SUMMARY
Adenosine deaminases acting on RNA (ADARs) convert adenosine to inosine, which is then recognized as guanosine. To study the role of ADAR proteins in RNA editing and gene regulation, we sequenced and compared the DNA and RNA of human B cells. Then, we followed up the findings experimentally with siRNA knockdown and RNA and protein immunoprecipitations. The results uncovered over 60,000 A-to-G editing sites and several thousand genes whose expression levels are influenced by ADARs. Of these ADAR targets, 90% were identified. Our results also reveal that ADAR regulates transcript stability and gene expression through interaction with HuR (ELAVL1). These findings extend the role of ADAR and show that it cooperates with other RNA-processing proteins to regulate the sequence and expression of transcripts in human cells.
INTRODUCTION
Molecular studies and, more recently, genome and transcriptome sequencing have uncovered the complexity of RNA processing. From the same DNA templates, events such as RNA editing generate different forms of transcripts. In this study, we focused on ADAR-mediated RNA editing and its interactions with other RNA processing steps to regulate gene expression. In human cells, two classes of proteins are known to be involved in RNA editing: the ADAR and APOBEC families. ADARs, which are expressed in a wide variety of cell types, deaminate adenosine to inosine, which is then recognized by the translation and splicing machineries as guanosine (Bass and Weintraub, 1988; Kim et al., 1994; Rueter et al., 1995; Yang et al., 1995). APOBEC1 is expressed predominantly in human liver and converts cytidine to uridine (C-to-U) (Chen et al., 1987; Powell et al., 1987). There are only a few characterized targets of human APOBEC1, the APOB and NF1 genes.
Recent work has uncovered many more RNA-editing events mediated by ADAR proteins. These findings led to new questions. Most of the A-to-G editing sites were identified by computational analysis of sequence data without experimental validation. Some of the findings were based on a comparison of RNA sequences with reference DNA sequences that were not derived from the same cells. In addition, it has been suggested that ADAR plays a role in other biological processes in an editing-independent manner (Clerzius et al., 2009; Heale et al., 2009), but the extent of these processes is not known. Lastly, it is not clear whether ADAR1 and ADAR2 play the same role or different roles in human cells. To address these issues, we sought to answer three main questions: (1) What sites do ADAR proteins edit? (2) Do ADAR proteins regulate gene expression, and if so, is this regulation dependent on editing? (3) What other proteins interact with ADARs in RNA processing?
We compared DNA and RNA sequences in human B cells from two individuals to identify RNA-DNA sequence differences (RDDs). We validated the findings by RNAi and RNA immunoprecipitation (RNA-IP). The results uncovered ~10,000 known and ~50,000 unknown ADAR-mediated A-to-G editing sites in premature and mature mRNAs and long noncoding RNAs (lncRNAs). We also found that ADAR proteins have an editing-independent effect on gene expression. Our results showed that ADAR1 interacts with HuR (ELAVL1) to regulate transcript stability. Together, these results provided us with a deeper understanding of ADAR proteins in RNA editing and gene regulation.
RESULTS
DNA and RNA Sequencing
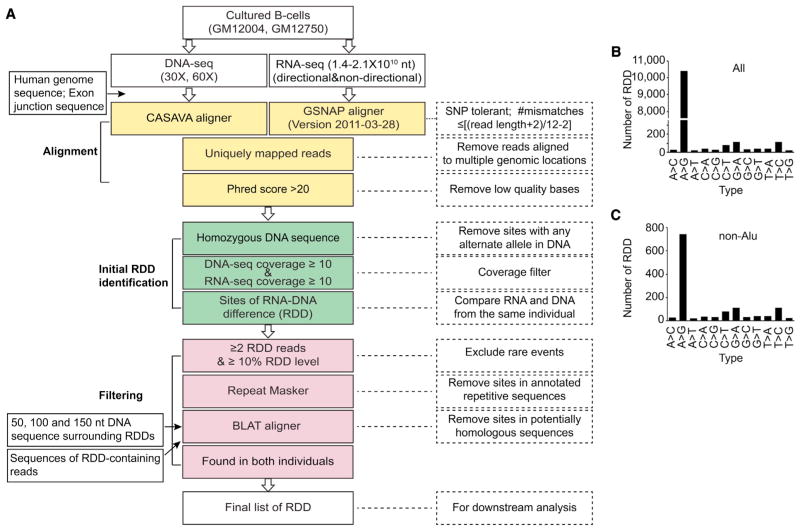
We sequenced the DNA and mRNA from cultured B cells of two individuals using Illumina-based next-generation sequencing (NGS) (Bentley et al., 2008). We conducted DNA sequencing (DNA-seq) to >30× coverage and obtained >140 million RNA sequencing (RNA-seq) reads for each sample. At least 80% of the sequence reads mapped to the reference genome sequence (Table S1). For each individual, we compared their DNA and mRNA sequences to identify editing and other types of RDDs (Chen et al., 2012; Ju et al., 2011; Li et al., 2011). Data from strand-specific (directional) sequencing allowed us to annotate all 12 types of possible mismatches between DNA and RNA sequences. To simplify the mapping of the sequence reads, repetitive sequences are often excluded. However, since most of the ADAR-mediated A-to-G editing sites were found in Alu repeats (Athanasiadis et al., 2004; Kim et al., 2004; Levanon et al., 2004; Peng et al., 2012), we retained Alu sequences (but excluded other sequence repeats) in our analysis. Using stringent thresholds, we identified 10,992 sites where the RNA sequences were discordant from the corresponding DNA sequences in both individuals (Figure 1A; Table S2). All 12 types of RDDs (A-to-C, A-to-G, etc.) were found (Figure 1B). These included 9,675 sites in Alu-containing regions and 1,317 sites in nonrepetitive regions of the genome. The distributions of the 12 types of RDDs were very different for Alu-containing and Alu-free regions of the genome. Most (99%) of the sites in Alu regions were A-to-G editing sites, whereas in regions without Alu repeats, only 57% were A-to-G sites (Figure 1C). We then validated the results by Sanger sequencing and emulsion-based droplet digital PCR (Figures 2 and S1). Twenty-four out of 25 sites were validated by Sanger sequencing, and five out of six sites were validated by droplet digital PCR. Thus, the false discovery rate (FDR) is approximately 6.5% (Figure S1).
Figure 1. Identification of RDDs.
(A) Analysis steps to identify RDDs (see also the Supplemental Experimental Procedures). All 12 types of RDDs were found.
(B) Sites detected genome wide.
(C) Sites detected in non-Alu regions.
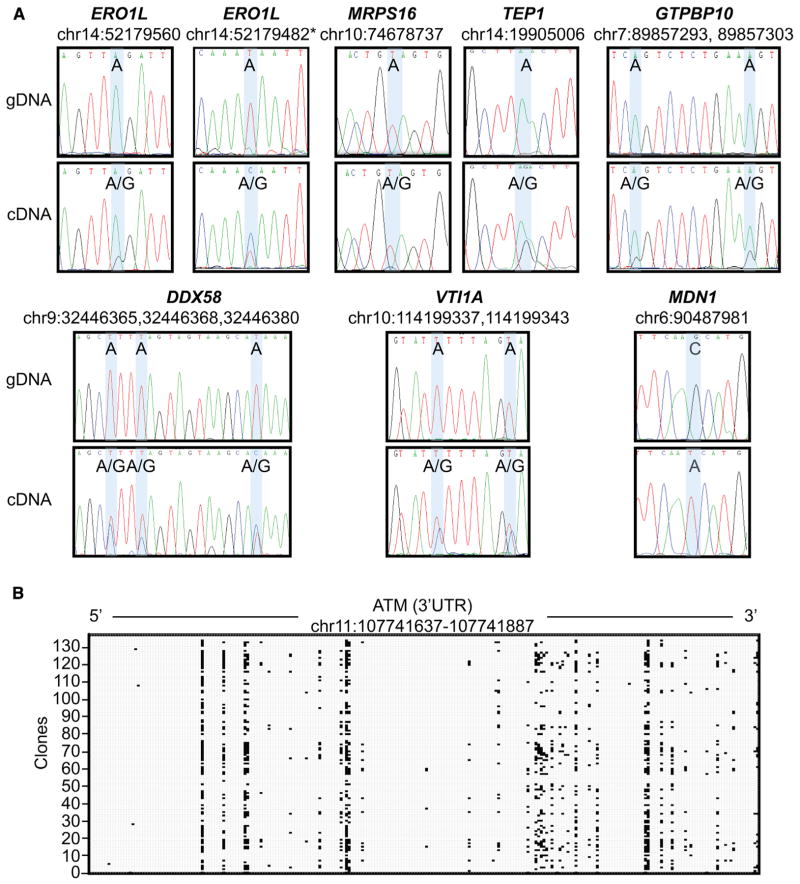
Figure 2. Validation of A-to-G Editing and RDD Sites by Sanger Sequencing.
(A) Sequences surrounding editing or RDD sites were amplified by PCR using genomic DNA or cDNA from the same two individuals as templates. The sites validated by Sanger sequencing are highlighted in blue and the corresponding nucleotide changes are labeled. Some samples were sequenced from the reverse strand, and the nucleotides are labeled according to the forward strand. *An example of an editing site in ERO1L that did not meet our inclusion criteria but nonetheless was validated by Sanger sequencing.
(B) Hyperedited region in ATM transcript. 3′ UTR of ATM was PCR amplified from cDNA and cloned. Sequences from 137 individual clones are illustrated. Each black dot represents an A-to-G site detected in a clone by Sanger sequencing.
See also Figure S1.
ADAR1 Plays a Major Role in A-to-G RNA Editing in Human B Cells
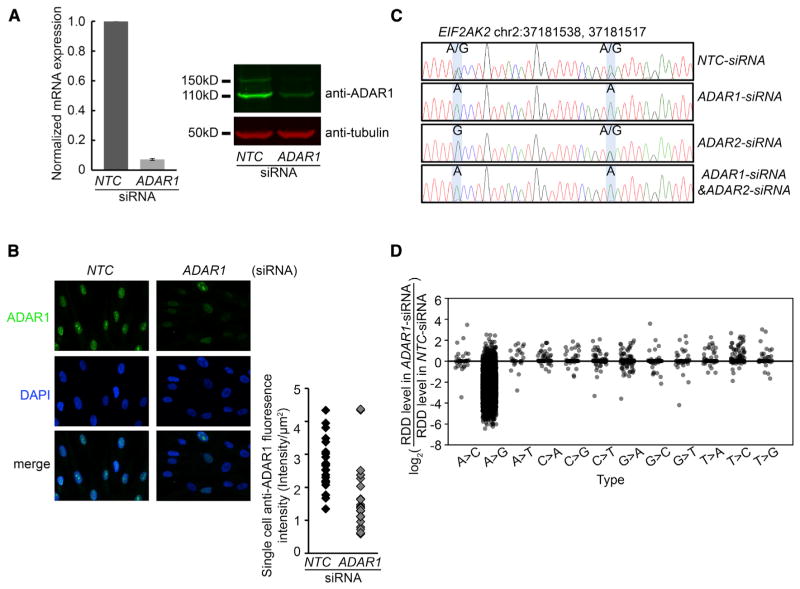
To assess the extent to which the ADAR family of deaminases contributes to mismatches between RNA and corresponding DNA sequences, we carried out RNAi-mediated gene knockdowns and deep sequencing of the resulting cells. Human B cells possess three members of the ADAR family: ADAR1, ADAR2, and ADAR3. ADAR1 and ADAR2 are functional deaminases (Bass and Weintraub, 1988; Kim et al., 1994), whereas ADAR3 does not have a known enzymatic function (Chen et al., 2000). The expression level of ADAR1 is >20 times higher than that of ADAR2 and ADAR3 (reads per kilobase of transcript per million mapped reads [RPKM] of ADAR1 = 7 compared with RPKM of ADAR2 and ADAR3 < 0.3), suggesting that ADAR1 is the predominant form of ADARs in human B cells. Following gene knockdown with four independent siRNAs and a pool comprising the four siRNAs, ADAR1 was reduced by >50% at mRNA and protein levels (Figures 3A, 3B, S2A, and S2B). The editing activities were also reduced, as A-to-G editing in EIF2AK2 mRNA, a known target of ADAR1 (Blow et al., 2004), was abolished following ADAR1 knockdown (Figures 3C and S2C). Similar results were obtained from the different siRNAs; for subsequent experiments, we used the pooled siRNAs to minimize off-target effects (Supplemental Experimental Procedures; Figure S2; Grimson et al., 2007).
Figure 3. siRNA Knockdown of ADAR1 Resulted in Reduced A-to-G Levels.
(A) Left panel: real-time RT-PCR shows the decrease in the ADAR1 mRNA level following knockdown using pooled siRNA. The average fold change from triplicates is shown. Error bar indicates SEM. Right panel: western blot shows the decrease of ADAR1 protein following knockdown.
(B) Immunofluorescence staining of primary fibroblast confirmed that siRNA knockdown results in a decrease of ADAR1 expression. Left panel: representative immunofluorescence image of primary fibroblasts treated with nontargeting control siRNA (NTC) or ADAR1-siRNA. Right panel: fluorescence quantification of ADAR1 expression in 24 cells treated with NTC-siRNA or ADAR1-siRNA, respectively.
(C) Editing levels at two A-to-G sites in EIF2AK2 were reduced following ADAR1 knockdown, but the levels increased following ADAR2 knockdown and were abolished following double knockdown.
(D) ADAR1 knockdown led to reduced levels in 96% A-to-G sites, but had a minimal effect on other types of RDDs.
See also Figures S2–S4 and Tables S3, S6, and S7.
Next, we sequenced and compared the DNA and RNA of the siRNA-treated B cells. This allowed us to experimentally validate the editing sites and determine the effect of ADAR1 on editing. False-positive results due to misalignment of sequence reads or other artifacts would not “respond” to siRNA treatments.
ADAR and RNAi pathways work cooperatively (Scadden and Smith, 2001; Wu et al., 2011; Yang et al., 2005), so the double-stranded RNAs (dsRNAs) used in gene knockdown likely have effects on ADAR function other than knockdown of its expression level. To study the specific effects of ADAR1 knockdown, we compared the sequences of cells transfected with control siRNAs with those of cells treated with pooled ADAR1-specific siRNAs. In the cells treated with control siRNA, we found 6,996 sites where the RNA and DNA sequences were discordant, including 6,524 A-to-G editing sites. In the ADAR1 knockdown cells, the editing level of 6,258 (96%) sites decreased by 20% or more in samples from both individuals, whereas only 43 sites of the other 11 types of RDDs decreased by the same extent (Figure 3D; Table S3). A small number of sites (91 of the A-to-G sites and 125 of the other RDDs) showed increased levels following ADAR1 knockdown. The editing levels of >2,000 A-to-G sites were reduced to zero following ADAR1 knockdown. These included sites in genes that encode caspases (CASP8 and CASP10) and the von Hippel-Lindau (VHL) tumor suppressor, which have been implicated in various cancers. In contrast, the levels of the other types of RDDs did not change or decreased very modestly. This suggests that ADAR1 mediates the majority of A-to-G editing in B cells and does not contribute to the other types of RDDs. In addition, these results show that the FDR of A-to-G editing is no more than 4%.
The above data were obtained at one time point. In order to study the kinetics of A-to-G editing, we carried out RNA-seq on the cells at several time points after siRNA transfection. The expression level of ADAR1 and the editing levels of its many targets remained low throughout the time course (Figures S3A and S3B). For instance, the A-to-G editing levels in TRAF1, CENPH, and USP46 were less than 5% of those in control samples 96 hr after siRNA transfection. Gene Ontology analysis (Ashburner et al., 2000; Huang et al., 2009a, 2009b) showed that editing targets are enriched for genes that encode zinc-finger proteins (p < 0.05), as well as proteins that are involved in chromosomal organization (p < 10−5) and antiviral defense (p < 10−3).
Role of ADAR2 in RNA Editing in Human B Cells
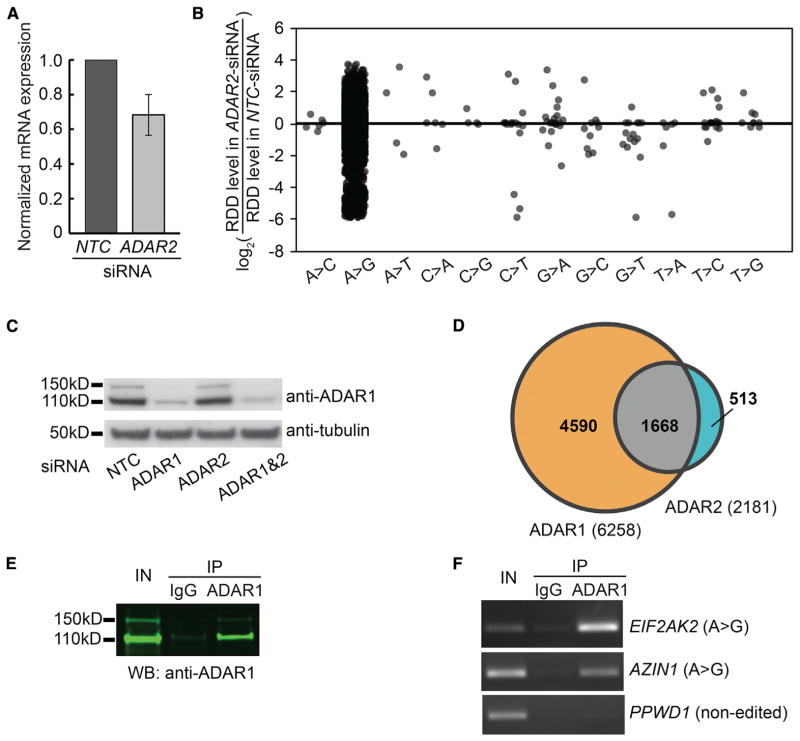
Next, we carried out siRNA knockdown of ADAR2 (ADARB1) followed by nucleic acid sequencing. The ADAR2 mRNA level was reduced by 25% (Figures 4A and S2A). The lack of specific antibodies prevented us from measuring ADAR2 protein expression. Following ADAR2 knockdown, we observed a decrease in its activity: the editing levels of 2,181 of 6,084 A-to-G sites (Table S4), and 32 of the other types of RDDs decreased by at least 20%. In contrast to ADAR1 knockdown, after ADAR2 knockdown, the levels of 2,240 A-to-G sites increased by 20% or more (Figure 4B). We reasoned that these sites (e.g., those in EIF2AK2) are mainly targeted by ADAR1; therefore, following ADAR2 knockdown, a compensatory increase in ADAR1 binding or activity would lead to higher editing levels, which would be abolished by the simultaneous silencing of ADAR1 and ADAR2. This hypothesis was confirmed by a decrease in EIF2AK2 editing following double knockdown of ADAR1 and ADAR2 (Figure 3C). The compensation is not due to higher ADAR1 protein expression, since it increased only minimally following ADAR2 knockdown (Figure 4C). These results suggest that the increase in editing levels following ADAR2 knockdown could be due to increased availability of the sites to ADAR1 and/or homodimerization of ADAR1, a more active form of ADAR1 (Chilibeck et al., 2006; Lehmann and Bass, 2000).
Figure 4. Role of ADAR2 in RNA Editing.
(A) Real-time RT-PCR shows that the ADAR2 mRNA level is downregulated following siRNA knockdown. We were unable to assess changes in the ADAR2 protein level because none of the antibodies we tested gave a specific ADAR2 signal in western blot. Error bar indicates SEM.
(B) ADAR2 knockdown led to changes of editing levels in ~2,000 A-to-G sites. See also Table S4.
(C) Western blot shows that the ADAR1 protein level is not upregulated following ADAR2 knockdown.
(D) ADAR1 targets more editing sites than ADAR2. The Venn diagram shows shared and unique editing sites targeted by ADAR1 and ADAR2.
(E) Anti-ADAR1 RNA-IP pulled down ADAR1 protein and its associated editing targets specifically. Western blot shows that anti-ADAR1 pulled down ADAR1 protein.
(F) RT-PCR shows that ADAR1 antibody pulled down transcripts of the editing targets, EIF2AK2 and AZIN1, but not the negative control transcript, PPWD1.
Shared Editing Targets of ADAR1 and ADAR2
Next, we examined the specificity of ADAR1 and ADAR2 by comparing editing sites identified from the knockdown experiments described above. We found that the editing levels of 6,771 sites decreased after at least one of the ADAR proteins was silenced. Of these, 1,668 sites showed a reduction in editing levels by ≥20% following knockdown of ADAR1 and ADAR2, suggesting they are targets of both enzymes (Figure 4D; Tables S3 and S4). These included sites in genes that encode the DNA damage repair protein ERCC4 and the telomerase-associated protein TEP1. Other targets appeared to be specific to ADAR1 or ADAR2: 4,590 sites showed a decrease in levels following only ADAR1 silencing, and 513 sites showed a decrease only in ADAR2 knockdown (Figure 4D). The extent of ADAR2 knockdown is smaller than that of ADAR1 knockdown, which could account for the more modest decrease in A-to-G editing following ADAR2 knockdown.
RNA-IP Uncovered Many Additional A-to-G Editing Sites
ADAR deaminases are RNA-binding proteins that interact directly with their substrates (Klaue et al., 2003). To understand the RNA-binding activity of ADAR1, we carried out native IP of ADAR1 in B cells and sequenced the RNA that coprecipitated with the ADAR1 protein (Figure 4E). Previously, we selected polyadenylated mRNAs for analysis in order to obtain adequate sequence coverage. Here, we targeted the IP to RNAs that are specifically bound to ADAR1 in vivo without selecting for polyadenylated mRNAs. This allowed us to study the effects of ADAR1 on a broader set of RNAs, including immature transcripts whose introns have yet to be spliced out. To test the quality of the ADAR RNA-IP, we showed that known ADAR1 substrates, such as EIF2AK2 and AZIN1, were bound by ADAR1 protein, in contrast to the control transcript PPWD1, which is not edited (Figure 4F). We next carried out RNA-seq analysis and identified edited transcripts that were pulled down by ADAR1 antibody but not by negative-control immunoglobulin G (IgG). Using the same thresholds as above, we identified 55,719 A-to-G sites in the two individuals, which is far more than the 10,412 editing sites identified from the mRNA samples of the same individuals (Table S5). Transcripts that are bound and edited by ADAR1 protein include those that encode WEE1, a protein kinase that plays a role in DNA replication, and COPB1, a member of the coatomer protein complex that is involved in trafficking between the Golgi and the endoplasmic reticulum.
Of these 55,719 sites, fewer than 4,500 sites have been previously reported (Bahn et al., 2012; Carmi et al., 2011; Kiran and Baranov, 2010; Li et al., 2009; Peng et al., 2012). The majority (81%) of the sites were found in introns and some were found in lncRNAs, including LINC00265 and LINC00476. The transcripts from the RNA-IP were hyperedited: >30% of the editing sites clustered in 224 transcripts, each of which had >50 A-to-G editing sites (Table 1). More than 97% of the 55,719 editing sites were in Alu repeats that promote dsRNA formation and therefore binding and hyperediting by ADAR proteins (Osenberg et al., 2009). When we examined a hyperedited region of ATM more closely, we found that each adenosine was deaminated. However, the editing level at a given site ranged from 1% to 99%, and within a given transcript there was no obvious pattern as to which adenosine was edited (Figure 2B).
Table 1.
Hyperedited Transcripts
| Hyperedited Region | Gene Symbol | Number of Edited Sites |
|---|---|---|
| chr9:131701274-131841654 | FNBP1 | 291 |
| chr3:47608175-47795690 | SMARCC1 | 218 |
| chr1:1713762-1810015 | GNB1 | 214 |
| chr4:39379937-39452078 | UBE2K | 167 |
| chr5:138923557-138985907 | UBE2D2 | 162 |
| chr8:98728784-98810463 | MTDH | 154 |
| chr15:42553696-42603223 | CTDSPL2 | 148 |
| chr1:149438531-149485085 | PIP5K1A | 141 |
| chr10:70152450-70219274 | CCAR1 | 138 |
| chr17:24746313-24892874 | TAOK1 | 136 |
| chr16:68968176-69027669 | ST3GAL2 | 134 |
| chr5:176497466-176651020 | NSD1 | 134 |
| chr2:61559886-61613430 | XPO1 | 133 |
| chr3:49046623-49101020 | QRICH1 | 131 |
| chr12:49088991-49144511 | LARP4 | 128 |
| chr16:15655703-15700735 | NDE1 | 128 |
| chr1:149652924-149695116 | POGZ | 127 |
| chr19:17076438-17180447 | MYO9B | 127 |
| chr19:16604467-16625697 | C19orf42 | 125 |
| chr16:88337701-88409128 | FANCA | 123 |
Features Differ between A-to-G Editing Sites and Other Types of RDDs
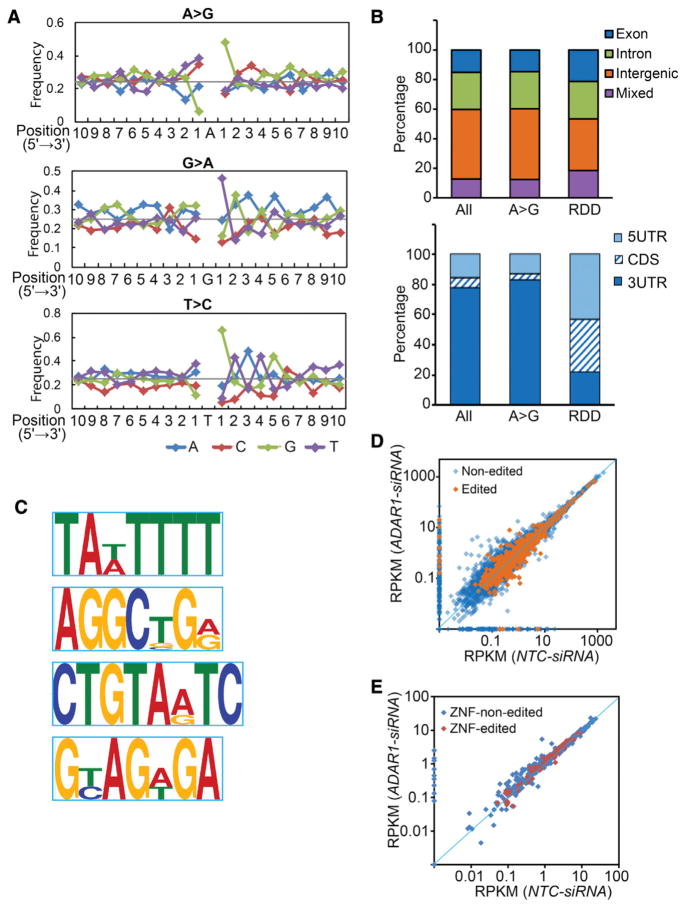
The results from ADAR knockdown and RNA-IP suggest that although ADARs mediate A-to-G editing, they do not mediate other types of RDDs. The levels of other types of differences were largely unaffected by ADAR knockdown, and the transcripts that showed those differences were not bound by ADAR. This prompted us to compare the genomic features surrounding the A-to-G editing sites and other types of RDDs. First, the sequence contexts of A-to-G and non-A-to-G sites are different. The base 5′ adjacent to the adenosine in A-to-G sites is depleted of guanosine (G) and the base 3′ to A-to-G editing sites is enriched for G (Figure 5A), consistent with previous reports (Lehmann and Bass, 2000). This sequence feature is specific to A-to-G editing because it is not present in random adenosines within nonedited Alu repeats (data not shown). This sequence motif was also not found for any of the RDDs. We identified sequence motifs for G-to-A and T-to-C sites, and they differed from the motif around the A-to-G sites (Figure 5A). Second, the A-to-G sites were more clustered than the non-A-to-G sites (67% of A-to-G sites were found within 25 nt of each other, compared with 14% of non-A-to-G RDDs). Third, most of the A-to-G sites were within or near inverted repeats, which form dsRNA and are preferentially recognized and bound by ADAR enzymes. Nearly 45% of the A-to-G sites resided within inverted repeats and another 30% were found near inverted repeats (<1 kb). In contrast, very few (0.9%) of the non-A-to-G sites were found in inverted repeats. Lastly, A-to-G sites and RDD sites were found in different regions of genes. A-to-G sites were found mostly in the 3′ UTRs, whereas RDDs were found mainly in the 5′ UTRs and in coding exons. Only 4% of the A-to-G sites (compared with 35% of RDDs) were in coding exons (Figure 5B). The differences between A-to-G editing sites and the other types of RDDs suggest that they are mediated by different mechanisms. Biochemically, this is expected since some of the RDDs are transversion events that cannot be explained simply by deamination.
Figure 5. Features of A-to-G and RDD Sites.
(A) The nucleotide 5′ to A-to-G sites is depleted of G, and the nucleotide 3′ to A-to-G sites is enriched for G. In contrast, the nucleotide 3′ to G-to-A sites is enriched for T, and the nucleotide 3′ to T-to-C sites is enriched for G. Sequences for 10 nt upstream and downstream of A-to-G or RDD sites were analyzed and the frequencies of A, C, T, and G at each position are shown. The horizontal line at a frequency of 0.25 indicates the expected frequency if the four nucleotides are represented equally.
(B) A-to-G and other RDD sites are found in different genomic regions. Upper panel: genome-wide distribution (“Mixed” indicates regions with multiple or ambiguous annotation). Lower panel: distribution in exonic regions.
(C) Sequence motifs for editing targets pulled down in anti-ADAR RNA-IP assays. The MEME program was used to analyze DNA sequences corresponding to 100 nt upstream and downstream of editing sites. The four motifs that are most significantly enriched in input sequences are shown (p < 10−10, Fisher’s exact test). Scrambled sequences were used as negative-control sequences.
(D and E) Expression levels of transcripts do not correlate with editing levels. RPKM values of transcripts measured in an ADAR1 knockdown sample and a negative-control sample (NTC) are plotted. Edited and nonedited transcripts are indicated in different colors.
(D) All transcripts.
(E) Genes encoding zinc-finger proteins whose expression levels changed by ≥20%.
See also Table S8.
Sequence Motifs near A-to-G Editing Sites
The large number of RNA editing sites in our study gave us an opportunity to uncover characteristics of the editing targets. We expanded our sequence analysis to 100 nt upstream and downstream of A-to-G sites using the motif discovery tool MEME (Bailey et al., 2009). MEME identified four motifs that are significantly enriched in the sequences surrounding the A-to-G editing sites compared with control sequences (p < 10−10, Fisher’s exact test; Figure 5C). One of these motifs (TA(T/A)TTTT) corresponds to the binding motif of HuR, an RNA-binding protein that regulates mRNA turnover (Myer et al., 1997). Other studies have also investigated the sequence and structure specificity of targeted sites of ADAR enzymes (Bahn et al., 2012; Daniel et al., 2012; Dawson et al., 2004; Kuttan and Bass, 2012; Lehmann and Bass, 2000; Wong et al., 2001). However, the sequence motifs we described here have not been previously reported in ADAR editing targets. This is likely because we searched more distant sequences surrounding editing targets in a larger number of editing sites of various types of RNAs, whereas most previous studies focused on immediately adjacent sequences on fewer targets.
Finding the HuR motif near ADAR-binding sites led us to reason that ADAR interacts with other RNA-binding proteins. The sequence motifs for RNA-binding proteins in edited transcripts suggest cooperative binding among RNA processing proteins, akin to the coupling seen in regulation of gene expression by multiple transcription factors. This finding prompted us to study the interactions between ADAR1 and HuR proteins (see below).
ADAR Regulates Gene Expression
After examining how ADAR proteins affect RNA sequences, we turned to study their effects on gene expression and to determine the relationship between RNA editing and gene expression. We found that ADAR1 and ADAR2 affect the expression of thousands of genes and their transcripts in human B cells. We looked for genes that showed changes in the total gene-expression level. Following ADAR1 knockdown, 635 genes showed significant changes in gene expression in two individuals (p < 0.05; Table S6). The RNA-seq data allowed us to analyze the effect of ADAR on gene expression at single-nucleotide resolution to quantify changes of transcript expression in addition to total gene expression following ADAR1 knockdown. Many genes demonstrate “isoform switching” under physiological or experimental perturbations (Trapnell et al., 2013). The expression levels of 1,238 transcripts showed significant changes in expression (Table S6). Nearly half of these transcripts (579) belong to the genes that changed the total expression level. However, changes in 659 transcripts were not reflected at the total gene-expression level. For some transcripts, such as VNN2 and ARH-GAP19, two isoforms showed changes in opposite directions, and thus the total gene levels that are the sums of isoforms did not show change (Figure S3C). Gene Ontology analysis (Huang et al., 2009a) showed that these ADAR-regulated genes are enriched in kinase (p < 10−9), DNA damage response proteins (p < 10−10), and zinc-finger proteins (p < 10−6; Table S7).
RNA-seq data provide information on editing and gene expression in the same samples, and thus allow us to assess the connection between the two. We examined the levels of ADAR1-dependent editing and transcript expression, and found that they were not correlated (r < 0.05 for both individuals). Following ADAR1 knockdown, changes in expression level were independent of the editing status of the target genes (Figure 5D). For example, among the 263 zinc-finger protein genes whose expression levels changed following ADAR1 knockdown, only 40% (104 genes) were editing targets of ADAR1. ADAR1 regulated the expression of zinc-finger proteins regardless of whether they were editing targets or not (Figure 5E). For instance, the expression levels of ZNF16 decreased and those of ZNF432 increased following ADAR1 knockdown; however, even though they both had multiple Alu repeats, neither gene was edited. Therefore, editing of Alu is not required for ADAR1 to regulate the expression of zinc-finger proteins (Shen et al., 2011).
Another way to investigate the relationship between RNA editing and gene-expression regulation is to study the 106 genes that are both edited and regulated by ADAR1 at the mRNA expression level (Table S8). Among these, following ADAR1 knockdown, the expression levels of 67 genes increased and those of 39 genes decreased. Changes in editing levels and gene expression following ADAR1 knockdown were not significantly correlated (r < 0.05). For example, IKZF3, a transcription factor that regulates proliferation and differentiation of B lymphocytes, has 68 A-to-G editing sites. Its expression level increased by 1.3-fold, whereas its editing level decreased by >7-fold following ADAR1 knockdown. In contrast, both the editing and expression levels of CENPN (43 A-to-G editing sites) decreased following ADAR1 knockdown. The positions of edited sites within genes (such as coding exons, 3′ UTRs) and the number of edited sites per transcript also did not correlate with changes in expression following ADAR1 knockdown. These results further suggest that ADAR1 can affect gene expression independently of its deamination activity.
We also examined the editing and gene-expression regulatory roles of ADAR2. Although ADAR2 has fewer editing targets than ADAR1, it regulates the expression levels of more genes. Following ADAR2 knockdown, the expression levels of 4,154 transcripts (in 3,379 genes) increased by 2-fold, and those of 872 transcripts (in 734 genes) decreased by 2-fold (Table S9). Thus, ADAR2 has a broader effect on gene expression even though it plays a lesser role in editing compared with ADAR1. This further implies that ADAR proteins affect editing and gene expression independently.
ADAR1 Interacts with HuR to Regulate Transcript Stability
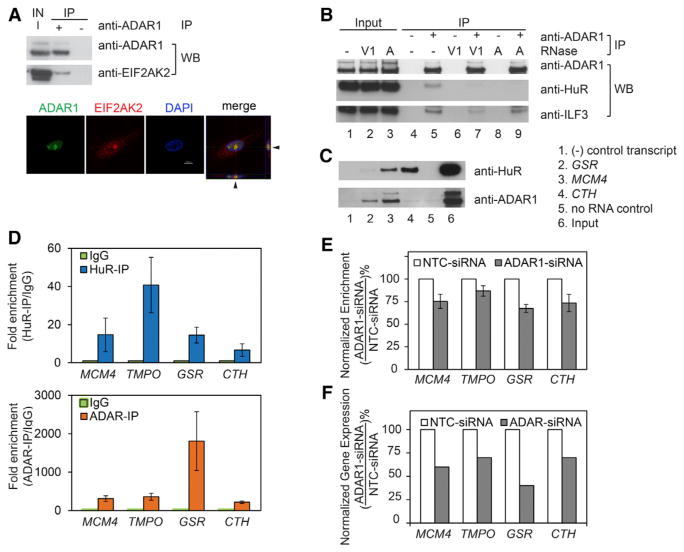
Our analysis of sequence motifs around editing sites identified an enrichment of HuR-binding motifs. This motivated us to study whether HuR and ADAR1 function cooperatively. HuR binds to single-stranded RNA (ssRNA) and regulates transcript stability and gene expression (Fan and Steitz, 1998). We carried out protein IP using anti-ADAR1 and negative-control IgG. We confirmed specific pull-down of ADAR1 by immunoblotting, and identification of transcripts and protein of EIF2AK2, a known editing target and interacting partner of ADAR, in the immunoprecipitates (Figure 6A; Clerzius et al., 2009). Using antibody against HuR, we found that HuR was pulled down with ADAR1, suggesting these two proteins interact in vivo (Figure 6B, lanes 4 and 5). As a control, ILF3, a protein that is known to interact with ADAR1 in a dsRNA-dependent manner, was also pulled down (Nie et al., 2005). Next, we asked whether the interaction between ADAR1 and HuR is dependent on scaffold RNAs. We carried out ADAR1-IP using RNase A- and RNase V1-treated whole-cell lysates. RNase A treatment, which digests ssRNA, abolished the interactions between HuR and ADAR1, but not the interactions between ILF3 and ADAR1. In contrast, the dsRNA-specific RNase V1 reduced the interactions between HuR and ADAR1, and between ILF3 and ADAR1 (Figure 6B, lanes 6–9). These results show that the interaction between HuR and ADAR1 is dependent on both ssRNA and dsRNA.
Figure 6. ADAR1 and HuR Proteins Interact in an RNA-Dependent Manner and Coregulate Common Transcripts.
(A) Anti-ADAR1-IP of ADAR1 and its interacting protein EIF2AK2. Western blot analysis shows that ADAR1 and EIF2AK2 are pulled down by anti-ADAR1, but not by negative controls. Confocal immunofluorescence analysis confirms the interaction between ADAR1 and EIF2AK2 in the nucleus. Arrows indicate orthogonal views of colocalized ADAR1 and EIF2AK2.
(B) ADAR1 and HuR interact in vivo in an RNA-dependent manner. RNase A and V1 treatment before IP abolishes the interaction between ADAR1 and HuR.
(C) RNA pull-down experiments showed that HuR (top panel) and ADAR (bottom panel) bind to the same target transcripts. A (polyA)25 RNA was used as the negative-control transcript. Cell lysate incubated with mock solution before pull-down was included as the no-RNA control.
(D) ADAR1 and HuR antibodies, but not control IgG, pulled down the same transcripts. Following anti-ADAR1 and anti-HuR RNA-IP, quantitative RT-PCR was carried out to measure the levels of various transcripts. RNA levels bound by negative-control IgG were normalized to one.
(E) ADAR1 knockdown leads to reduced binding of HuR to their target transcripts. HuR RNA-IP was carried out in cells treated with ADAR1-siRNA or NTC-siRNA, and the HuR-associated transcript level was measured by quantitative RT-PCR.
(F) The gene expression of the target transcripts of HuR and ADAR1 was reduced following ADAR1 knockdown. Gene expression levels from RNA-seq data (RPKM) were normalized to those obtained from NTC-siRNA samples.
To examine how ADAR and HuR interact with their RNA targets, we carried out additional analyses. First, we studied the HuR-binding sites in ADAR-bound transcripts. As mentioned above, we found that sequences of transcripts bound by ADARs were enriched for AU-rich elements (AREs), which are HuR-binding sites. Among the 4,279 ADAR-bound transcripts, 4,198 (98%) had at least one and often many HuR-binding sites. There were 172,000 TA(T/A)TTTT sites in ADAR-bound transcripts, significantly more (χ2, p < 0.0001) than in control transcripts (68% of 4,279 random control transcripts contain 79,084 AREs). Similarly, other HuR-binding sequences (including (U/A) UUUA, (U/C)UUUA, and AUUU(U/C); Mukherjee et al., 2011) were also enriched in ADAR-bound transcripts. Second, since the presence of AREs does not mean that HuR binds to them, we confirmed the binding using PAR-CLIP data (Kishore et al., 2011; Lebedeva et al., 2011; Mukherjee et al., 2011). Among the 4,279 transcripts bound by ADAR1, 2,866 (67%) were also bound by HuR in PAR-CLIP, which is significantly more than observed in random transcripts (36%; χ2, p < 0.0001), showing that HuR binds to ADAR1 targets in vivo. These common binding targets of HuR and ADAR1 include MCM4, which plays a key role in DNA replication; TMPO, which encodes a nuclear membrane protein; and GSR, which encodes the enzyme glutathione reductase in the antioxidative stress pathway.
The enrichment of HuR-binding sites in ADAR targets and the identification of an RNA-dependent HuR-ADAR complex led us to reason that HuR and ADAR bind to common transcripts and regulate them cooperatively. We confirmed our hypothesis by employing two experimental approaches. First, we carried out RNA pull-down assays. We prepared in vitro synthesized and biotinylated RNA for three transcripts (MCM4, CTH, and GSR) that we previously identified as shared targets of ADAR and HuR. After incubating these transcripts with B cell lysates, we pulled down the transcripts using their biotin tags and immunoblotted for ADAR and HuR proteins. Our results showed that ADAR and HuR are specifically pulled down on MCM4 and GSR transcripts, confirming concurrent ADAR and HuR binding (Figure 6C). Although it binds strongly to HuR, the CTH transcript pulled down less ADAR1, suggesting its weaker interaction with ADAR1 compared with MCM4 and GSR. Second, we carried out HuR RNA-IP to confirm that HuR binds to the same transcripts that ADAR1 targets, and then examined the effects of such binding. Using HuR antibody, we pulled down HuR protein and tested whether ADAR1-targeted transcripts were pulled down with HuR in human B cells. The results showed that HuR antibody, but not negative-control IgG, pulled down the same transcripts that immunoprecipitated with ADAR1 antibody, including MCM4, TMPO, GSR, and CTH (Figure 6D).
We then asked whether HuR and ADAR1 depend on each other for binding to their common targets. Previous studies have found that HuR and other RNA-binding proteins cooperate by binding to the same RNA substrates (Chang et al., 2010; Lal et al., 2004). We carried out HuR RNA-IP following siRNA knockdown of ADAR1 and found that following ADAR1 knockdown, binding of HuR to its target transcripts is greatly reduced (Figure 6E). In cells transfected with ADAR1 siRNAs, the protein level in HuR is the same as that in controls (Figure S2B), confirming that the decrease in HuR binding is not due to decreased HuR protein expression.
After identifying that ADAR is required for HuR binding to transcripts, we examined the effects of ADAR and HuR on transcript levels. Since HuR regulates gene expression by stabilizing mRNAs (Myer et al., 1997), we examined whether ADAR binding affects transcript stability through HuR. Among the 775 genes whose expression levels decreased following ADAR1 knockdown, there were significantly more genes containing HuR-binding sites than genes whose expression levels increased following ADAR1 knockdown (χ2, p < 0.01). For example, the expression levels of MCM4, TMPO, GSR, and CTH transcripts were reduced in both individuals following ADAR1 knockdown, consistent with binding of their transcripts by both HuR and ADAR1 (Figure 6F). These results support the notion that in the absence of ADAR1, HuR binding decreased; thus, the target genes were not stabilized, resulting in lower gene expression. Lastly, these data suggest that ADAR1 and HuR expression levels should correlate with the expression levels of their target genes. Using results from another study in our lab (Cheung et al., 2010), we compared the expression levels of the target genes with ADAR1 and HuR in cultured B cells from 41 unrelated individuals and found that they were significantly correlated (p ≪ 0.01). Correlation plots for MCM4 and TMPO with ADAR1 and HuR are shown in Figure S4A.
Our findings suggest that ADAR1 and HuR proteins cooperate to regulate RNA processing through editing and mRNA turnover. These proteins coregulate transcripts by binding to specific sequences and secondary structures that mediate these processing steps.
DISCUSSION
In this study, we uncovered ~60,000 A-to-G RNA editing sites mediated by ADAR1 and ADAR2 proteins in human B cells. We show that ADAR proteins are involved in gene regulation, particularly in regulating RNA stability and processing.
Prior to our study, many A-to-G editing sites had been identified. Here, we added to the list of such sites by using gene knockdown and RNA-IP, and we validated experimentally that our sites are direct targets of ADAR1 and ADAR2 proteins. Traditionally, editing sites are identified by comparing DNA and RNA sequences. Often the DNA sequences used for comparisons are those from the reference genome. We extracted the DNA and RNA from the same cells and subjected them to deep sequencing, which allowed a direct comparison of RNA sequences and their corresponding DNA. Although NGS provides sequence information with unprecedented coverage, there are hundreds of millions of sequence reads that have to be mapped correctly for proper interpretation. To have confidence in our sequence mapping, we set stringent analysis thresholds that required uniquely mapped reads from two different sequence alignment algorithms (GSNAP and blat) and at least ten sequence reads at each site. However, computational analysis alone may not be adequate. To determine a list of high-confidence ADAR targets, we coupled deep sequencing with ADAR gene knockdowns and ADAR RNA-IP. The same analysis method was used to analyze sequence reads from all samples, and thus the sites in which editing is responsive to gene knockdown, or that are bound specifically to ADAR proteins, cannot be artifacts of computational analyses. In a recent study on RNA editing in Drosophila (Rodriguez et al., 2012), RNA-seq of nascent RNA from an ADAR null strain was compared with that of a wild-type strain. The results were used to estimate an FDR of ~5%. In our study, we used a similar approach and estimated our FDR to be ~4%.
The large number of sites in which RNA sequences differed from the underlying DNA sequences is surprising and requires further attention in genetic studies. Results from this and other studies show that there are likely many thousands of A-to-G editing sites in each individual. Previously, we showed that there are individual differences in the number of RDDs (Li et al., 2011). Here, in our two subjects, we also observed differences in the number of editing sites and the level of editing. These results indicate that genetic variation can extend beyond DNA sequence variation. Even though two individuals may have the same DNA sequences at a site, their RNA sequences may differ. To date, most genetic studies have focused on DNA sequence variation in looking for disease-susceptibility alleles. As it becomes clear that RNA sequence variation extends beyond DNA sequence polymorphism, RNA editing and other types of RDDs will have to be considered in studies to identify the genetic basis of human diseases and traits. Comprehensive lists of editing and RDD sites, such as those presented in this study, are important for facilitating the inclusion of RNA variants in genetic studies.
RNA transcripts are tethered to regulatory factors, and the combinatorial binding of RBPs to transcripts coordinates different steps of RNA processing (Hogan et al., 2008; Licatalosi and Darnell, 2010; Maniatis and Reed, 2002). We found enrichment of binding sequences for HuR in transcripts edited by ADAR. Computational and experimental evidence from HuR RNA-IP in human B cells and cells transfected with ADAR siRNAs showed that HuR binding is facilitated by ADAR binding to RNAs. Our results are consistent with a model in which binding of ADAR to RNA forms secondary structures that are then recognized by HuR proteins. Thus, RNA sequences and structures allow gene regulation by a combination of different RNA processing proteins. Transcription factors cooperate to mediate gene regulation; similarly, RNA processing proteins coordinate to affect gene expression. The complex regulatory codes involve RNA sequences and structures that are facilitated by different combinations of RNA-binding proteins. Therefore, to understand co- and posttranscriptional regulation of gene expression, we need to go beyond studying single proteins. Experimental methods that examine protein complexes and their target RNAs are needed to enhance our understanding of gene regulation.
In summary, in this work we studied ADAR-mediated RNA editing and gene-expression regulation. Our findings uncover editing targets, reveal ADARs’ role in mediating RNA editing and regulation of gene expression, and show that the ADAR protein complex coordinates multiple steps in RNA processing. However, they also raise new questions. Our findings suggest that other mechanisms, such as those that mediate non-A-to-G type RDDs, remain to be identified. In addition, the RNA sequence and structural signatures of the regulatory codes for co- and posttranscriptional processing are largely unknown. Elucidating ADAR’s functions will further our understanding of RNA processing and provide insights into human diseases.
EXPERIMENTAL PROCEDURES
Identification of Editing and RDDs
B cell lines from two individuals in the Centre d’Étude du Polymorphisme Humain database were cultured and genomic DNA and RNA were extracted. DNA-seq and RNA-seq libraries were prepared and sequenced on a HiSeq 2000 instrument (Illumina). DNA-seq and RNA-seq data were aligned to the reference genome (HG18) using CASAVA and GSNAP, respectively. To identify RDDs, we compared each RNA sequence with its corresponding DNA sequence. We required an editing site or RDD site to be covered by a minimum of 10 total DNA-seq and RNA-seq reads, 100% concordance in the DNA sequence, an RDD level ≥ 10%, and an RDD event to be found in both individuals. Potential sites were then filtered using stringent thresholds.
Validation of RDDs using Sanger Sequencing and Droplet Digital PCR
Cultured B cells were transfected with Accell siRNAs (Thermo Scientific) against ADAR1 and ADAR2. Sequences surrounding RDD sites were PCR amplified using genomic DNA or cDNA as the template, and PCR products were sequenced. The 3′ UTR of ATM was amplified from cDNA of B cells and cloned into TOPO vector (Invitrogen).
For droplet digital PCR, DNA probes specific to the DNA and RNA variants at RDD sites were synthesized and labeled by VIC and FAM, respectively (ABI Biosystems). Emulsion PCR was carried out and quantified on a QuantaLIfe Droplet Reader (Bio-Rad Laboratories).
RNA-IP
Anti-ADAR1 and anti-HuR RNA-IP was carried out with a Magna RNA-Binding Protein Immunoprecipitation Kit (Millipore). Quantitative PCR and RNA-seq of immunoprecipitated transcripts were carried out. RNA-editing sites that were detected in transcripts pulled down by ADAR1 antibody, but not by negative-control IgG, were identified as ADAR1-specific targets.
RNA-Protein Pull-Down Assays
Transcripts of HuR and ADAR1 targets were synthesized and biotin labeled in vitro, and incubated with whole-cell lysates. RNA-protein complexes were pulled down and analyzed by western blot (Pierce).
Protein IP of the ADAR-HuR Complex
B cell lysates were incubated with anti-ADAR1 or negative-control rabbit IgG at 4°C overnight. The immunocomplex was pulled down using Protein A agarose (Roche), washed, and finally eluted in 20 mM Tris/7.5, 150 mM NaCl, 2.5 mM MgCl2, 0.2% SDS. To examine RNA-dependent interactions, whole-cell lysates were diluted to 1 μg/μl, RNase A or RNase V1 was added, and lysates were incubated at room temperature for 15 min. Protein samples were analyzed by western blot.
A detailed description of the materials and methods used in this work is provided in the Supplemental Experimental Procedures.
Supplementary Material
Acknowledgments
We thank Dr. Alan Bruzel, Susannah Elwyn, Zhengwei Zhu, Allison Richards, and Jonathan Toung for discussions and technical support and Colleen McGarry for manuscript preparation. This work was supported by grants from the National Institutes of Health (to V.G.C.) and by the Howard Hughes Medical Institute.
Footnotes
ACCESSION NUMBERS
Sequencing data have been deposited in the NCBI Gene Expression Omnibus under accession numbers ERP001478 (DNA-seq) and GSE38233 (RNA-seq).
Supplemental Information includes Supplemental Experimental Procedures, four figures, and ten tables and can be found with this article online at http://dx.doi.org/10.1016/j.celrep.2013.10.002.
References
- Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, et al. Gene ontology: tool for the unification of biology. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanasiadis A, Rich A, Maas S. Widespread A-to-I RNA editing of Alu-containing mRNAs in the human transcriptome. PLoS Biol. 2004;2:e391. doi: 10.1371/journal.pbio.0020391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahn JH, Lee JH, Li G, Greer C, Peng G, Xiao X. Accurate identification of A-to-I RNA editing in human by transcriptome sequencing. Genome Res. 2012;22:142–150. doi: 10.1101/gr.124107.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, Ren J, Li WW, Noble WS. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res. 2009;37(Web Server issue):W202–W208. doi: 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass BL, Weintraub H. An unwinding activity that covalently modifies its double-stranded RNA substrate. Cell. 1988;55:1089–1098. doi: 10.1016/0092-8674(88)90253-x. [DOI] [PubMed] [Google Scholar]
- Bentley DR, Balasubramanian S, Swerdlow HP, Smith GP, Milton J, Brown CG, Hall KP, Evers DJ, Barnes CL, Bignell HR, et al. Accurate whole human genome sequencing using reversible terminator chemistry. Nature. 2008;456:53–59. doi: 10.1038/nature07517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blow M, Futreal PA, Wooster R, Stratton MR. A survey of RNA editing in human brain. Genome Res. 2004;14:2379–2387. doi: 10.1101/gr.2951204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmi S, Borukhov I, Levanon EY. Identification of widespread ultra-edited human RNAs. PLoS Genet. 2011;7:e1002317. doi: 10.1371/journal.pgen.1002317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang N, Yi J, Guo G, Liu X, Shang Y, Tong T, Cui Q, Zhan M, Gorospe M, Wang W. HuR uses AUF1 as a cofactor to promote p16INK4 mRNA decay. Mol Cell Biol. 2010;30:3875–3886. doi: 10.1128/MCB.00169-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SH, Habib G, Yang CY, Gu ZW, Lee BR, Weng SA, Silberman SR, Cai SJ, Deslypere JP, Rosseneu M, et al. Apolipoprotein B-48 is the product of a messenger RNA with an organ-specific in-frame stop codon. Science. 1987;238:363–366. doi: 10.1126/science.3659919. [DOI] [PubMed] [Google Scholar]
- Chen CX, Cho DS, Wang Q, Lai F, Carter KC, Nishikura K. A third member of the RNA-specific adenosine deaminase gene family, ADAR3, contains both single- and double-stranded RNA binding domains. RNA. 2000;6:755–767. doi: 10.1017/s1355838200000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Mias GI, Li-Pook-Than J, Jiang L, Lam HYK, Chen R, Miriami E, Karczewski KJ, Hariharan M, Dewey FE, et al. Personal omics profiling reveals dynamic molecular and medical phenotypes. Cell. 2012;148:1293–1307. doi: 10.1016/j.cell.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung VG, Nayak RR, Wang IX, Elwyn S, Cousins SM, Morley M, Spielman RS. Polymorphic cis- and trans-regulation of human gene expression. PLoS Biol. 2010;8:e1000480. doi: 10.1371/journal.pbio.1000480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilibeck KA, Wu T, Liang C, Schellenberg MJ, Gesner EM, Lynch JM, MacMillan AM. FRET analysis of in vivo dimerization by RNA-editing enzymes. J Biol Chem. 2006;281:16530–16535. doi: 10.1074/jbc.M511831200. [DOI] [PubMed] [Google Scholar]
- Clerzius G, Gélinas JF, Daher A, Bonnet M, Meurs EF, Gatignol A. ADAR1 interacts with PKR during human immunodeficiency virus infection of lymphocytes and contributes to viral replication. J Virol. 2009;83:10119–10128. doi: 10.1128/JVI.02457-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel C, Venø MT, Ekdahl Y, Kjems J, Öhman M. A distant cis acting intronic element induces site-selective RNA editing. Nucleic Acids Res. 2012;40:9876–9886. doi: 10.1093/nar/gks691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson TR, Sansam CL, Emeson RB. Structure and sequence determinants required for the RNA editing of ADAR2 substrates. J Biol Chem. 2004;279:4941–4951. doi: 10.1074/jbc.M310068200. [DOI] [PubMed] [Google Scholar]
- Fan XC, Steitz JA. Overexpression of HuR, a nuclear-cytoplasmic shuttling protein, increases the in vivo stability of ARE-containing mRNAs. EMBO J. 1998;17:3448–3460. doi: 10.1093/emboj/17.12.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimson A, Farh KKH, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell. 2007;27:91–105. doi: 10.1016/j.molcel.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heale BSE, Keegan LP, McGurk L, Michlewski G, Brindle J, Stanton CM, Caceres JF, O’Connell MA. Editing independent effects of ADARs on the miRNA/siRNA pathways. EMBO J. 2009;28:3145–3156. doi: 10.1038/emboj.2009.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan DJ, Riordan DP, Gerber AP, Herschlag D, Brown PO. Diverse RNA-binding proteins interact with functionally related sets of RNAs, suggesting an extensive regulatory system. PLoS Biol. 2008;6:e255. doi: 10.1371/journal.pbio.0060255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009a;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Huang W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009b;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju YS, Kim JI, Kim S, Hong D, Park H, Shin JY, Lee S, Lee WC, Kim S, Yu SB, et al. Extensive genomic and transcriptional diversity identified through massively parallel DNA and RNA sequencing of eighteen Korean individuals. Nat Genet. 2011;43:745–752. doi: 10.1038/ng.872. [DOI] [PubMed] [Google Scholar]
- Kim U, Wang Y, Sanford T, Zeng Y, Nishikura K. Molecular cloning of cDNA for double-stranded RNA adenosine deaminase, a candidate enzyme for nuclear RNA editing. Proc Natl Acad Sci USA. 1994;91:11457–11461. doi: 10.1073/pnas.91.24.11457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DDY, Kim TTY, Walsh T, Kobayashi Y, Matise TC, Buyske S, Gabriel A. Widespread RNA editing of embedded alu elements in the human transcriptome. Genome Res. 2004;14:1719–1725. doi: 10.1101/gr.2855504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiran A, Baranov PV. DARNED: a DAtabase of RNa EDiting in humans. Bioinformatics. 2010;26:1772–1776. doi: 10.1093/bioinformatics/btq285. [DOI] [PubMed] [Google Scholar]
- Kishore S, Jaskiewicz L, Burger L, Hausser J, Khorshid M, Zavolan M. A quantitative analysis of CLIP methods for identifying binding sites of RNA-binding proteins. Nat Methods. 2011;8:559–564. doi: 10.1038/nmeth.1608. [DOI] [PubMed] [Google Scholar]
- Klaue Y, Källman AM, Bonin M, Nellen W, Ohman M. Biochemical analysis and scanning force microscopy reveal productive and nonproductive ADAR2 binding to RNA substrates. RNA. 2003;9:839–846. doi: 10.1261/rna.2167603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuttan A, Bass BL. Mechanistic insights into editing-site specificity of ADARs. Proc Natl Acad Sci USA. 2012;109:E3295–E3304. doi: 10.1073/pnas.1212548109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal A, Mazan-Mamczarz K, Kawai T, Yang X, Martindale JL, Gorospe M. Concurrent versus individual binding of HuR and AUF1 to common labile target mRNAs. EMBO J. 2004;23:3092–3102. doi: 10.1038/sj.emboj.7600305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebedeva S, Jens M, Theil K, Schwanhäusser B, Selbach M, Landthaler M, Rajewsky N. Transcriptome-wide analysis of regulatory interactions of the RNA-binding protein HuR. Mol Cell. 2011;43:340–352. doi: 10.1016/j.molcel.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Lehmann KA, Bass BL. Double-stranded RNA adenosine deaminases ADAR1 and ADAR2 have overlapping specificities. Biochemistry. 2000;39:12875–12884. doi: 10.1021/bi001383g. [DOI] [PubMed] [Google Scholar]
- Levanon EY, Eisenberg E, Yelin R, Nemzer S, Hallegger M, Shemesh R, Fligelman ZY, Shoshan A, Pollock SR, Sztybel D, et al. Systematic identification of abundant A-to-I editing sites in the human transcriptome. Nat Biotechnol. 2004;22:1001–1005. doi: 10.1038/nbt996. [DOI] [PubMed] [Google Scholar]
- Li JB, Levanon EY, Yoon JK, Aach J, Xie B, Leproust E, Zhang K, Gao Y, Church GM. Genome-wide identification of human RNA editing sites by parallel DNA capturing and sequencing. Science. 2009;324:1210–1213. doi: 10.1126/science.1170995. [DOI] [PubMed] [Google Scholar]
- Li M, Wang IX, Li Y, Bruzel A, Richards AL, Toung JM, Cheung VG. Widespread RNA and DNA sequence differences in the human transcriptome. Science. 2011;333:53–58. doi: 10.1126/science.1207018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licatalosi DD, Darnell RB. RNA processing and its regulation: global insights into biological networks. Nat Rev Genet. 2010;11:75–87. doi: 10.1038/nrg2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T, Reed R. An extensive network of coupling among gene expression machines. Nature. 2002;416:499–506. doi: 10.1038/416499a. [DOI] [PubMed] [Google Scholar]
- Mukherjee N, Corcoran DL, Nusbaum JD, Reid DW, Georgiev S, Hafner M, Ascano M, Jr, Tuschl T, Ohler U, Keene JD. Integrative regulatory mapping indicates that the RNA-binding protein HuR couples pre-mRNA processing and mRNA stability. Mol Cell. 2011;43:327–339. doi: 10.1016/j.molcel.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myer VE, Fan XC, Steitz JA. Identification of HuR as a protein implicated in AUUUA-mediated mRNA decay. EMBO J. 1997;16:2130–2139. doi: 10.1093/emboj/16.8.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Y, Ding L, Kao PN, Braun R, Yang JH. ADAR1 interacts with NF90 through double-stranded RNA and regulates NF90-mediated gene expression independently of RNA editing. Mol Cell Biol. 2005;25:6956–6963. doi: 10.1128/MCB.25.16.6956-6963.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osenberg S, Dominissini D, Rechavi G, Eisenberg E. Widespread cleavage of A-to-I hyperediting substrates. RNA. 2009;15:1632–1639. doi: 10.1261/rna.1581809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z, Cheng Y, Tan BCM, Kang L, Tian Z, Zhu Y, Zhang W, Liang Y, Hu X, Tan X, et al. Comprehensive analysis of RNA-seq data reveals extensive RNA editing in a human transcriptome. Nat Biotechnol. 2012;30:253–260. doi: 10.1038/nbt.2122. [DOI] [PubMed] [Google Scholar]
- Powell LM, Wallis SC, Pease RJ, Edwards YH, Knott TJ, Scott J. A novel form of tissue-specific RNA processing produces apolipopro-tein-B48 in intestine. Cell. 1987;50:831–840. doi: 10.1016/0092-8674(87)90510-1. [DOI] [PubMed] [Google Scholar]
- Rodriguez J, Menet JS, Rosbash M. Nascent-seq indicates widespread cotranscriptional RNA editing in Drosophila. Mol Cell. 2012;47:27–37. doi: 10.1016/j.molcel.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueter SM, Burns CM, Coode SA, Mookherjee P, Emeson RB. Glutamate receptor RNA editing in vitro by enzymatic conversion of adenosine to inosine. Science. 1995;267:1491–1494. doi: 10.1126/science.7878468. [DOI] [PubMed] [Google Scholar]
- Scadden AD, Smith CW. Specific cleavage of hyper-edited dsRNAs. EMBO J. 2001;20:4243–4252. doi: 10.1093/emboj/20.15.4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Lin L, Cai JJ, Jiang P, Kenkel EJ, Stroik MR, Sato S, Davidson BL, Xing Y. Widespread establishment and regulatory impact of Alu exons in human genes. Proc Natl Acad Sci USA. 2011;108:2837–2842. doi: 10.1073/pnas.1012834108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Hendrickson DG, Sauvageau M, Goff L, Rinn JL, Pachter L. Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat Biotechnol. 2013;31:46–53. doi: 10.1038/nbt.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SK, Sato S, Lazinski DW. Substrate recognition by ADAR1 and ADAR2. RNA. 2001;7:846–858. doi: 10.1017/s135583820101007x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Lamm AT, Fire AZ. Competition between ADAR and RNAi pathways for an extensive class of RNA targets. Nat Struct Mol Biol. 2011;18:1094–1101. doi: 10.1038/nsmb.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JH, Sklar P, Axel R, Maniatis T. Editing of glutamate receptor subunit B pre-mRNA in vitro by site-specific deamination of adenosine. Nature. 1995;374:77–81. doi: 10.1038/374077a0. [DOI] [PubMed] [Google Scholar]
- Yang W, Wang Q, Howell KL, Lee JT, Cho DSC, Murray JM, Nishikura K. ADAR1 RNA deaminase limits short interfering RNA efficacy in mammalian cells. J Biol Chem. 2005;280:3946–3953. doi: 10.1074/jbc.M407876200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.