Abstract
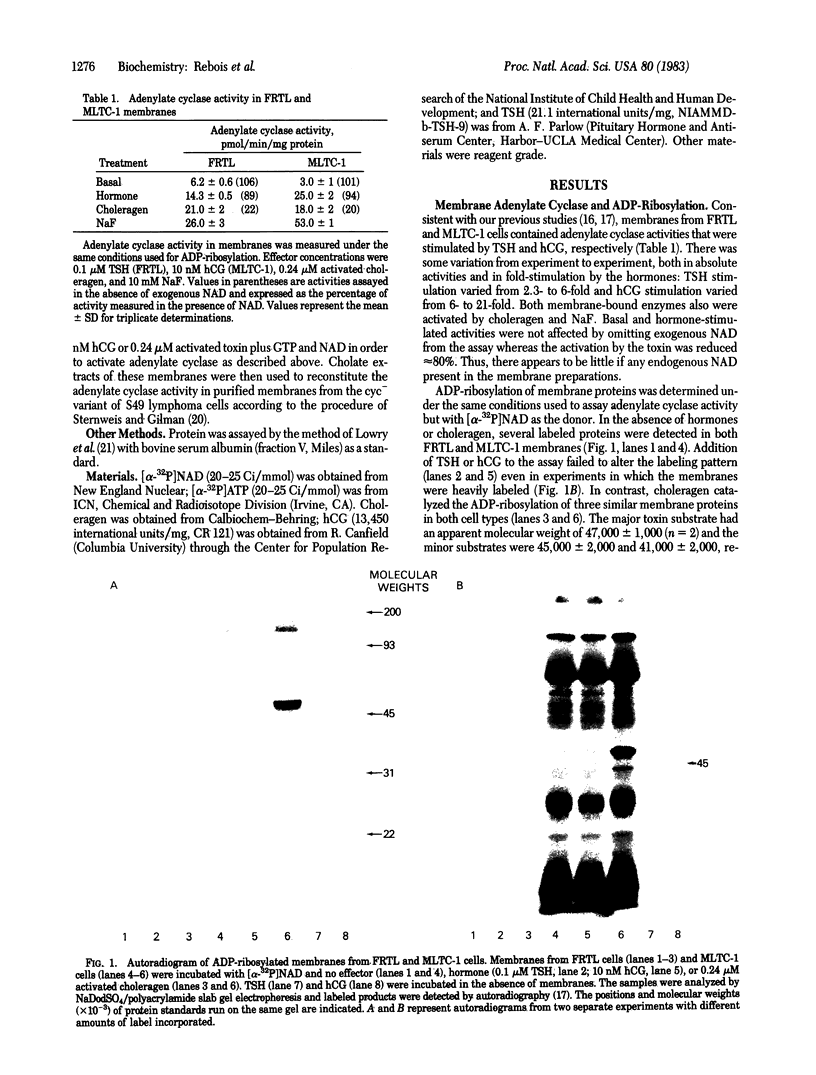
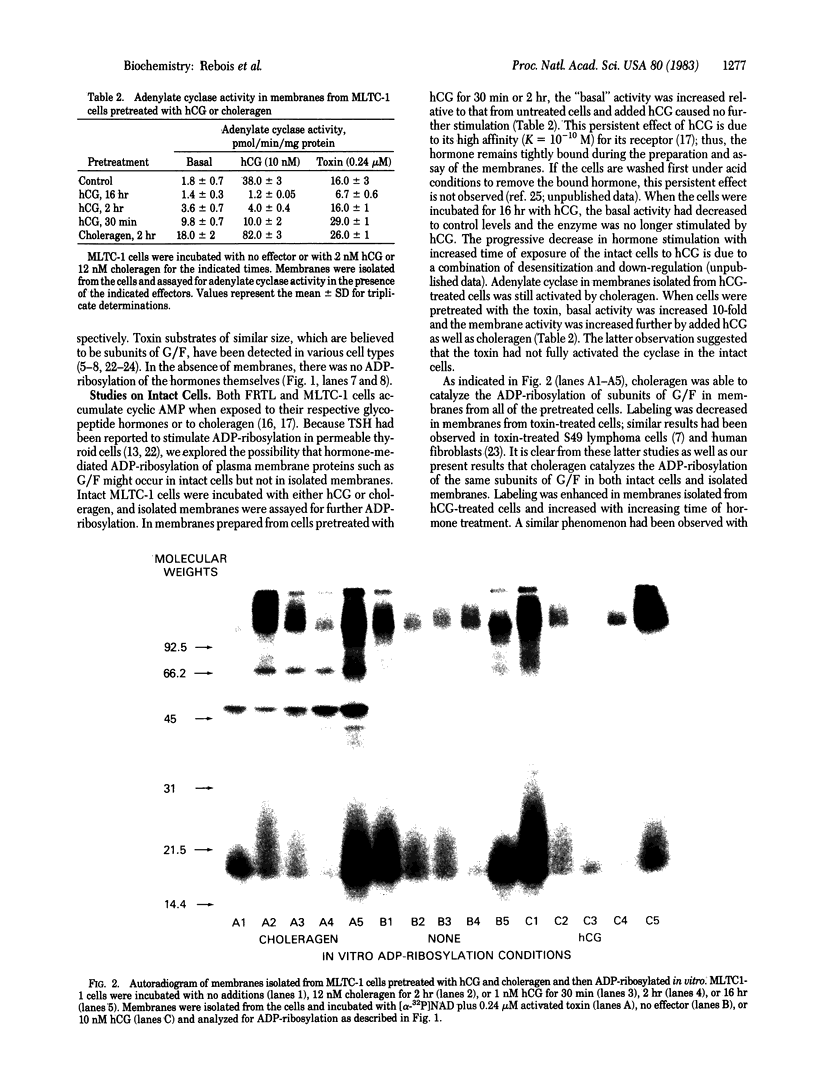
The cultured murine Leydig tumor cell line MLTC-1 and the normal rat thyroid strain FRTL have adenylate cyclase activities that are stimulated by human chorionic gonadotropin (hCG) and thyrotropin, respectively. Both cell types also respond to choleragen. Activation of adenylate cyclase in membranes by choleragen required NAD whereas stimulation of the enzyme by hormones did not. With [alpha-32P]NAD as a donor, ADP-ribosylation of membrane proteins was determined under the same conditions used to assay adenylate cyclase activity. Under these conditions, choleragen, but not the hormones, caused the ADP-ribosylation of subunits of the regulatory component (G/F) of adenylate cyclase in both FRTL and MLTC-1 membranes. In the absence of any effectors, several membrane proteins became labeled but the hormones did not cause the specific labeling of these or any other membrane proteins. Pretreatment of intact MLTC-1 cells with hCG did not block the ability of choleragen to ADP-ribosylate G/F in isolated membranes; labeling was actually enhanced in a manner related to the length of exposure to hCG. In contrast, pretreatment of the cells with choleragen inhibited ADP-ribosylation of G/F by the toxin in isolated membranes. Extracts of membranes from untreated, hCG-treated, and choleragen-treated MLTC-1 cells were used to reconstitute adenylate cyclase activity in membranes from the cyc- variant of S49 lymphoma cells which lacks a functional G/F. Toxin but not hormone treatment caused an increase in the basal activity of adenylate cyclase in the reconstituted system. Our results indicate that ADP-ribosylation of the regulatory component of adenylate cyclase is required for choleragen action but not for hormone action.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambesi-Impiombato F. S., Parks L. A., Coon H. G. Culture of hormone-dependent functional epithelial cells from rat thyroids. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3455–3459. doi: 10.1073/pnas.77.6.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckner S. K., Brady R. O., Fishman P. H. Reevaluation of the role of gangliosides in the binding and action of thyrotropin. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4848–4852. doi: 10.1073/pnas.78.8.4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel D., Pfeuffer T. Mechanism of cholera toxin action: covalent modification of the guanyl nucleotide-binding protein of the adenylate cyclase system. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2669–2673. doi: 10.1073/pnas.75.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wolf M. J., Vitti P., Ambesi-Impiombato F. S., Kohn L. D. Thyroid membrane ADP ribosyltransferase activity. Stimulation by thyrotropin and activity in functioning and nonfunctioning rat thyroid cells in culture. J Biol Chem. 1981 Dec 10;256(23):12287–12296. [PubMed] [Google Scholar]
- Filetti S., Rapoport B. Hormonal stimulation of eucaryotic cell ADP-ribosylation. J Clin Invest. 1981 Aug;68(2):461–467. doi: 10.1172/JCI110276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filetti S., Takai N. A., Rapoport B. Prevention by nicotinamide of desensitization to thyrotropin stimulation in cultured human thyroid cells. J Biol Chem. 1981 Feb 10;256(3):1072–1075. [PubMed] [Google Scholar]
- Gill D. M., Meren R. ADP-ribosylation of membrane proteins catalyzed by cholera toxin: basis of the activation of adenylate cyclase. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3050–3054. doi: 10.1073/pnas.75.7.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. L., Kaslow H. R., Bourne H. R. Genetic evidence that cholera toxin substrates are regulatory components of adenylate cyclase. J Biol Chem. 1978 Oct 25;253(20):7120–7123. [PubMed] [Google Scholar]
- Johnson G. L., Kaslow H. R., Farfel Z., Bourne H. R. Genetic analysis of hormone-sensitive adenylate cyclase. Adv Cyclic Nucleotide Res. 1980;13:1–37. [PubMed] [Google Scholar]
- Kassis S., Fishman P. H. Different mechanisms of desensitization of adenylate cyclase by isoproterenol and prostaglandin E1 in human fibroblasts. Role of regulatory components in desensitization. J Biol Chem. 1982 May 10;257(9):5312–5318. [PubMed] [Google Scholar]
- Kurosky A., Markel D. E., Peterson J. W., Fitch W. M. Primary structure of cholera toxin beta-chain: a glycoprotein hormone analog? Science. 1977 Jan 21;195(4275):299–301. doi: 10.1126/science.831277. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ledley F. D., Mullin B. R., Lee G., Aloj S. M., Fishman P. H., Hunt L. T., Dayhoff M. O., Kohn L. D. Sequence similarity between cholera toxin and glycoprotein hormones: implications for structure activity relationship and mechanism of action. Biochem Biophys Res Commun. 1976 Apr 19;69(4):852–859. doi: 10.1016/0006-291x(76)90452-6. [DOI] [PubMed] [Google Scholar]
- Moss J., Ross P. S., Agosto G., Birken S., Canfield R. E., Vaughan M. Mechanism of action of choleragen and the glycopeptide hormones: is the nicotinamide adenine dinucleotide glycohydrolase activity observed in purified hormone preparations intrinsic to the hormone? Endocrinology. 1978 Feb;102(2):415–419. doi: 10.1210/endo-102-2-415. [DOI] [PubMed] [Google Scholar]
- Moss J., Vaughan M. Mechanism of action of choleragen. Evidence for ADP-ribosyltransferase activity with arginine as an acceptor. J Biol Chem. 1977 Apr 10;252(7):2455–2457. [PubMed] [Google Scholar]
- Mullin B. R., Aloj S. M., Fishman P. H., Lee G., Kohn L. D., Brady R. O. Cholera toxin interactions with thyrotropin receptors on thyroid plasma membranes. Proc Natl Acad Sci U S A. 1976 May;73(5):1679–1683. doi: 10.1073/pnas.73.5.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northup J. K., Sternweis P. C., Smigel M. D., Schleifer L. S., Ross E. M., Gilman A. G. Purification of the regulatory component of adenylate cyclase. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6516–6520. doi: 10.1073/pnas.77.11.6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce J. G., Parsons T. F. Glycoprotein hormones: structure and function. Annu Rev Biochem. 1981;50:465–495. doi: 10.1146/annurev.bi.50.070181.002341. [DOI] [PubMed] [Google Scholar]
- Rapoport B., Filetti S., Takai N., Seto P. Studies on the desensitization of the cyclic AMP response to thyrotropin in thyroid tissue. FEBS Lett. 1982 Sep 6;146(1):23–27. doi: 10.1016/0014-5793(82)80697-2. [DOI] [PubMed] [Google Scholar]
- Rebois R. V. Establishment of gonadotropin-responsive murine leydig tumor cell line. J Cell Biol. 1982 Jul;94(1):70–76. doi: 10.1083/jcb.94.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodbell M. The role of hormone receptors and GTP-regulatory proteins in membrane transduction. Nature. 1980 Mar 6;284(5751):17–22. doi: 10.1038/284017a0. [DOI] [PubMed] [Google Scholar]
- Ross E. M., Gilman A. G. Biochemical properties of hormone-sensitive adenylate cyclase. Annu Rev Biochem. 1980;49:533–564. doi: 10.1146/annurev.bi.49.070180.002533. [DOI] [PubMed] [Google Scholar]
- Salomon Y. Adenylate cyclase assay. Adv Cyclic Nucleotide Res. 1979;10:35–55. [PubMed] [Google Scholar]
- Segaloff D. L., Ascoli M. Removal of the surface-bound human choriogonadotropin results in the cessation of hormonal responses in cultured Leydig tumor cells. J Biol Chem. 1981 Nov 25;256(22):11420–11423. [PubMed] [Google Scholar]
- Sternweis P. C., Gilman A. G. Reconstitution of catecholamine-sensitive adenylate cyclase. Reconstitution of the uncoupled variant of the S40 lymphoma cell. J Biol Chem. 1979 May 10;254(9):3333–3340. [PubMed] [Google Scholar]
- Vitti P., De Wolf M. J., Acquaviva A. M., Epstein M., Kohn L. D. Thyrotropin stimulation of the ADP-ribosyltransferase activity of bovine thyroid membranes. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1525–1529. doi: 10.1073/pnas.79.5.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins P. A., Moss J., Vaughan M. ADP ribosylation of membrane proteins from human fibroblasts. Effect of prior exposure of cells to choleragen. J Biol Chem. 1981 May 25;256(10):4895–4899. [PubMed] [Google Scholar]