Abstract
Background
The functional disability and high costs of treating anterior cruciate ligament (ACL) injuries have generated a great deal of interest in understanding the mechanism of noncontact ACL injuries. Secondary bone bruises have been reported in over 80% of partial and complete ACL ruptures.
Purpose
The objectives of this study were (1) to quantify ACL strain under a range of physiologically relevant loading conditions and (2) to evaluate soft tissue and bony injury patterns associated with applied loading conditions thought to be responsible for many noncontact ACL injuries.
Study Design
Controlled laboratory study.
Methods
Seventeen cadaveric legs (age, 45 ± 7 years; 9 female and 8 male) were tested utilizing a custom-designed drop stand to simulate landing. Specimens were randomly assigned between 2 loading groups that evaluated ACL strain under either knee abduction or internal tibial rotation moments. In each group, combinations of anterior tibial shear force, and knee abduction and internal tibial rotation moments under axial impact loading were applied sequentially until failure. Specimens were tested at 25° of flexion under simulated 1200-N quadriceps and 800-N hamstring loads. A differential variable reluctance transducer was used to calculate ACL strain across the anteromedial bundle. A general linear model was used to compare peak ACL strain at failure. Correlations between simulated knee injury patterns and loading conditions were evaluated by the χ2 test for independence.
Results
Anterior cruciate ligament failure was generated in 15 of 17 specimens (88%). A clinically relevant distribution of failure patterns was observed including medial collateral ligament tears and damage to the menisci, cartilage, and subchondral bone. Only abduction significantly contributed to calculated peak ACL strain at failure (P = .002). While ACL disruption patterns were independent of the loading mechanism, tibial plateau injury patterns (locations) were significantly (P = .002) dependent on the applied loading conditions. Damage to the articular cartilage along with depression of the midlateral tibial plateau was primarily associated with knee abduction moments, while cartilage damage with depression of the posterolateral tibial plateau was primarily associated with internal tibial rotation moments.
Conclusion
The current findings demonstrate the relationship between the location of the tibial plateau injury and ACL injury mechanisms. The resultant injury locations were similar to the clinically observed bone bruises across the tibial plateau during a noncontact ACL injury. These findings indicate that abduction combined with other modes of loading (multiplanar loading) may act to produce ACL injuries.
Clinical Relevance
A better understanding of ACL injury mechanisms and associated risk factors may improve current preventive, surgical, and rehabilitation strategies and limit the risk of ACL and secondary injuries, which may in turn minimize the future development of posttraumatic osteoarthritis of the knee.
Keywords: anterior cruciate ligament, knee, injury mechanism, bone bruise
An acute anterior cruciate ligament (ACL) injury can be devastating and often results in clinical sequelae including meniscal tears, chondral lesions, and osteoarthritis.1-3,8,9,12,26,43 The majority of these injuries are defined as noncontact (without a direct blow to the knee joint),16,18 resulting from jump landings5,45 and lateral cutting maneuvers that may occur in different athletic activities such as volleyball, basketball, and soccer.1,3,40,46 The high number of ACL injuries (more than 120,000 in the United States annually),31 associated costs, and resulting long-term disability have generated significant interest in the investigation of noncontact ACL injury mechanisms.
Many investigators have developed experimental strategies to reproduce ACL injuries.11,35,39,44,58 Such experiments have focused on a variety of causative factors including muscle loading11,58 and axial compression39 to generate ACL strain. However, these and other studies have failed to consistently produce ACL injuries with a clinically relevant distribution of soft tissue and bony injuries commonly reported to occur in patients who have sustained ACL injuries.
Clinical, biomechanical, and video analysis studies have demonstrated that an anterior tibial shear force,6,13,34,36 and knee abduction5,15,32,33,45 and internal tibial rotation moments4,37,38,44 are the most likely loading conditions responsible for noncontact ACL injuries. However, there is still controversy and debate about the exact mechanism responsible for noncontact ACL injuries. Axial compression across the knee joint caused by ground-reaction force during initial landing may generate severe tibiofemoral articular cartilage loading.38,47 Subsequently, load transferred from articular cartilage to subchondral bone may often result in bone bruises associated with microfractures of the subchondral or trabecular bone or, in severe cases, tibial plateau fractures.19,25,41,42,48,54,56 Various bone bruise patterns of the tibia and femur, and bone contusions of the lateral tibial plateau, as seen on magnetic resonance imaging (MRI) scans,†† have been observed in more than 80% of partial or complete ACL tears.41,54,55 Understanding the relationship between bone bruise patterns (locations) and loading conditions may help identify ACL injury mechanisms.47
The objectives of this biomechanical laboratory study were (1) to quantify ACL strain under a range of physiologically relevant loading conditions to identify high-risk loading factors for ACL injuries and (2) to evaluate both soft tissue and bony injury patterns associated with applied loading conditions thought to be responsible for many non-contact ACL injuries with the goal of determining the relationship between loading mechanisms and resultant injury patterns. To accomplish this goal, an experimental model capable of generating an ACL injury by reproducing a range of loading conditions experienced during high-risk activities was developed.
The current study employed a unique custom-designed drop stand with physiologically relevant drop weights and drop heights in an effort to apply various combinations of axial compression, anterior tibial shear force, and knee abduction and internal tibial rotation moments that have been associated with landing. The complete loading path from ground contact with the shoe through the foot and knee has been incorporated into this model. In this way, load transmission and associated damping maintain fidelity with real-world conditions. We hypothesized that identified high-risk loading factors would cause different injury/disruption patterns across the ACL and secondary anatomic structures, which are correlated with applied physiologically relevant loading conditions during a simulated landing from a jump. These established correlations would further help evaluate the mechanism of noncontact ACL injuries and potentially help with the development of better prevention strategies.
MATERIALS AND METHODS
Specimen Preparation
Seventeen unembalmed, fresh-frozen cadaveric lower limbs (age, 45 ± 7 years; 9 female and 8 male) were used in this study. Each specimen was inspected visually and by computed tomography and MRI for signs of soft or hard tissue injuries including ACL disruption. No obvious signs of injury were observed. Specimens were sectioned at the mid-femur (30 cm above the joint line). The proximal femur (up to 15 cm superior to the joint line) of each specimen was potted in polyester resin for rigid attachment to the testing frame. The quadriceps (rectus femoris) and hamstring (semitendinosus, biceps femoris, and semimembranosus) ten-dons were isolated and captured inside metal tendon grips to allow for the application of simulated muscle loads. Otherwise, the skin, musculature, and other soft tissue structures were not disrupted. The foot and ankle joint were maintained intact to provide a realistic load-transfer interface. Specimens were stored at –20°C. Before testing, specimens were slowly thawed to room temperature.
Testing Apparatus
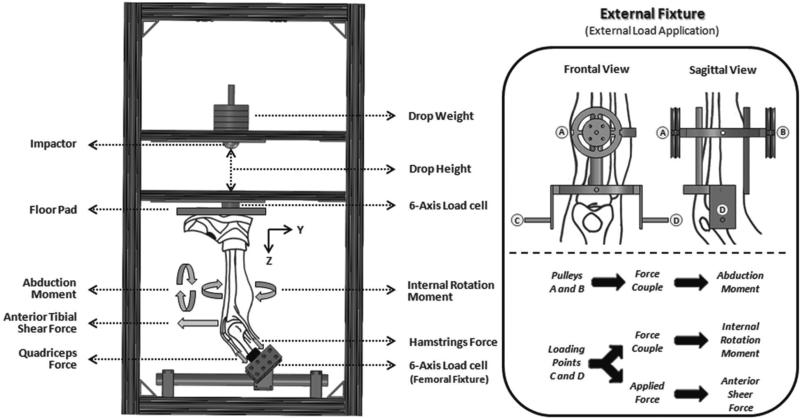
A custom-designed testing apparatus was used to simulate landing from a jump under a wide range of loading conditions (Figure 1).30 Each specimen was rigidly fixed at the proximal femur to a 6-axis load cell (B9401, RA Denton Corp, Rochester Hills, Michigan), while the tibia was orientated vertically with the foot positioned superiorly. All specimens were tested at 25° of flexion. This flexion angle is typical of injurious events based on video analysis studies of ACL injuries.32 The load cell was suspended such that the orientation of the femur could be rotated about all 3 axes to align the tibia with the vertical loading axis. A mass pulley system was used to apply 1200 N to the quadriceps tendons and 800 N to the hamstring tendons, while maintaining the physiological line of action of each tendon. To apply anterior tibial shear force and internal tibial rotation moment, an external fixation frame was rigidly attached to the tibia such that points of load application were located about the approximate knee center of rotation. Also, an integrated pulley system was placed on the midtibial shaft to apply knee abduction moments. The combination of static hanging weights and cable pulley system produced forces to generate an anterior tibial shear force and produced force couples to generate pure knee abduction and internal tibial rotation moments (Figure 1). As unconstrained force couples (free to move with the specimens) were used to generate pure moments in this study, their action was independent of the point of application. Thus, the abduction moment could be applied at any point along the tibia. The set value of the externally applied loads was maintained constant throughout each test. To change the load magnitude, different static weights were used.
Figure 1.
Testing apparatus: custom-designed drop stand (left) and external fixture (right).
While the proximal potted femur was rigidly fixed, the distal extremity was not constrained until it was placed in contact with the floor pad after loading and positioning the specimen. This allowed for the unconstrained application of external loads. After all external loads were applied, the tibia was vertically aligned by rotating the femoral fixture, and an athletic shoe was placed on the foot. Floor contact was simulated by an inverted floor pad set upon the shoe that was maintained in a horizontal position by a series of 3 vertically aligned linear bearings. Subsequent to alignment of the tibia, the floor pad was set upon the shoe to simulate a foot-planted position, maintaining the ankle in the neutral position. The foot–shoe–floor plate complex served to simulate a realistic load transfer. An impactor was designed, utilizing a series of 3 vertically oriented linear bearings to guide a mass into the floor plate in line with the tibia. Impacts were initiated by releasing either half body weight (350 N) or full body weight (700 N) from 30 or 60 cm above the foot to simulate a vertical landing15 by impacting the floor pad in a standard drop-stand configuration. A second 6-axis load cell (2586, RA Denton Corp) embedded in the floor plate was used to capture all forces and moments applied to the specimen during impact.
Analog data were collected at 4 kHz. Two arrays of 3 infrared light-emitting diode markers were rigidly attached to the femur and tibia to capture knee kinematics using an Optotrak 3020 (Northern Digital, Waterloo, Ontario, Canada) 3-dimensional motion capture system at 400 Hz. The ACL strain was calculated based on measurements from a differential variable reluctance transducer (DVRT) (MicroStrain Inc, Williston, Vermont) with a linear range of ±3 mm that was arthroscopically placed on the anteromedial (AM) bundle of the ACL.14 Average strain across the transducer was calculated based on the change in length of the measured segment using the following equation:
where L is the instantaneous length measured across the DVRT, and L0 is the length measured across the DVRT at the reference length of the ligament. Reference length was calculated as the distinct inflection point in the force versus DVRT displacement during 4 continuous cycles of anterior-posterior shear loading.13,22 The selected inflection point was chosen as the proper reference between ligament-taut and -slack states. Therefore, the reference length is not dependent on the initial gauge length of the DVRT at the time of insertion.
Loading Protocol
Multiple combinations of knee abduction and internal tibial rotation moments with or without anterior tibial shear force under axial impact were used to establish 2 loading groups: 1 that focused on the effects of the knee abduction moments, and 1 that focused on the effects of the internal tibial rotation moments on ACL strain (Table 1). Specimens were randomly assigned to each loading group and sequentially tested from condition 1 through 20 or until hard or soft tissue failure was observed. Observations of the mechanical response during testing were considered as indicators of ACL failure. Such observations included but were not limited to a sudden and unrecovered shift in anterior tibial translation (unrecovered anterior tibial shift >5 mm) and ACL strain (unrecovered elevated strain >2%). Subsequently, manual evaluation of joint laxity was followed by arthroscopic inspection to confirm ACL failure. Both loading protocols have similar starting and ending combinations to facilitate comparisons between specimens, while evaluating a broad range of injurious conditions.
TABLE 1.
Loading Protocola
| Knee Abduction Loading Group |
IR Loading Group |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Drop Weight, ×BW | Drop Height, cm | Anterior Shear Force, N | Abduction, N·m | Internal Rotation, N·m | Drop Weight, ×BW | Drop Height, cm | Anterior Shear Force, N | Abduction, N·m | Internal Rotation, N·m |
| 0.5 | 30 | 0 | 0 | 0 | 0.5 | 30 | 0 | 0 | 0 |
| 0.5 | 30 | 134 | 0 | 0 | 0.5 | 30 | 134 | 0 | 0 |
| 0.5 | 30 | 268 | 0 | 0 | 0.5 | 30 | 268 | 0 | 0 |
| 0.5 | 30 | 0 | 25 | 0 | 0.5 | 30 | 0 | 0 | 10 |
| 0.5 | 30 | 134 | 25 | 0 | 0.5 | 30 | 0 | 0 | 20 |
| 0.5 | 30 | 0 | 50 | 0 | 0.5 | 30 | 268 | 0 | 20 |
| 0.5 | 30 | 134 | 50 | 0 | 0.5 | 30 | 0 | 0 | 40 |
| 0.5 | 30 | 268 | 50 | 0 | 0.5 | 30 | 268 | 0 | 40 |
| 0.5 | 30 | 0 | 75 | 0 | 0.5 | 30 | 0 | 0 | 60 |
| 0.5 | 30 | 268 | 75 | 0 | 0.5 | 30 | 268 | 0 | 60 |
| 0.5 | 30 | 0 | 75 | 20 | 0.5 | 30 | 0 | 0 | 80 |
| 0.5 | 30 | 0 | 75 | 60 | 0.5 | 30 | 268 | 0 | 80 |
| 0.5 | 30 | 268 | 75 | 60 | 0.5 | 30 | 268 | 75 | 60 |
| 0.5 | 30 | 268 | 100 | 60 | 0.5 | 30 | 268 | 100 | 60 |
| 0.5 | 30 | 268 | 125 | 60 | 0.5 | 30 | 268 | 125 | 60 |
| 0.5 | 30 | 268 | 150 | 60 | 0.5 | 30 | 268 | 150 | 60 |
| 0.5 | 60 | 268 | 150 | 60 | 0.5 | 60 | 268 | 150 | 60 |
| 1 | 60 | 268 | 150 | 60 | 1 | 60 | 268 | 150 | 60 |
| 1 | 60 | 268 | 150 | 80 | 1 | 60 | 268 | 150 | 80 |
| 1 | 60 | 268 | 150 | 100 | 1 | 60 | 268 | 150 | 100 |
BW, body weight; IR, internal rotation.
Clinical Evaluation
After dynamic testing (simulated landings), a board-certified orthopaedic surgeon, fellowship trained in sports medicine, carefully dissected each specimen to evaluate the injury to the anatomic structures about the knee joint. Tissue damage and general conditions were photographically documented during dissection. Thorough anatomic inspection included the cruciate ligaments (anterior and posterior), collateral ligaments (medial and lateral), posterolateral corner (PLC) (popliteal tendon and popliteofibular ligament), menisci (medial and lateral), articular cartilage (femoral condyle and tibial plateau), bony structures (femur and tibia), and knee capsule. Care was taken to document the type and location of the injuries to address the hypotheses.
Statistical Analysis
A general linear model was used to evaluate the effects of anterior tibial shear force, and knee abduction and internal tibial rotation moments on peak ACL strain under failure loading conditions. The general linear model is an extension of the multifactorial analysis of variance (ANOVA), which is used to investigate the complex relationships between dependent and multiple independent variables. This approach was employed to statistically quantify the effect of different loading factors on calculated peak ACL strain at failure over a range of the treatment group sizes. A χ2 test for independence was conducted on the clinical observations of resultant injuries to investigate the relationship between injury patterns and loading mechanisms. This test was used to examine whether specific factors affect the distribution of binomial (ie, yes or no) outcomes.
RESULTS
Anterior cruciate ligament failure was generated in 15 of 17 specimens (88%). Details of ACL strain at failure and associated loading conditions are shown in Table 2. In 4 of 17 specimens (23%), the medial collateral ligament (MCL) was also injured. However, complete disruption was not observed. Inspection of the lateral collateral ligament (LCL) and PLC revealed that in only 1 of 17 specimens (6%), the LCL was injured. Only 1 specimen sustained a posterior cruciate ligament (PCL) rupture. Of the ACL failures observed, 11 of 15 specimens (73%) primarily demonstrated soft tissue failure, while 4 of 15 specimens (27%) primarily demonstrated bony avulsions. Three of the 15 specimens with ACL failure (20%) failed under axial impact with additional anterior tibial shear forces. Only 1 specimen (7%) sustained ACL failure under an internal tibial rotation moment along with axial impact. Four (27%) sustained ACL failure under a knee abduction moment (2 with and 2 without anterior tibial shear force) along with axial impact. Finally, 7 of 15 specimens (47%) sustained an ACL injury because of combined knee abduction and internal tibial rotation moments, and anterior tibial shear force under axial impact. A summary of the observed injury patterns across the knee joint is provided in Table 3. The general linear model showed that only knee abduction moment significantly contributed to calculated peak ACL strain at failure (P = .002), while neither additional anterior tibial shear force (P = .41) nor internal tibial rotation moment (P = .76) affected the peak ACL strain at failure significantly.
TABLE 2.
Peak ACL Strain at Failure and Associated Loading Conditionsa
| Loading at Failure |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Specimens |
Loading Group | Peak ACL Strain, % | Drop Weight, ×BW | Drop Height, cm | Anterior Shear Force, N | Abduction, N·m | Internal Rotation, N·m | |||
| ID | Sex | Age, y | Side | |||||||
| 1-C080044 | F | 52 | L | Abduction | 13.5 | 0.5 | 30 | 0 | 75 | 0 |
| 2-C090105 | M | 38 | L | IR | 34.5 | 1 | 60 | 268 | 150 | 60 |
| 3-C080033 | M | 51 | L | Abduction | 34.6 | 1 | 60 | 268 | 150 | 80 |
| 4-S090574 | F | 49 | R | Abduction | 28.4 | 0.5 | 30 | 268 | 50 | 0 |
| 5-C090155 | M | 46 | L | Abduction | 36 | 0.5 | 60 | 268 | 150 | 60 |
| 6-C090105 | M | 38 | R | Abduction | 12.7 | 0.5 | 30 | 0 | 75 | 20 |
| 7-C090155 | M | 46 | R | IR | 17.2 | 0.5 | 30 | 268 | 75 | 60 |
| 8-C090361 | M | 34 | R | Abduction | 15.9 | 0.5 | 30 | 0 | 25 | 0 |
| 9-C090508 | F | 45 | L | Abduction | 20.7 | 0.5 | 60 | 268 | 150 | 60 |
| 10-C090508 | F | 45 | R | IR | 13 | 0.5 | 30 | 0 | 0 | 10 |
| 11-C080044 | F | 52 | R | Abduction | 9.7 | 0.5 | 30 | 268 | 0 | 0 |
| 12-C090552 | M | 45 | R | IR | — | 0.5 | 30 | 0 | 0 | 20 |
| 13-1007889 | F | 29 | R | IR | — | 0.5 | 30 | 0 | 0 | 40 |
| 14-1008352 | F | 54 | L | Abduction | 7 | 0.5 | 30 | 134 | 25 | 0 |
| 15-1008352 | F | 54 | R | IR | 11.3 | 0.5 | 30 | 134 | 0 | 0 |
| 16-C090552 | M | 45 | L | Abduction | 18.9 | 0.5 | 60 | 268 | 150 | 60 |
| 17-S090706 | F | 50 | L | IR | 7.1 | 0.5 | 30 | 134 | 0 | 0 |
ID, identification number; ACL, anterior cruciate ligament; BW, body weight; F, female; M, male; L, left; R, right; IR, internal rotation.
TABLE 3.
Observed Injury Patterns Across the Knee Joint After Failurea
| Specimens |
Loading Group | ACL Disruption Pattern | Articular Cartilage Damage Location |
Tibial Plateau Depression Location | Damage to Other Structures | ||
|---|---|---|---|---|---|---|---|
| ID | Side | Femoral | Tibial | ||||
| 1-C080044 | L | Abduction | Complete tear at F | ML, MM | ML | ML | Partial MCL tear at J |
| 2-C090105 | L | IR | Avulsion at T | ML | PL | PL | |
| 3-C080033 | L | Abduction | AM bundle tear at T | Intact | ML | ML | |
| 4-S090574 | R | Abduction | Complete tear at F | Intact | ML | ML | |
| 5-C090155 | L | Abduction | AM bundle tear at T | Intact | Intact | Intact | |
| 6-C090105 | R | Abduction | Complete tear at M | Intact | ML | ML | |
| 7-C090155 | R | IR | Complete tear at F | ML | PL | PL | Partial MCL tear at J, damage to PL corner of the lateral meniscus |
| 8-C090361 | R | Abduction | Avulsion at T | ML, MM | Intact | Intact | |
| 9-C090508 | L | Abduction | Avulsion at T | ML | ML, MM | PM, PL | |
| 10-C090508 | R | IR | Partial tear at T | Intact | PL | PL | Damage to PL corner of the lateral meniscus |
| 11-C080044 | R | Abduction | Complete tear at M | Intact | ML | ML | |
| 12-C090552 | R | IR | Intact | Intact | PL | Intact | Femoral shaft fracture, partial MCL tear at J, damage to PL corner of the lateral meniscus |
| 13-1007889 | R | IR | Intact | Intact | Intact | Intact | Tibial shaft fracture, damage to PL corner of the lateral meniscus |
| 14-1008352 | L | Abduction | Complete tear at T | Intact | ML | ML | |
| 15-1008352 | R | IR | AM bundle tear at T | Intact | Intact | Intact | |
| 16-C090552 | L | Abduction | Avulsion at T | Intact | Intact | Intact | LCL tear at F |
| 17-S090706 | L | IR | Complete tear at M | Intact | PL | PL | Partial MCL tear at T, PCL complete rupture at T, damage to PL corner of the lateral meniscus |
ID, identification number; ACL, anterior cruciate ligament; L, left; R, right; IR, internal rotation; F, femoral insertion; T, tibial insertion; M, midsubstance; AM, anteromedial; ML, midlateral; MM, midmedial; PL, posterolateral; PM, posteromedial; MCL, medial collateral ligament; J, joint line; LCL, lateral collateral ligament; PCL, posterior cruciate ligament.
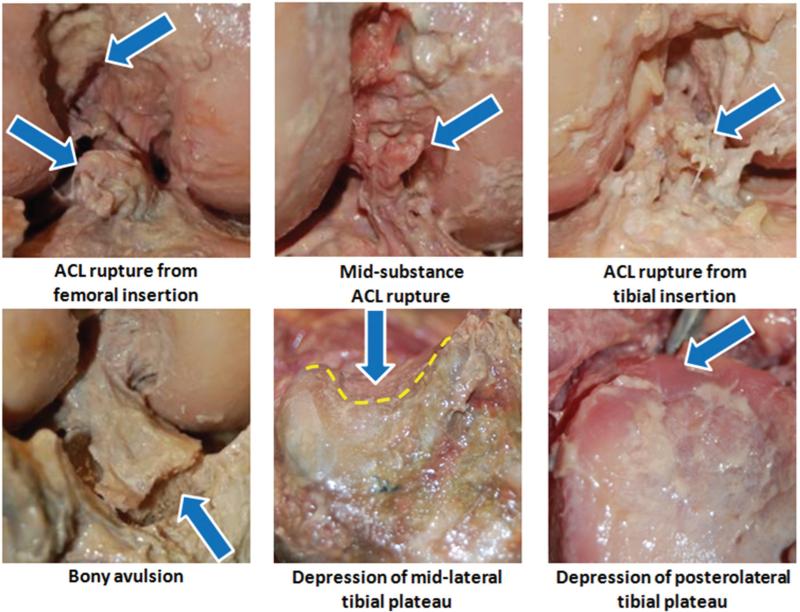
Tibial plateau damage (both articular cartilage and subchondral bone) was sustained in 11 of 17 cases (65%). Substantial damage to the articular cartilage without observable bony damage was seen in the tibial plateau for 1 of 17 cases (6%) and in the femoral condyles for 5 of 17 cases (29%). Representative photographs are provided to demonstrate common injury patterns in Figure 2. Of 11 tibial plateau injuries, cartilage damage along with depression of the subchondral bone occurred across the midlateral tibial plateau (group I) in 6 specimens, posterolateral aspect of the tibial plateau (group II) in 4 specimens, and bicondylar across the posterior aspect of the tibial plateau (group III) in 1 specimen. Group I injuries were associated with knee abduction moment alone (with and without anterior tibial shear force) in 3 cases, combined knee abduction and internal tibial rotation moments (with anterior tibial shear force) in 2 cases, and anterior tibial shear force alone in 1 case. Group II injuries occurred under an internal tibial rotation moment (without anterior tibial shear force) in 3 cases and anterior tibial shear force alone in 1 case. The χ2 test for independence showed that ACL disruption patterns (type: partial/complete, AM/posterolateral [PL] bundle; location: femoral insertion/mid-substance/tibial insertion/avulsion) were independent of applied loading conditions. No statistically significant relationship was observed between injury patterns across the MCL, LCL, PCL, menisci and femoral condyles, and loading conditions. Only the tibial plateau injury patterns (locations) were demonstrated to be significantly dependent on applied loading conditions for both cartilage damage (P = .002) and depression of the subchondral bone (P = .005).
Figure 2.
Representative images of structural damages about the knee joint following failure.
DISCUSSION
Bone bruise patterns of the femur and tibia as well as bone contusions of the tibial plateau are common complications associated with partial or complete ACL ruptures in over 80% of patients.‡‡ Knowledge of the relationship between injury locations on bony structures and underlying applied loading conditions may help to identify mechanisms of an ACL injury.
In this study, simulated landings from a jump were conducted on 17 cadaveric specimens using a custom-designed drop stand. The current findings were not able to support the first part of our hypothesis that a relationship exists between ACL disruption patterns (type: partial/complete, AM/PL bundle; location: femoral insertion/midsubstance/tibial insertion) and loading mechanisms, leading to injury, as the χ2 test demonstrated that ACL disruption patterns were independent of applied loading conditions. Statistical findings demonstrated that tibial plateau injury patterns (locations) were significantly dependent on applied loading conditions. These findings support our second hypothesis that resultant injury patterns across the secondary knee structures are correlated with loading conditions during a simulated landing. Tests were designed to evaluate the effects of anterior tibial shear force and knee abduction and internal tibial rotation moments under dynamic axial loading on ACL biomechanics as well as the type of secondary injuries that occur in other anatomic structures during ACL injuries. Anterior cruciate ligament failures were produced in 88% of specimens, demonstrating a clinically relevant distribution of ACL failure patterns that are commonly observed in patients experiencing noncontact ACL injuries. Injuries to the MCL, articular cartilage, and subchondral bone across the tibial plateau were observed, as are often associated with ACL injuries. The relevance and reproducibility of results in this study demonstrate that our testing protocol closely simulates the complex and sometimes violent environment that leads to ACL injuries in vivo.
Our findings demonstrate the significant role of the knee abduction moment on ACL strain under different modes of simulated landing employing a novel method. While no specific relationship was discovered between ACL disruption patterns and landing mechanisms, a strong correlation was observed between tibial plateau injury (both articular cartilage and subchondral bone) patterns (locations) and landing mechanisms.
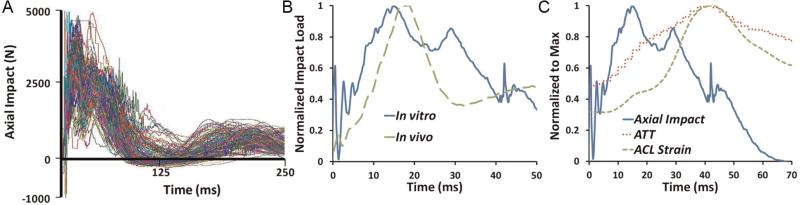
Biomechanical data from video analyses and in vivo studies demonstrate an increase in the risk of ACL injury under valgus rotation of the tibia.5,21,32,33 Our data support abduction as an important component of the ACL injury mechanism, as 67% of specimens failed under a loading condition that included an abduction moment. Using a general linear model, data demonstrate that only knee abduction moment played a significant role in peak ACL strains preceding injury under axial impact as tested in this study. Anterior tibial shear force used in the statistical model represents the externally applied shear force that was used to simulate the effects of different factors such as excessive quadriceps contraction,11,59 acceleration and deceleration,51 or other factors that may lead to anterior tibial shear force. This applied anterior tibial shear force should not be mistaken for the total shear force experienced across the knee joint, including that generated under axial impact.39 A post hoc power analysis employing average ACL strain levels for all experiments (a total of 163 tests including conditions that led to failure) demonstrated a statistical power of .9 with a nominal α of .05 by testing 17 specimens randomly assigned to 2 loading groups. However, the subset analysis of failure loading conditions alone resulted in limited power because of a lower number of data points. This lower number of data points at failure may limit our ability to discern a significant contribution of internal tibial rotation moment and anterior tibial shear force in generating ACL strain at failure. However, additional analysis utilizing the general linear model on all 163 loading conditions (subfailure and failure) resulted in similar trends, suggesting that abduction may be the only significant factor in elevated levels of ACL strain under simulated landings.28 Findings indicate that the primary factor that determines ACL strain in these experiments is axial impact. This cause and effect relationship is demonstrated by a predictable spike in ACL strain following axial impact in all cases tested (Figure 3), reaching the peak value. Other axes of loading certainly act to mediate the magnitude of ACL strain under axial impact, as described.
Figure 3.
(A) Axial impacts that simulated the ground-reaction force during landing were reproducible, as shown for all 17 specimens and 163 total impacts plotted together. (B) Axial load time history at the floor pad compared favorably with (unpublished) in vivo data. (C) Synchronization of axial loading history, anterior tibial translation, and ACL strain.
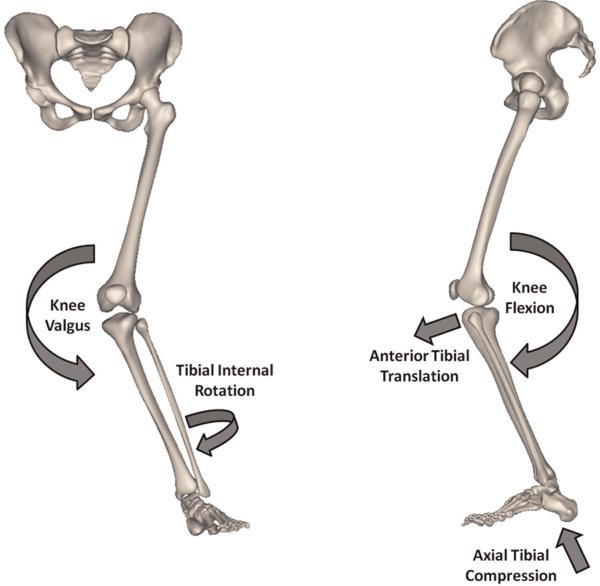
Knee abduction load is an important factor in ACL injuries.15,20,32,50,58 In our study, 47% of ACL injuries occurred under combined loading conditions (anterior tibial shear, knee abduction, and internal tibial rotation), indicating that knee abduction in combination with other modes of loading leads to high ACL strain and injury. These findings reinforce the assertion that multiplanar loading is a likely mechanism of ACL injuries (Figure 4). Previous studies have also considered combined multiplanar loading to be the worst-case scenario for ACL injuries.26,52
Figure 4.
Multiplanar loading mechanism of a noncontact anterior cruciate ligament injury.
Evaluation of injury patterns in the femur and tibia demonstrated damage to the midlateral femoral condyle in 5 cases in addition to 3 specific groups of tibial plateau injuries (to both cartilage and subchondral bone) in 11 cases. These injuries were all located at the middle to posterior portion of the lateral tibial plateau with only 1 bicondylar injury.
Valgus rotation of the tibia will increase the lateral compression of the femur and tibia,29,47 which can often lead to serious damage of the cartilage and subchondral bone between the femur and tibia.25 Excessive abduction moments across the knee joint during impact because of landing may cause extensive bony damage in the tibial plateau, as demonstrated by specimens tested in this study. Knee abduction moment seems to be the causative factor in specimens with cartilage damage and bony depression located in the midlateral tibial plateau, as all the specimens with group I injuries were tested under the loading group focused on knee abduction moment. Internal rotation of the tibia acts to translate the position of contact between the lateral femoral condyle and tibial plateau toward the posterolateral tibial plateau.29 Also, coupled valgus rotation of the internally rotated tibia53 will act to shift loading to the lateral side of the tibiofemoral joint. Combined knee abduction and internal tibial rotation result in compressive impact loading of the posterolateral corner on the tibial plateau against the lateral femoral condyle.47 This compression combined with axial impact loading across the joint during vertical landing can generate bone bruises on the posterolateral corner of the tibia47,49 or even bony fractures under more severe conditions. This theory was supported by the cartilage damage and depression of the subchondral bone across the posterolateral tibial plateau in specimens that tested under the loading group focused on the internal tibial rotation moment. Damage to the posterior aspect of the lateral meniscus was also observed in these specimens. These findings, in addition to the results of χ2 statistical analysis, support a strong association between the location of the tibial plateau injuries and ACL injury mechanisms. It must be noted that in our work, depression of the subchondral bone occurs with elevated frequency to what might be expected clinically. Bony depressions documented in this work were observed as osteochondral disruptions, resulting in depression of the surface of the tibial plateau without bony fragments or obvious fissures. It is uncertain whether this phenomenon is because of compromised mechanical properties of cadaveric tissue or increased severity of loading. In either case, it is probable that what appears as a subchondral bone depression in this work represents bone bruises or contusions in an in vivo model. Such observed tissue damage may correspond to microfractures of subchondral bone48,54 that may be difficult to detect under current imaging modalities and may well be interpreted as bone bruises. Further, the detection of tissue damage indicative of a bone bruise would be challenging in the absence of the in vivo tissue response to trauma. The correlation between the location of tissue damage observed in this study and that observed clinically is highly compelling evidence that can be derived from this experimental model.
A primary strength of this study is the development and utilization of a novel testing method that can accurately and reproducibly generate a range of clinically relevant ACL injuries. A clinically relevant17 distribution of soft and hard tissue failure characteristic of ACL injuries including partial and complete tears of the ACL and MCL as well as damage to the menisci, articular cartilage, and PLC was observed. Detailed attention to realistic impact parameters relative to sports injuries including body mass, drop height, and loading interface helped to generate an in vitro load time history similar to in vivo ground-reaction force data (Figure 3) measured by force plates during a landing from a jump task (unpublished data collected by Hewett et al).
As with all experimental studies, this work does have inherent limitations. While bony injuries were consistently seen in clinically relevant locations associated with tibial bone bruises, injury severity indicates that in some cases, higher magnitudes of loading may have been applied than are typically observed in real-world injuries. Shin et al52 have reported knee abduction moments up to 51 N·m and internal tibial rotation moments up to 30 N·m during noninjurious landings.52 However, higher magnitudes of the abovementioned loads were applied to simulate the high-risk injury conditions. Further, mechanical properties of the cadaveric tissue may have led to increased severity of tissue damage; thus, depression of subchondral bone in our cadaveric model may represent bone bruises under in vivo conditions. Further refinement of our testing protocol may help to more closely reproduce clinically observed injury patterns. Care was taken to understand these limitations during the interpretation of our findings. Avulsion failure of the ACL at the tibial attachment is not reflective of commonly reported injuries among young athletes but tends to be more common in studies that employ cadaveric tissue.38 While care was taken to identify any obvious damage to the specimens between loading conditions, inherent cumulative microdamage associated with injury biomechanics may have resulted in increased severity of tissue damage and elevated ACL strain levels at failure. Moreover, because of challenges associated with the determination of the exact yield (failure) of ACL strain during tested loading conditions, the peak calculated strain value under the loading that leads to failure was reported as the most appropriate measure of ACL strain at failure. Further, the high magnitudes of calculated peak ACL strain at failure may be indicative of complete tissue disruption or accumulated microdamage across the ACL prior to failure. However, calculated ACL strains before and after testing (subfailure) were not observed to differ by greater than 2% strain, suggesting that no substantial damage was sustained by the ACL before the failure loading condition. These factors present challenges in conducting robust parametric studies for high-rate mechanical characterization on any single specimen. As a result, increasing the sample size may improve our ability to resolve trends within our results. Ongoing efforts to utilize an experimentally validated finite element model27 will help us to further interpret these findings where experimental measurements were not possible such as stress and strain distributions under injurious conditions.
CONCLUSION
To the authors’ knowledge, this work represents the first study to both consistently generate ACL failures under impact conditions and to generate a physiological distribution of tissue damage realistic of clinically observed injuries. Data from this study indicate that the most critical dynamic loading conditions that lead to ACL failure include a combination of anterior tibial shear force, and knee abduction and internal tibial rotation moments under axial impact. The current findings highlight the significant role of knee abduction moment on ACL injury. While no distinct relationship between ACL disruption patterns and loading conditions was observed, the location of the tibial plateau injuries was shown to be significantly dependent on applied loading conditions. It was demonstrated that cartilage damage along with depression of the subchondral bone across the midlateral tibial plateau is mainly associated with knee abduction moments. Further, damage to the articular cartilage in addition to subchondral bone depression across the posterolateral aspect of the tibial plateau is mainly associated with internal tibial rotation moments. Resultant injury (both articular cartilage and subchondral bone) locations were similar to clinically observed bone bruise patterns across the tibial plateau during actual cases of noncontact ACL injuries. A better understanding of ACL injury mechanisms may provide insight that would improve current preventive, surgical, and rehabilitation strategies, thus limiting the risk of ACL and secondary injuries. Such measures may in turn minimize associated posttraumatic knee osteoarthritis.
ACKNOWLEDGMENT
The authors thank Dr Michael Dennis for assistance with specimen imaging (computed tomography and MRI) and Dr Scott Molitor for assistance with statistical analysis.
One or more of the authors has declared the following potential conflict of interest or source of funding: This study was funded by the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (grants R01-AR049735 and R01-AR056259).
Footnotes
REFERENCES
- 1.Agel J, Arendt EA, Bershadsky B. Anterior cruciate ligament injury in National Collegiate Athletic Association basketball and soccer: a 13-year review. Am J Sports Med. 2005;33(4):524–530. doi: 10.1177/0363546504269937. [DOI] [PubMed] [Google Scholar]
- 2.Andriacchi TP, Briant PL, Bevill SL, Koo S. Rotational changes at the knee after ACL injury cause cartilage thinning. Clin Orthop Relat Res. 2006;442:39–44. doi: 10.1097/01.blo.0000197079.26600.09. [DOI] [PubMed] [Google Scholar]
- 3.Arendt E, Dick R. Knee injury patterns among men and women in collegiate basketball and soccer: NCAA data and review of literature. Am J Sports Med. 1995;23(6):694–701. doi: 10.1177/036354659502300611. [DOI] [PubMed] [Google Scholar]
- 4.Arnold JA, Coker TP, Heaton LM, Park JP, Harris WD. Natural history of anterior cruciate tears. Am J Sports Med. 1979;7(6):305–313. doi: 10.1177/036354657900700601. [DOI] [PubMed] [Google Scholar]
- 5.Boden BP, Dean GS, Feagin JA, Jr, Garrett WE., Jr Mechanisms of anterior cruciate ligament injury. Orthopedics. 2000;23(6):573–578. doi: 10.3928/0147-7447-20000601-15. [DOI] [PubMed] [Google Scholar]
- 6.Butler DL, Noyes FR, Grood ES. Ligamentous restraints to anterior-posterior drawer in the human knee: a biomechanical study. J Bone Joint Surg Am. 1980;62(2):259–270. [PubMed] [Google Scholar]
- 7.Chan KK, Resnick D, Goodwin D, Seeger LL. Posteromedial tibial plateau injury including avulsion fracture of the semimembranous tendon insertion site: ancillary sign of anterior cruciate ligament tear at MR imaging. Radiology. 1999;211(3):754–758. doi: 10.1148/radiology.211.3.r99jn16754. [DOI] [PubMed] [Google Scholar]
- 8.Chaudhari AM, Briant PL, Bevill SL, Koo S, Andriacchi TP. Knee kinematics, cartilage morphology, and osteoarthritis after ACL injury. Med Sci Sports Exerc. 2008;40(2):215–222. doi: 10.1249/mss.0b013e31815cbb0e. [DOI] [PubMed] [Google Scholar]
- 9.Chu CR, Beynnon BD, Buckwalter JA, et al. Closing the gap between bench and bedside research for early arthritis therapies (EARTH): report from the AOSSM/NIH U-13 Post-Joint Injury Osteoarthritis Conference II. Am J Sports Med. 2011;39(7):1569–1578. doi: 10.1177/0363546511411654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis DS, Post WR. Segond fracture: lateral capsular ligament avulsion. J Orthop Sports Phys Ther. 1997;25(2):103–106. doi: 10.2519/jospt.1997.25.2.103. [DOI] [PubMed] [Google Scholar]
- 11.DeMorat G, Weinhold P, Blackburn T, Chudik S, Garrett W. Aggressive quadriceps loading can induce noncontact anterior cruciate ligament injury. Am J Sports Med. 2004;32(2):477–483. doi: 10.1177/0363546503258928. [DOI] [PubMed] [Google Scholar]
- 12.Fink C, Hoser C, Hackl W, Navarro RA, Benedetto KP. Long-term outcome of operative or nonoperative treatment of anterior cruciate ligament rupture: is sports activity a determining variable? Int J Sports Med. 2001;22(4):304–309. doi: 10.1055/s-2001-13823. [DOI] [PubMed] [Google Scholar]
- 13.Fleming BC, Beynnon BD, Nichols CE, Johnson RJ, Pope MH. An in vivo comparison of anterior tibial translation and strain in the anteromedial band of the anterior cruciate ligament. J Biomech. 1993;26(1):51–58. doi: 10.1016/0021-9290(93)90612-i. [DOI] [PubMed] [Google Scholar]
- 14.Fleming BC, Renstrom PA, Beynnon BD, et al. The effect of weight-bearing and external loading on anterior cruciate ligament strain. J Biomech. 2001;34(2):163–170. doi: 10.1016/s0021-9290(00)00154-8. [DOI] [PubMed] [Google Scholar]
- 15.Ford KR, Myer GD, Hewett TE. Valgus knee motion during landing in high school female and male basketball players. Med Sci Sports Exerc. 2003;35(10):1745–1750. doi: 10.1249/01.MSS.0000089346.85744.D9. [DOI] [PubMed] [Google Scholar]
- 16.Griffin LY, Agel J, Albohm MJ, et al. Noncontact anterior cruciate ligament injuries: risk factors and prevention strategies. J Am Acad Orthop Surg. 2000;8(3):141–150. doi: 10.5435/00124635-200005000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Guenoun D, Le Corroller T, Amous Z, et al. The contribution of MRI to the diagnosis of traumatic tears of the anterior cruciate ligament. Diagn Interv Imaging. 2012;93(5):331–341. doi: 10.1016/j.diii.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Henrichs A. A review of knee dislocations. J Athl Train. 2004;39(4):365–369. [PMC free article] [PubMed] [Google Scholar]
- 19.Hess T, Rupp S, Hopf T, Gleitz M, Liebler J. Lateral tibial avulsion fractures and disruptions to the anterior cruciate ligament: a clinical study of their incidence and correlation. Clin Orthop Relat Res. 1994;303:193–197. [PubMed] [Google Scholar]
- 20.Hewett TE, Myer GD, Ford KR, et al. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: a prospective study. Am J Sports Med. 2005;33(4):492–501. doi: 10.1177/0363546504269591. [DOI] [PubMed] [Google Scholar]
- 21.Hewett TE, Torg JS, Boden BP. Video analysis of trunk and knee motion during non-contact anterior cruciate ligament injury in female athletes: lateral trunk and knee abduction motion are combined components of the injury mechanism. Br J Sports Med. 2009;43(6):417–422. doi: 10.1136/bjsm.2009.059162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Howe JG, Wertheimer C, Johnson RJ, et al. Arthroscopic strain gauge measurement of the normal anterior cruciate ligament. Arthroscopy. 1990;6(3):198–204. doi: 10.1016/0749-8063(90)90075-o. [DOI] [PubMed] [Google Scholar]
- 23.Johnson DL, Urban WP, Jr, Caborn DN, Vanarthos WJ, Carlson CS. Articular cartilage changes seen with magnetic resonance imaging-detected bone bruises associated with acute anterior cruciate ligament rupture. Am J Sports Med. 1998;26(3):409–414. doi: 10.1177/03635465980260031101. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan PA, Gehl RH, Dussault RG, Anderson MW, Diduch DR. Bone contusions of the posterior lip of the medial tibial plateau (contrecoup injury) and associated internal derangements of the knee at MR imaging. Radiology. 1999;211(3):747–753. doi: 10.1148/radiology.211.3.r99jn30747. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan PA, Walker CW, Kilcoyne RF, et al. Occult fracture patterns of the knee associated with anterior cruciate ligament tears: assessment with MR imaging. Radiology. 1992;183(3):835–838. doi: 10.1148/radiology.183.3.1584943. [DOI] [PubMed] [Google Scholar]
- 26.Kessler MA, Behrend H, Henz S, et al. Function, osteoarthritis and activity after ACL-rupture: 11 years follow-up results of conservative versus reconstructive treatment. Knee Surg Sports Traumatol Arthrosc. 2008;16(5):442–448. doi: 10.1007/s00167-008-0498-x. [DOI] [PubMed] [Google Scholar]
- 27.Kiapour A, Kiapour AM, Kaul V, et al. Finite element model of the knee for investigation of high rate injury mechanisms: development and validation.. Paper presented at: Orthopaedic Research Society Annual Meeting; San Francisco, California. February 4-7, 2012; ( https://online.ors.org/web/Transactions/58/0101.pdf) [Google Scholar]
- 28.Kiapour AM, Demetropoulos CK, Quatman CE, et al. Effects of single- and multi-axis loading conditions on ACL strain: an indication of ACL injury mechanism.. Paper presented at: Orthopaedic Research Society Annual Meeting; San Francisco, California. February 4-7, 2012; ( https://online.ors.org/web/Transactions/58/0857.pdf) [Google Scholar]
- 29.Kiapour AM, Quatman CE, Goel VK, et al. Knee articular cartilage pressure distribution under single- and multi-axis loading conditions: implications for ACL injury mechanism.. Paper presented at: American Society of Biomechanics Annual Meeting; Gainesville, Florida. August 15-18, 2012; ( http://www.asbweb.org/conferences/2012/topics/ASB%202012%20oral.pdf) [Google Scholar]
- 30.Kiapour AM, Quatman CE, Goel VK, et al. A novel technique to simulate landing biomechanics: a cadaveric model of ACL injury.. Paper presented at: Orthopaedic Research Society Annual Meeting; San Francisco, California. February 4-7, 2012; ( https://online.ors.org/web/Transactions/58/0858.pdf) [Google Scholar]
- 31.Kim S, Bosque J, Meehan JP, Jamali A, Marder R. Increase in outpatient knee arthroscopy in the United States: a comparison of National Surveys of Ambulatory Surgery, 1996 and 2006. J Bone Joint Surg Am. 2011;93(11):994–1000. doi: 10.2106/JBJS.I.01618. [DOI] [PubMed] [Google Scholar]
- 32.Koga H, Nakamae A, Shima Y, et al. Mechanisms for noncontact anterior cruciate ligament injuries: knee joint kinematics in 10 injury situations from female team handball and basketball. Am J Sports Med. 2010;38(11):2218–2225. doi: 10.1177/0363546510373570. [DOI] [PubMed] [Google Scholar]
- 33.Krosshaug T, Nakamae A, Boden BP, et al. Mechanisms of anterior cruciate ligament injury in basketball: video analysis of 39 cases. Am J Sports Med. 2007;35(3):359–367. doi: 10.1177/0363546506293899. [DOI] [PubMed] [Google Scholar]
- 34.Markolf KL, Burchfield DM, Shapiro MM, et al. Combined knee loading states that generate high anterior cruciate ligament forces. J Orthop Res. 1995;13(6):930–935. doi: 10.1002/jor.1100130618. [DOI] [PubMed] [Google Scholar]
- 35.Markolf KL, O'Neill G, Jackson SR, McAllister DR. Effects of applied quadriceps and hamstrings muscle loads on forces in the anterior and posterior cruciate ligaments. Am J Sports Med. 2004;32(5):1144–1149. doi: 10.1177/0363546503262198. [DOI] [PubMed] [Google Scholar]
- 36.McLean SG, Oh YK, Palmer ML, et al. The relationship between anterior tibial acceleration, tibial slope, and ACL strain during a simulated jump landing task. J Bone Joint Surg Am. 2011;93(14):1310–1317. doi: 10.2106/JBJS.J.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McNair PJ, Marshall RN, Matheson JA. Important features associated with acute anterior cruciate ligament injury. N Z Med J. 1990;103(901):537–539. [PubMed] [Google Scholar]
- 38.Meyer EG, Baumer TG, Slade JM, Smith WE, Haut RC. Tibiofemoral contact pressures and osteochondral microtrauma during anterior cruciate ligament rupture due to excessive compressive loading and internal torque of the human knee. Am J Sports Med. 2008;36(10):1966–1977. doi: 10.1177/0363546508318046. [DOI] [PubMed] [Google Scholar]
- 39.Meyer EG, Haut RC. Excessive compression of the human tibiofemoral joint causes ACL rupture. J Biomech. 2005;38(11):2311–2316. doi: 10.1016/j.jbiomech.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 40.Mihata LC, Beutler AI, Boden BP. Comparing the incidence of anterior cruciate ligament injury in collegiate lacrosse, soccer, and basketball players: implications for anterior cruciate ligament mechanism and prevention. Am J Sports Med. 2006;34(6):899–904. doi: 10.1177/0363546505285582. [DOI] [PubMed] [Google Scholar]
- 41.Mink JH, Deutsch AL. Radiology. 1989;170(3 Pt 1):823–829. doi: 10.1148/radiology.170.3.2916038. Occult cartilage and bone injuries of the knee: detection, classification, and assessment with MR imaging [DOI] [PubMed] [Google Scholar]
- 42.Murphy BJ, Smith RL, Uribe JW, et al. Bone signal abnormalities in the posterolateral tibia and lateral femoral condyle in complete tears of the anterior cruciate ligament: a specific sign? Radiology. 1992;182(1):221–224. doi: 10.1148/radiology.182.1.1727286. [DOI] [PubMed] [Google Scholar]
- 43.Nebelung W, Wuschech H. Thirty-five years of follow-up of anterior cruciate ligament-deficient knees in high-level athletes. Arthroscopy. 2005;21(6):696–702. doi: 10.1016/j.arthro.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 44.Oh YK, Kreinbrink JL, Wojtys EM, Ashton-Miller JA. Effect of axial tibial torque direction on ACL relative strain and strain rate in an in vitro simulated pivot landing. J Orthop Res. 2012;30(4):528–534. doi: 10.1002/jor.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Olsen OE, Myklebust G, Engebretsen L, Bahr R. Injury mechanisms for anterior cruciate ligament injuries in team handball: a systematic video analysis. Am J Sports Med. 2004;32(4):1002–1012. doi: 10.1177/0363546503261724. [DOI] [PubMed] [Google Scholar]
- 46.Prodromos CC, Han Y, Rogowski J, et al. A meta-analysis of the incidence of anterior cruciate ligament tears as a function of gender, sport, and a knee injury-reduction regimen. Arthroscopy. 2007;23(12):1320–1325. e6. doi: 10.1016/j.arthro.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 47.Quatman CE, Kiapour A, Myer GD, et al. Cartilage pressure distributions provide a footprint to define female anterior cruciate ligament injury mechanisms. Am J Sports Med. 2011;39(8):1706–1713. doi: 10.1177/0363546511400980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosen MA, Jackson DW, Berger PE. Occult osseous lesions documented by magnetic resonance imaging associated with anterior cruciate ligament ruptures. Arthroscopy. 1991;7(1):45–51. doi: 10.1016/0749-8063(91)90077-b. [DOI] [PubMed] [Google Scholar]
- 49.Sanders TG, Medynski MA, Feller JF, Lawhorn KW. Bone contusion patterns of the knee at MR imaging: footprint of the mechanism of injury. Radiographics. 2000;20 doi: 10.1148/radiographics.20.suppl_1.g00oc19s135. Spec No:S135-S151. [DOI] [PubMed] [Google Scholar]
- 50.Shin CS, Chaudhari AM, Andriacchi TP. The effect of isolated valgus moments on ACL strain during single-leg landing: a simulation study. J Biomech. 2009;42(3):280–285. doi: 10.1016/j.jbiomech.2008.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shin CS, Chaudhari AM, Andriacchi TP. The influence of deceleration forces on ACL strain during single-leg landing: a simulation study. J Biomech. 2007;40(5):1145–1152. doi: 10.1016/j.jbiomech.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 52.Shin CS, Chaudhari AM, Andriacchi TP. Valgus plus internal rotation moments increase anterior cruciate ligament strain more than either alone. Med Sci Sports Exerc. 2011;43(8):1484–1491. doi: 10.1249/MSS.0b013e31820f8395. [DOI] [PubMed] [Google Scholar]
- 53.Shultz SJ, Beynnon BD, Schmitz RJ. Sex differences in coupled knee motions during the transition from non-weight bearing to weight bearing. J Orthop Res. 2009;27(6):717–723. doi: 10.1002/jor.20810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Speer KP, Spritzer CE, Bassett FH, 3rd, Feagin JA, Jr, Garrett WE., Jr Osseous injury associated with acute tears of the anterior cruciate ligament. Am J Sports Med. 1992;20(4):382–389. doi: 10.1177/036354659202000403. [DOI] [PubMed] [Google Scholar]
- 55.Spindler KP, Schils JP, Bergfeld JA, et al. Prospective study of osseous, articular, and meniscal lesions in recent anterior cruciate ligament tears by magnetic resonance imaging and arthroscopy. Am J Sports Med. 1993;21(4):551–557. doi: 10.1177/036354659302100412. [DOI] [PubMed] [Google Scholar]
- 56.Stallenberg B, Gevenois PA, Sintzoff SA, Jr, et al. Fracture of the posterior aspect of the lateral tibial plateau: radiographic sign of anterior cruciate ligament tear. Radiology. 1993;187(3):821–825. doi: 10.1148/radiology.187.3.8497638. [DOI] [PubMed] [Google Scholar]
- 57.Weber WN, Neumann CH, Barakos JA, et al. Lateral tibial rim (Segond) fractures: MR imaging characteristics. Radiology. 1991;180(3):731–734. doi: 10.1148/radiology.180.3.1871286. [DOI] [PubMed] [Google Scholar]
- 58.Withrow TJ, Huston LJ, Wojtys EM, Ashton-Miller JA. The effect of an impulsive knee valgus moment on in vitro relative ACL strain during a simulated jump landing. Clin Biomech (Bristol, Avon) 2006;21(9):977–983. doi: 10.1016/j.clinbiomech.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 59.Withrow TJ, Huston LJ, Wojtys EM, Ashton-Miller JA. The relationship between quadriceps muscle force, knee flexion, and anterior cruciate ligament strain in an in vitro simulated jump landing. Am J Sports Med. 2006;34(2):269–274. doi: 10.1177/0363546505280906. [DOI] [PubMed] [Google Scholar]