Abstract
During singing in songbirds, the extent of beak opening, like the extent of mouth opening in human singers, is partially correlated with the fundamental frequency of the sounds emitted. Since song in songbirds is under the control of “the song system” (a collection of interconnected forebrain nuclei dedicated to the learning and production of song), it might be expected that beak movements during singing would also be controlled by this system. However, direct neural connections between the telencephalic output of the song system and beak muscle motor neurons in the brainstem are conspicuous by their absence, leaving unresolved the question of how beak movements are affected during singing. By using standard tract tracing methods, we sought to answer this question by defining beak premotor neurons and examining their afferent projections. In the caudal medulla, jaw premotor cell bodies were located adjacent to the terminal field of the output of the song system, into which many premotor neurons extended their dendrites. The premotor neurons also received a novel input from the trigeminal ganglion and an overlapping input from a lateral arcopallial component of a trigeminal sensorimotor circuit that traverses the forebrain. The ganglionic input in songbirds, which is not present in doves and pigeons that vocalize with a closed beak, may modulate the activity of beak premotor neurons in concert with the output of the song system. These inputs to jaw premotor neurons could, together, affect beak movements as a means of modulating filter properties of the upper vocal tract during singing.
INDEXING TERMS: vocal control, gape modulation, canary, zebra finch, tract tracing
Songbirds engage in extensive opening-closing movements of the beak during vocal production (Westneat et al., 1993; Podos et al.; 1995; Williams, 2001; Goller et al., 2004). Using reconstructions of video films of singing swamp sparrows and white-throated sparrows, Westneat et al. (1993) showed that there was a positive correlation between the extent of beak opening (gape) and the fundamental frequency of the sounds emitted, but there was no correlation with sound amplitude. A positive correlation between gape, in this case measured online with a magneto-sensitive system, and fundamental frequency was also found in northern cardinals (Suthers et al., 1996; Suthers and Goller, 1997) and for notes sung by zebra finches (Goller et al., 2004), but for cardinals this relationship was found to break down above ~3.5 kHz, and for zebra finches it was not present for the harmonic stacks that characterize much of this species’ song. During the production of these atonal syllables, the correlation of gape is with peak frequency (Williams, 2001). In any case, all these and related observations (Hoese et al., 2000; Goller et al., 2004) suggest that, contrary to earlier formulations (Greenwalt, 1968; see Nowicki and Marler, 1988), beak movements play an important role modulating the acoustic properties of the vocal tract during singing in songbirds and probably during the vocalizations of many nonsongbirds as well (cf. Hausberger et al., 1991), except in those species such as doves and pigeons that vocalize with a closed beak (Riede et al., 2004).
The function of beak gape modulation during singing is controversial (Nelson et al., 2005), and the mechanisms underlying the relation between beak gape and sound frequency are unclear. As pointed out by Hoese et al. (2000), the resonance properties of the bird’s vocal tract could be modified by changes in tracheal length (Daley and Goller, 2004), glottal narrowing and tongue movements (Beckers et al., 2004), and opening–closing movements of the beak. In addition, gape is associated with changes in oropharyngeal shape and size, thereby altering filter properties of the upper vocal tract (Riede et al., 2006). Whatever the mechanisms, sensory feedback from the beak and pharynx, either from associated muscles and/or from other tissues, could well be involved in the modulation of vocal output via reflex circuitry that engages the brainstem and, ultimately, output to the jaw muscles via premotor and motor neurons. Although this feedback could reach the brainstem over several cranial nerves, e.g., trigeminal, glossopharyngeal, or vagus, any mechanosensory input would likely terminate in some part of the trigeminal brainstem complex (TBC). However, because singing in songbirds is under the control of the forebrain (Nottebohm et al., 1976), it is conceivable that activity in the TBC is modulated during singing in a fashion analogous to the way in which brainstem respiratory circuitry is modulated by the forebrain during singing (Sturdy et al., 2003). In the latter case, descending projections from the output of the telencephalic song control circuitry, nucleus robustus arcopallialis (RA), terminate directly on respiratory premotor neurons in nucleus parambigualis (PAm) and nucleus retroambigualis (RAm) in the caudal medulla, as well as on vocal motoneurons in nucleus tracheosyringealis (XIIts), thereby modulating the rhythmic activity characteristic of quiet breathing and effecting a precise coordination of respiratory and syringeal muscles during singing (Wild, 1993a,b; Sturdy et al., 2003; Kubke et al., 2005; Wild et al., 2009). However, as far as can be determined at the light microscopic level, RA axons do not appear to terminate upon jaw motoneurons, nor do they terminate in relation to the TBC (Wild, 1993a). Whether they terminate on jaw premotor neurons is also unknown, because these neurons have not been identified in songbirds. The purpose of the present study, therefore, is to determine this, by retrogradely labeling these premotor neurons from the jaw motor nuclei and determining their relation to trigeminal afferents, on the one hand, and to descending projections from the arcopallium, on the other. In the latter case, we reassessed descending projections not only from RA but also from the lateral part of the intermediate arcopallium (Ail), because a previous study of the zebra finch found this to be a major source of projections to the lateral reticular formation of the pons and medulla (Wild and Farabaugh, 1996), although the significance of some of these projections with reference to jaw premotor neurons was not then fully appreciated.
MATERIALS AND METHODS
Subjects were 62 songbirds (eight adult male canaries, Serinus canaria, and 54 adult male zebra finches, Taeniopygia guttata) and two nonsongbirds (pigeons, Columba livia). Each was anesthetized with an intramuscular injection of an equal-parts mixture of ketamine (50 mg/kg) and xylazine (20 mg/kg) and fixed in a Kopf stereotaxic apparatus with the beak tilted downward at 45° to the horizontal (Stokes et al., 1974).
To locate putative jaw premotor neurons, stereotaxically guided (Stokes et al., 1974) injections of retrograde tracers were made into the trigeminal motor nucleus (MV) and the caudomedially adjacent dorsal facial motor nucleus (MVIId) in five canaries and 12 zebra finches. In nonsongbirds, different subnuclei of the main (intermediate), trigeminal motor nucleus (MVi) innervate all the jawclosing muscles and the protractor of the independently mobile upper jaw (Bock, 1964), whereas the dorsal facial nucleus innervates the depressor of the lower jaw (Wild and Zeigler, 1980; den Boer et al., 1986). A similar arrangement appears to be present for the trigeminal motor complex in songbirds (Wild, 1999, and unpublished observations). The injections were made using glass micro-pipettes (outer diameter 10–25 µm) filled with either 1% unconjugated cholera toxin B subunit (CTB; List Biological Laboratories, Campbell, CA) in 0.01M phosphate-buffered saline (PBS), pH 7.4, or 10% biotinylated dextran amine in PBS (BDA; Invitrogen, Eugene, OR; 10k MW in 0.01 M PBS). In two zebra finches, the fluorescent tracer dextran Alexa 488 was used (see below; Invitrogen; 10% in PBS). The tracers were injected using either air pressure (Picospritzer; General Valve, Fairfield, NJ) or iontophoresis (2–4 µA, 7 seconds on, 7 seconds off, 10–20 minutes total).
To confirm the projections of the putative premotor neurons upon the jaw and other upper vocal tract motor nuclei, three canaries and 15 zebra finches received unilateral iontophoretic injections of BDA at various locations in the caudal medulla. In four cases, the BDA injections were located in the ventromedial part of the parvocellular reticular formation (RPcvm), which corresponded to specific regions containing cells retrogradely labeled from injections in the jaw motor nuclei. In 14 other cases, the injections were made in nearby parts of the caudal medulla, e.g., the tracheosyringeal motor nucleus (XIIts), PAm, or RAm (Wild, 1993a; Wild et al., 2000). To verify and clarify the source of projections upon RPcvm from MV/MVIId and to discover other sources of projections upon RPcvm, iontophoretic injections of CTB were also made in RPcvm in another four zebra finches.
Because of the small size of the finch medulla and the close proximity of the nuclear groups involved, all the injections in the medulla were made using electrophysiological guidance, i.e., with reference to multiunit recordings of neural activity in the nucleus of the descending trigeminal tract (TTD; evoked by tactile stimulation of the beak) or to respiratory-related neuronal activity in XIIts or the adjacent PAm and RAm (Manogue and Paton, 1982; Wild, 1993b; 1994; Reinke and Wild, 1998; Sturdy et al., 2003) using either tungsten microelectrodes (Frederick Haer and Company; 3–5 MΩ) or tracer filled glass micropipettes. In six zebra finches, the injection of BDA in the medulla was combined with an injection of CTB into the jaw muscles in the base of the orbit or into m. depressor mandibulae (the opener of the lower jaw) or m. geniohyoideus (the tongue protruder) or the tongue retractors (mm. serpihyoideus and stylohyoideus). These combined injections served to identify unequivocally the motor nuclei upon which the premotor neurons projected.
To determine the relation of arcopallial projections (Wild, 1993a; Wild and Farabaugh, 1996) to upper vocal tract premotor neurons, injections of BDA were made into either medial (RA, n = 5) or lateral (Ail, n = 4) parts of the intermediate arcopallium. In four of these cases (two canaries and two zebra finches), these injections were combined with an injection of CTB into the contralateral MV/MVIId to relate more easily the largely ipsilateral medullary terminations of RA or Ail (Wild, 1993a; Vicario, 1993; Wild and Farabaugh, 1996; Wild et al., 2000) to retrogradely labeled premotor neurons, which preliminary experiments showed were largely contralateral to the motor nuclei that they innervated.
To determine the relation between the central projections of primary trigeminal afferents and upper vocal tract premotor neurons, the trigeminal ganglion in six male zebra finches was exposed by retraction of the anterior tectum and injected using a glass micropipette (outer diameter ~20 µm) under visual guidance with ~50 nl of either BDA or CTB. In two CTB cases, an injection of dextran Alexa 488 was made into MV/MVIId on the same side (see above). In another eight zebra finches, the sensory mandibular branch (n = 3) or the ophthalmic branch (n = 3) or the skin of the lower eyelid (innervated by the maxillary branch; n = 2) was injected with CTB using air pressure. The ophthalmic branch was located medial to the globe of the eye; the sensory mandibular branch was located at the base of the lower beak (Wild and Zeigler, 1996). In two pigeons, the trigeminal ganglion was injected with BDA to compare the projections directly with those observed in zebra finches and with those previously reported for pigeons (Wild and Zeigler, 1996) with CTB-horseradish peroxidase (HRP) as the tracer.
Survival times ranged from 4 to 6 days, after which the birds were deeply anesthetized with ketamine and xylazine and perfused through the left ventricle with saline, followed by 100 ml of 4% paraformaldehyde (PFA) in 0.01 M phosphate buffer. The trigeminal ganglia and 1–2 mm of attached trigeminal nerves were dissected free from their bony and membranous surrounds, the brains were blocked transversely in the stereotaxic plane, and both brain and ganglia were postfixed for 3–5 hours and equilibrated in 30% sucrose buffer overnight. Thirty-five-micrometer-thick sections were cut from the ganglia and brains on a freezing microtome, those from the ganglia in a quasihorizontal plane and those from the brain in the transverse plane. Brain sections were collected in three or four series, and all sections were treated for the demonstration of transported label. BDA and CTB were visualized in free-floating sections after they had been placed for 20 minutes in 50% methanol and 1% H2O2 and washed thoroughly in 0.01 M phosphate buffered saline (PBS). BDA was visualized by incubating sections for 1 hour in 1:1,000 streptavidin conjugated to HRP (Invitrogen) in 0.3% Triton X-100 in PBS, followed by diaminobenzidene (DAB; 0.02%) for 1–5 minutes. CTB was visualized by sequential incubations in a primary goat anti-CTB antibody (List Laboratories; 1:30,000 in 0.3% Triton X-100 in PBS). This antibody was raised against purified choleragenoid and does not result in labeling following preabsorption of the antibody with excess concentration of choleragenoid (Stocker et al., 2006), and no labeling is seen in material in which a CTB injection has not been performed (Kubke et al., 2004). Incubation in the primary antibody was followed by a biotinylated rabbit anti-goat antibody (Sigma-Aldrich, St. Louis, MO) diluted 1:300 for 1 hour. Streptavidin HRP diluted 1:1,000 was used to bind to the secondary antibody before incubation in the chromogen solution. If BDA and CTB were visualized in the same sections, BDA was visualized first, and the reaction intensified and colored black by the addition of 0.002% cobalt chloride to the DAB mixture, and CTB was visualized second, with no cobalt in the DAB, which left the reaction product brown (Wild, 1993a). Visualization of CTB in the two ganglionic cases also receiving an injection of dextran Alexa 488 into MV/MVIId was accomplished using a donkey anti-goat secondary antibody coupled to Alexa 568 (Invitrogen). In many cases, at least one series of sections was counterstained on the slide with Giemsa, neutral red, or cresyl violet for the identification of nuclear groups.
Sections were viewed via brightfield or fluorescent optics with a Nikon 80i microscope equipped with appropriate filters. Anterogradely and retrogradely labeled projections were drawn with the aid of a drawing tube and/ or digitally photographed using a 5-megapixel Nikon color camera. Images were entered into a computer and traced and labeled in Photoshop and CorelDraw software and adjusted for brightness and/or contrast.
RESULTS
Identification of putative jaw premotor neurons
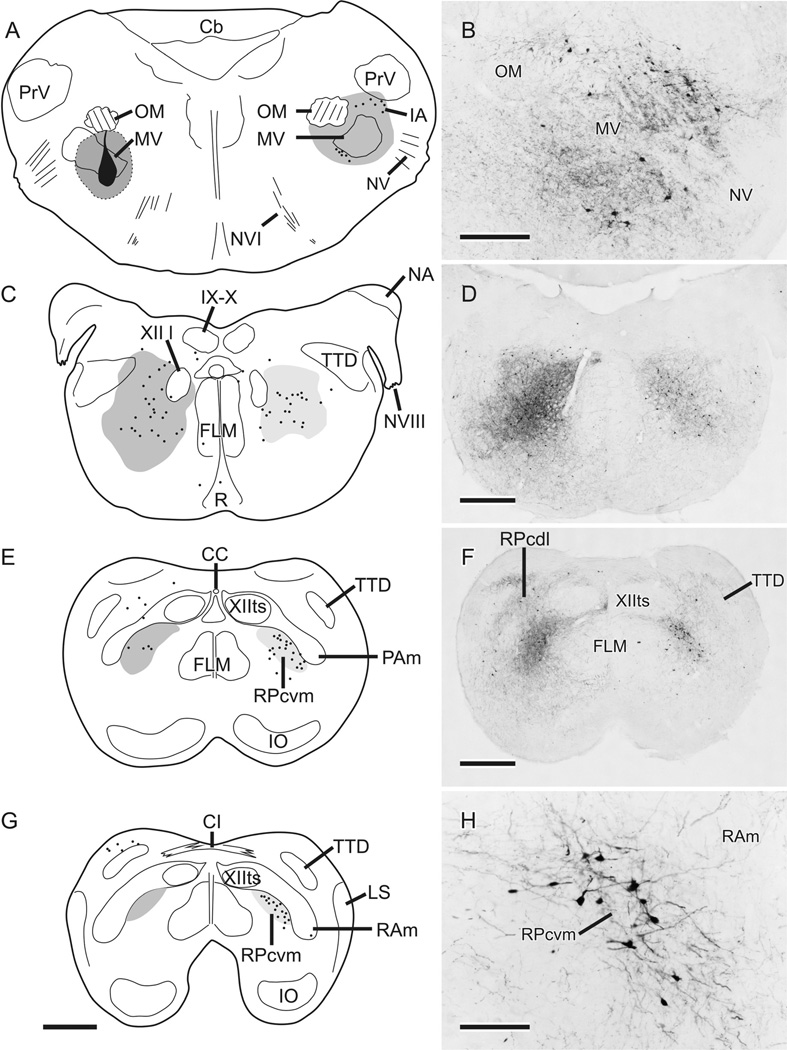
The results from canaries and zebra finches were indistinguishable in terms of the general pattern of retrograde labeling from injections in jaw motor nuclei. Injections were considered to be on target if labeled axons were visible in the exiting trigeminal and facial nerve roots and if labeled neurons were present in the mesencephalic trigeminal nucleus (MesV). Figure 1 charts the distribution within the pons and medulla of retrogradely labeled neurons following a typical injection of BDA centered on MV. In an attempt to involve most of MV, which is a complex nucleus with several subnuclei having various degrees of separation at different rostrocaudal levels, as well as MVIId, there was inevitable spread of tracer beyond the confines of both trigeminal and dorsal facial motor nuclei, but in each case some parts of MV (e.g., lateral MV in the case shown in Fig. 1) were relatively free of label. At rostrocaudal levels of the injection, there were retrogradely labeled neurons in the contralateral intertrigeminal area (IA) and medial to MV (Fig. 1A,B). Farther caudally, throughout the caudal pons and upper medulla, labeled neurons were scattered widely throughout central parts of the tegmentum on both sides (Fig. 1C,D), but, caudal to the level of the eighth nerve, the labeled neurons began to cluster in the lateral parvocellular reticular formation (RPc), especially on the side contralateral to the injection (Fig. 1E,F), finally to be largely confined to the ventromedial part of RPc, i.e., RPcvm (Fig. 1G,H). RPcvm is separated from a dorsolateral part of RPc (RPcdl) by nuclei parambigualis (PAm) and retroambigualis (RAm), which were previously defined in terms of their projections to spinal motor neurons innervating inspiratory or expiratory muscles, respectively (Wild, 1993b; Reinke and Wild, 1998) and by the terminal field of nucleus robustus arcopallialis (RA), the output of the telencephalic song control system (Nottebohm et al., 1976; Wild, 1993a; present study; see also Wild et al., 2009). Thus, in the present study, neurons in the caudal medulla retrogradely labeled from injections in MV/MVII were clustered medially adjacent to PAm and RAm and ventrolateral to XIIts, the vocal motor nucleus, as seen in cresyl violet-counterstained sections and, like PAm and RAm, defined by the RA terminal field (Wild, 1993a). In cases such as that depicted in Figure 1, in which RA was not also injected, the dendrites of many of the labeled neurons in RPcvm could be seen to extend laterally into the unlabeled RA terminal field in PAm and RAm (Fig. 1H).
Figure 1.
A,C,E,G: Schematic representations of retrograde and anterograde labeling resulting from an injection of BDA (shown in black in A) centered on MV but also including MVIId located slightly more caudal to the level depicted. Spread from the injection center is indicated by dark gray shading; dots represent single retrogradely labeled neurons; medium gray shading represents dense fiber and terminal labeling; light gray shading represents light fiber and terminal labeling. B,D,F,H: Photomicrographs of retrograde and anterograde labeling in regions roughly corresponding to those indicated schematically at left. Note the laterally directed processes of some of the retrogradely labeled neurons in RPcvm in H. Scale bars = 500 µm in G (applies to A,C,E,G); 250 µm in B; 500 µm in D,F; 125 µm in H.
In cases receiving an injection of CTB in MV/MVIId, there were many more retrogradely labeled neurons throughout the hindbrain compared with cases with BDA injections. Thus, in addition to retrogradely labeled cells in the locations described above, neurons were labeled dorsolateral to RAm (i.e., in the dorsolateral parvocellular reticular formation, RPcdl; see Fig. 4A), in the nucleus of the descending trigeminal tract (TTD), and in the raphe (not shown).
In those cases that received an injection of BDA in RA combined with an injection of CTB into the contralateral MV/MVIId (e.g., Fig. 4A), PAm and RAm were densely innervated by anterogradely labeled fibers, some of which infiltrated the retrogradely labeled cluster of neurons in RPcvm, but evidence of an RA terminal field in RPcvm was unimpressive.
Anterograde labeling resulting from MV/MVIId injections
A consistent feature of the projections resulting from the injections centered on MV/MVIId was that dense anterograde fiber and terminal labeling was present in and around the contralateral MV (Fig. 1A,B) and, farther caudally, ipsilaterally throughout the central tegmental area of the lower pons and upper medulla (Fig. 1C,D). In the caudal medulla, the dense ipsilateral labeling was concentrated specifically in RPcvm (Fig. 1E – G), whereas much lighter and more restricted anterograde labeling was present contralaterally (Fig. 1C – G). Thus, in the caudal medulla, retrograde cell body labeling was predominantly contralateral to the injection in MV/MVIId, whereas anterograde labeling was predominantly ipsilateral throughout the hindbrain caudal to the injection. The origin of this anterograde labeling was likely trigeminal interneurons inadvertently involved in the injection (see below).
From this series of MV/MVIId injections, some of which were combined with injections in the contralateral RA, it is clear that the cell bodies of putative jaw premotor neurons do not, at least for the most part, receive terminations from descending RA fibers; these course through the hindbrain and terminate massively in XIIts, PAm, and RAm (see also Wild, 1993a,b). However, it is additionally clear that RA projections could gain access to at least some putative jaw premotor neurons, namely, those within RPcvm medially adjacent to PAm and RAm and ventrolaterally adjacent toXIIts, either directly by way of RA axonal terminations on their dendrites within RAm or indirectly via local projections of RAm neurons that receive RA terminations (Wild, 1993a). Which of these scenarios is likely is considered below.
Projections of putative jaw and other upper vocal tract premotor neurons
Efferent projections
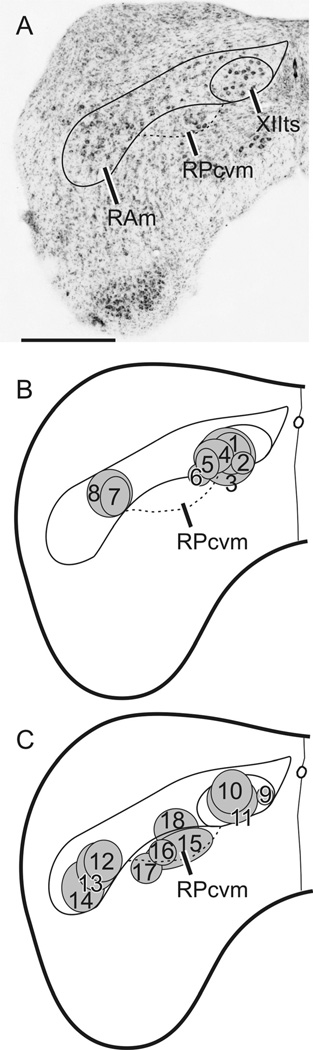
Figure 2 shows the location of 18 injections of BDA, each one in a different case, made into various locations in the caudal medulla. Numbers 15–17 were centered on RPcvm (e.g., Fig. 3A), and number 18 involved RPcvm to a significant extent. All four of these injections resulted in substantial anterograde fiber and terminal labeling of jaw and other upper vocal tract motor nuclei, as depicted in Figure 3.
Figure 2.
A: Nissl-counterstained left hemisection of the caudal medulla in a zebra finch showing XIIts, RAm, and RPcvm. B,C: Schematic representations of the location of 18 injections of BDA, each in a different case and separated into two schematics for illustrative purposes. Scale bar = 500 µm.
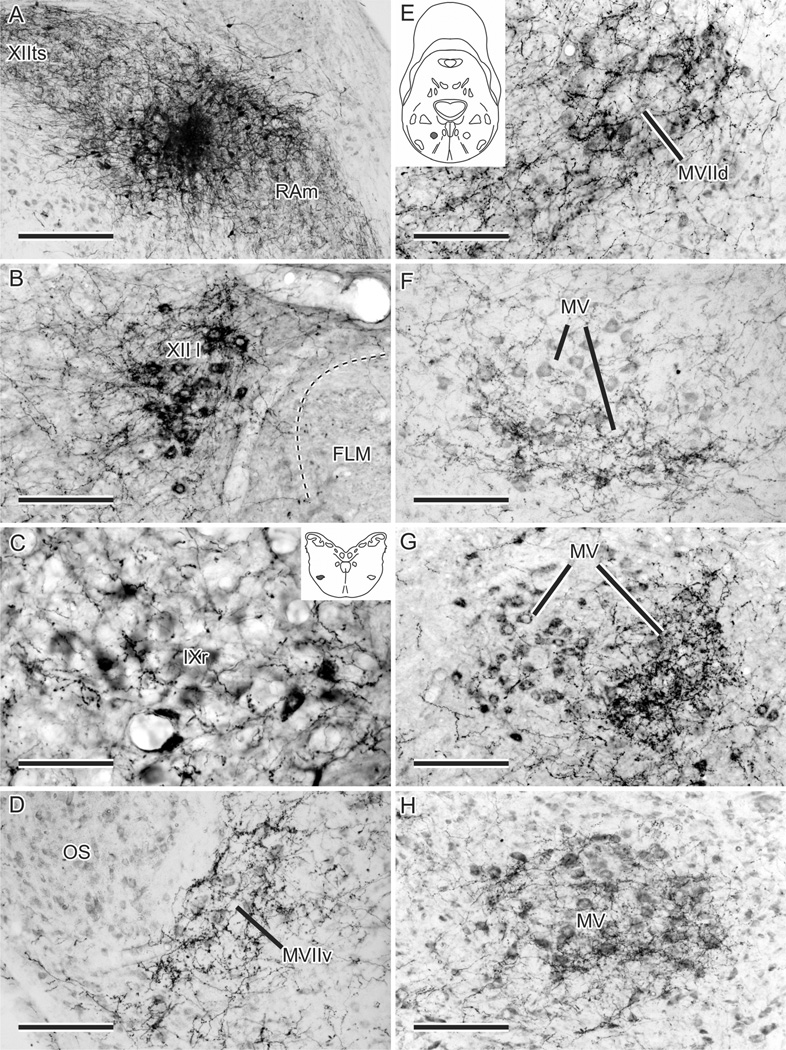
Figure 3.
A–H: Anterograde fiber and terminal labeling in left upper vocal tract motor nuclei resulting from an injection of BDA (A; number 18 in Fig. 2) centered on the right RPcvm, with spread into RAm and intra-axonal transport within RAm and XIIts (see Wild et al., 2009). B,C,G: The motor neurons in XII l, IXr, and MV, respectively, have been retrogradely labeled by CTB injected into relevant muscles (see Materials and Methods). The ventrolateral position of IXr is shaded in the inset in C and similarly for MVIId in the inset in E. Note how the fiber and terminal labeling is concentrated ventrally in MV in F and medially in MV in G but extends throughout much of MV in H. Scale bars = 300 µm in A; 150 µm in B,D–H; 75 µm in C.
Confirming the results of retrograde labeling from MV/ MVIId injections, most projections were contralateral. In ascending order, terminations were first seen in the lingual hypoglossal nucleus (XII l; Fig. 3B), which innervates intrinsic tongue muscles. Next there was labeling around the cell bodies of retrofacial glossopharyngeal motor neurons (IXr) in the ventrolateral caudal pons (Fig. 3C), which innervate the tongue protruder muscle of the hyoid horn. Farther rostrally, there was clear and specific labeling of the ventral facial nucleus (MVIIv; Fig. 3D), which innervates tongue retractor muscles, and less dense labeling of MVIId (Fig. 3E), which innervates the opener (depressor) of the lower jaw. Finally, in different cases, there was specific labeling of different parts of MV (Fig. 3F – H). This did not necessarily indicate subnuclear-specific labeling but rather was the result of injections in RPcvm that inadvertently involved different parts of the nucleus; subnuclear specific projections were not attempted in the present study. The only evidence of the topography of premotor projections was in the rostrocaudal direction, where more rostrally placed injections in RPcvm produced heavier labeling in rostral MV, whereas very caudally placed injections produced terminal fields that were confined to a single, more caudal motor subnucleus, e.g., MVIIv.
Injections such as numbers 3 and 5 (Fig. 2) that were centered ventrolaterally in XIIts with some ventral spread outside the nucleus involving RPcvm, or in the “neck” part of RAm adjacent to XIIts and PRcvm (number 6), somatopetally labeled the cell bodies of RPcvm neurons and produced anterograde (terminal) labeling in several or all of the upper vocal tract motor nuclei (XII l, IXr, VIIv, VIId, and V), always heavier on the contralateral side. In contrast, injections confined to XIIts (numbers 1, 2, 4, and 9–11) did not produce anterograde labeling in upper vocal tract motor nuclei, nor did injections that were centered within and largely confined to the more ventrolateral (i.e., the “body”) parts of RAm (Wild et al; 2009; numbers 7, 8, and 12–14), although these produced massive anterograde labeling throughout the rest of RAm, XIIts, and PAm as well as other respiratory-vocal nuclei in the brainstem (see Wild et al., 2009). Furthermore, these RAm injections did not give rise to any anterograde (terminal) labeling within Rpcvm. Summarizing these results, we can say that any injection that was either centered on or partially involved RPcvm or involved the dendrites of RPcvm neurons within parts of PAm or RAm resulted in the anterograde labeling of one or more of the upper vocal tract motor nuclei, predominantly contralaterally.
Putative sources of RPcvm afferents
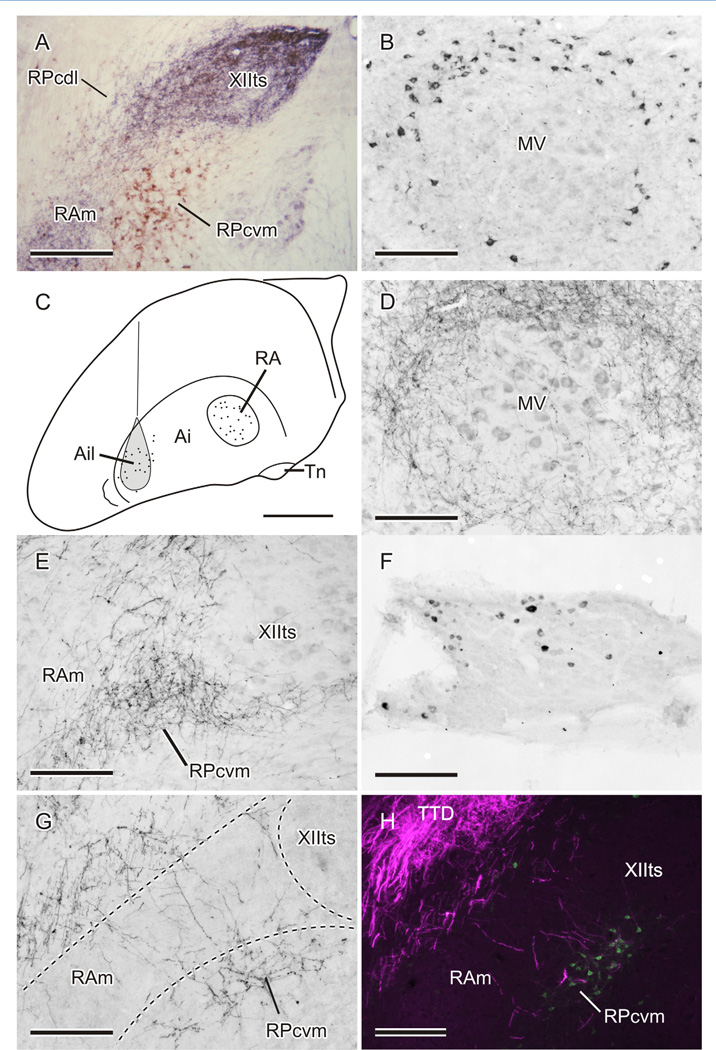
Injections of CTB into RPcvm produced extensive retrograde labeling of neurons throughout the medullary and pontine lateral reticular formation and, at the level of rostral MV, where the nucleus presents a circular cross-section, produced a rim of labeled reticular neurons around the ipsilateral MV (Fig. 4B). Presumably these were the origin of at least some of the anterograde labeling in RPc, including RPcvm, following injections centered on MV, but with spread outside the nucleus (see above). Both CTB and BDA injections in RPcvm also retrogradely labeled neurons in the lateral part of the ipsilateral intermediate arcopallium (Ail; Fig. 4C). In cases in which the injection included RPcvm and adjoining parts of RAm, retrogradely labeled neurons were found both in Ail and in RA (Fig. 4C). In contrast, injections confined to XIIts, PAm, or RAm did not retrogradely label any neurons in the lateral arcopallium, only within the ipsilateral RA and other respiratory-vocal nuclei in the brainstem, as documented previously and hence not shown here (Wild, 1993a;b; Reinke and Wild, 1998; Wild et al., 2001).
Figure 4.
A: Photomicrograph showing anterograde labeling (black) in XIIts and RAm and retrograde labeling (brown) in RPcvm in a canary receiving one injection of BDA into RA and another of CTB into MV/MVIId. B: Retrograde labeling around MV resulting from an injection of CTB in the ipsilateral RPcvm. C: Schematic depiction of retrograde labeling in the arcopallium in different cases: cells (dots) in the lateral (Ail) and medial (RA) arcopallium retrogradely labeled from BDA injections in either RPcvm or RAm/XIIts, respectively. Also shown as shaded in C is the location of a typical injection of BDA in Ail (see text). D,E: Fiber and terminal labeling around MV (D) and in RPcvm (E) following an injection of BDA in Ail, shaded in C. F: Retrogradely labeled neurons in the trigeminal ganglion following an injection of CTB in the ipsilateral RPcvm. G: Fiber and terminal labeling in RPcvm following an injection of BDA in the ipsilateral trigeminal ganglion. H: Fiber and terminal labeling in TTD (red fluorescence, rendered magenta) and fibers crossing through the width of RAm to terminate in relation to retrogradely labeled neurons (green) in RPcvm (see text). Scale bars = 300 µm in A; 150 µm in B,D–G; 1 mm in C; 200 µm in H.
Descending projections from the arcopallium
The descending projections of RA in the present study (e.g., Fig. 4A) were very similar to those previously defined (Nottebohm et al., 1976; Vicario, 1993; Wild, 1993a; Wild et al., 2001). Here we confirm that, although RA terminates densely in XIIts, PAm, and RAm, specific terminal fields in relation to upper vocal tract motor nuclei receiving projections from RPcvm are conspicuous by their absence. Projections from the lateral arcopallium have also been previously documented in finches (Wild and Farabaugh, 1996), but the relation of some of these to jaw premotor neurons was then unknown. In the present study, injections in Ail (Fig. 4C) gave rise to a bilateral, but predominantly ipsilateral, projection that coursed caudally through the brainstem in the occipitomesencephalic tract (OM). Within the pons, OM lies immediately dorsal to MV and gives off projections that surrounded the nucleus (Fig. 4D). Caudal to MV, OM terminates in extensive parts of the lateral brainstem, and particularly in the nuclei of TTD. In the caudal medulla, a contingent of labeled fibers left OM, crossed through the neck of RAm, and terminated specifically in RPcvm (Fig. 4E). Labeled fibers reached the same part of RPcvm on the contralateral side via the commissura infima. No terminations were observed in RPcdl dorsolaterally adjacent to PAm or RAm, although there was a distinct terminal field in the nucleus caudalis of TTD at these levels. Together, the retrograde and anterograde results involving the widely separated arcopallial nuclei RA and Ail strongly suggest that the projections to the medulla are differentiated according to whether they arise either medially (from RA) or laterally (from Ail) and target either respiratory-vocal (XIIts, PAm, and RAm) or jaw and tongue premotor and other trigeminal regions, respectively.
Projections resulting from injections in the trigeminal ganglion
In zebra finches, injections of CTB in RPcvm were also found to label cells retrogradely in the ipsilateral trigeminal ganglion (Fig. 4F), but not in MesV. To confirm these findings, injections of either BDA or CTB were made into the ganglion in six cases, and two of the CTB cases also received an injection of dextran Alexa 488 in MV/MVIId on the same side. A detailed, full description of the ganglionic projections in the zebra finch is unnecessary for the purposes of the present study. Suffice it to say that the injections in the trigeminal ganglion all gave similar results, with major ipsilateral projections to the principal and spinal trigeminal tract nuclei and a minor contralateral component in the trigeminal dorsal horn, as found in pigeons, ducks, and chickens (Dubbeldam and Karten, 1978; Arends and Dubbeldam, 1984; Wild and Zeigler, 1996; Wild et al., 2010). Unlike the case in nonsongbirds (confirmed in the two pigeons receiving ganglionic injections of BDA in the present study), labeled fibers caudal to the obex in zebra finches were observed to leave the descending trigeminal tract ventromedially to cross the dorsomedial (neck) part of RAm and terminate specifically within RPcvm medially adjacent to RAm (Fig. 4G). This was unequivocally visualized in the two fluorescent cases in which jaw premotor neurons within RPcvm were retrogradely labeled from an injection of dextran Alexa 488 in MV/MVIId, combined with an injection of CTB into the trigeminal ganglion, visualized with a direct secondary Alexa 568 antibody (Fig. 4H). Thus, though examined in different cases, the terminal field resulting from injections in the lateral arcopallium and that resulting from injections in the trigeminal ganglion appeared to overlap in RPcvm. However, because ganglionic injections result in uptake of tracer by both cell bodies and fibres, the terminal field in RPcvm could in principle have resulted from the labeling MesV (proprioceptive) neurons that innervate jaw closer muscles (Arends and Dubbeldam, 1982; Bout et al., 1997). These neurons were invariably labeled by ganglionic injections, but in ducks they are known to project upon RPcdl and not upon RPcvm (Bout et al., 1997). In any case, because injections in RPcvm did not retrogradely label MesV neurons, but did retrogradely label ganglionic neurons (see above), it can be deduced that the source of the terminal field in RPcvm following ganglionic injections is, in fact, ganglionic neurons. In an attempt to discover the specific source of these afferents, injections of CTB were made into trigeminal sensory nerve branches or territories or into the jaw muscles (see Materials and Methods), but in none of these cases were projections upon RPcvm observed, although dense terminal fields were observed throughout the topographic territories of these nerve branches in the nucleus of the descending trigeminal tract, as in other birds (Wild and Zeigler, 1980; Wild et al., 2010).
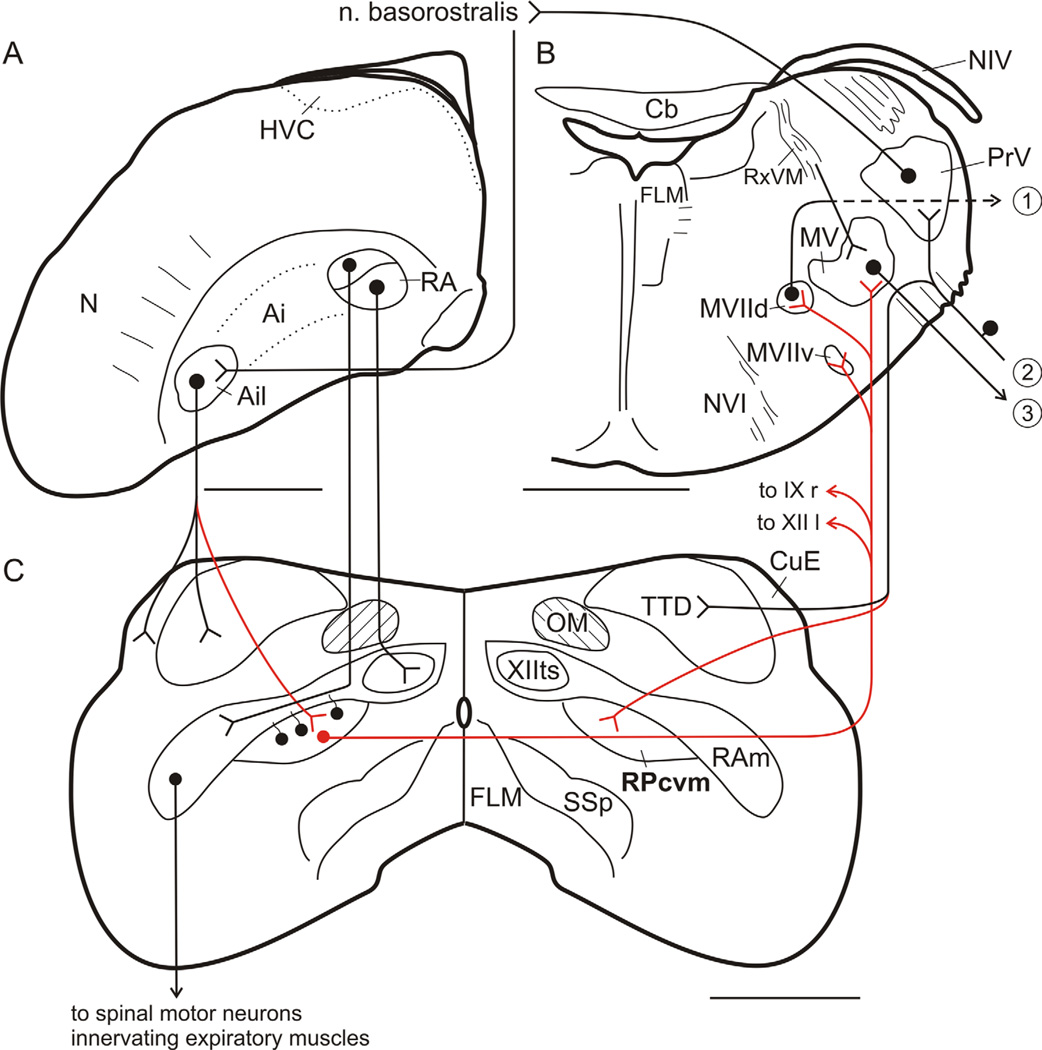
Figure 5 summarizes the pathways defined in the present study in the context of related circuitry previously described, and Figure 6 schematically illustrates the direct and indirect, predominantly ipsilateral projections of Ail upon RPcvm and the predominantly contralateral projections of RPcvm upon MV/MVIId.
Figure 5.
Circuit diagram showing inputs and outputs of jaw and other upper vocal tract premotor neurons in RPcvm in the canary and zebra finch. Dots represent neuronal cell bodies; inverted arrowheads represent terminations; red lines indicate projections identified in the present study. A: Left caudal hemisphere. HVC controls song via its output to RA. B: Right hemisection through the pons. 1, Output to opener muscle of the lower jaw; 2, sensory inputs arriving over the trigeminal nerve. 3, output to jaw closer muscles of the lower jaw and the opener muscle of the upper jaw. C: Caudal medulla. Rostral telencephalic levels of the circuitry are represented by nucleus basoros-tralis, which receives a major input from PrV and projects indirectly upon Ail. See text and Literature Cited. For clarity, some crossed projections, e.g., of Ail, have not been included. Scale bars = 1 mm.
Figure 6.
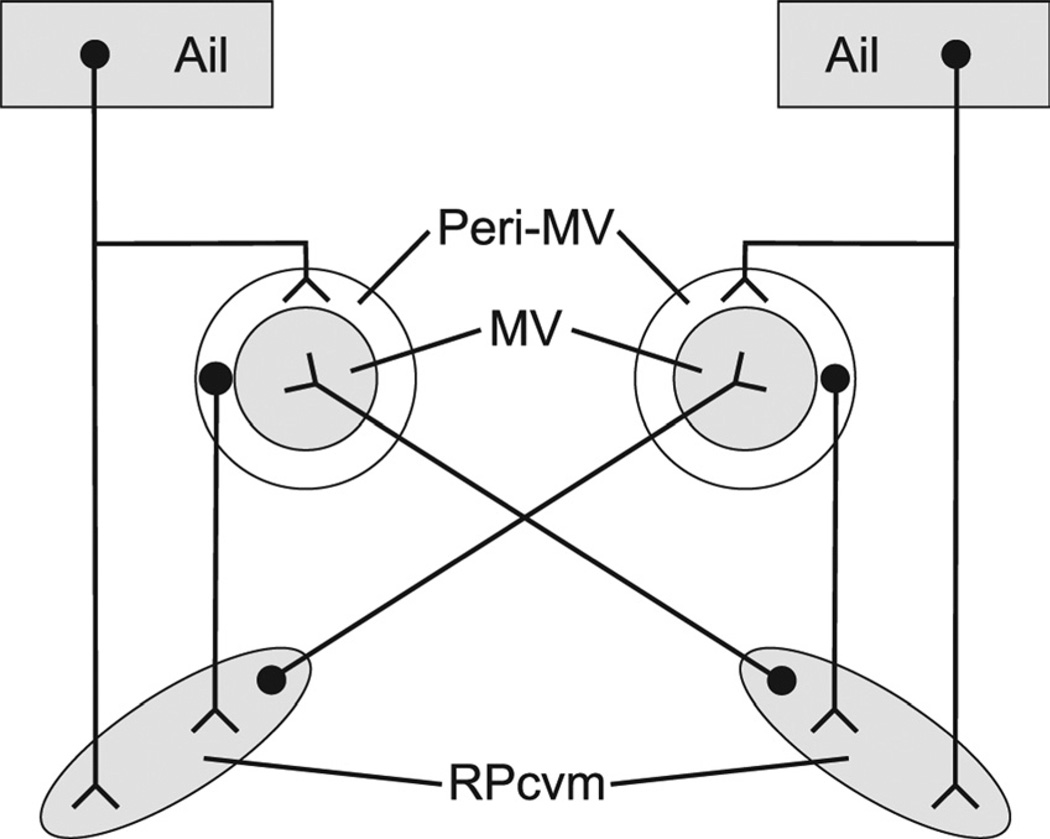
Simplified schematic illustrating the predominantly ipsi-lateral projections of Ail upon the peri-MV region and upon RPcvm, the predominantly ipsilateral projections of peri-MV upon RPcvm, and the predominantly contralateral projections of RPcvm upon MV. The weaker contralateral projections of Ail and peri-MV and the weaker ipsilateral projections of RPcvm have been omitted for clarity.
DISCUSSION
The purpose of the present study was to identify neural circuitry that could account for the positive correlation observed between beak gape and sound frequency during singing. The findings show that the only likely contact between the respiratory-vocal and jaw control systems is at the level of the caudal medulla. There, a group of jaw and other upper vocal tract premotor neurons in RPcvm was identified in proximity to the respiratory-vocal premotor nuclei RAm and PAm. Many neurons in RPcvm had dendrites that extended dorsally and laterally into RAm, where they could receive axodendritic contacts either directly from RA and/or indirectly by way of local RAm neurons that receive RA projections (Wild, 1993a). No evidence from anterograde tracing studies was found for direct projections from either PAm or RAm to RPcvm cell bodies lying medially adjacent to PAm and RAm (Wild et al., 2009; present study).
Although premotor neurons in RPcvm apparently project to several motor nuclei, in addition to those of MV and MVIId that innervate jaw closer and opener muscles, viz., XII l, which innervates the intrinsic tongue muscles (Wild and Zeigler, 1980; Wild, 1981, 1990); MVIIv, which innervates extrinsic tongue retractor muscles (Wild and Zeigler, 1980; Arends and Dubbeldam, 1982; Bout, 1987; Dubbeldam and Bout, 1990); and IXr, which innervates the tongue protractor muscle, M. geniohyoideus (Wild, 1981; Bout, 1987), the only trigeminal motor subnucleus that did not appear to receive a projection from RPcvm was that innervating the muscles of the lower eyelid (den Boer et al., 1986; Wild, 1999). Thus, the jaw muscle motor nuclei receive the heaviest innervation from RPcvm, in keeping with the vital role of the jaw muscles in feeding, preening, and gape modulation during vocalization. The roles in vocalization, if any, of muscles acting on the tongues of birds await electromyographic elucidation, but M geniohyoideus may be involved in changing the shape and size of the oropharynx and upper part of the esophagus by way of its control of the movements of the hyoid apparatus (Homberger, 1999; Riede et al., 2006). The role of the larynx in songbird vocalizations is unknown, although Homberger (1999) has suggested that the larynx pl.ays “a crucial role as a modifiable resonating cavity for vocalizing birds” (p 4), a position receiving some support from the suggestion that laryngeal motor neurons in songbirds receive a direct projection from RA (Wild, 1993a). As a corollory, a dense projection from RPcvm upon nucleus ambiguus, which innervates the laryngeal muscles, was not found in the present study.
For finches, our findings with respect to the projections of RPcvm upon jaw and tongue motor nuclei suggested a crude rostrocaudal topography or functionally differentiated pattern of projections of jaw and tongue premotor nuclei. In the pigeon, no evidence of premotor topography was found on the basis of EMG-controlled injections of HRP into the jaw motor subnuclei (Berkhoudt et al., 1982), and, in the mallard (also a nonsongbird, like pigeons), neurons in a single premotor area could innervate either functionally agonistic or functionally antagonistic motor subnuclei (Bout and Dubbeldam, 1994). Similarly in monkeys and rats, anterograde and double retrograde labeling studies also suggest the absence of a clearcut topographic organization of premotor projections (Thoms and Ju¨rgens, 1987; Li et al., 1993). These observations could suggest either that the premotor organization as depicted in the various studies reflects a real situation that likely underlies the concurrent physiological activation of several different motor nuclei and subnuclei involved in the control of feeding and vocalization or that the methods employed are inadequate to the task of revealing a more detailed organization of premotor neurons and their projections involved in these complex behaviors.
Predominantly contralateral RPcvm efferents and ipsilateral afferents
In the zebra finch, the projections of jaw premotor neurons were bilateral but distinctly heavier on the contralateral side, a pattern opposite that found for the ascending projections of RAm (Wild et al., 2009). A contralateral predominance of jaw premotor projections was not observed in the mallard (Bout, 1987; Bout and Dubbeldam, 1994), and, in cats and rats, the premotor projections are primarily bilateral with an ipsilateral predominance (Holstege et al., 1977; Li et al., 1993, 1995; Yamamoto et al., 2007). In finches, the pattern of predominantly crossed projections of premotor nuclei (RPcvm) upon upper vocal tract motor nuclei stands in contrast to the predominantly ipsilateral projections of peri-MV interneurons upon RPcvm. It is interesting, too, that the ipsilateral descending projections from Ail encircle MV in a manner very similar to the distribution of neurons around MV retrogradely labeled from injections in the ipsilateral RPcvm (see Fig. 4B,D). Further experiments are required to determine whether these Ail projections actually target these peri-MV RPcvm-projecting neurons and to determine the functional nature of this interesting pattern of ascending, predominantly crossed projections of premotor neurons and their predominantly ipsilateral descending inputs from peri-MV interneurons. These ipsi- and contralateral projections to and from RPcvm are schematically illustrated in Figure 6.
Comparison with mallards
In the mallard, premotor neurons for jaw and tongue motor subnuclei are distributed throughout two longitudinal columns of the parvocellular reticular formation, dorsolateral (RPcdl) and ventromedial (RPcvm; Arends and Dubbeldam, 1982; Bout, 1987; Bout and Dubbeldam, 1994; Dubbeldam, 1998). Jaw opener subnuclei of the MV/VIId complex receive projections both from the caudal part of RPcdl and from RPcvm, whereas the jaw closer (adductor) and pterygoid subnuclei of MV receive projections both from the rostral part of RPcdl (i.e., the intertrigeminal area; Dubbeldam, 1998) and from RPcvm (Bout, 1987; Bout and Dubbeldam, 1994). A comparison of the present results with those in the mallard is complicated by the fact that PAm and RAm have not been identified in the mallard, whereas, in finches, these respiratory-vocal nuclei appear to separate RPcvm ventromedially from RPcdl dorsolaterally. Also, in the mallard, the projections of the respiratory-vocal dorsomedial nucleus of the intercollicular complex (DM) has not been defined, although in another nonsongbird (pigeon) the terminal field of DM appears identical to that of RA in songbirds (Wild et al., 1997a). If a similar situation holds true for the projections of DM in mallards, we can suggest that, in finches, the RPcvm described here likely corresponds to a caudal part of RPcvm in the mallard (and to a similar region in pigeons; Berkhoudt et al., 1982), whereas the caudal part of RPcdl of mallards probably includes a region in finches and other birds that lies dorsolateral to PAm and RAm and medial to the spinal trigeminal nucleus (Figs. 1F, 4A). The equivalent of the rostral part of RPcdl in mallards seems likely to be the intertrigeminal area (AI) in finches, because there were retrogradely labeled neurons in this region following injections in the (contralateral) jaw motor nuclei (see Fig. 1B). That is, AI is probably a rostral component of the premotor input to jaw motor neurons. In the present study, BDA injections in the jaw motor nuclei retrogradely labeled very few neurons dorsolateral to PAm and RAm (Fig. 1), unlike CTB injections, and BDA injections in RPcvm tended to produce less heavy terminal labeling in MVIId than in MV (see Results). With reference to the situation in mallards, these observations could suggest that our BDA injections in the motor nuclei were more concentrated in jaw closer than in jaw opener subnuclei and that our CTB injections, which tended to be larger than our BDA injections, were more likely to have involved MVIId.
Trigeminal ganglionic input to RPcvm
Another similarity between RPcvm in mallards and finches is that in neither is there a projection from MesV (Bout, 1987; present study), unlike the case for RPcdl (Bout, 1987). However, a distinct difference in the RPcvm between finches and mallards (and other nonsongbirds such as pigeons; Wild and Zeigler, 1996; present results) is that, in finches, RPcvm receives a projection from ganglionic neurons. The peripheral structures innervated by these ganglionic neurons were not identified by injecting sensory nerve branches, nerve territories, or muscles, but the specificity of their central projections upon jaw premotor nuclei nevertheless supports the idea of a proprioceptive sensorimotor circuit for the control of gape in singing birds. Previous discussions of the role of a jaw proprioceptive input in the control of gape have considered MesV input only in the context of feeding (Dubbeldam, 1984; Bout and Dubbeldam, 1991), and jaw joint proprioceptors have been recorded in the spinal trigeminal nucleus in pigeons (Silver and Witkovsky, 1973), yet MesV inputs do not appear to project upon RPcvm in either ducks or finches, so the ganglionic projections upon RPcvm in songbirds presumably convey information of a different kind and from a source other than jaw closer muscle spindles, possibly from jaw joint receptors. Whether the circuit described in the present study is specifically involved in the control of gape during singing is a crucial but unanswered question. However, the close relation of RPcvm and RAm in songbirds described here, the absence of a ganglionic projection to RPcvm in nonsongbirds such as pigeons and ducks (Arends and Dubbeldam, 1984; Wild and Zeigler, 1996; present study), and the closed beak vocalizations of doves (Riede et al., 2004) support the possibility of proprioceptive gape modulation in songbirds.
Descending influences from the lateral arcopallium
RPcvm in both zebra finches and ducks also receives descending projections from the lateral arcopallium (Ail; Wild and Farabaugh, 1996; Dubbeldam et al., 1997; present study) and in both species the lateral arcopallium receives indirect projections from nucleus basorostralis (Bas; Dubbeldam and Visser, 1987; Wild and Farabaugh, 1996). Bas is the direct forebrain recipient of a major ascending sensory system that originates in the beak and principal sensory trigeminal nucleus, traverses the forebrain, and terminates in the trigeminal brainstem complex and in part on jaw premotor nuclei (Wallenberg, 1903; Dubbeldam et al., 1981; Wild et al., 1985, 1997b, 2001; Wild and Farabaugh, 1996). Thus, this long loop circuitry provides for the telencephalic modulation of basic brainstem circuitry involved in the control of beak movements in relation to sensory input, e.g., during feeding (Berkhoudt et al., 1982; Wild et al., 1985). Indeed, in pigeons, lesions of the lateral arcopallium have been found to produce deficits in grasp efficiency and pecking accuracy (Levine and Zeigler, 1981). In the present study, a potentially new finding was that Ail projects upon RPcvm both directly and indirectly via the peri-MV region (see Fig. 6). The functional significance of this pattern of projections is presently unknown, but, if Ail has access, via these projections, to the dendrites of ipsilateral MV motor neurons, in addition to ipsilateral premotor neurons in RPcvm (and in AI), which then project predominantly to the contralateral MV, then Ail could play an important role in the bilateral coordination of jaw muscle activity. Another possibility, however, is that the descending projections from Ail mediate auditory feedback during singing, because the intermediate lateral lemniscal nucleus, which receives a dedicated input from the cochlear nuclei (Kru¨tzfeldt et al., 2010), has a distinct projection upon an auditory component of Bas, the output of which is relayed to Ail through the telencephalon in a manner similar to the somatosensory projections of Bas (Wild and Farabaugh, 1996). Whether lesions of the lateral arcopallium in songbirds would interfere with the relation of gape size to sound frequency during singing is, therefore, an interesting question, although Goller and Cooper (2005) found that zebra finches whose acoustic output had been experimentally altered retained a normal pattern of beak movements during singing. Perhaps auditory feedback is used during development to establish the pattern of beak movements associated with sound frequencies in adult song (cf. Podos et al., 1995). In the present context, it is pertinent to note that there appear to be no connections between either the trigeminal or the Bas auditory sensorimotor system and the song control system at forebrain levels. Any telencephalic modulation of gape during singing, therefore, must be effected at brainstem levels, possibly via the direct or indirect connections of RA axons with the dendrites of jaw premotor neurons in RAm.
In any case, descending systems such as those originating in RA or the lateral arcopallium are likely to affect motor output by modulating the activity in reflex circuits in the brainstem, a theme common to the tunes called by upper motor neurons (Dubbeldam, 1984; Zeigler, 1989). The feeding circuit takes developmental and possibly evolutionary precedence over a vocalization circuit in which gape modulation may be used for a variety of acoustic purposes (Larsen and Dabelsteen, 1990; Westneat et al., 1993; Suthers and Goller, 1997; Nelson et al., 2005). As shown by Podos et al. (1995), the correlation between gape and sound frequency in songbirds develops after self-feeding is well under way and then only with the advent of tonality, so perhaps, just as respiratory circuitry in the brainstem has been co-opted by a telencephalic (RA) or a midbrain (DM) center for the control of vocalization (Sturdy et al., 2003; Wild et al., 1997a), the feeding circuit might have been co-opted by the same vocal control centers for the modulation of gape during vocalization in general and singing in particular. It is perhaps not surprising, therefore, to find that beak gape in zebra finches is positively correlated with respiratory (air sac) pressure, which itself is a function of RAm activity and, in the words of Goller et al. (2004) “suggests a ‘reflexive’ interaction between respiratory and mandibular control.”
Interclass comparative comments
Dubbeldam (1998) has commented on the comparative nature of the control of the jaw, especially as this relates to feeding, but the linked premotor control of structures used in both feeding and vocalization in mammals was indicated by the report of Holstege (1989) that in cats injections of tritiated amino acids into nucleus retroambiguus (NRA) led to the labeling not only of expiratory-related motor nuclei in the spinal cord but also of mouth opening, facial, lingual, pharyngeal, and possibly laryngeal motor nuclei as well. The similarity of NRA in mammals to RAm birds has been noted, in terms of both their relative positions and some of their projections, especially those from the midbrain (Gerrits and Holstege, 1996; Reinke and Wild, 1997; Wild et al., 1997a, 2009; Vanderhorst et al., 2000), but in cats the failure of NRA chemical stimulation to pattern orofacial activity appropriately during vocalization (Zhang et al., 1992; Davis et al., 1996) suggests that the relation of NRA to articulatory (orofacial) premotor and motoneurons is not as direct as is that of NRA to vocal (laryngeal) motoneurons (Holstege, 1989; Vanderhorst et al., 2001; Boers et al., 2002). For the zebra finch, the predominant location of jaw premotor neurons adjacent to, but not within, RAm and their dendritic extensions into the RA (and DM) terminal fields within RAm supply a comparative comment on this situation and underscore the basic similarity in the structural and functional organization of premotor control of vocalization and its jawed articulation in birds and mammals.
A more detailed investigation of this similarity, however, awaits studies in birds like the studies of Hage and Ju¨rgens (2006a,b) in squirrel monkeys, in which a region in the ventrolateral pons has been characterized physiologically as a vocal pattern generator, specifically for FM calls. This VOC region, as it is called, lies immediately dorsal to the superior olivary complex; receives projections from the PAG; and projects upon jaw, facial, and laryngeal motor nuclei. In birds, a region in a comparable position is occupied in part by jaw opener and tongue retractor facial motor neurons, but it does not receive a specific projection from DM, the proposed equivalent of the vocally related part of PAG, and it was not identified in the present study as a specific source of projections upon jaw motor nuclei. The possible presence and location of premotor neurons specifically encoding FM calls or song syllables in birds would nevertheless be interesting to determine.
Acknowledgments
Grant sponsor: Royal Society of New Zealand Marsden Fund (to J.M.W.); Grant sponsor: National Institutes of Health; Grant number: RO1 NSo29467 (to R.A. Suthers and J.M.W.).
Abbreviations
- Ai
intermediate arcopallium
- Ail
lateral part of intermediate arcopallium
- Cb
cerebellum
- CI
commissura infima
- CuE
external cuneate nucleus
- FLM
medial longitudinal fasciculus
- HVC
HVC abbreviation used as a proper noun
- IA
intertrigeminal area
- IO
inferior olive
- LS
spinal lemniscus
- MV
trigeminal motor nucleus
- MVi
main (intermediate) trigeminal motor nucleus
- MVIId
dorsal subnucleus of the facial motor nucleus
- MVIIi
intermediate subnucleus of the facial motor nucleus
- MVIIv
ventral subnucleus of the facial motor nucleus
- NA
nucleus angularis
- N
nidopallium
- NIV
trochlear nerve
- NV
trigeminal nerve
- NVI
abducent nerve
- NVIII
eighth (vestibulocochlear) nerve
- OM
occipitomesencephalic tract
- OS
superior olive
- Pam
parambigual nucleus (parambigualis)
- PrV
principal trigeminal sensory nucleus
- R
raphe nucleus
- RA
robust nucleus of the arcopallium
- RAm
retroambigual nucleus (retroambigualis)
- RPc
parvocellular (lateral) part of reticular formation
- RPcdl
dorsolateral part of RPc
- RPcvm
ventromedial part of RPc
- RxVM
radix of mesencephalic trigeminal nerve
- SSp
supraspinal nucleus
- TBC
trigeminal brainstem complex
- Tn
taeniael nucleus of the amygdale
- TTD
nucleus and tract of the descending trigeminal nerve
- IXr
retrofacial glossopharyngeal motor nucleus
- IX–X
dorsal glossopharyngeal-vagal nucleus
- XII l
lingual part of hypoglossal nucleus
- XIIts
tracheosyringeal part of hypoglossal nucleus
LITERATURE CITED
- Arends JJA, Dubbeldam JL. Exteroceptive and proprioceptive afferents of the trigeminal and facial motor nuclei in the mallard (Anas platyrhynchos L.) J Comp Neurol. 1982;209:313–329. doi: 10.1002/cne.902090309. [DOI] [PubMed] [Google Scholar]
- Arends JJA, Dubbeldam JL. The subnuclei and primary afferents of the descending trigeminal system in the mallard (Anas platyrhynchos L.) Neuroscience. 1984;13:781–795. doi: 10.1016/0306-4522(84)90096-4. [DOI] [PubMed] [Google Scholar]
- Beckers GJ, Nelson BS, Suthers RA. Vocal-tract filtering by lingual articulation in a parrot. Curr Biol. 2004;14:1592–1597. doi: 10.1016/j.cub.2004.08.057. [DOI] [PubMed] [Google Scholar]
- Berkhoudt H, Klein BG, Zeigler HP. Afferents to the trigeminal and facial motor nuclei in pigeon (Columba livia L.): central connections of jaw motoneurons. J Comp Neu-rol. 1982;209:301–312. doi: 10.1002/cne.902090308. [DOI] [PubMed] [Google Scholar]
- Bock W. Kinetics of the avian skull. J Morphol. 1964;114:1–41. [Google Scholar]
- Boers J, Klop EM, Hulshoff AC, de Weerd H, Holstege G. Direct projections from the nucleus retroambiguus to cricothyroid motoneurons in the cat. Neurosci Lett. 2002;319:5–8. doi: 10.1016/s0304-3940(01)02395-3. [DOI] [PubMed] [Google Scholar]
- Bout RG. Neuroanatomical circuits for proprioceptive and motor control of feeding movements in the mallard (Anas platyrhynchos L.) PhD thesis, Leiden University; 1987. [Google Scholar]
- Bout RG, Dubbeldam JL. Functional morphological interpretation of the distribution of muscle spindles in the jaw muscles of the mallard (Anas platyrhynchos) J Morphol. 1991;210:215–226. doi: 10.1002/jmor.1052100302. [DOI] [PubMed] [Google Scholar]
- Bout RG, Dubbeldam JL. The reticular premotor neurons of the jaw muscle motor nuclei in the mallard (Anas platyr hynchos L.) Eur J Morphol. 1994;32:134–137. [PubMed] [Google Scholar]
- Bout RG, Tellegen AJ, Dubbeldam JL. Central connections of the nucleus mesencephalicus nervi trigemini in the mallard (Anas platyrhynchos L.) Anat Rec. 1997;248:554–565. doi: 10.1002/(SICI)1097-0185(199708)248:4<554::AID-AR7>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Daley M, Goller F. Tracheal length changes during zebra finch song and their possible role in upper vocal tract filtering. J Neurobiol. 2004;59:319–330. doi: 10.1002/neu.10332. [DOI] [PubMed] [Google Scholar]
- Davis PJ, Zhang SP, Winkworth A, Bandler R. Neural control of vocalization: respiratory and emotional influences. J Voice. 1996;10:23–38. doi: 10.1016/s0892-1997(96)80016-6. [DOI] [PubMed] [Google Scholar]
- den Boer PJ, Bout RG, Dubbeldam LJ. Topographical representation of the jaw muscles within the trigeminal motor nucleus. An HRP study in the mallard, Anas platyrhynchos . Acta Morphol Neerl Scand. 1986;24:1–17. [PubMed] [Google Scholar]
- Dubbeldam JL. Brainstem mechanisms for feeding in birds: interaction or plasticity. Brain Behav Evol. 1984;25:85–98. doi: 10.1159/000118854. [DOI] [PubMed] [Google Scholar]
- Dubbeldam JL. The neural substrate for “learned” and “nonlearned” activities in birds: a discussion of the organization of bulbar reticular premotor systems with side-lights on the mammalian situation. Acta Anat. 1998;163:157–172. doi: 10.1159/000046494. [DOI] [PubMed] [Google Scholar]
- Dubbeldam JL, Bout RG. The identification of the motor nuclei innervating the tongue muscles in the mallard (Anas platyrhynchos): an HRP study. Neurosci Lett. 1990;119:223–227. doi: 10.1016/0304-3940(90)90839-2. [DOI] [PubMed] [Google Scholar]
- Dubbeldam JL, Karten HJ. The trigeminal system in the pigeon (Columba livia). I. Projections of the gasserian ganglion. J Comp Neurol. 1978;180:661–678. doi: 10.1002/cne.901800402. [DOI] [PubMed] [Google Scholar]
- Dubbeldam JL, Visser AM. The organization of the nucleus basalis-neostriatum complex of the mallard (Anas platyrhynchos L.) and its connections with the archistriatum and the paleostriatum complex. Neuroscience. 1987;21:487–517. doi: 10.1016/0306-4522(87)90137-0. [DOI] [PubMed] [Google Scholar]
- Dubbeldam JL, Brauch CS, Don A. Studies on the soma-totopy of the trigeminal system in the mallard, Anas platyrhynchos L. III. Afferents and organization of the nucleus basalis. J Comp Neurol. 1981;196:391–405. doi: 10.1002/cne.901960304. [DOI] [PubMed] [Google Scholar]
- Dubbeldam JL, den Boer-Visser AM, Bout RG. Organization and efferent connections of the archistriatum of the mallard, Anas platyrhynchos L: an anterograde and retrograde tracing study. J Comp Neurol. 1997;388:632–657. doi: 10.1002/(sici)1096-9861(19971201)388:4<632::aid-cne10>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Gerrits PO, Holstege G. Pontine and medullary projections to the nucleus retroambiguus: a wheat germ agglutinin-horseradish peroxidase and autoradiographic tracing study in the cat. J Comp Neurol. 1996;373:173–185. doi: 10.1002/(SICI)1096-9861(19960916)373:2<173::AID-CNE2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Goller F, Cooper BG. Altered acoustic feedback does not affect patterns of beak movement during song in the zebra finch. Soc Neurosci Abstr. 2005;1002:13. [Google Scholar]
- Goller F, Mallinckrodt MJ, Torti SD. Beak gape dynamics during song in the zebra finch. J Neurobiol. 2004;59:289–303. doi: 10.1002/neu.10327. [DOI] [PubMed] [Google Scholar]
- Greenwalt C. Bird song: acoustics and physiology. Washington, DC: Smithsonian Institution Press; 1968. [Google Scholar]
- Hage SR, Jürgens U. Localization of a vocal pattern generator in the pontine brainstem of the squirrel monkey. Eur J Neurosci. 2006a;23:840–844. doi: 10.1111/j.1460-9568.2006.04595.x. [DOI] [PubMed] [Google Scholar]
- Hage SR, Jürgens U. On the role of the pontine brainstem in vocal pattern generation: a telemetric single-unit recording study in the squirrel monkey. J Neurosci. 2006b;26:7105–7115. doi: 10.1523/JNEUROSCI.1024-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausberger M, Black JM, Richard JP. Bill opening and sound spectrum in barnacle goose loud calls: individuals with “wide mouths” have higher pitched voices. Anim. Behav. 1991;42:319–322. [Google Scholar]
- Hoese WJ, Podos J, Boetticher NC, Nowicki S. Vocal tract function in birdsong production: experimental manipulation of beak movements. J Exp Biol. 2000;203:1845–1855. doi: 10.1242/jeb.203.12.1845. [DOI] [PubMed] [Google Scholar]
- Holstege G. Anatomical study of the final common pathway for vocalization in the cat. J Comp Neurol. 1989;284:242–252. doi: 10.1002/cne.902840208. [DOI] [PubMed] [Google Scholar]
- Holstege G, Kuypers HGJM, Dekker JJ. The organization of the bulbar fibre connections to the trigeminal, facial and hypoglossal motor nuclei. II. An autoradiogrtaphic tracing study in cat. Brain. 1977;100:265–286. [PubMed] [Google Scholar]
- Homberger DG. The avian tongue and larynx: multiple functions in nutrition and vocalisation. In: Adams NJ, Slo-tow RH, editors. Proc 22 Int Ornithol Congr, Durban. BirdLife South Africa: Johannesburg; 1999. pp. 94–113. [Google Scholar]
- Krützfeldt NOE, Logerot P, Kubke MF, Wild JM. Connections of the auditory brainstem in a songbird, Taeniopygia guttata. II. Projections of nucleus angularis and nucleus laminaris to the superior olive and lateral lemniscal nuclei. J Comp Neurol. 2010;518:2135–22148. doi: 10.1002/cne.22324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubke MF, Ross JM, Wild JM. Vagal innervation of the air sacs in a songbird, Taenopygia guttata . J Anat. 2004;204:283–292. doi: 10.1111/j.0021-8782.2004.00286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubke MF, Yazaki-Sugiyama Y, Mooney R, Wild JM. Physiology of neuronal subtypes in the respiratory-vocal integration nucleus retroambigualis of the male zebra finch. J Neurophysiol. 2005;94:2379–2390. doi: 10.1152/jn.00257.2005. [DOI] [PubMed] [Google Scholar]
- Larsen ON, Dabelsteen T. Directionality of blackbird vocalization, implications for vocal communication and its further study. Ornis Scand. 1990;21:37–45. [Google Scholar]
- Levine RR, Zeigler HP. Extratelencephalic pathways and feeding behavior in the pigeon (Columba livia) Brain Behav Evol. 1981;19:56–92. doi: 10.1159/000121634. [DOI] [PubMed] [Google Scholar]
- Li Y-Q, Takada M, Mizuno N. Identification of premotor interneurons which project bilaterally to the trigeminal motor, facial or hypoglossal nuclei: a fluorescent retrograde double-labeling study in the rat. Brain Res. 1993;611:16–164. doi: 10.1016/0006-8993(93)91789-u. [DOI] [PubMed] [Google Scholar]
- Li Y-Q, Takada M, Kaneko T, Mizuno N. Premotor neurons for trigeminal motor nucleus neurons innervating the jawclosing and jaw-opening muscles: differential distribution in the lower brainstem of the rat. J Comp Neurol. 1995;356:563–579. doi: 10.1002/cne.903560407. [DOI] [PubMed] [Google Scholar]
- Manogue KR, Paton JA. Respiratory gating of activity in the avian vocal control system. Brain Res. 1982;247:383–387. doi: 10.1016/0006-8993(82)91265-3. [DOI] [PubMed] [Google Scholar]
- Nelson BS, Beckers GJ, Suthers RA. Vocal tract filtering and sound radiation in a songbird. J Exp Biol. 2005;208:297–308. doi: 10.1242/jeb.01378. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Stokes TM, Leonard CM. Central control of song in the canary, Serinus canarius . J Comp Neurol. 1976;165:457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- Nowicki S, Marler P. How do birds sing? Music Perception. 1988;5:391–426. [Google Scholar]
- Podos J, Sherer JK, Peters S, Nowicki S. Ontogeny of vocal-tract movements during song production in song sparrows. Anim Behav. 1995;50:1287–1296. [Google Scholar]
- Reinke H, Wild JM. Distribution and connections of inspiratory premotor neurons in the brainstem of the pigeon (Columba livia) J Comp Neurol. 1997;379:347–362. [PubMed] [Google Scholar]
- Reinke H, Wild JM. Identification and connections of inspiratory premotor neurons in songbirds and budgerigar. J Comp Neurol. 1998;391:147–163. [PubMed] [Google Scholar]
- Riede T, Beckers GJ, Blevins W, Suthers RA. Inflation of the esophagus and vocal tract filtering in ring doves. J Exp Biol. 2004;207:4025–4036. doi: 10.1242/jeb.01256. [DOI] [PubMed] [Google Scholar]
- Riede T, Suthers RA, Fletcher NH, Blevins WE. Songbirds tune their vocal tract to the fundamental frequency of their song. Proc Natl Acad Sci U S A. 2006;103:5543–5548. doi: 10.1073/pnas.0601262103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver R, Witkovsky P. Functional characteristics of single units in the spinal trigeminal nucleus of the pigeon. Brain Behav Evol. 1973;8:287–303. doi: 10.1159/000124359. [DOI] [PubMed] [Google Scholar]
- Stocker SD, Simmons JR, Stornetta RL, Toney GM, Guyenet PG. Water deprivation activates a glutamatergic projection from the hypothalamic paraventricular nucleus to the rostral ventrolateral medulla. J Comp Neurol. 2006;494:673–685. doi: 10.1002/cne.20835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes TM, Leonard CM, Nottebohm F. The telencephalon, diencephalon, and mesencephalon of the canary, Serinus canaria, in stereotaxic coordinates. J Comp Neurol. 1974;15:337–374. doi: 10.1002/cne.901560305. [DOI] [PubMed] [Google Scholar]
- Sturdy CB, Wild JM, Mooney R. Respiratory and telence-phalic modulation of vocal motor neurons in the zebra finch. J Neurosci. 2003;23:1072–1086. doi: 10.1523/JNEUROSCI.23-03-01072.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthers RA, Goller F. Motor correlates of vocal diversity in songbirds. Curr Ornithol. 1997;14:235–288. [Google Scholar]
- Suthers RA, Goller F, Bermejo R, Wild JM, Zeigler HP. Relationship of beak gape to the lateralization, acoustics and motor dynamics of song in cardinals. In: Abstracts of the Nineteenth Midwinter Meeting of the Association for Research in Otolaryngology. Des Moines, IA. 1996:p158. [Google Scholar]
- Thoms G, Ju¨rgens U. Common input of the cranial motor nuclei involved in phonation in squirrel monkey. Exp Neurol. 1987;95:85–99. doi: 10.1016/0014-4886(87)90009-4. [DOI] [PubMed] [Google Scholar]
- Vanderhorst VG, Terasawa E, Ralston HJ, 3rd, Holstege G. Monosynaptic projections from the nucleus retroambiguus to motoneurons supplying the abdominal wall, axial, hindlimb, and pelvic floor muscles in the female rhesus monkey. J Comp Neurol. 2000;424:233–250. doi: 10.1002/1096-9861(20000821)424:2<233::aid-cne4>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Vanderhorst VG, Terasawa E, Ralston HJ., 3rd Monosynaptic projections from the nucleus retroambiguus region to laryngeal motoneurons in the rhesus monkey. Neuro-science. 2001;107:117–125. doi: 10.1016/s0306-4522(01)00343-8. [DOI] [PubMed] [Google Scholar]
- Vicario DS. A new brain stem pathway for vocal control in the zebra finch song system. Neuroreport. 1993;4:983–986. doi: 10.1097/00001756-199307000-00037. [DOI] [PubMed] [Google Scholar]
- Wallenberg A. Der Ursprung des Tractus isthmostriatus (oder bulbostriatus) der Taube. Neurol Zbl. 1903;22:98–101. [Google Scholar]
- Westneat MW, Long JH, Jr, Hoese W, Nowicki S. Kinematics of birdsong: functional correlation of cranial movements and acoustic features in sparrows. J Exp Biol. 1993;182:147–171. doi: 10.1242/jeb.182.1.147. [DOI] [PubMed] [Google Scholar]
- Wild JM. Identification and localization of the motor nuclei and sensory projections of the glossopharyngeal, vagus, and hypoglossal nerves of the cockatoo (Cacatua roseicapilla), Cacatuidae. J Comp Neurol. 1981;203:351–377. doi: 10.1002/cne.902030304. [DOI] [PubMed] [Google Scholar]
- Wild JM. Peripheral and central terminations of hypoglossal afferents innervating lingual tactile mechanoreceptor complexes in Fringillidae. J Comp Neurol. 1990;298:157–171. doi: 10.1002/cne.902980203. [DOI] [PubMed] [Google Scholar]
- Wild JM. Descending projections of the songbird nucleus robustus archistriatalis. J Comp Neurol. 1993a;338:225–241. doi: 10.1002/cne.903380207. [DOI] [PubMed] [Google Scholar]
- Wild JM. The avian nucleus retroambigualis: a nucleus for breathing, singing and calling. Brain Res. 1993b;606:319–324. doi: 10.1016/0006-8993(93)91001-9. [DOI] [PubMed] [Google Scholar]
- Wild JM. The auditory-vocal-respiratory axis in birds. Brain Behav Evol. 1994;44:192–209. doi: 10.1159/000113577. [DOI] [PubMed] [Google Scholar]
- Wild JM. Trigeminal disynaptic circuit mediating corneal afferent input to M. depressor palpebrae inferioris motoneurons in the pigeon (Columba livia) J Comp Neurol. 1999;403:391–406. doi: 10.1002/(sici)1096-9861(19990118)403:3<391::aid-cne8>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Wild JM, Farabaugh SM. Organization of afferent and efferent projections of the nucleus basalis prosencephali in a passerine, Taeniopygia guttata . J Comp Neurol. 1996;365:306–328. doi: 10.1002/(SICI)1096-9861(19960205)365:2<306::AID-CNE8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Wild JM, Zeigler HP. Central representation and somatotopic organization of the jaw muscles within the facial and trigeminal nuclei of the pigeon (Columba livia) J Comp Neurol. 1980;192:175–201. doi: 10.1002/cne.901920112. [DOI] [PubMed] [Google Scholar]
- Wild JM, Zeigler HP. Central projections and somato-topic organisation of trigeminal primary afferents in pigeon (Columba livia) J Comp Neurol. 1996;368:136–152. doi: 10.1002/(SICI)1096-9861(19960422)368:1<136::AID-CNE9>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Wild JM, Arends JJ, Zeigler HP. Telencephalic connections of the trigeminal system in the pigeon (Columba livia): a trigeminal sensorimotor circuit. J Comp Neurol. 1985;234:441–464. doi: 10.1002/cne.902340404. [DOI] [PubMed] [Google Scholar]
- Wild JM, Li D, Eagleton C. Projections of the dorsomedial nucleus of the intercollicular complex (DM) in relation to respiratory-vocal nuclei in the brainstem of pigeon (Columba livia) and zebra finch (Taeniopygia guttata) J Comp Neurol. 1997a;377:392–413. doi: 10.1002/(sici)1096-9861(19970120)377:3<392::aid-cne7>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Wild JM, Reinke H, Farabaugh SM. A non-thalamic pathway contributes to a whole body map in the brain of the budgerigar. Brain Res. 1997b;755:137–141. doi: 10.1016/s0006-8993(97)00026-7. [DOI] [PubMed] [Google Scholar]
- Wild JM, Williams MN, Suthers RA. Neural pathways for bilateral vocal control in songbirds. J Comp Neurol. 2000;423:413–426. doi: 10.1002/1096-9861(20000731)423:3<413::aid-cne5>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Wild JM, Williams MN, Suthers RA. Parvalbumin-positive projection neurons characterise the vocal premotor pathway in male, but not female, zebra finches. Brain Res. 2001;917:235–252. doi: 10.1016/s0006-8993(01)02938-9. [DOI] [PubMed] [Google Scholar]
- Wild JM, Kubke MF, Mooney R. Avian nucleus retroambigualis: cell types and projections to other respiratoryvocal nuclei in the brain of the zebra finch (Taeniopygia guttata) J Comp Neurol. 2009;512:768–783. doi: 10.1002/cne.21932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild JM, Kru¨tzfeldt NOE, Altshuler DL. Trigeminal and spinal dorsal horn (dis)continuity and avian evolution. Brain Behav Evol. 2010;76:11–19. doi: 10.1159/000319239. [DOI] [PubMed] [Google Scholar]
- Williams H. Choreography of song, dance and beak movements in the zebra finch (Taeniopygia guttata) J Exp Biol. 2001;204:3497–3506. doi: 10.1242/jeb.204.20.3497. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Moritani M, Chang Z, Taki I, Tomita A, Ono T, Bae Y-C, Shigenaga Y, Yoshida A. The somatotopic organization of trigeminal premotoneurons in the cat brain-stem. Brain Res. 2007;1149:111–117. doi: 10.1016/j.brainres.2007.02.072. [DOI] [PubMed] [Google Scholar]
- Zeigler HP. Neural control of the jaw and ingestive behavior: anatomical and neurobehavioral studies of a trigeminal sensorimotor circuit: modulation of defined vertebrate neural circuits. Ann N Y Acad Sci. 1989;563:69–86. doi: 10.1111/j.1749-6632.1989.tb42191.x. [DOI] [PubMed] [Google Scholar]
- Zhang SP, Davis PJ, Carrive P, Bandler R. Vocalization and marked pressor effect evoked from the region of the nucleus retroambigualis in the caudal ventrolateral medulla of the cat. Lett. 1992;140:103–107. doi: 10.1016/0304-3940(92)90692-z. [DOI] [PubMed] [Google Scholar]