Abstract
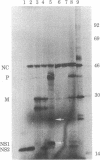
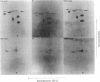
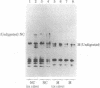
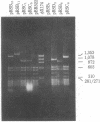
Cytoplasmic poly(A)-containing RNA from respiratory syncytial virus-infected cells was used as a template to synthesize oligo(dT)-primed cDNAs. Discrete size classes of single-stranded cDNAs, resolved by alkali agarose gel electrophoresis, were used separately to construct double-stranded cDNAs that were subsequently inserted into the plasmid vector pBR322 at the Pst I site by means of oligo(dG)oligo(dC) tailing. After transfection of Escherichia coli, recombinant plasmids were screened mostly by serial rounds of hybrid selection of mRNAs from virus-infected cells and subsequent in vitro translation of the selected mRNAs. Comparative peptide mapping of the translation products with those of authentic virion proteins served to establish the viral origin of the cDNA recombinants. In this manner, four distinct classes of recombinant plasmids were identified. These encode sequences corresponding to those of respiratory syncytial virus nucleocapsid protein, matrix protein, phosphoprotein, and a nonstructural protein.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belshe R. B., Van Voris L. P., Mufson M. A. Parenteral administration of live respiratory syncytial virus vaccine: results of a field trial. J Infect Dis. 1982 Mar;145(3):311–319. doi: 10.1093/infdis/145.3.311. [DOI] [PubMed] [Google Scholar]
- Bernstein J. M., Hruska J. F. Respiratory syncytial virus proteins: identification by immunoprecipitation. J Virol. 1981 Apr;38(1):278–285. doi: 10.1128/jvi.38.1.278-285.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J. J., Cosby S. L., Scott J. K., Rima B. K., Martin S. J., Appel M. A comparison of measles and canine distemper virus polypeptides. J Gen Virol. 1980 May;48(1):149–159. doi: 10.1099/0022-1317-48-1-149. [DOI] [PubMed] [Google Scholar]
- Cash P., Pringle C. R., Preston C. M. The polypeptides of human respiratory syncytial virus: products of cell-free protein synthesis and post-translational modifications. Virology. 1979 Jan 30;92(2):375–384. doi: 10.1016/0042-6822(79)90142-9. [DOI] [PubMed] [Google Scholar]
- Collins P. L., Hightower L. E., Ball L. A. Transcriptional map for Newcastle disease virus. J Virol. 1980 Sep;35(3):682–693. doi: 10.1128/jvi.35.3.682-693.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P. L., Wertz G. W., Ball L. A., Hightower L. E. Coding assignments of the five smaller mRNAs of Newcastle disease virus. J Virol. 1982 Sep;43(3):1024–1031. doi: 10.1128/jvi.43.3.1024-1031.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Moss B. In vitro translation of immediate early, early, and late classes of RNA from vaccinia virus-infected cells. Virology. 1979 Jul 30;96(2):368–380. doi: 10.1016/0042-6822(79)90095-3. [DOI] [PubMed] [Google Scholar]
- Deng G., Wu R. An improved procedure for utilizing terminal transferase to add homopolymers to the 3' termini of DNA. Nucleic Acids Res. 1981 Aug 25;9(16):4173–4188. doi: 10.1093/nar/9.16.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkind P. R., Cross R. K., Lamb R. A., Merz D. C., Choppin P. W. In vitro synthesis of structural and nonstructural proteins of Sendai and SV5 viruses. Virology. 1980 Jan 15;100(1):22–33. doi: 10.1016/0042-6822(80)90548-6. [DOI] [PubMed] [Google Scholar]
- Fernie B. F., Gerin J. L. Immunochemical identification of viral and nonviral proteins of the respiratory syncytial virus virion. Infect Immun. 1982 Jul;37(1):243–249. doi: 10.1128/iai.37.1.243-249.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gergen J. P., Stern R. H., Wensink P. C. Filter replicas and permanent collections of recombinant DNA plasmids. Nucleic Acids Res. 1979 Dec 20;7(8):2115–2136. doi: 10.1093/nar/7.8.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez H. B., Pringle C. R. Seven complementation groups of respiratory syncytial virus temperature-sensitive mutants. J Virol. 1978 Sep;27(3):459–464. doi: 10.1128/jvi.27.3.459-464.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazier K., Raghow R., Kingsbury D. W. Regulation of Sendai virus transcription: evidence for a single promoter in vivo. J Virol. 1977 Mar;21(3):863–871. doi: 10.1128/jvi.21.3.863-871.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Hall W. W., Lamb R. A., Choppin P. W. The polypeptides of canine distemper virus: synthesis in infected cells and relatedness to the polypeptides of other morbilliviruses. Virology. 1980 Jan 30;100(2):433–449. doi: 10.1016/0042-6822(80)90534-6. [DOI] [PubMed] [Google Scholar]
- Hershfield V., Boyer H. W., Yanofsky C., Lovett M. A., Helinski D. R. Plasmid ColEl as a molecular vehicle for cloning and amplification of DNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3455–3459. doi: 10.1073/pnas.71.9.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y. T., Wertz G. W. The genome of respiratory syncytial virus is a negative-stranded RNA that codes for at least seven mRNA species. J Virol. 1982 Jul;43(1):150–157. doi: 10.1128/jvi.43.1.150-157.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isle H. B., Venkatesan S., Moss B. Cell-free translation of early and late mRNAs selected by hybridization to cloned DNA fragments derived from the left 14 million to 72 million daltons of the vaccinia virus genome. Virology. 1981 Jul 15;112(1):306–317. doi: 10.1016/0042-6822(81)90636-x. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W. Determination by peptide mapping of the unique polypeptides in Sendai virions and infected cells. Virology. 1978 Feb;84(2):469–478. doi: 10.1016/0042-6822(78)90263-5. [DOI] [PubMed] [Google Scholar]
- Land H., Grez M., Hauser H., Lindenmaier W., Schütz G. 5'-Terminal sequences of eucaryotic mRNA can be cloned with high efficiency. Nucleic Acids Res. 1981 May 25;9(10):2251–2266. doi: 10.1093/nar/9.10.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzarini R. A., Keene J. D., Schubert M. The origins of defective interfering particles of the negative-strand RNA viruses. Cell. 1981 Oct;26(2 Pt 2):145–154. doi: 10.1016/0092-8674(81)90298-1. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McCarthy M., Lazzarini R. A. Intracellular nucleocapsid RNA of mumps virus. J Gen Virol. 1982 Jan;58(Pt 1):205–209. doi: 10.1099/0022-1317-58-1-205. [DOI] [PubMed] [Google Scholar]
- Méchali M., Girard M., de Recondo A. M. DNA polymerase activities in growing cells infected with simian virus 40. J Virol. 1977 Jul;23(1):117–125. doi: 10.1128/jvi.23.1.117-125.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeples M., Levine S. Respiratory syncytial virus polypeptides: their location in the virion. Virology. 1979 May;95(1):137–145. doi: 10.1016/0042-6822(79)90408-2. [DOI] [PubMed] [Google Scholar]
- Rima B. K., Roberts M. W., McAdam W. D., Martin S. J. Polypeptide synthesis i mumps virus-infected cells. J Gen Virol. 1980 Feb;46(2):501–505. doi: 10.1099/0022-1317-46-2-501. [DOI] [PubMed] [Google Scholar]
- Thayer R. E. An improved method for detecting foreign DNA in plasmids of Escherichia coli. Anal Biochem. 1979 Sep 15;98(1):60–63. doi: 10.1016/0003-2697(79)90705-x. [DOI] [PubMed] [Google Scholar]
- Treuhaft M. W., Beem M. O. Defective interfering particles of respiratory syncytial virus. Infect Immun. 1982 Aug;37(2):439–444. doi: 10.1128/iai.37.2.439-444.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan S., Baroudy B. M., Moss B. Distinctive nucleotide sequences adjacent to multiple initiation and termination sites of an early vaccinia virus gene. Cell. 1981 Sep;25(3):805–813. doi: 10.1016/0092-8674(81)90188-4. [DOI] [PubMed] [Google Scholar]
- Venkatesan S., Moss B. Eukaryotic mRNA capping enzyme-guanylate covalent intermediate. Proc Natl Acad Sci U S A. 1982 Jan;79(2):340–344. doi: 10.1073/pnas.79.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winberg G., Hammarskjöld M. L. Isolation of DNA from agarose gels using DEAE-paper. Application to restriction site mapping of adenovirus type 16 DNA. Nucleic Acids Res. 1980 Jan 25;8(2):253–264. doi: 10.1093/nar/8.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittek R., Cooper J. A., Barbosa E., Moss B. Expression of the vaccinia virus genome: analysis and mapping of mRNAs encoded within the inverted terminal repetition. Cell. 1980 Sep;21(2):487–493. doi: 10.1016/0092-8674(80)90485-7. [DOI] [PubMed] [Google Scholar]
- Wright P. F., Gharpure M. A., Hodes D. S., Chanock R. M. Genetic studies of respiratory syncytial virus temperature-sensitive mutants. Arch Gesamte Virusforsch. 1973;41(3):238–247. doi: 10.1007/BF01252771. [DOI] [PubMed] [Google Scholar]
- Wunner W. H., Pringle C. R. Respiratory syncytial virus proteins. Virology. 1976 Aug;73(1):228–243. doi: 10.1016/0042-6822(76)90077-5. [DOI] [PubMed] [Google Scholar]