso A, Haack T, Kmiec T, Jurkiewicz E, Heim K, Roeber S, Tarabin V, Dusi S, Krajewska-Walasek M, Jozwiak S, Hempel M, Winkelmann J, Elstner M, Oexle K, Klopstock T, Mueller-Felber W, Gasser T, Trenkwalder C, Tiranti V, Kretzschmar H, Schmitz G, Strom TM, Meitinger T, and Prokisch H. Absence of an orphan mitochondrial protein, c19orf12, causes a distinct clinical subtype of neurodegeneration with brain iron accumulation. Am J Hum Genet 89: 543–550, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hauser DN. and Hastings TG. Mitochondrial dysfunction and oxidative stress in Parkinson's disease and monogenic parkinsonism. Neurobiol Dis: 35–42, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hayflick SJ, Westaway SK, Levinson B, Zhou B, Johnson MA, Ching KH, and Gitschier J. Genetic, clinical, and radiographic delineation of Hallervorden-Spatz syndrome. N Engl J Med 348: 33–40, 2003 [DOI] [PubMed] [Google Scholar]
- 195.He Y, Alam SL, Proteasa SV, Zhang Y, Lesuisse E, Dancis A, and Stemmler TL. Yeast frataxin solution structure, iron binding, and ferrochelatase interaction. Biochemistry 43: 16254–16262, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hentze MW. and Kuhn LC. Molecular control of vertebrate iron metabolism: mRNA-based regulatory circuits operated by iron, nitric oxide, and oxidative stress. Proc Natl Acad Sci U S A 93: 8175–8182, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hentze MW, Muckenthaler MU, Galy B, and Camaschella C. Two to tango: regulation of Mammalian iron metabolism. Cell 142: 24–38, 2010 [DOI] [PubMed] [Google Scholar]
- 198.Hesketh S, Sassoon J, Knight R, and Brown DR. Elevated manganese levels in blood and CNS in human prion disease. Mol Cell Neurosci 37: 590–598, 2008 [DOI] [PubMed] [Google Scholar]
- 199.Hida A, Kowa H, Iwata A, Tanaka M, Kwak S, and Tsuji S. Aceruloplasminemia in a Japanese woman with a novel mutation of CP gene: clinical presentations and analysis of genetic and molecular pathogenesis. J Neurol Sci 298: 136–139, 2010 [DOI] [PubMed] [Google Scholar]
- 200.Hintze KJ. and Theil EC. DNA and mRNA elements with complementary responses to hemin, antioxidant inducers, and iron control ferritin-L expression. Proc Natl Acad Sci U S A 102: 15048–15052, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hochstrasser H, Tomiuk J, Walter U, Behnke S, Spiegel J, Kruger R, Becker G, Riess O, and Berg D. Functional relevance of ceruloplasmin mutations in Parkinson's disease. FASEB J 19: 1851–1853, 2005 [DOI] [PubMed] [Google Scholar]
- 202.Huang ML, Becker EM, Whitnall M, Suryo Rahmanto Y, Ponka P, and Richardson DR. Elucidation of the mechanism of mitochondrial iron loading in Friedreich's ataxia by analysis of a mouse mutant. Proc Natl Acad Sci U S A 106: 16381–16386, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Huang XD, Atwood CS, Moir RD, Hartshorn MA, Tanzi RE, and Bush AI. Trace metal contamination initiates the apparent auto-aggregation, amyloidosis, and oligomerization of Alzheimer's A beta peptides. J Biol Inorg Chem 9: 954–960, 2004 [DOI] [PubMed] [Google Scholar]
- 204.Hughes AJ, Daniel SE, Kilford L, and Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55: 181–184, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hui Y, Wang D, Li W, Zhang L, Jin J, Ma N, Zhou L, Nakajima O, Zhao W, and Gao X. Long-term overexpression of heme oxygenase 1 promotes tau aggregation in mouse brain by inducing tau phosphorylation. J Alzheimers Dis 26: 299–313, 2011 [DOI] [PubMed] [Google Scholar]
- 206.Hunot S. and Hirsch EC. Neuroinflammatory processes in Parkinson's disease. Ann Neurol 53Suppl 3: S49–S58; discussion S58–S60, 2003. [DOI] [PubMed] [Google Scholar]
- 207.Hur K, Kim JI, Choi SI, Choi EK, Carp RI, and Kim YS. The pathogenic mechanisms of prion diseases. Mech Ageing Dev 123: 1637–1647, 2002 [DOI] [PubMed] [Google Scholar]
- 208.Hwang D, Lee IY, Yoo H, Gehlenborg N, Cho JH, Petritis B, Baxter D, Pitstick R, Young R, Spicer D, Price ND, Hohmann JG, DeArmond SJ, Carlson GA, and Hood LE. A systems approach to prion disease. Mol Syst Biol 5: 252, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Jeong SY, Crooks DR, Wilson-Ollivierre H, Ghosh MC, Sougrat R, Lee J, Cooperman S, Mitchell JB, Beaumont C, and Rouault TA. Iron insufficiency compromises motor neurons and their mitochondrial function in Irp2-null mice. PLoS One 6: e25404, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Jeong SY. and David S. Glycosylphosphatidylinositol-anchored ceruloplasmin is required for iron efflux from cells in the central nervous system. J Biol Chem 278: 27144–27148, 2003 [DOI] [PubMed] [Google Scholar]
- 211.Jeong SY. and David S. Age-related changes in iron homeostasis and cell death in the cerebellum of ceruloplasmin-deficient mice. J Neurosci 26: 9810–9819, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Jiao Y, Wilkinson Jt, Di X, Wang W, Hatcher H, Kock ND, D'Agostino R, Jr., Knovich MA, Torti FM, and Torti SV. Curcumin, a cancer chemopreventive and chemotherapeutic agent, is a biologically active iron chelator. Blood 113: 462–469, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Johansson C, Roos AK, Montano SJ, Sengupta R, Filippakopoulos P, Guo K, von Delft F, Holmgren A, Oppermann U, and Kavanagh KL. The crystal structure of human GLRX5: iron-sulfur cluster co-ordination, tetrameric assembly and monomer activity. Biochem J 433: 303–311, 2011 [DOI] [PubMed] [Google Scholar]
- 214.Jones CE, Abdelraheim SR, Brown DR, and Viles JH. Preferential Cu2+ coordination by His96 and His111 induces beta-sheet formation in the unstructured amyloidogenic region of the prion protein. J Biol Chem 279: 32018–32027, 2004 [DOI] [PubMed] [Google Scholar]
- 215.Jones CE, Klewpatinond M, Abdelraheim SR, Brown DR, and Viles JH. Probing copper2+ binding to the prion protein using diamagnetic nickel2+ and 1H NMR: the unstructured N terminus facilitates the coordination of six copper2+ ions at physiological concentrations. J Mol Biol 346: 1393–1407, 2005 [DOI] [PubMed] [Google Scholar]
- 216.Joshi JG, Fleming JT, Dhar M, and Chauthaiwale V. A novel ferritin heavy chain messenger ribonucleic acid in the human brain. J Neurol Sci 134: 52–56, 1995 [DOI] [PubMed] [Google Scholar]
- 217.Kannengiesser C, Jouanolle AM, Hetet G, Mosser A, Muzeau F, Henry D, Bardou-Jacquet E, Mornet M, Brissot P, Deugnier Y, Grandchamp B, and Beaumont C. A new missense mutation in the L ferritin coding sequence associated with elevated levels of glycosylated ferritin in serum and absence of iron overload. Haematologica 94: 335–339, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kaplan J, Ward DM, and De Domenico I. The molecular basis of iron overload disorders and iron-linked anemias. Int J Hematol 93: 14–20, 2011 [DOI] [PubMed] [Google Scholar]
- 219.Kato J, Fujikawa K, Kanda M, Fukuda N, Sasaki K, Takayama T, Kobune M, Takada K, Takimoto R, Hamada H, Ikeda T, and Niitsu Y. A mutation, in the iron-responsive element of H ferritin mRNA, causing autosomal dominant iron overload. Am J Hum Genet 69: 191–197, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Kaur D, Peng J, Chinta SJ, Rajagopalan S, Di Monte DA, Cherny RA, and Andersen JK. Increased murine neonatal iron intake results in Parkinson-like neurodegeneration with age. Neurobiol Aging 28: 907–913, 2007 [DOI] [PubMed] [Google Scholar]
- 221.Kaur D, Yantiri F, Rajagopalan S, Kumar J, Mo JQ, Boonplueang R, Viswanath V, Jacobs R, Yang L, Beal MF, DiMonte D, Volitaskis I, Ellerby L, Cherny RA, Bush AI, and Andersen JK. Genetic or pharmacological iron chelation prevents MPTP-induced neurotoxicity in vivo: a novel therapy for Parkinson's disease. Neuron 37: 899–909, 2003 [DOI] [PubMed] [Google Scholar]
- 222.Kawabata H, Fleming RE, Gui D, Moon SY, Saitoh T, O'Kelly J, Umehara Y, Wano Y, Said JW, and Koeffler HP. Expression of hepcidin is down-regulated in TfR2 mutant mice manifesting a phenotype of hereditary hemochromatosis. Blood 105: 376–381, 2005 [DOI] [PubMed] [Google Scholar]
- 223.Kawabata H, Yang R, Hirama T, Vuong PT, Kawano S, Gombart AF, and Koeffler HP. Molecular cloning of transferrin receptor 2. A new member of the transferrin receptor-like family. J Biol Chem 274: 20826–20832, 1999 [DOI] [PubMed] [Google Scholar]
- 224.Ke Y. and Qian ZM. Brain iron metabolism: neurobiology and neurochemistry. Prog Neurobiol 83: 149–173, 2007 [DOI] [PubMed] [Google Scholar]
- 225.Kell D. Journal club. A systems biologist ponders how disparate ideas can sometimes come together beautifully. Nature 460: 669, 2009 [DOI] [PubMed] [Google Scholar]
- 226.Kell DB. Towards a unifying, systems biology understanding of large-scale cellular death and destruction caused by poorly liganded iron: Parkinson's, Huntington's, Alzheimer's, prions, bactericides, chemical toxicology and others as examples. Arch Toxicol 84: 825–889, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Kidane TZ, Sauble E, and Linder MC. Release of iron from ferritin requires lysosomal activity. Am J Physiol Cell Physiol 291: C445–C455, 2006 [DOI] [PubMed] [Google Scholar]
- 228.Kielar F, Helsel ME, Wang Q, and Franz KJ. Prochelator BHAPI protects cells against paraquat-induced damage by ROS-triggered iron chelation. Metallomics 4: 899–909, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Kikuchi G, Yoshida T, and Noguchi M. Heme oxygenase and heme degradation. Biochem Biophys Res Commun 338: 558–567, 2005 [DOI] [PubMed] [Google Scholar]
- 230.Kim NH, Park SJ, Jin JK, Kwon MS, Choi EK, Carp RI, and Kim YS. Increased ferric iron content and iron-induced oxidative stress in the brains of scrapie-infected mice. Brain Res 884: 98–103, 2000 [DOI] [PubMed] [Google Scholar]
- 231.Kispal G, Csere P, Guiard B, and Lill R. The ABC transporter Atm1p is required for mitochondrial iron homeostasis. FEBS Lett 418: 346–350, 1997 [DOI] [PubMed] [Google Scholar]
- 232.Kispal G, Csere P, Prohl C, and Lill R. The mitochondrial proteins Atm1p and Nfs1p are essential for biogenesis of cytosolic Fe/S proteins. EMBO J 18: 3981–3989, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, and Shimizu N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392: 605–608, 1998 [DOI] [PubMed] [Google Scholar]
- 234.Klamt F, Dal-Pizzol F, Conte da Frota ML, Jr., Walz R, Andrades ME, da Silva EG, Brentani RR, Izquierdo I, and Fonseca Moreira JC. Imbalance of antioxidant defense in mice lacking cellular prion protein. Free Radic Biol Med 30: 1137–1144, 2001 [DOI] [PubMed] [Google Scholar]
- 235.Klomp LW, Farhangrazi ZS, Dugan LL, and Gitlin JD. Ceruloplasmin gene expression in the murine central nervous system. J Clin Invest 98: 207–215, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Klomp LW. and Gitlin JD. Expression of the ceruloplasmin gene in the human retina and brain: implications for a pathogenic model in aceruloplasminemia. Hum Mol Genet 5: 1989–1996, 1996 [DOI] [PubMed] [Google Scholar]
- 237.Koeppen AH, Michael SC, Knutson MD, Haile DJ, Qian J, Levi S, Santambrogio P, Garrick MD, and Lamarche JB. The dentate nucleus in Friedreich's ataxia: the role of iron-responsive proteins. Acta Neuropathol 114: 163–173, 2007 [DOI] [PubMed] [Google Scholar]
- 238.Koeppen AH, Morral JA, Davis AN, Qian J, Petrocine SV, Knutson MD, Gibson WM, Cusack MJ, and Li D. The dorsal root ganglion in Friedreich's ataxia. Acta Neuropathol 118: 763–776, 2009 [DOI] [PubMed] [Google Scholar]
- 239.Kotzbauer PT, Truax AC, Trojanowski JQ, and Lee VM. Altered neuronal mitochondrial coenzyme A synthesis in neurodegeneration with brain iron accumulation caused by abnormal processing, stability, and catalytic activity of mutant pantothenate kinase 2. J Neurosci 25: 689–698, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Koutnikova H, Campuzano V, Foury F, Dolle P, Cazzalini O, and Koenig M. Studies of human, mouse and yeast homologues indicate a mitochondrial function for frataxin. Nat Genet 16: 345–351, 1997 [DOI] [PubMed] [Google Scholar]
- 241.Kramer MF, Gunaratne P, and Ferreira GC. Transcriptional regulation of the murine erythroid-specific 5-aminolevulinate synthase gene. Gene 247: 153–166, 2000 [DOI] [PubMed] [Google Scholar]
- 242.Krishnamurthi RV, Mathai S, Kim AH, Zhang R, and Guan J. A novel diketopiperazine improves functional recovery given after the onset of 6-OHDA-induced motor deficit in rats. Br J Pharmacol 156: 662–672, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Kruer MC, Boddaert N, Schneider SA, Houlden H, Bhatia KP, Gregory A, Anderson JC, Rooney WD, Hogarth P, and Hayflick SJ. Neuroimaging features of neurodegeneration with brain iron accumulation. AJNR Am J Neuroradiol 33: 407–414, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Kruer MC, Paisan-Ruiz C, Boddaert N, Yoon MY, Hama H, Gregory A, Malandrini A, Woltjer RL, Munnich A, Gobin S, Polster BJ, Palmeri S, Edvardson S, Hardy J, Houlden H, and Hayflick SJ. Defective FA2H leads to a novel form of neurodegeneration with brain iron accumulation (NBIA). Ann Neurol 68: 611–618, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kruer MC, Paudel R, Wagoner W, Sanford L, Kara E, Gregory A, Foltynie T, Lees A, Bhatia K, Hardy J, Hayflick SJ, and Houlden H. Analysis of ATP13A2 in large neurodegeneration with brain iron accumulation (NBIA) and dystonia-parkinsonism cohorts. Neurosci Lett 523: 35–38, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Kubota A, Hida A, Ichikawa Y, Momose Y, Goto J, Igeta Y, Hashida H, Yoshida K, Ikeda S, Kanazawa I, and Tsuji S. A novel ferritin light chain gene mutation in a Japanese family with neuroferritinopathy: description of clinical features and implications for genotype-phenotype correlations. Mov Disord 24: 441–445, 2009 [DOI] [PubMed] [Google Scholar]
- 247.Kupershmidt L, Weinreb O, Amit T, Mandel S, Bar-Am O, and Youdim MB. Novel molecular targets of the neuroprotective/neurorescue multimodal iron chelating drug M30 in the mouse brain. Neuroscience 189: 345–358, 2011 [DOI] [PubMed] [Google Scholar]
- 248.Kurian MA, McNeill A, Lin JP, and Maher ER. Childhood disorders of neurodegeneration with brain iron accumulation (NBIA). Dev Med Child Neurol 53: 394–404, 2011 [DOI] [PubMed] [Google Scholar]
- 249.Kuriyama S, Shimazu T, Ohmori K, Kikuchi N, Nakaya N, Nishino Y, Tsubono Y, and Tsuji I. Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: the Ohsaki study. J Am Med Assoc 296: 1255–1265, 2006 [DOI] [PubMed] [Google Scholar]
- 250.Kurtz JE, Dufour P, Duclos B, Bergerat JP, and Exinger F. [Saccharomyces cerevisiae: an efficient tool and model system for anticancer research]. Bull Cancer 91: 133–139, 2004 [PubMed] [Google Scholar]
- 251.Labunskyy VM. and Gladyshev VN. Role of reactive oxygen species-mediated signaling in aging. Antioxid Redox Signal 19: 1362–1372, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Land T. and Rouault TA. Targeting of a human iron-sulfur cluster assembly enzyme, nifs, to different subcellular compartments is regulated through alternative AUG utilization. Mol Cell 2: 807–815, 1998 [DOI] [PubMed] [Google Scholar]
- 253.Lane DJ, Robinson SR, Czerwinska H, Bishop GM, and Lawen A. Two routes of iron accumulation in astrocytes: ascorbate-dependent ferrous iron uptake via the divalent metal transporter (DMT1) plus an independent route for ferric iron. Biochem J 432: 123–132, 2010 [DOI] [PubMed] [Google Scholar]
- 254.Lang AE. and Lozano AM. Parkinson's disease. Second of two parts. N Engl J Med 339: 1130–1143, 1998 [DOI] [PubMed] [Google Scholar]
- 255.LaVaute T, Smith S, Cooperman S, Iwai K, Land W, Meyron-Holtz E, Drake SK, Miller G, Abu-Asab M, Tsokos M, Switzer R, 3rd, Grinberg A, Love P, Tresser N, and Rouault TA. Targeted deletion of the gene encoding iron regulatory protein-2 causes misregulation of iron metabolism and neurodegenerative disease in mice. Nat Genet 27: 209–214, 2001 [DOI] [PubMed] [Google Scholar]
- 256.Layer G, Ollagnier-de Choudens S, Sanakis Y, and Fontecave M. Iron-sulfur cluster biosynthesis: characterization of Escherichia coli CYaY as an iron donor for the assembly of [2Fe-2S] clusters in the scaffold IscU. J Biol Chem 281: 16256–16263, 2006 [DOI] [PubMed] [Google Scholar]
- 257.Lazarou M, Narendra DP, Jin SM, Tekle E, Banerjee S, and Youle RJ. PINK1 drives Parkin self-association and HECT-like E3 activity upstream of mitochondrial binding. J Cell Biol 200: 163–172, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Leggett BA, Fletcher LM, Ramm GA, Powell LW, and Halliday JW. Differential regulation of ferritin H and L subunit mRNA during inflammation and long-term iron overload. J Gastroenterol Hepatol 8: 21–27, 1993 [DOI] [PubMed] [Google Scholar]
- 259.Lei P, Ayton S, Finkelstein DI, Spoerri L, Ciccotosto GD, Wright DK, Wong BX, Adlard PA, Cherny RA, Lam LQ, Roberts BR, Volitakis I, Egan GF, McLean CA, Cappai R, Duce JA, and Bush AI. Tau deficiency induces parkinsonism with dementia by impairing APP-mediated iron export. Nat Med 18: 291–295, 2012 [DOI] [PubMed] [Google Scholar]
- 260.Leidgens S, De Smet S, and Foury F. Frataxin interacts with Isu1 through a conserved tryptophan in its beta-sheet. Hum Mol Genet 19: 276–286, 2010 [DOI] [PubMed] [Google Scholar]
- 261.Lesbordes-Brion JC, Viatte L, Bennoun M, Lou DQ, Ramey G, Houbron C, Hamard G, Kahn A, and Vaulont S. Targeted disruption of the hepcidin 1 gene results in severe hemochromatosis. Blood 108: 1402–1405, 2006 [DOI] [PubMed] [Google Scholar]
- 262.Levi S, Corsi B, Bosisio M, Invernizzi R, Volz A, Sanford D, Arosio P, and Drysdale J. A human mitochondrial ferritin encoded by an intronless gene. J Biol Chem 276: 24437–24440, 2001 [DOI] [PubMed] [Google Scholar]
- 263.Levi S. and Rovida E. The role of iron in mitochondrial function. Biochim Biophys Acta 1790: 629–636, 2009 [DOI] [PubMed] [Google Scholar]
- 264.Levy JE, Jin O, Fujiwara Y, Kuo F, and Andrews NC. Transferrin receptor is necessary for development of erythrocytes and the nervous system. Nat Genet 21: 396–399, 1999 [DOI] [PubMed] [Google Scholar]
- 265.Li A, Dong J, and Harris DA. Cell surface expression of the prion protein in yeast does not alter copper utilization phenotypes. J Biol Chem 279: 29469–29477, 2004 [DOI] [PubMed] [Google Scholar]
- 266.Li K, Besse EK, Ha D, Kovtunovych G, and Rouault TA. Iron-dependent regulation of frataxin expression: implications for treatment of Friedreich ataxia. Hum Mol Genet 17: 2265–2273, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Li Y, Tian X, Qian L, Yu X, and Jiang W. Anodal transcranial direct current stimulation relieves the unilateral bias of a rat model of Parkinson's disease. Conf Proc IEEE Eng Med Biol Soc 2011: 765–768, 2011 [DOI] [PubMed] [Google Scholar]
- 268.Lim CK, Kalinowski DS, and Richardson DR. Protection against hydrogen peroxide-mediated cytotoxicity in Friedreich's ataxia fibroblasts using novel iron chelators of the 2-pyridylcarboxaldehyde isonicotinoyl hydrazone class. Mol Pharmacol 74: 225–235, 2008 [DOI] [PubMed] [Google Scholar]
- 269.Lim JE, Jin O, Bennett C, Morgan K, Wang F, Trenor CC, 3rd, Fleming MD, and Andrews NC. A mutation in Sec15l1 causes anemia in hemoglobin deficit (hbd) mice. Nat Genet 37: 1270–1273, 2005 [DOI] [PubMed] [Google Scholar]
- 270.Lim SK, Kim H, bin Ali A, Lim YK, Wang Y, Chong SM, Costantini F, and Baumman H. Increased susceptibility in Hp knockout mice during acute hemolysis. Blood 92: 1870–1877, 1998 [PubMed] [Google Scholar]
- 271.Lin AM, Yang CH, and Chai CY. Striatal dopamine dynamics are altered following an intranigral infusion of iron in adult rats. Free Radic Biol Med 24: 988–993, 1998 [DOI] [PubMed] [Google Scholar]
- 272.Liochev SI. and Fridovich I. The role of O2.- in the production of HO.: in vitro and in vivo. Free Radic Biol Med 16: 29–33, 1994 [DOI] [PubMed] [Google Scholar]
- 273.Liu X, Jin W, and Theil EC. Opening protein pores with chaotropes enhances Fe reduction and chelation of Fe from the ferritin biomineral. Proc Natl Acad Sci U S A 100: 3653–3658, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Liu XS, Patterson LD, Miller MJ, and Theil EC. Peptides selected for the protein nanocage pores change the rate of iron recovery from the ferritin mineral. J Biol Chem 282: 31821–31825, 2007 [DOI] [PubMed] [Google Scholar]
- 275.Liuzzi JP, Aydemir F, Nam H, Knutson MD, and Cousins RJ. Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proc Natl Acad Sci U S A 103: 13612–13617, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Lotharius J. and Brundin P. Pathogenesis of Parkinson's disease: dopamine, vesicles and alpha-synuclein. Nat Rev Neurosci 3: 932–942, 2002 [DOI] [PubMed] [Google Scholar]
- 277.Lovell MA, Robertson JD, Teesdale WJ, Campbell JL, and Markesbery WR. Copper, iron and zinc in Alzheimer's disease senile plaques. J Neurol Sci 158: 47–52, 1998 [DOI] [PubMed] [Google Scholar]
- 278.Lv Z, Jiang H, Xu H, Song N, and Xie J. Increased iron levels correlate with the selective nigral dopaminergic neuron degeneration in Parkinson's disease. J Neural Transm 118: 361–369, 2011 [DOI] [PubMed] [Google Scholar]
- 279.Lyoumi S, Abitbol M, Andrieu V, Henin D, Robert E, Schmitt C, Gouya L, de Verneuil H, Deybach JC, Montagutelli X, Beaumont C, and Puy H. Increased plasma transferrin, altered body iron distribution, and microcytic hypochromic anemia in ferrochelatase-deficient mice. Blood 109: 811–818, 2007 [DOI] [PubMed] [Google Scholar]
- 280.Maciel P, Cruz VT, Constante M, Iniesta I, Costa MC, Gallati S, Sousa N, Sequeiros J, Coutinho P, and Santos MM. Neuroferritinopathy: missense mutation in FTL causing early-onset bilateral pallidal involvement. Neurology 65: 603–605, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.MacKenzie EL, Iwasaki K, and Tsuji Y. Intracellular iron transport and storage: from molecular mechanisms to health implications. Antioxid Redox Signal 10: 997–1030, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Mackova E, Hruskova K, Bendova P, Vavrova A, Jansova H, Haskova P, Kovarikova P, Vavrova K, and Simunek T. Methyl and ethyl ketone analogs of salicylaldehyde isonicotinoyl hydrazone: novel iron chelators with selective antiproliferative action. Chem Biol Interact 197: 69–79, 2012 [DOI] [PubMed] [Google Scholar]
- 283.Madsen E. and Gitlin JD. Copper and iron disorders of the brain. Annu Rev Neurosci 30: 317–337, 2007 [DOI] [PubMed] [Google Scholar]
- 284.Magness ST, Maeda N, and Brenner DA. An exon 10 deletion in the mouse ferrochelatase gene has a dominant-negative effect and causes mild protoporphyria. Blood 100: 1470–1477, 2002 [DOI] [PubMed] [Google Scholar]
- 285.Maines MD. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J 2: 2557–2568, 1988 [PubMed] [Google Scholar]
- 286.Malecki EA, Devenyi AG, Beard JL, and Connor JR. Existing and emerging mechanisms for transport of iron and manganese to the brain. J Neurosci Res 56: 113–122, 1999 [DOI] [PubMed] [Google Scholar]
- 287.Malik I, Turk J, Mancuso DJ, Montier L, Wohltmann M, Wozniak DF, Schmidt RE, Gross RW, and Kotzbauer PT. Disrupted membrane homeostasis and accumulation of ubiquitinated proteins in a mouse model of infantile neuroaxonal dystrophy caused by PLA2G6 mutations. Am J Pathol 172: 406–416, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Mallucci GR, White MD, Farmer M, Dickinson A, Khatun H, Powell AD, Brandner S, Jefferys JG, and Collinge J. Targeting cellular prion protein reverses early cognitive deficits and neurophysiological dysfunction in prion-infected mice. Neuron 53: 325–335, 2007 [DOI] [PubMed] [Google Scholar]
- 289.Mancuso C. and Barone E. Curcumin in clinical practice: myth or reality? Trends Pharmacol Sci 30: 333–334, 2009 [DOI] [PubMed] [Google Scholar]
- 290.Mancuso C. and Barone E. The heme oxygenase/biliverdin reductase pathway in drug research and development. Curr Drug Metab 10: 579–594, 2009 [DOI] [PubMed] [Google Scholar]
- 291.Mancuso C, Siciliano R, and Barone E. Curcumin and Alzheimer disease: this marriage is not to be performed. J Biol Chem 286: le3; author reply le4, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Mancuso M, Davidzon G, Kurlan RM, Tawil R, Bonilla E, Di Mauro S, and Powers JM. Hereditary ferritinopathy: a novel mutation, its cellular pathology, and pathogenetic insights. J Neuropathol Exp Neurol 64: 280–294, 2005 [DOI] [PubMed] [Google Scholar]
- 293.Marro S, Barisani D, Chiabrando D, Fagoonee S, Muckenthaler MU, Stolte J, Meneveri R, Haile D, Silengo L, Altruda F, and Tolosano E. Lack of haptoglobin affects iron transport across duodenum by modulating ferroportin expression. Gastroenterology 133: 1261–1271, 2007 [DOI] [PubMed] [Google Scholar]
- 294.Martin GG, Atshaves BP, McIntosh AL, Mackie JT, Kier AB, and Schroeder F. Liver fatty acid-binding protein gene-ablated female mice exhibit increased age-dependent obesity. J Nutr 138: 1859–1865, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Martin JB. Molecular basis of the neurodegenerative disorders. N Engl J Med 340: 1970–1980, 1999 [DOI] [PubMed] [Google Scholar]
- 296.Martin LJ, Pan Y, Price AC, Sterling W, Copeland NG, Jenkins NA, Price DL, and Lee MK. Parkinson's disease alpha-synuclein transgenic mice develop neuronal mitochondrial degeneration and cell death. J Neurosci 26: 41–50, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Masison DC. and Wickner RB. Prion-inducing domain of yeast Ure2p and protease resistance of Ure2p in prion-containing cells. Science 270: 93–95, 1995 [DOI] [PubMed] [Google Scholar]
- 298.Mastroberardino PG, Hoffman EK, Horowitz MP, Betarbet R, Taylor G, Cheng D, Na HM, Gutekunst CA, Gearing M, Trojanowski JQ, Anderson M, Chu CT, Peng J, and Greenamyre JT. A novel transferrin/TfR2-mediated mitochondrial iron transport system is disrupted in Parkinson's disease. Neurobiol Dis 34: 417–431, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.McKie AT, Marciani P, Rolfs A, Brennan K, Wehr K, Barrow D, Miret S, Bomford A, Peters TJ, Farzaneh F, Hediger MA, Hentze MW, and Simpson RJ. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol Cell 5: 299–309, 2000 [DOI] [PubMed] [Google Scholar]
- 300.McLachlan DR, Smith WL, and Kruck TP. Desferrioxamine and Alzheimer's disease: video home behavior assessment of clinical course and measures of brain aluminum. Ther Drug Monit 15: 602–607, 1993 [PubMed] [Google Scholar]
- 301.Mena NP, Bulteau AL, Salazar J, Hirsch EC, and Nunez MT. Effect of mitochondrial complex I inhibition on Fe-S cluster protein activity. Biochem Biophys Res Commun 409: 241–246, 2011 [DOI] [PubMed] [Google Scholar]
- 302.Meriin AB, Zhang X, He X, Newnam GP, Chernoff YO, and Sherman MY. Huntington toxicity in yeast model depends on polyglutamine aggregation mediated by a prion-like protein Rnq1. J Cell Biol 157: 997–1004, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Messa E, Pellegrino RM, Palmieri A, Carturan S, Cilloni D, Saglio G, and Roetto A. Identification of a novel mutation in the L ferritin iron-responsive element causing hereditary hyperferritinemia-cataract syndrome. Acta Haematol 122: 223–225, 2009 [DOI] [PubMed] [Google Scholar]
- 304.Meynard D, Kautz L, Darnaud V, Canonne-Hergaux F, Coppin H, and Roth MP. Lack of the bone morphogenetic protein BMP6 induces massive iron overload. Nat Genet 41: 478–481, 2009 [DOI] [PubMed] [Google Scholar]
- 305.Miller-Fleming L, Giorgini F, and Outeiro TF. Yeast as a model for studying human neurodegenerative disorders. Biotechnol J 3: 325–338, 2008 [DOI] [PubMed] [Google Scholar]
- 306.Miller LL, Miller SC, Torti SV, Tsuji Y, and Torti FM. Iron-Independent Induction of Ferritin-H Chain by Tumor-Necrosis-Factor. Proc Natl Acad Sci U S A 88: 4946–4950, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Miura T, Sasaki S, Toyama A, and Takeuchi H. Copper reduction by the octapeptide repeat region of prion protein: pH dependence and implications in cellular copper uptake. Biochemistry 44: 8712–8720, 2005 [DOI] [PubMed] [Google Scholar]
- 308.Miyajima H. Aceruloplasminemia, an iron metabolic disorder. Neuropathology 23: 345–350, 2003 [DOI] [PubMed] [Google Scholar]
- 309.Miyajima H, Nishimura Y, Mizoguchi K, Sakamoto M, Shimizu T, and Honda N. Familial apoceruloplasmin deficiency associated with blepharospasm and retinal degeneration. Neurology 37: 761–767, 1987 [DOI] [PubMed] [Google Scholar]
- 310.Moore DJ, West AB, Dawson VL, and Dawson TM. Molecular pathophysiology of Parkinson's disease. Annu Rev Neurosci 28: 57–87, 2005 [DOI] [PubMed] [Google Scholar]
- 311.Moos T. Brain iron homeostasis. Dan Med Bull 49: 279–301, 2002 [PubMed] [Google Scholar]
- 312.Moos T, Rosengren Nielsen T, Skjorringe T, and Morgan EH. Iron trafficking inside the brain. J Neurochem 103: 1730–1740, 2007 [DOI] [PubMed] [Google Scholar]
- 313.Muckenthaler MU. Fine tuning of hepcidin expression by positive and negative regulators. Cell Metab 8: 1–3, 2008 [DOI] [PubMed] [Google Scholar]
- 314.Muhlenhoff U, Richhardt N, Ristow M, Kispal G, and Lill R. The yeast frataxin homolog Yfh1p plays a specific role in the maturation of cellular Fe/S proteins. Hum Mol Genet 11: 2025–2036, 2002 [DOI] [PubMed] [Google Scholar]
- 315.Muhlenhoff U, Stadler JA, Richhardt N, Seubert A, Eickhorst T, Schweyen RJ, Lill R, and Wiesenberger G. A specific role of the yeast mitochondrial carriers Mrs3/4p in mitochondrial iron acquisition under iron-limiting conditions. J Biol Chem 278: 40612–40620, 2003 [DOI] [PubMed] [Google Scholar]
- 316.Muhoberac BB, Baraibar MA, and Vidal R. Iron loading-induced aggregation and reduction of iron incorporation in heteropolymeric ferritin containing a mutant light chain that. BBA Mol Basis Dis 1812: 544–548, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Mumford AD, Cree IA, Arnold JD, Hagan MC, Rixon KC, and Harding JJ. The lens in hereditary hyperferritinaemia cataract syndrome contains crystalline deposits of L-ferritin. Br J Ophthalmol 84: 697–700, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Musco G, Stier G, Kolmerer B, Adinolfi S, Martin S, Frenkiel T, Gibson T, and Pastore A. Towards a structural understanding of Friedreich's ataxia: the solution structure of frataxin. Structure 8: 695–707, 2000 [DOI] [PubMed] [Google Scholar]
- 319.Napier I, Ponka P, and Richardson DR. Iron trafficking in the mitochondrion: novel pathways revealed by disease. Blood 105: 1867–1874, 2005 [DOI] [PubMed] [Google Scholar]
- 320.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, and Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306: 2090–2093, 2004 [DOI] [PubMed] [Google Scholar]
- 321.Nicolas G, Bennoun M, Devaux I, Beaumont C, Grandchamp B, Kahn A, and Vaulont S. Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proc Natl Acad Sci U S A 98: 8780–8785, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Nie GJ, Sheftel AD, Kim SF, and Ponka P. Overexpression of mitochondrial ferritin causes cytosolic iron depletion and changes cellular iron homeostasis. Blood 105: 2161–2167, 2005 [DOI] [PubMed] [Google Scholar]
- 323.Nutt JG. and Wooten GF. Clinical practice. Diagnosis and initial management of Parkinson's disease. N Engl J Med 353: 1021–1027, 2005 [DOI] [PubMed] [Google Scholar]
- 324.Nuytemans K, Theuns J, Cruts M, and Van Broeckhoven C. Genetic etiology of Parkinson disease associated with mutations in the SNCA, PARK2, PINK1, PARK7, and LRRK2 genes: a mutation update. Hum Mutat 31: 763–780, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Oakley AE, Collingwood JF, Dobson J, Love G, Perrott HR, Edwardson JA, Elstner M, and Morris CM. Individual dopaminergic neurons show raised iron levels in Parkinson disease. Neurology 68: 1820–1825, 2007 [DOI] [PubMed] [Google Scholar]
- 326.Oexle H, Gnaiger E, and Weiss G. Iron-dependent changes in cellular energy metabolism: influence on citric acid cycle and oxidative phosphorylation. Biochim Biophys Acta 1413: 99–107, 1999 [DOI] [PubMed] [Google Scholar]
- 327.Ohgami RS, Campagna DR, Greer EL, Antiochos B, McDonald A, Chen J, Sharp JJ, Fujiwara Y, Barker JE, and Fleming MD. Identification of a ferrireductase required for efficient transferrin-dependent iron uptake in erythroid cells. Nat Genet 37: 1264–1269, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Ohshima K, Montermini L, Wells RD, and Pandolfo M. Inhibitory effects of expanded GAA.TTC triplet repeats from intron I of the Friedreich ataxia gene on transcription and replication in vivo. J Biol Chem 273: 14588–14595, 1998 [DOI] [PubMed] [Google Scholar]
- 329.Ohta E, Nagasaka T, Shindo K, Toma S, Nagasaka K, Ohta K, and Shiozawa Z. Neuroferritinopathy in a Japanese family with a duplication in the ferritin light chain gene. Neurology 70: 1493–1494, 2008 [DOI] [PubMed] [Google Scholar]
- 330.Olivieri S, Conti A, Iannaccone S, Cannistraci CV, Campanella A, Barbariga M, Codazzi F, Pelizzoni I, Magnani G, Pesca M, Franciotta D, Cappa SF, and Alessio M. Ceruloplasmin oxidation, a feature of Parkinson's disease CSF, inhibits ferroxidase activity and promotes cellular iron retention. J Neurosci 31: 18568–18577, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Osaki S. and Johnson DA. Mobilization of Liver Iron by Ferroxidase (Ceruloplasmin). J Biol Chem 244: 5757–5758, 1969 [PubMed] [Google Scholar]
- 332.Ostrerova-Golts N, Petrucelli L, Hardy J, Lee JM, Farer M, and Wolozin B. The A53T alpha-synuclein mutation increases iron-dependent aggregation and toxicity. J Neurosci 20: 6048–6054, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Outeiro TF. and Lindquist S. Yeast cells provide insight into alpha-synuclein biology and pathobiology. Science 302: 1772–1775, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Pagon RA, Bird TD, Detter JC, and Pierce I. Hereditary sideroblastic anaemia and ataxia: an X linked recessive disorder. J Med Genet 22: 267–273, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Paisan-Ruiz C, Li A, Schneider SA, Holton JL, Johnson R, Kidd D, Chataway J, Bhatia KP, Lees AJ, Hardy J, Revesz T, and Houlden H. Widespread Lewy body and tau accumulation in childhood and adult onset dystonia-parkinsonism cases with PLA2G6 mutations. Neurobiol Aging 33: 814–823, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Pandey A, Yoon H, Lyver ER, Dancis A, and Pain D. Isd11p protein activates the mitochondrial cysteine desulfurase Nfs1p protein. J Biol Chem 286: 38242–38252, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 337.Pandolfo M. Friedreich ataxia: the clinical picture. J Neurol 256Suppl 1: 3–8, 2009 [DOI] [PubMed] [Google Scholar]
- 338.Paradkar PN, Zumbrennen KB, Paw BH, Ward DM, and Kaplan J. Regulation of mitochondrial iron import through differential turnover of mitoferrin 1 and mitoferrin 2. Mol Cell Biol 29: 1007–1016, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Park S, Gakh O, O'Neill HA, Mangravita A, Nichol H, Ferreira GC, and Isaya G. Yeast frataxin sequentially chaperones and stores iron by coupling protein assembly with iron oxidation. J Biol Chem 278: 31340–31351, 2003 [DOI] [PubMed] [Google Scholar]
- 340.Patel BN, Dunn RJ, and David S. Alternative RNA splicing generates a glycosylphosphatidylinositol-anchored form of ceruloplasmin in mammalian brain. J Biol Chem 275: 4305–4310, 2000 [DOI] [PubMed] [Google Scholar]
- 341.Patel BN, Dunn RJ, Jeong SY, Zhu Q, Julien JP, and David S. Ceruloplasmin regulates iron levels in the CNS and prevents free radical injury. J Neurosci 22: 6578–6586, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Pauly PC. and Harris DA. Copper stimulates endocytosis of the prion protein. J Biol Chem 273: 33107–33110, 1998 [DOI] [PubMed] [Google Scholar]
- 343.Peng J, Peng L, Stevenson FF, Doctrow SR, and Andersen JK. Iron and paraquat as synergistic environmental risk factors in sporadic Parkinson's disease accelerate age-related neurodegeneration. J Neurosci 27: 6914–6922, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Perez CA, Tong Y, and Guo M. Iron chelators as potential therapeutic agents for Parkinson's disease. Curr Bioact Compd 4: 150–158, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Petersen RB, Siedlak SL, Lee HG, Kim YS, Nunomura A, Tagliavini F, Ghetti B, Cras P, Moreira PI, Castellani RJ, Guentchev M, Budka H, Ironside JW, Gambetti P, Smith MA, and Perry G. Redox metals and oxidative abnormalities in human prion diseases. Acta Neuropathol 110: 232–238, 2005 [DOI] [PubMed] [Google Scholar]
- 346.Picard V, Epsztejn S, Santambrogio P, Cabantchik ZI, and Beaumont C. Role of ferritin in the control of the labile iron pool in murine erythroleukemia cells. J Biol Chem 273: 15382–15386, 1998 [DOI] [PubMed] [Google Scholar]
- 347.Pierre JL, Baret P, and Serratrice G. Hydroxyquinolines as iron chelators. Curr Med Chem 10: 1077–1084, 2003 [DOI] [PubMed] [Google Scholar]
- 348.Pietsch EC, Chan JY, Torti FM, and Torti SV. Nrf2 mediates the induction of ferritin H in response to xenobiotics and cancer chemopreventive dithiolethiones. J Biol Chem 278: 2361–2369, 2003 [DOI] [PubMed] [Google Scholar]
- 349.Pilsl A. and Winklhofer KF. Parkin, PINK1 and mitochondrial integrity: emerging concepts of mitochondrial dysfunction in Parkinson's disease. Acta Neuropathol 123: 173–188, 2012 [DOI] [PubMed] [Google Scholar]
- 350.Poli M, Derosas M, Luscieti S, Cavadini P, Campanella A, Verardi R, Finazzi D, and Arosio P. Pantothenate kinase-2 (Pank2) silencing causes cell growth reduction, cell-specific ferroportin upregulation and iron deregulation. Neurobiol Dis 39: 204–210, 2010 [DOI] [PubMed] [Google Scholar]
- 351.Pollak Y, Mechlovich D, Amit T, Bar-Am O, Manov I, Mandel SA, Weinreb O, Meyron-Holtz EG, Iancu TC, and Youdim MB. Effects of novel neuroprotective and neurorestorative multifunctional drugs on iron chelation and glucose metabolism. J Neural Transm 120: 37–48, 2013 [DOI] [PubMed] [Google Scholar]
- 352.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, and Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science 276: 2045–2047, 1997 [DOI] [PubMed] [Google Scholar]
- 353.Pondarre C, Antiochos BB, Campagna DR, Greer EL, Deck KM, McDonald A, Han AP, Medlock A, Kutok JL, Anderson SA, Eisenstein RS, and Fleming MD. The mitochondrial ATP-binding cassette transporter Abcb7 is essential in mice and participates in cytosolic iron-sulfur cluster biogenesis. Hum Mol Genet 15: 953–964, 2006 [DOI] [PubMed] [Google Scholar]
- 354.Poss KD. and Tonegawa S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc Natl Acad Sci U S A 94: 10919–10924, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Powers JM. p53-mediated apoptosis, neuroglobin overexpression, and globin deposits in a patient with hereditary ferritinopathy. J Neuropathol Exp Neurol 65: 716–721, 2006 [DOI] [PubMed] [Google Scholar]
- 356.Prasanthi JR, Schrag M, Dasari B, Marwarha G, Dickson A, Kirsch WM, and Ghribi O. Deferiprone reduces amyloid-beta and tau phosphorylation levels but not reactive oxygen species generation in hippocampus of rabbits fed a cholesterol-enriched diet. J Alzheimers Dis 30: 167–182, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Primosigh JV. and Thomas ED. Studies on the partition of iron in bone marrow cells. J Clin Invest 47: 1473–1482, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Prischi F, Konarev PV, Iannuzzi C, Pastore C, Adinolfi S, Martin SR, Svergun DI, and Pastore A. Structural bases for the interaction of frataxin with the central components of iron-sulphur cluster assembly. Nat Commun 1: 95, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Prusiner SB. The prion diseases. Brain Pathol 8: 499–513, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Prusiner SB. Prions. Proc Natl Acad Sci U S A 95: 13363–13383, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Puccio H, Simon D, Cossee M, Criqui-Filipe P, Tiziano F, Melki J, Hindelang C, Matyas R, Rustin P, and Koenig M. Mouse models for Friedreich ataxia exhibit cardiomyopathy, sensory nerve defect and Fe-S enzyme deficiency followed by intramitochondrial iron deposits. Nat Genet 27: 181–186, 2001 [DOI] [PubMed] [Google Scholar]
- 362.Radford HE. and Mallucci GR. The role of GPI-anchored PrP C in mediating the neurotoxic effect of scrapie prions in neurons. Curr Issues Mol Biol 12: 119–127, 2010 [PubMed] [Google Scholar]
- 363.Radisky DC, Babcock MC, and Kaplan J. The yeast frataxin homologue mediates mitochondrial iron efflux. Evidence for a mitochondrial iron cycle. J Biol Chem 274: 4497–4499, 1999 [DOI] [PubMed] [Google Scholar]
- 364.Ramazzotti A, Vanmansart V, and Foury F. Mitochondrial functional interactions between frataxin and Isu1p, the iron-sulfur cluster scaffold protein, in Saccharomyces cerevisiae. FEBS Lett 557: 215–220, 2004 [DOI] [PubMed] [Google Scholar]
- 365.Ramirez A, Heimbach A, Grundemann J, Stiller B, Hampshire D, Cid LP, Goebel I, Mubaidin AF, Wriekat AL, Roeper J, Al-Din A, Hillmer AM, Karsak M, Liss B, Woods CG, Behrens MI, and Kubisch C. Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat Genet 38: 1184–1191, 2006 [DOI] [PubMed] [Google Scholar]
- 366.Religa D, Strozyk D, Cherny RA, Volitakis I, Haroutunian V, Winblad B, Naslund J, and Bush AI. Elevated cortical zinc in Alzheimer disease. Neurology 67: 69–75, 2006 [DOI] [PubMed] [Google Scholar]
- 367.Rhodes SL. and Ritz B. Genetics of iron regulation and the possible role of iron in Parkinson's disease. Neurobiol Dis 32: 183–195, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Richardson DR, Lane DJR, Becker EM, Huang MLH, Whitnall M, Rahmanto YS, Sheftel AD, and Ponka P. Mitochondrial iron trafficking and the integration of iron metabolism between the mitochondrion and cytosol. Proc Natl Acad Sci U S A 107: 10775–10782, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Richardson DR, Ponka P, and Vyoral D. Distribution of iron in reticulocytes after inhibition of heme synthesis with succinylacetone: examination of the intermediates involved in iron metabolism. Blood 87: 3477–3488, 1996 [PubMed] [Google Scholar]
- 370.Ristow M, Mulder H, Pomplun D, Schulz T, Muller-Schmehl K, Krause A, Fex M, Puccio H, Muller J, Isken F, Spranger J, Muller-Wieland D, Magnuson MA, Mohlig M, Koenig M, and Pfeiffer AFH. Frataxin deficiency in pancreatic islets causes diabetes due to loss of beta cell mass. J Clin Invest 112: 527–534, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Ritchie CW, Bush AI, Mackinnon A, Macfarlane S, Mastwyk M, MacGregor L, Kiers L, Cherny R, Li QX, Tammer A, Carrington D, Mavros C, Volitakis I, Xilinas M, Ames D, Davis S, Beyreuther K, Tanzi RE, and Masters CL. Metal-protein attenuation with iodochlorhydroxyquin (clioquinol) targeting Abeta amyloid deposition and toxicity in Alzheimer disease: a pilot phase 2 clinical trial. Arch Neurol 60: 1685–1691, 2003 [DOI] [PubMed] [Google Scholar]
- 372.Rogers JT, Bush AI, Cho HH, Smith DH, Thomson AM, Friedlich AL, Lahiri DK, Leedman PJ, Huang XD, and Cahill CM. Iron and the translation of the amyloid precursor protein (APP) and ferritin mRNAs: riboregulation against neural oxidative damage in Alzheimer's disease. Biochem Soc Trans 36: 1282–1287, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Rogers JT, Randall JD, Cahill CM, Eder PS, Huang XD, Gunshin H, Leiter L, McPhee J, Sarang SS, Utsuki T, Greig NH, Lahiri DK, Tanzi RE, Bush AI, Giordano T, and Gullans SR. An iron-responsive element type II in the 5′-untranslated region of the Alzheimer's amyloid precursor protein transcript. J Biol Chem 277: 45518–45528, 2002 [DOI] [PubMed] [Google Scholar]
- 374.Roth JA, Singleton S, Feng J, Garrick M, and Paradkar PN. Parkin regulates metal transport via proteasomal degradation of the 1B isoforms of divalent metal transporter 1. J Neurochem 113: 454–464, 2010 [DOI] [PubMed] [Google Scholar]
- 375.Rotig A, deLonlay P, Chretien D, Foury F, Koenig M, Sidi D, Munnich A, and Rustin P. Aconitase and mitochondrial iron-sulphur protein deficiency in Friedreich ataxia. Nat Genet 17: 215–217, 1997 [DOI] [PubMed] [Google Scholar]
- 376.Rouault TA, Zhang DL, and Jeong SY. Brain iron homeostasis, the choroid plexus, and localization of iron transport proteins. Metab Brain Dis 24: 673–684, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Saitsu H, Nishimura T, Muramatsu K, Kodera H, Kumada S, Sugai K, Kasai-Yoshida E, Sawaura N, Nishida H, Hoshino A, Ryujin F, Yoshioka S, Nishiyama K, Kondo Y, Tsurusaki Y, Nakashima M, Miyake N, Arakawa H, Kato M, Mizushima N, and Matsumoto N. De novo mutations in the autophagy gene WDR45 cause static encephalopathy of childhood with neurodegeneration in adulthood. Nat Genet 45: 445–449; 449e441, 2013 [DOI] [PubMed] [Google Scholar]
- 378.Sakamoto N, Ohshima K, Montermini L, Pandolfo M, and Wells RD. Sticky DNA, a self-associated complex formed at long GAA center dot TTC repeats in intron 1 of the frataxin gene, inhibits transcriptions. J Biol Chem 276: 27171–27177, 2001 [DOI] [PubMed] [Google Scholar]
- 379.Salahudeen AA, Thompson JW, Ruiz JC, Ma HW, Kinch LN, Li Q, Grishin NV, and Bruick RK. An E3 ligase possessing an iron-responsive hemerythrin domain is a regulator of iron homeostasis. Science 326: 722–726, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Salazar J, Mena N, Hunot S, Prigent A, Alvarez-Fischer D, Arredondo M, Duyckaerts C, Sazdovitch V, Zhao L, Garrick LM, Nunez MT, Garrick MD, Raisman-Vozari R, and Hirsch EC. Divalent metal transporter 1 (DMT1) contributes to neurodegeneration in animal models of Parkinson's disease. Proc Natl Acad Sci U S A 105: 18578–18583, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Santambrogio P, Biasiotto G, Sanvito F, Olivieri S, Arosio P, and Levi S. Mitochondrial ferritin expression in adult mouse tissues. J Histochem Cytochem 55: 1129–1137, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Santos MM, de Sousa M, Rademakers LHPM, Clevers H, Marx JJM, and Schilham MW. Iron overload and heart fibrosis in mice deficient for both beta 2-microglobulin and Rag1. Am J Pathol 157: 1883–1892, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Santos R, Lefevre S, Sliwa D, Seguin A, Camadro JM, and Lesuisse E. Friedreich ataxia: molecular mechanisms, redox considerations, and therapeutic opportunities. Antioxid Redox Signal 13: 651–690, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Savitt JM, Dawson VL, and Dawson TM. Diagnosis and treatment of Parkinson disease: molecules to medicine. J Clin Invest 116: 1744–1754, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Scapagnini G, Butterfield DA, Colombrita C, Sultana R, Pascale A, and Calabrese V. Ethyl ferulate, a lipophilic polyphenol, induces HO-1 and protects rat neurons against oxidative stress. Antioxid Redox Signal 6: 811–818, 2004 [DOI] [PubMed] [Google Scholar]
- 386.Scapagnini G, Vasto S, Abraham NG, Caruso C, Zella D, and Fabio G. Modulation of Nrf2/ARE pathway by food polyphenols: a nutritional neuroprotective strategy for cognitive and neurodegenerative disorders. Mol Neurobiol 44: 192–201, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Schmucker S, Martelli A, Colin F, Page A, Wattenhofer-Donze M, Reutenauer L, and Puccio H. Mammalian Frataxin: An Essential Function for Cellular Viability through an Interaction with a Preformed ISCU/NFS1/ISD11 Iron-Sulfur Assembly Complex. PLoS One 6: e16199, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Schneider SA. and Bhatia KP. Syndromes of neurodegeneration with brain iron accumulation. Semin Pediatr Neurol 19: 57–66, 2012 [DOI] [PubMed] [Google Scholar]
- 389.Schroder JM. Ferritinopathy: diagnosis by muscle or nerve biopsy, with a note on other nuclear inclusion body diseases. Acta Neuropathol 109: 109–114, 2005 [DOI] [PubMed] [Google Scholar]
- 390.Schroeder EK, Kelsey NA, Doyle J, Breed E, Bouchard RJ, Loucks FA, Harbison RA, and Linseman DA. Green tea epigallocatechin 3-gallate accumulates in mitochondria and displays a selective antiapoptotic effect against inducers of mitochondrial oxidative stress in neurons. Antioxid Redox Signal 11: 469–480, 2009 [DOI] [PubMed] [Google Scholar]
- 391.Schulz JB, Dehmer T, Schols L, Mende H, Hardt C, Vorgerd M, Burk K, Matson W, Dichgans J, Beal MF, and Bogdanov MB. Oxidative stress in patients with Friedreich ataxia. Neurology 55: 1719–1721, 2000 [DOI] [PubMed] [Google Scholar]
- 392.Seguin A, Bayot A, Dancis A, Rogowska-Wrzesinska A, Auchere F, Camadro JM, Bulteau AL, and Lesuisse E. Overexpression of the yeast frataxin homolog (Yfh1): contrasting effects on iron-sulfur cluster assembly, heme synthesis and resistance to oxidative stress. Mitochondrion 9: 130–138, 2009 [DOI] [PubMed] [Google Scholar]
- 393.Senbongi H, Ling F, and Shibata T. A mutation in a mitochondrial ABC transporter results in mitochondrial dysfunction through oxidative damage of mitochondrial DNA. Mol Gen Genet 262: 426–436, 1999 [DOI] [PubMed] [Google Scholar]
- 394.Sharma N, Brandis KA, Herrera SK, Johnson BE, Vaidya T, Shrestha R, and Debburman SK. alpha-Synuclein budding yeast model: toxicity enhanced by impaired proteasome and oxidative stress. J Mol Neurosci 28: 161–178, 2006 [DOI] [PubMed] [Google Scholar]
- 395.Shaw GC, Cope JJ, Li LT, Corson K, Hersey C, Ackermann GE, Gwynn B, Lambert AJ, Wingert RA, Traver D, Trede NS, Barut BA, Zhou Y, Minet E, Donovan A, Brownlie A, Balzan R, Weiss MJ, Peters LL, Kaplan J, Zon LI, and Paw BH. Mitoferrin is essential for erythroid iron assimilation. Nature 440: 96–100, 2006 [DOI] [PubMed] [Google Scholar]
- 396.Sheftel AD, Richardson DR, Prchal J, and Ponka P. Mitochondrial iron metabolism and sideroblastic anemia. Acta Haematol 122: 120–133, 2009 [DOI] [PubMed] [Google Scholar]
- 397.Sheftel AD, Zhang AS, Brown C, Shirihai OS, and Ponka P. Direct interorganellar transfer of iron from endosome to mitochondrion. Blood 110: 125–132, 2007 [DOI] [PubMed] [Google Scholar]
- 398.Shi HF, Bencze KZ, Stemmler TL, and Philpott CC. A cytosolic iron chaperone that delivers iron to ferritin. Science 320: 1207–1210, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Shi R, Proteau A, Villarroya M, Moukadiri I, Zhang L, Trempe JF, Matte A, Armengod ME, and Cygler M. Structural basis for Fe-S cluster assembly and tRNA thiolation mediated by IscS protein-protein interactions. PLoS Biol 8: e1000354, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Shimada Y, Okuno S, Kawai A, Shinomiya H, Saito A, Suzuki M, Omori Y, Nishino N, Kanemoto N, Fujiwara T, Horie M, and Takahashi E. Cloning and chromosomal mapping of a novel ABC transporter gene (hABC7), a candidate for X-linked sideroblastic anemia with spinocerebellar ataxia. J Hum Genet 43: 115–122, 1998 [DOI] [PubMed] [Google Scholar]
- 401.Shirihai OS, Gregory T, Yu CN, Orkin SH, and Weiss MJ. ABC-me: a novel mitochondrial transporter induced by GATA-1 during erythroid differentiation. EMBO J 19: 2492–2502, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Shvartsman M, Kikkeri R, Shanzer A, and Cabantchik ZI. Non-transferrin-bound iron reaches mitochondria by a chelator-inaccessible mechanism: biological and clinical implications. Am J Physiol Cell Physiol 293: C1383–C1394, 2007 [DOI] [PubMed] [Google Scholar]
- 403.Sian-Hulsmann J, Mandel S, Youdim MB, and Riederer P. The relevance of iron in the pathogenesis of Parkinson's disease. J Neurochem 118: 939–957, 2011 [DOI] [PubMed] [Google Scholar]
- 404.Sigurdsson EM, Brown DR, Alim MA, Scholtzova H, Carp R, Meeker HC, Prelli F, Frangione B, and Wisniewski T. Copper chelation delays the onset of prion disease. J Biol Chem 278: 46199–46202, 2003 [DOI] [PubMed] [Google Scholar]
- 405.Singh A, Beveridge AJ, and Singh N. Decreased CSF transferrin in sCJD: a potential pre-mortem diagnostic test for prion disorders. PLoS One 6: e16804, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Singh A, Haldar S, Horback K, Tom C, Zhou L, Meyerson H, and Singh N. Prion protein regulates iron transport by functioning as a ferrireductase. J Alzheimers Dis 35: 541–552, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Singh A, Isaac AO, Luo X, Mohan ML, Cohen ML, Chen F, Kong Q, Bartz J, and Singh N. Abnormal brain iron homeostasis in human and animal prion disorders. PLoS Pathog 5: e1000336, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Singh A, Kong Q, Luo X, Petersen RB, Meyerson H, and Singh N. Prion protein (PrP) knock-out mice show altered iron metabolism: a functional role for PrP in iron uptake and transport. PLoS One 4: e6115, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Singh A, Mohan ML, Isaac AO, Luo X, Petrak J, Vyoral D, and Singh N. Prion protein modulates cellular iron uptake: a novel function with implications for prion disease pathogenesis. PLoS One 4: e4468, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Singh A, Qing L, Kong Q, and Singh N. Change in the characteristics of ferritin induces iron imbalance in prion disease affected brains. Neurobiol Dis 45: 930–938, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Singh BN, Shankar S, and Srivastava RK. Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications. Biochem Pharmacol 82: 1807–1821, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Singh N, Singh A, Das D, and Mohan ML. Redox control of prion and disease pathogenesis. Antioxid Redox Signal 12: 1271–1294, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Sipe JC, Lee P, and Beutler E. Brain iron metabolism and neurodegenerative disorders. Dev Neurosci 24: 188–196, 2002 [DOI] [PubMed] [Google Scholar]
- 414.Smith CD, Chebrolu H, Wekstein DR, Schmitt FA, Jicha GA, Cooper G, and Markesbery WR. Brain structural alterations before mild cognitive impairment. Neurology 68: 1268–1273, 2007 [DOI] [PubMed] [Google Scholar]
- 415.Smith MA, Harris PLR, Sayre LM, and Perry G. Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc Natl Acad Sci U S A 94: 9866–9868, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Snyder AM, Wang XS, Patton SM, Arosio P, Levi S, Earley CJ, Allen RP, and Connor JR. Mitochondrial ferritin in the substantia nigra in restless legs syndrome. J Neuropathol Exp Neurol 68: 1193–1199, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Sofic E, Lange KW, Jellinger K, and Riederer P. Reduced and oxidized glutathione in the substantia nigra of patients with Parkinson's disease. Neurosci Lett 142: 128–130, 1992 [DOI] [PubMed] [Google Scholar]
- 418.Song N, Jiang H, Wang J, and Xie JX. Divalent metal transporter 1 up-regulation is involved in the 6-hydroxydopamine-induced ferrous iron influx. J Neurosci Res 85: 3118–3126, 2007 [DOI] [PubMed] [Google Scholar]
- 419.Stearman R, Yuan DS, Yamaguchi-Iwai Y, Klausner RD, and Dancis A. A permease-oxidase complex involved in high-affinity iron uptake in yeast. Science 271: 1552–1557, 1996 [DOI] [PubMed] [Google Scholar]
- 420.Stocker R. Antioxidant activities of bile pigments. Antioxid Redox Signal 6: 841–849, 2004 [DOI] [PubMed] [Google Scholar]
- 421.Subramanian P, Rodrigues AV, Ghimire-Rijal S, and Stemmler TL. Iron chaperones for mitochondrial Fe-S cluster biosynthesis and ferritin iron storage. Curr Opin Chem Biol 15: 312–318, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Taketani S, Kakimoto K, Ueta H, Masaki R, and Furukawa T. Involvement of ABC7 in the biosynthesis of heme in erythroid cells: interaction of ABC7 with ferrochelatase. Blood 101: 3274–3280, 2003 [DOI] [PubMed] [Google Scholar]
- 423.Tapryal N, Mukhopadhyay C, Das D, Fox PL, and Mukhopadhyay CK. Reactive Oxygen Species Regulate Ceruloplasmin by a Novel mRNA Decay Mechanism Involving Its 3′-Untranslated Region: Implications in Neurodegenerative Diseases. J Biol Chem 284: 1873–1883, 2009 [DOI] [PubMed] [Google Scholar]
- 424.Tardiff DF, Tucci ML, Caldwell KA, Caldwell GA, and Lindquist S. Different 8-hydroxyquinolines protect models of TDP-43 protein, alpha-synuclein, and polyglutamine proteotoxicity through distinct mechanisms. J Biol Chem 287: 4107–4120, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Theil EC. Ferritin: at the crossroads of iron and oxygen metabolism. J Nutr 133: 1549s–1553s, 2003 [DOI] [PubMed] [Google Scholar]
- 426.Theil EC, Chen HJ, Miranda C, Janser H, Elsenhans B, Nunez MT, Pizarro F, and Schumann K. Absorption of iron from ferritin is independent of heme iron and ferrous salts in women and rat intestinal segments. J Nutr 142: 478–483, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Theil EC, Liu XFS, and Tosha T. Gated pores in the ferritin protein nanocage. Inorganica Chimica Acta 361: 868–874, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Thompson K, Menzies S, Muckenthaler M, Torti FM, Wood T, Torti SV, Hentze MW, Beard J, and Connor J. Mouse brains deficient in H-ferritin have normal iron concentration but a protein profile of iron deficiency and increased evidence of oxidative stress. J Neurosci Res 71: 46–63, 2003 [DOI] [PubMed] [Google Scholar]
- 429.Todorich B, Zhang XS, Slagle-Webb B, Seaman WE, and Connor JR. Tim-2 is the receptor for H-ferritin on oligodendrocytes. J Neurochem 107: 1495–1505, 2008 [DOI] [PubMed] [Google Scholar]
- 430.Toliyat T, Jorjani M, and Khorasanirad Z. An extended-release formulation of desferrioxamine for subcutaneous administration. Drug Deliv 16: 416–421, 2009 [DOI] [PubMed] [Google Scholar]
- 431.Torti FM. and Torti SV. Regulation of ferritin genes and protein. Blood 99: 3505–3516, 2002 [DOI] [PubMed] [Google Scholar]
- 432.Torti SV, Kwak EL, Miller SC, Miller LL, Ringold GM, Myambo KB, Young AP, and Torti FM. The molecular-cloning and characterization of murine ferritin heavy-chain, a tumor necrosis factor-inducible gene. J Biol Chem 263: 12638–12644, 1988 [PubMed] [Google Scholar]
- 433.Trancikova A, Tsika E, and Moore DJ. Mitochondrial dysfunction in genetic animal models of Parkinson's disease. Antioxid Redox Signal 16: 896–919, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Trenor CC, 3rd, Campagna DR, Sellers VM, Andrews NC, and Fleming MD. The molecular defect in hypotransferrinemic mice. Blood 96: 1113–1118, 2000 [PubMed] [Google Scholar]
- 435.Treusch S, Hamamichi S, Goodman JL, Matlack KE, Chung CY, Baru V, Shulman JM, Parrado A, Bevis BJ, Valastyan JS, Han H, Lindhagen-Persson M, Reiman EM, Evans DA, Bennett DA, Olofsson A, DeJager PL, Tanzi RE, Caldwell KA, Caldwell GA, and Lindquist S. Functional links between Abeta toxicity, endocytic trafficking, and Alzheimer's disease risk factors in yeast. Science 334: 1241–1245, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Trinder D. and Baker E. Transferrin receptor 2: a new molecule in iron metabolism. Int J Biochem Cell Biol 35: 292–296, 2003 [DOI] [PubMed] [Google Scholar]
- 437.Ulubayram K, Kiziltay A, Yilmaz E, and Hasirci N. Desferrioxamine release from gelatin-based systems. Biotechnol Appl Biochem 42: 237–245, 2005 [DOI] [PubMed] [Google Scholar]
- 438.Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, Gonzalez-Maldonado R, Deller T, Salvi S, Cortelli P, Gilks WP, Latchman DS, Harvey RJ, Dallapiccola B, Auburger G, and Wood NW. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science 304: 1158–1160, 2004 [DOI] [PubMed] [Google Scholar]
- 439.Vashisht AA, Zumbrennen KB, Huang X, Powers DN, Durazo A, Sun D, Bhaskaran N, Persson A, Uhlen M, Sangfelt O, Spruck C, Leibold EA, and Wohlschlegel JA. Control of iron homeostasis by an iron-regulated ubiquitin ligase. Science 326: 718–721, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Velasco-Sanchez D, Aracil A, Montero R, Mas A, Jimenez L, O'Callaghan M, Tondo M, Capdevila A, Blanch J, Artuch R, and Pineda M. Combined therapy with idebenone and deferiprone in patients with Friedreich's ataxia. Cerebellum 10: 1–8, 2011 [DOI] [PubMed] [Google Scholar]
- 441.Venti A, Giordano T, Eder P, Bush AI, Lahiri DK, Greig NH, and Rogers JT. The integrated role of desferrioxamine and phenserine targeted to an iron-responsive element in the APP-mRNA 5′-untranslated region. Ann N Y Acad Sci 1035: 34–48, 2004 [DOI] [PubMed] [Google Scholar]
- 442.Vidal R, Delisle MB, and Ghetti B. Neurodegeneration caused by proteins with an aberrant carboxyl-terminus. J Neuropathol Exp Neurol 63: 787–800, 2004 [DOI] [PubMed] [Google Scholar]
- 443.Vidal R, Delisle MB, Rascol O, and Ghetti B. Hereditary ferritinopathy. J Neurol Sci 207: 110–111, 2003 [DOI] [PubMed] [Google Scholar]
- 444.Vidal R, Miravalle L, Gao XY, Barbeito AG, Baraibar MA, Hekmatyar SK, Widel M, Bansal N, Delisle MB, and Ghetti B. Expression of a mutant form of the ferritin light chain gene induces neurodegeneration and iron overload in transgenic mice. J Neurosci 28: 60–67, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Vives-Bauza C. and Przedborski S. Mitophagy: the latest problem for Parkinson's disease. Trends Mol Med 17: 158–165, 2011 [DOI] [PubMed] [Google Scholar]
- 446.von Bernhardi R. and Eugenin J. Alzheimer's disease: redox dysregulation as a common denominator for diverse pathogenic mechanisms. Antioxid Redox Signal 16: 974–1031, 2012 [DOI] [PubMed] [Google Scholar]
- 447.Vulpe CD, Kuo YM, Murphy TL, Cowley L, Askwith C, Libina N, Gitschier J, and Anderson GJ. Hephaestin, a ceruloplasmin homologue implicated in intestinal iron transport, is defective in the sla mouse. Nat Genet 21: 195–199, 1999 [DOI] [PubMed] [Google Scholar]
- 448.Walden WE, Selezneva AI, Dupuy J, Volbeda A, Fontecilla-Camps JC, Theil EC, and Volz K. Structure of dual function iron regulatory protein 1 complexed with ferritin IRE-RNA. Science 314: 1903–1908, 2006 [DOI] [PubMed] [Google Scholar]
- 449.Wang F, Wang X, Yuan CG, and Ma J. Generating a prion with bacterially expressed recombinant prion protein. Science 327: 1132–1135, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Wang J, Jiang H, and Xie JX. Ferroportin1 and hephaestin are involved in the nigral iron accumulation of 6-OHDA-lesioned rats. Eur J Neurosci 25: 2766–2772, 2007 [DOI] [PubMed] [Google Scholar]
- 451.Wang JA. and Pantopoulos K. Regulation of cellular iron metabolism. Biochem J 434: 365–381, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Wang RH, Li CL, Xu XL, Zheng Y, Xiao CY, Zerfas P, Cooperman S, Eckhaus M, Rouault T, Mishra L, and Deng CX. A role of SMAD4 in iron metabolism through the positive regulation of hepicidin expression. Cell Metab 2: 399–409, 2005 [DOI] [PubMed] [Google Scholar]
- 453.Wang T, Wang CY, Shan ZY, Teng WP, and Wang ZY. Clioquinol reduces zinc accumulation in neuritic plaques and inhibits the amyloidogenic pathway in AbetaPP/PS1 transgenic mouse brain. J Alzheimers Dis 29: 549–559, 2012 [DOI] [PubMed] [Google Scholar]
- 454.Waters BM. and Eide DJ. Combinatorial control of yeast FET4 gene expression by iron, zinc, and oxygen. J Biol Chem 277: 33749–33757, 2002 [DOI] [PubMed] [Google Scholar]
- 455.Waxman EA. and Giasson BI. Induction of Intracellular Tau Aggregation Is Promoted by alpha-Synuclein Seeds and Provides Novel Insights into the Hyperphosphorylation of Tau. J Neurosci 31: 7604–7618, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Wei X, Roettger Y, Tan B, He Y, Dodel R, Hampel H, Wei G, Haney J, Gu H, Johnstone BH, Liu J, Farlow MR, and Du Y. Human anti-prion antibodies block prion peptide fibril formation and neurotoxicity. J Biol Chem 287: 12858–12866, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Wei Y, Miller SC, Tsuji Y, Torti SV, and Torti FM. Interleukin 1 induces ferritin heavy chain in human muscle cells. Biochem Biophys Res Commun 169: 289–296, 1990 [DOI] [PubMed] [Google Scholar]
- 458.Weinreb O, Amit T, Mandel S, Kupershmidt L, and Youdim MB. Neuroprotective multifunctional iron chelators: from redox-sensitive process to novel therapeutic opportunities. Antioxid Redox Signal 13: 919–949, 2010 [DOI] [PubMed] [Google Scholar]
- 459.Weinreb O, Amit T, Mandel S, and Youdim MB. Neuroprotective molecular mechanisms of (−)-epigallocatechin-3-gallate: a reflective outcome of its antioxidant, iron chelating and neuritogenic properties. Genes Nutr 4: 283–296, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Weiss G. Iron metabolism in the anemia of chronic disease. Biochim Biophys Acta 1790: 682–693, 2009 [DOI] [PubMed] [Google Scholar]
- 461.Wessling-Resnick M. Iron Homeostasis and the Inflammatory Response. Ann Rev Nutr 30: 105–122, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.West AR. and Oates PS. Mechanisms of heme iron absorption: current questions and controversies. World J Gastroenterol 14: 4101–4110, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.White AR, Bush AI, Beyreuther K, Masters CL, and Cappai R. Exacerbation of copper toxicity in primary neuronal cultures depleted of cellular glutathione. J Neurochem 72: 2092–2098, 1999 [DOI] [PubMed] [Google Scholar]
- 464.White RA, Boydston LA, Brookshier TR, McNulty SG, Nsumu NN, Brewer BP, and Blackmore K. Iron metabolism mutant hbd mice have a deletion in Sec15l1, which has homology to a yeast gene for vesicle docking. Genomics 86: 668–673, 2005 [DOI] [PubMed] [Google Scholar]
- 465.Whitnall M. and Richardson DR. Iron: a new target for pharmacological intervention in neurodegenerative diseases. Semin Pediatr Neurol 13: 186–197, 2006 [DOI] [PubMed] [Google Scholar]
- 466.Whitnall M, Suryo Rahmanto Y, Huang ML, Saletta F, Lok HC, Gutierrez L, Lazaro FJ, Fleming AJ, St Pierre TG, Mikhael MR, Ponka P, and Richardson DR. Identification of nonferritin mitochondrial iron deposits in a mouse model of Friedreich ataxia. Proc Natl Acad Sci U S A 109: 20590–20595, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Whitnall M, Suryo Rahmanto Y, Sutak R, Xu X, Becker EM, Mikhael MR, Ponka P, and Richardson DR. The MCK mouse heart model of Friedreich's ataxia: Alterations in iron-regulated proteins and cardiac hypertrophy are limited by iron chelation. Proc Natl Acad Sci U S A 105: 9757–9762, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Wickner RB. [URE3] as an altered URE2 protein: evidence for a prion analog in Saccharomyces cerevisiae. Science 264: 566–569, 1994 [DOI] [PubMed] [Google Scholar]
- 469.Wickner RB, Masison DC, and Edskes HK. [PSI] and [URE3] as yeast prions. Yeast 11: 1671–1685, 1995 [DOI] [PubMed] [Google Scholar]
- 470.Wilks A, Torpey J, and Ortiz de Montellano PR. Heme oxygenase (HO-1). Evidence for electrophilic oxygen addition to the porphyrin ring in the formation of alpha-meso-hydroxyheme. J Biol Chem 269: 29553–29556, 1994 [PubMed] [Google Scholar]
- 471.Wills AJ, Sawle GV, Guilbert PR, and Curtis AR. Palatal tremor and cognitive decline in neuroferritinopathy. J Neurol Neurosurg Psychiatry 73: 91–92, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Winzeler EA, Shoemaker DD, Astromoff A, Liang H, Anderson K, Andre B, Bangham R, Benito R, Boeke JD, Bussey H, Chu AM, Connelly C, Davis K, Dietrich F, Dow SW, El Bakkoury M, Foury F, Friend SH, Gentalen E, Giaever G, Hegemann JH, Jones T, Laub M, Liao H, Liebundguth N, Lockhart DJ, Lucau-Danila A, Lussier M, M'Rabet N, Menard P, Mittmann M, Pai C, Rebischung C, Revuelta JL, Riles L, Roberts CJ, Ross-MacDonald P, Scherens B, Snyder M, Sookhai-Mahadeo S, Storms RK, Veronneau S, Voet M, Volckaert G, Ward TR, Wysocki R, Yen GS, Yu K, Zimmermann K, Philippsen P, Johnston M, and Davis RW. Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science 285: 901–906, 1999 [DOI] [PubMed] [Google Scholar]
- 473.Wong A, Yang J, Cavadini P, Gellera C, Lonnerdal B, Taroni F, and Cortopassi G. The Friedreich's ataxia mutation confers cellular sensitivity to oxidant stress which is rescued by chelators of iron and calcium and inhibitors of apoptosis. Hum Mol Genet 8: 425–430, 1999 [DOI] [PubMed] [Google Scholar]
- 474.Wong BS, Brown DR, Pan T, Whiteman M, Liu T, Bu XD, Li RL, Gambetti P, Olesik J, Rubenstein R, and Sy MS. Oxidative impairment in scrapie-infected mice is associated with brain metals perturbations and altered antioxidant activities. J Neurochem 79: 689–698, 2001 [DOI] [PubMed] [Google Scholar]
- 475.Wong CS, Kwok JC, and Richardson DR. PCTH: a novel orally active chelator of the aroylhydrazone class that induces iron excretion from mice. Biochim Biophys Acta 1739: 70–80, 2004 [DOI] [PubMed] [Google Scholar]
- 476.Ye H. and Rouault TA. Human iron-sulfur cluster assembly, cellular iron homeostasis, and disease. Biochemistry 49: 4945–4956, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Yoon T. and Cowan JA. Iron-sulfur cluster biosynthesis. Characterization of frataxin as an iron donor for assembly of [2Fe-2S] clusters in ISU-type proteins. J Am Chem Soc 125: 6078–6084, 2003 [DOI] [PubMed] [Google Scholar]
- 478.Yoon T. and Cowan JA. Frataxin-mediated iron delivery to ferrochelatase in the final step ofheme biosynthesis. J Biol Chem 279: 25943–25946, 2004 [DOI] [PubMed] [Google Scholar]
- 479.Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, Llorens V, Gomez Tortosa E, del Ser T, Munoz DG, and de Yebenes JG. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol 55: 164–173, 2004 [DOI] [PubMed] [Google Scholar]
- 480.Zecca L, Youdim MB, Riederer P, Connor JR, and Crichton RR. Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci 5: 863–873, 2004 [DOI] [PubMed] [Google Scholar]
- 481.Zhang S, Wang J, Song N, Xie J, and Jiang H. Up-regulation of divalent metal transporter 1 is involved in 1-methyl-4-phenylpyridinium (MPP(+))-induced apoptosis in MES23.5 cells. Neurobiol Aging 30: 1466–1476, 2009 [DOI] [PubMed] [Google Scholar]
- 482.Zhang Y, Gao J, Chung KK, Huang H, Dawson VL, and Dawson TM. Parkin functions as an E2-dependent ubiquitin- protein ligase and promotes the degradation of the synaptic vesicle-associated protein, CDCrel-1. Proc Natl Acad Sci U S A 97: 13354–13359, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Zhou T, Ma Y, Kong X, and Hider RC. Design of iron chelators with therapeutic application. Dalton Trans 41: 6371–6389, 2012 [DOI] [PubMed] [Google Scholar]
- 484.Zhou XY, Tomatsu S, Fleming RE, Parkkila S, Waheed A, Jiang J, Fei Y, Brunt EM, Ruddy DA, Prass CE, Schatzman RC, O'Neill R, Britton RS, Bacon BR, and Sly WS. HFE gene knockout produces mouse model of hereditary hemochromatosis. Proc Natl Acad Sci U S A 95: 2492–2497, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]