Abstract
Using short-term cultures of hamster embryo cells, we have examined the effects of the free-radical scavenger superoxide dismutase (superoxide:superoxide oxidoreductase, EC 1.15.1.1) and the enzyme catalase (hydrogen-peroxide:hydrogenperoxide oxidoreductase, EC 1.11.1.6) on x-ray- and bleomycin-induced transformation and on the enhancement of radiogenic transformation by the tumor promoter 12-O-tetradecanoylphorbol 13-acetate (TPA). We find that superoxide dismutase inhibits (i) transformation induced by x-ray and bleomycin and (ii) promotional action of TPA in vitro. The results suggest that the oncogenic action of x-rays and bleomycin and the enhancement of oncogenic transformation by TPA are mediated in part by free radicals. The findings also suggest that superoxide dismutase can serve as an inhibitor of oncogenesis and that its actions, as seen in this in vitro system, are most predominantly on inhibiting late events in the progression of cellular transformation--those associated with promotion.
Full text
PDF
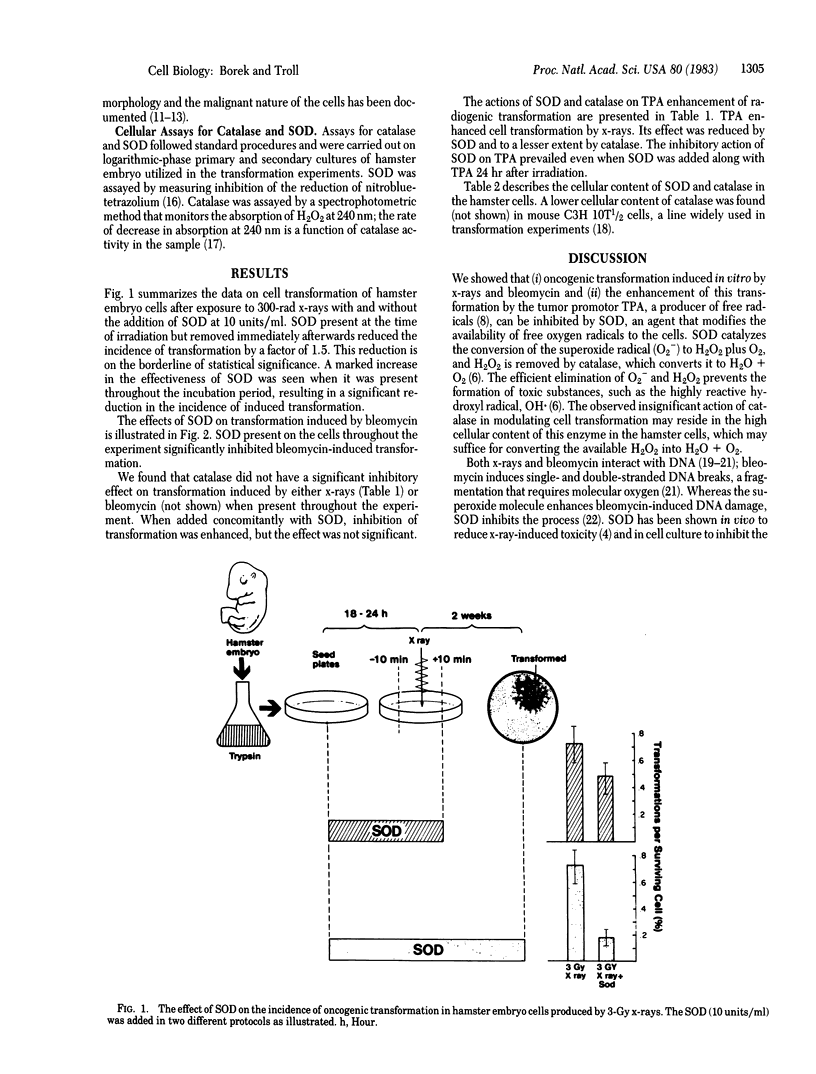
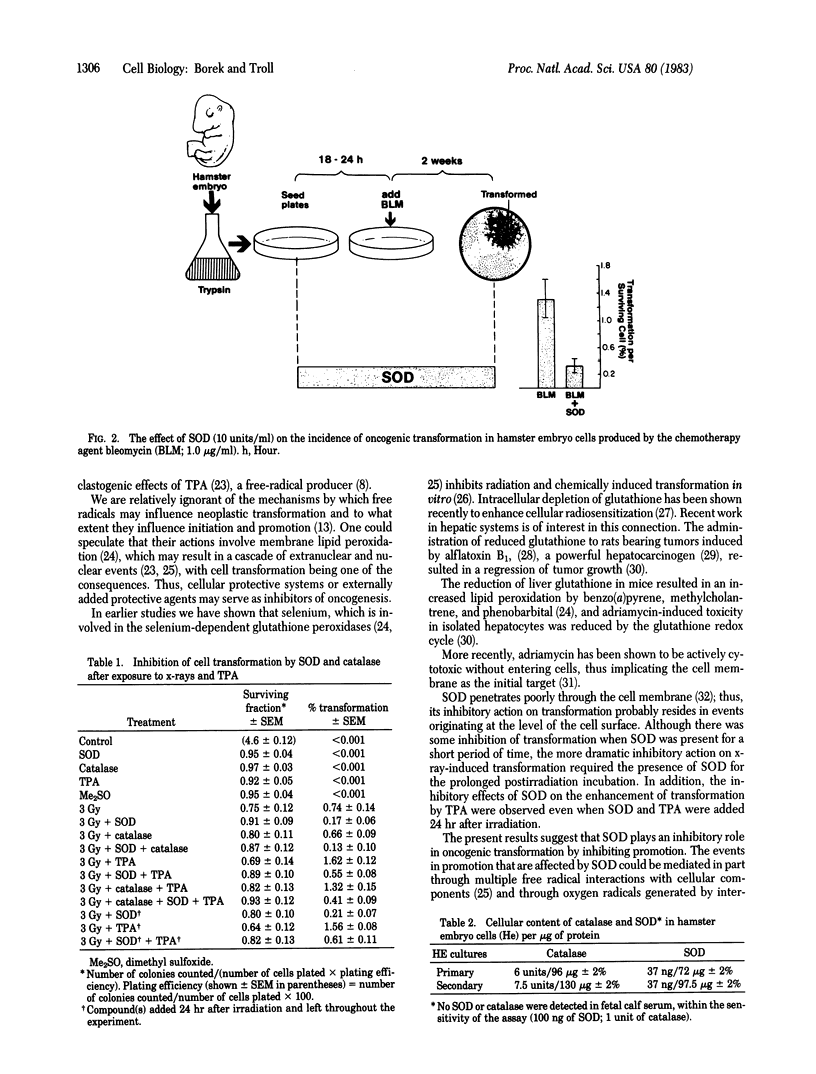

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEERS R. F., Jr, SIZER I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952 Mar;195(1):133–140. [PubMed] [Google Scholar]
- Babson J. R., Abell N. S., Reed D. J. Protective role of the glutathione redox cycle against adriamycin-mediated toxicity in isolated hepatocytes. Biochem Pharmacol. 1981 Aug 15;30(16):2299–2304. doi: 10.1016/0006-2952(81)90102-7. [DOI] [PubMed] [Google Scholar]
- Borek C., Hall E. J. Effect of split doses of x rays on neoplastic transformation of single cells. Nature. 1974 Dec 6;252(5483):499–501. doi: 10.1038/252499a0. [DOI] [PubMed] [Google Scholar]
- Borek C., Miller R., Pain C., Troll W. Conditions for inhibiting and enhancing effects of the protease inhibitor antipain on x-ray-induced neoplastic transformation in hamster and mouse cells. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1800–1803. doi: 10.1073/pnas.76.4.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borek C. Radiation oncogenesis in cell culture. Adv Cancer Res. 1982;37:159–232. doi: 10.1016/s0065-230x(08)60884-2. [DOI] [PubMed] [Google Scholar]
- Borek C., Sachs L. In vitro cell transformation by x-irradiation. Nature. 1966 Apr 16;210(5033):276–278. doi: 10.1038/210276a0. [DOI] [PubMed] [Google Scholar]
- Bump E. A., Yu N. Y., Brown J. M. Radiosensitization of hypoxic tumor cells by depletion of intracellular glutathione. Science. 1982 Aug 6;217(4559):544–545. doi: 10.1126/science.7089580. [DOI] [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978 Sep 8;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- Fujiki H., Mori M., Nakayasu M., Terada M., Sugimura T. A possible naturally occurring tumor promoter, teleocidin B from Streptomyces. Biochem Biophys Res Commun. 1979 Oct 12;90(3):976–983. doi: 10.1016/0006-291x(79)91923-5. [DOI] [PubMed] [Google Scholar]
- Goldstein B. D., Witz G., Amoruso M., Stone D. S., Troll W. Stimulation of human polymorphonuclear leukocyte superoxide anion radical production by tumor promoters. Cancer Lett. 1981 Jan;11(3):257–262. doi: 10.1016/0304-3835(81)90117-8. [DOI] [PubMed] [Google Scholar]
- Groopman J. D., Busby W. F., Jr, Wogan G. N. Nuclear distribution of aflatoxin B1 and its interaction with histones in rat liver in vivo. Cancer Res. 1980 Dec;40(12):4343–4351. [PubMed] [Google Scholar]
- Ishida R., Takahashi T. Increased DNA chain breakage by combined action of bleomycin and superoxide radical. Biochem Biophys Res Commun. 1975 Oct 27;66(4):1432–1438. doi: 10.1016/0006-291x(75)90519-7. [DOI] [PubMed] [Google Scholar]
- Kohn K. W., Ewig R. A. Effect of pH on the bleomycin-induced DNA single-strand scission in L1210 cells and the relation to cell survival. Cancer Res. 1976 Oct;36(10):3839–3841. [PubMed] [Google Scholar]
- Leibovitz B. E., Siegel B. V. Aspects of free radical reactions in biological systems: aging. J Gerontol. 1980 Jan;35(1):45–56. doi: 10.1093/geronj/35.1.45. [DOI] [PubMed] [Google Scholar]
- Novi A. M. Regression of aflatoxin B1-induced hepatocellular carcinomas by reduced glutathione. Science. 1981 May 1;212(4494):541–542. doi: 10.1126/science.6782675. [DOI] [PubMed] [Google Scholar]
- Painter R. B., Young B. R. Repair replication in mammalian cells after x-irradiation. Mutat Res. 1972 Feb;14(2):225–235. doi: 10.1016/0027-5107(72)90049-8. [DOI] [PubMed] [Google Scholar]
- Patt H. M., Tyree E. B., Straube R. L., Smith D. E. Cysteine Protection against X Irradiation. Science. 1949 Aug 26;110(2852):213–214. doi: 10.1126/science.110.2852.213. [DOI] [PubMed] [Google Scholar]
- Petkau A., Chelack W. S., Kelly K., Barefoot C., Monasterski L. Tissue distribution of bovine 125I-superoxide dismutase in mice. Res Commun Chem Pathol Pharmacol. 1976 Dec;15(4):641–654. [PubMed] [Google Scholar]
- Petkau A. Radiation protection by superoxide dismutase. Photochem Photobiol. 1978 Oct-Nov;28(4-5):765–774. doi: 10.1111/j.1751-1097.1978.tb07015.x. [DOI] [PubMed] [Google Scholar]
- Reznikoff C. A., Brankow D. W., Heidelberger C. Establishment and characterization of a cloned line of C3H mouse embryo cells sensitive to postconfluence inhibition of division. Cancer Res. 1973 Dec;33(12):3231–3238. [PubMed] [Google Scholar]
- Roizin-Towle L., Hall E. J. Studies with bleomycin and misonidazole on aerated and hypoxic cells. Br J Cancer. 1978 Feb;37(2):254–260. doi: 10.1038/bjc.1978.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaga T. J., Klein-Szanto A. J., Triplett L. L., Yotti L. P., Trosko K. E. Skin tumor-promoting activity of benzoyl peroxide, a widely used free radical-generating compound. Science. 1981 Aug 28;213(4511):1023–1025. doi: 10.1126/science.6791284. [DOI] [PubMed] [Google Scholar]
- Triton T. R., Yee G. The anticancer agent adriamycin can be actively cytotoxic without entering cells. Science. 1982 Jul 16;217(4556):248–250. doi: 10.1126/science.7089561. [DOI] [PubMed] [Google Scholar]
- Wendel A., Feuerstein S. Drug-induced lipid peroxidation in mice--I. Modulation by monooxygenase activity, glutathione and selenium status. Biochem Pharmacol. 1981 Sep 15;30(18):2513–2520. doi: 10.1016/0006-2952(81)90576-1. [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C., Hawkins R. E., Brian M., Carrell R. W. The estimation of red cell superoxide dismutase activity. J Lab Clin Med. 1975 Feb;85(2):337–341. [PubMed] [Google Scholar]



