Abstract
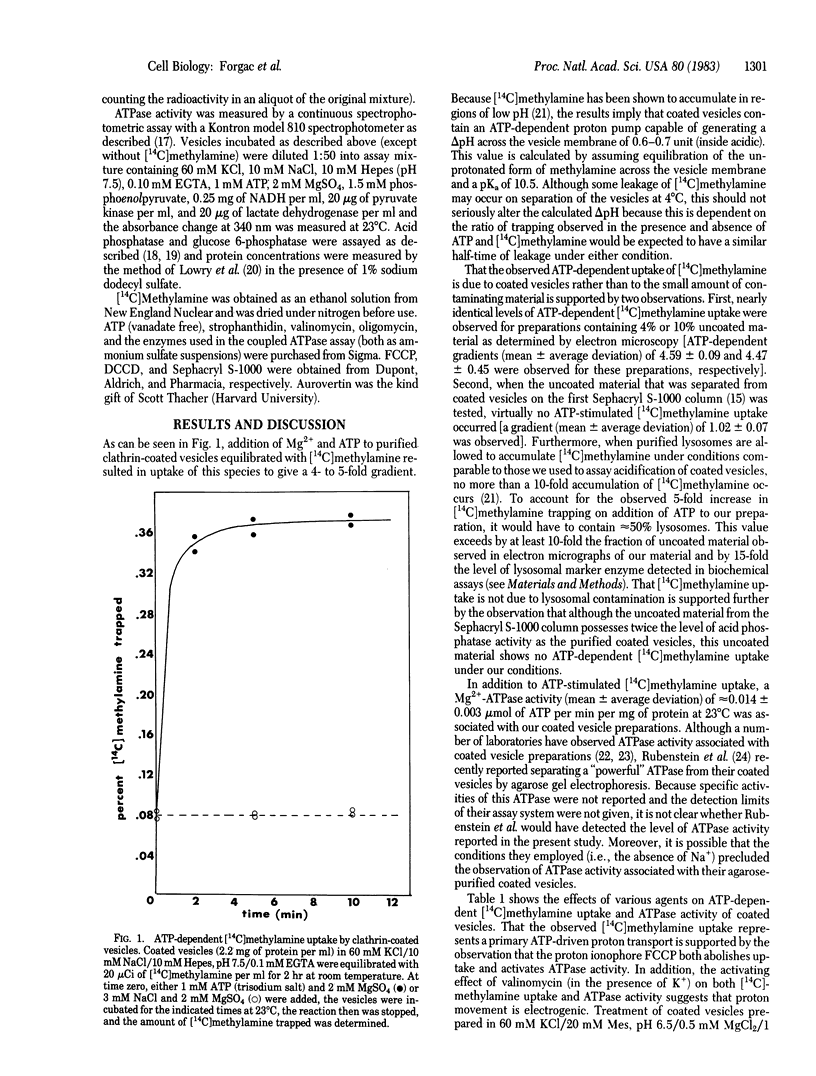
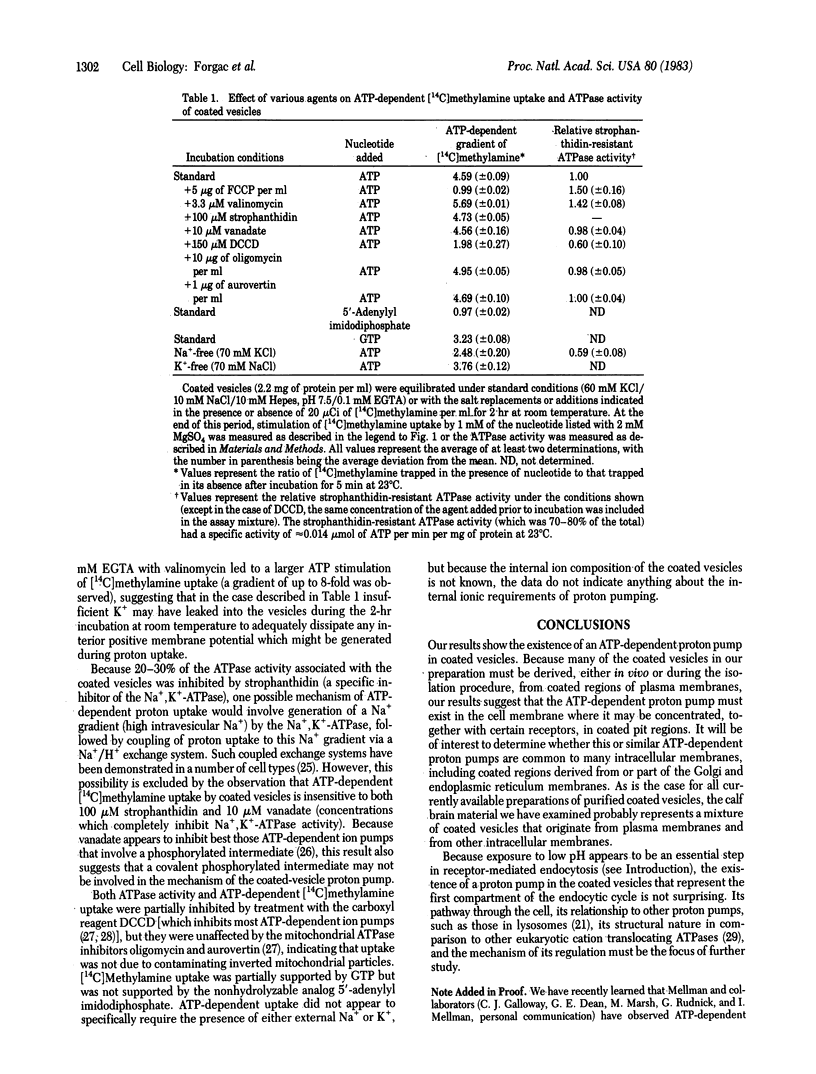
Clathrin-coated vesicles isolated from calf brain contain an ATP-dependent proton pump. Proton movement was monitored by measuring [14C]methylamine distribution. Addition of Mg2+ and ATP to coated vesicles equilibrated with [14C]methylamine resulted in the generation of a 4- to 5-fold concentration gradient, corresponding to a delta pH of 0.6-0.7 units between the medium and the acidic inside of the coated vesicles. ATP-dependent [14C]methylamine uptake was abolished by the proton ionophore carbonylcyanide p-trifluoromethoxyphenylhydrazone (FCCP) and partially inhibited by the carboxyl reagent N,N'-dicyclohexylcarbodiimide but was unaffected by the Na+, K+-ATPase inhibitors strophanthidin (100 microM) and vanadate (10 microM) and the mitochondrial ATPase inhibitors oligomycin (10 microgram/ml) and aurovertin (1 microgram/ml). GTP, but not the nonhydrolyzable analog 5'-adenylyl imidodiphosphate, could support [14C]methylamine uptake. Dissipation of the membrane potential with K+ and valinomycin resulted in stimulation of [14C]methylamine uptake, whereas both FCCP and valinomycin stimulated the strophanthidin-resistant ATPase activity. These results are consistent with the existence of an electrogenic, ATP-dependent proton pump in clathrin-coated vesicles. This proton pump may play a role in the acidification events that are essential in receptor-mediated endocytosis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basu S. K., Goldstein J. L., Anderson R. G., Brown M. S. Monensin interrupts the recycling of low density lipoprotein receptors in human fibroblasts. Cell. 1981 May;24(2):493–502. doi: 10.1016/0092-8674(81)90340-8. [DOI] [PubMed] [Google Scholar]
- Blitz A. L., Fine R. E., Toselli P. A. Evidence that coated vesicles isolated from brain are calcium-sequestering organelles resembling sarcoplasmic reticulum. J Cell Biol. 1977 Oct;75(1):135–147. doi: 10.1083/jcb.75.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantley L. C., Jr, Cantley L. G., Josephson L. A characterization of vanadate interactions with the (Na,K)-ATPase. Mechanistic and regulatory implications. J Biol Chem. 1978 Oct 25;253(20):7361–7368. [PubMed] [Google Scholar]
- Cantley L. C., Jr, Josephson L. A slow interconversion between active and inactive states of the (Na-K)ATPase. Biochemistry. 1976 Nov 30;15(24):5280–5287. doi: 10.1021/bi00669a013. [DOI] [PubMed] [Google Scholar]
- Forgac M., Chin G. Na+ transport by the (Na+)-stimulated adenosine triphosphatase. J Biol Chem. 1982 May 25;257(10):5652–5655. [PubMed] [Google Scholar]
- Goldstein J. L., Anderson R. G., Brown M. S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979 Jun 21;279(5715):679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Green A., Olefsky J. M. Evidence for insulin-induced internalization and degradation of insulin receptors in rat adipocytes. Proc Natl Acad Sci U S A. 1982 Jan;79(2):427–431. doi: 10.1073/pnas.79.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti G. The structure of intrinsic membrane proteins. J Supramol Struct. 1977;7(3-4):489–497. doi: 10.1002/jss.400070318. [DOI] [PubMed] [Google Scholar]
- Helenius A., Kartenbeck J., Simons K., Fries E. On the entry of Semliki forest virus into BHK-21 cells. J Cell Biol. 1980 Feb;84(2):404–420. doi: 10.1083/jcb.84.2.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matlin K. S., Reggio H., Helenius A., Simons K. Infectious entry pathway of influenza virus in a canine kidney cell line. J Cell Biol. 1981 Dec;91(3 Pt 1):601–613. doi: 10.1083/jcb.91.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlin K. S., Reggio H., Helenius A., Simons K. Pathway of vesicular stomatitis virus entry leading to infection. J Mol Biol. 1982 Apr 15;156(3):609–631. doi: 10.1016/0022-2836(82)90269-8. [DOI] [PubMed] [Google Scholar]
- Maxfield F. R. Weak bases and ionophores rapidly and reversibly raise the pH of endocytic vesicles in cultured mouse fibroblasts. J Cell Biol. 1982 Nov;95(2 Pt 1):676–681. doi: 10.1083/jcb.95.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz N., Schook W., Puszkin S. Comparison of calmodulin binding to brain synaptic and coated vesicles. Biochim Biophys Acta. 1982 Aug 12;689(3):523–530. doi: 10.1016/0005-2736(82)90310-8. [DOI] [PubMed] [Google Scholar]
- Murphy A. J. Kinetics of the inactivation of the ATPase of sarcoplasmic reticulum by dicyclohexylcarbodiimide. J Biol Chem. 1981 Dec 10;256(23):12046–12050. [PubMed] [Google Scholar]
- Pastan I. H., Willingham M. C. Journey to the center of the cell: role of the receptosome. Science. 1981 Oct 30;214(4520):504–509. doi: 10.1126/science.6170111. [DOI] [PubMed] [Google Scholar]
- Rindler M. J., Saier M. H., Jr Evidence for Na+/H+ antiport in cultured dog kidney cells (MDCK). J Biol Chem. 1981 Nov 10;256(21):10820–10825. [PubMed] [Google Scholar]
- Rubenstein J. L., Fine R. E., Luskey B. D., Rothman J. E. Purification of coated vesicles by agarose gel electrophoresis. J Cell Biol. 1981 May;89(2):357–361. doi: 10.1083/jcb.89.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K., Olsnes S. Rapid entry of nicked diphtheria toxin into cells at low pH. Characterization of the entry process and effects of low pH on the toxin molecule. J Biol Chem. 1981 Sep 10;256(17):9068–9076. [PubMed] [Google Scholar]
- Schneider D. L. ATP-dependent acidification of intact and disrupted lysosomes. Evidence for an ATP-driven proton pump. J Biol Chem. 1981 Apr 25;256(8):3858–3864. [PubMed] [Google Scholar]
- Tietze C., Schlesinger P., Stahl P. Mannose-specific endocytosis receptor of alveolar macrophages: demonstration of two functionally distinct intracellular pools of receptor and their roles in receptor recycling. J Cell Biol. 1982 Feb;92(2):417–424. doi: 10.1083/jcb.92.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouet A. Isolation of modified liver lysosomes. Methods Enzymol. 1974;31:323–329. doi: 10.1016/0076-6879(74)31034-8. [DOI] [PubMed] [Google Scholar]
- Tycko B., Maxfield F. R. Rapid acidification of endocytic vesicles containing alpha 2-macroglobulin. Cell. 1982 Mar;28(3):643–651. doi: 10.1016/0092-8674(82)90219-7. [DOI] [PubMed] [Google Scholar]
- Unanue E. R., Ungewickell E., Branton D. The binding of clathrin triskelions to membranes from coated vesicles. Cell. 1981 Nov;26(3 Pt 1):439–446. doi: 10.1016/0092-8674(81)90213-0. [DOI] [PubMed] [Google Scholar]
- van Renswoude J., Bridges K. R., Harford J. B., Klausner R. D. Receptor-mediated endocytosis of transferrin and the uptake of fe in K562 cells: identification of a nonlysosomal acidic compartment. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6186–6190. doi: 10.1073/pnas.79.20.6186. [DOI] [PMC free article] [PubMed] [Google Scholar]



