Abstract
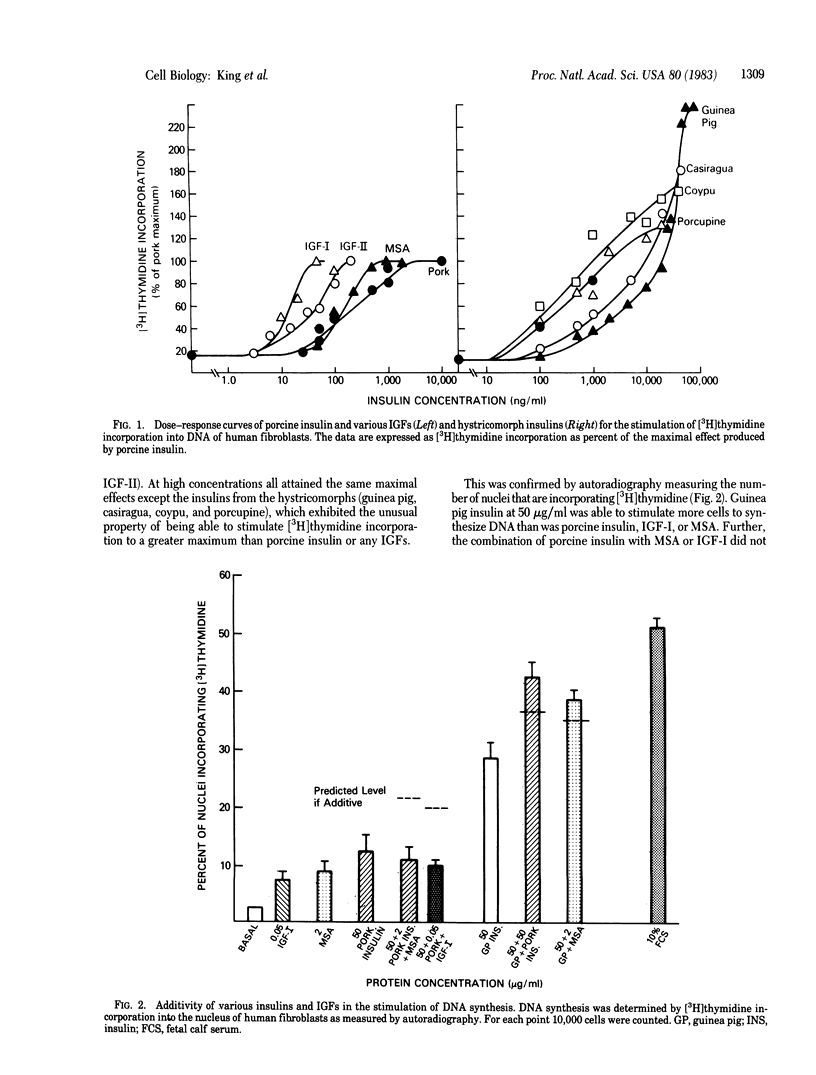
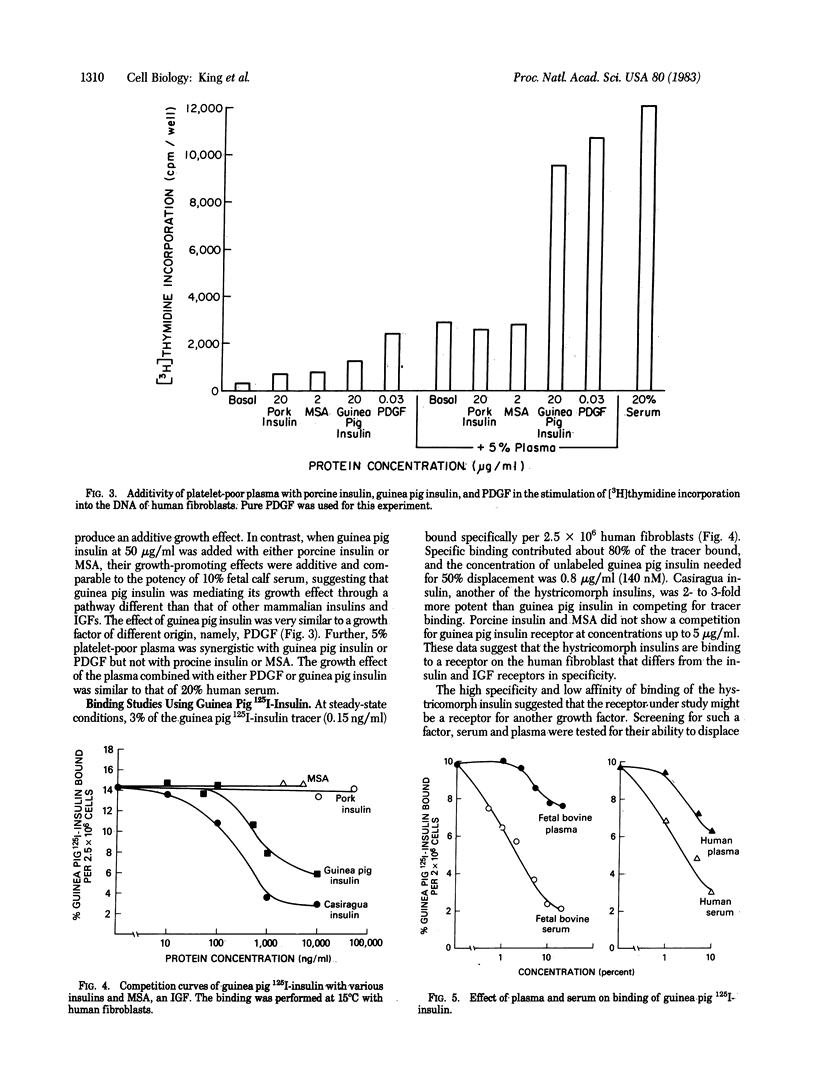
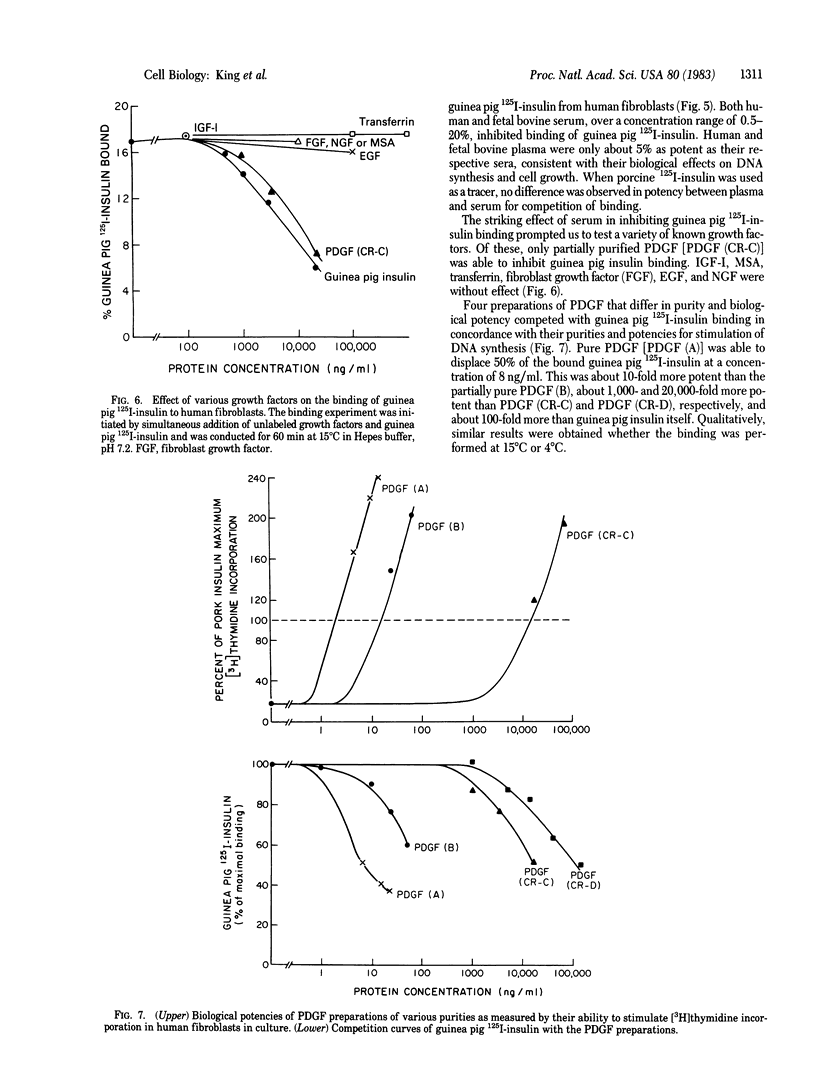
Insulins from the hystricomorphs (guinea pig, porcupine, coypu, and casiragua) at high concentration stimulate DNA synthesis in human fibroblasts to a greater level than other mammalian insulins or insulin-like growth factors (IGFs). 125I-Labeled guinea pig insulin binds to a specific receptor and this binding is competed for by hystricomorph insulins but not by porcine insulin or IGFs. Fetal bovine serum also inhibits the binding of 125I-labeled guinea pig insulin and is more potent than fetal bovine plasma, in concordance with their relative potencies for growth stimulation in human fibroblasts. Of several other known growth factors tested, only platelet-derived growth factor (PDGF) inhibits binding of 125I-labeled guinea pig insulin. Four preparations of PDGF that vary in purity and potency for the stimulation of DNA synthesis in human fibroblasts over a 1,000-fold range compete with binding of 125I-labeled guinea pig insulin in proportion to their biological potencies. The purest preparation of PDGF is able to inhibit binding of 125I-labeled guinea pig insulin by 50% at 15 ng/ml (0.25 nM). Biologically, guinea pig insulin, like PDGF, exhibits a synergistic effect with plasma in initiating DNA synthesis in human fibroblasts; this effect is not observed with other mammalian insulins or IGFs. Thus, hystricomorph insulins appear to be mediating their growth-promoting effect through a different receptor and mechanism than other mammalian insulins or IGFs. Further, hystricomorph insulins may be sharing the mechanism of action for their growth effects with PDGF, perhaps suggesting some relationship between these peptides from very different sources.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoniades H. N. Human platelet-derived growth factor (PDGF): purification of PDGF-I and PDGF-II and separation of their reduced subunits. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7314–7317. doi: 10.1073/pnas.78.12.7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniades H. N., Scher C. D., Stiles C. D. Purification of human platelet-derived growth factor. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1809–1813. doi: 10.1073/pnas.76.4.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell T. L., Cutfield J. F., Cutfield S. M., Dodson E. J., Dodson G. G., Hodgkin D. C., Mercola D. A. Three-dimensional atomic structure of insulin and its relationship to activity. Diabetes. 1972;21(2 Suppl):492–505. doi: 10.2337/diab.21.2.s492. [DOI] [PubMed] [Google Scholar]
- Clemmons D. R., Van Wyk J. J., Pledger W. J. Sequential addition of platelet factor and plasma to BALB/c 3T3 fibroblast cultures stimulates somatomedin-C binding early in cell cycle. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6644–6648. doi: 10.1073/pnas.77.11.6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuel T. F., Huang J. S., Proffitt R. T., Baenziger J. U., Chang D., Kennedy B. B. Human platelet-derived growth factor. Purification and resolution into two active protein fractions. J Biol Chem. 1981 Sep 10;256(17):8896–8899. [PubMed] [Google Scholar]
- Freychet P., Kahn R., Roth J., Neville D. M., Jr Insulin interactions with liver plasma membranes. Independence of binding of the hormone and its degradation. J Biol Chem. 1972 Jun 25;247(12):3953–3961. [PubMed] [Google Scholar]
- Heldin C. H., Wasteson A., Westermark B. Interaction of platelet-derived growth factor with its fibroblast receptor. Demonstration of ligand degradation and receptor modulation. J Biol Chem. 1982 Apr 25;257(8):4216–4221. [PubMed] [Google Scholar]
- Heldin C. H., Westermark B., Wasteson A. Platelet-derived growth factor. Isolation by a large-scale procedure and analysis of subunit composition. Biochem J. 1981 Mar 1;193(3):907–913. doi: 10.1042/bj1930907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B., Wasteson A. Platelet-derived growth factor: purification and partial characterization. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3722–3726. doi: 10.1073/pnas.76.8.3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horuk R., Goodwin P., O'connor K., Neville R. W., Lazarus N. R., Stone D. Evolutionary change in the insulin receptors of hystricomorph rodents. Nature. 1979 May 31;279(5712):439–440. doi: 10.1038/279439a0. [DOI] [PubMed] [Google Scholar]
- Hughes J. Opioid peptides and their relatives. Nature. 1979 Mar 29;278(5703):394–395. doi: 10.1038/278394a0. [DOI] [PubMed] [Google Scholar]
- Johnsson A., Heldin C. H., Westermark B., Wasteson A. Platelet-derived growth factor: identification of constituent polypeptide chains. Biochem Biophys Res Commun. 1982 Jan 15;104(1):66–74. doi: 10.1016/0006-291x(82)91941-6. [DOI] [PubMed] [Google Scholar]
- King G. L., Kahn C. R. Non-parallel evolution of metabolic and growth-promoting functions of insulin. Nature. 1981 Aug 13;292(5824):644–646. doi: 10.1038/292644a0. [DOI] [PubMed] [Google Scholar]
- King G. L., Kahn C. R., Rechler M. M., Nissley S. P. Direct demonstration of separate receptors for growth and metabolic activities of insulin and multiplication-stimulating activity (an insulinlike growth factor) using antibodies to the insulin receptor. J Clin Invest. 1980 Jul;66(1):130–140. doi: 10.1172/JCI109826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondschein J. S., Schomberg D. W. Growth factors modulate gonadotropin receptor induction in granulosa cell cultures. Science. 1981 Mar 13;211(4487):1179–1180. doi: 10.1126/science.6258228. [DOI] [PubMed] [Google Scholar]
- Raines E. W., Ross R. Platelet-derived growth factor. I. High yield purification and evidence for multiple forms. J Biol Chem. 1982 May 10;257(9):5154–5160. [PubMed] [Google Scholar]
- Rechler M. M., Podskalny J. M., Goldfine I. D., Wells C. A. DNA synthesis in human fibroblasts: stimulation by insulin and by nonsuppressible insulin-like activity (NSILA-S). J Clin Endocrinol Metab. 1974 Sep;39(3):512–521. doi: 10.1210/jcem-39-3-512. [DOI] [PubMed] [Google Scholar]
- Rechler M. M., Podskalny J. M. Insulin receptors in cultured human fibroblasts. Diabetes. 1976 Apr;25(4):250–255. doi: 10.2337/diab.25.4.250. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J., Kariya B., Harker L. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1207–1210. doi: 10.1073/pnas.71.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J. Methods for assessing immunologic and biologic properties of iodinated peptide hormones. Methods Enzymol. 1975;37:223–233. doi: 10.1016/s0076-6879(75)37018-3. [DOI] [PubMed] [Google Scholar]
- Wrann M., Fox C. F., Ross R. Modulation of epidermal growth factor receptors on 3T3 cells by platelet-derived growth factor. Science. 1980 Dec 19;210(4476):1363–1365. doi: 10.1126/science.6254158. [DOI] [PubMed] [Google Scholar]
- Zapf J., Rinderknecht E., Humbel R. E., Froesch E. R. Nonsuppressible insulin-like activity (NSILA) from human serum: recent accomplishments and their physiologic implications. Metabolism. 1978 Dec;27(12):1803–1828. doi: 10.1016/0026-0495(78)90267-6. [DOI] [PubMed] [Google Scholar]
- Zimmerman A. E., Moule M. L., Yip C. C. Guinea pig insulin. II. Biological activity. J Biol Chem. 1974 Jul 10;249(13):4026–4029. [PubMed] [Google Scholar]


