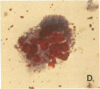
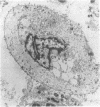
Abstract
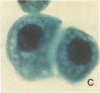
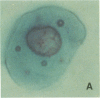
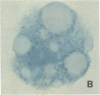
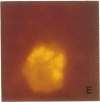
An average 15-fold increase in lactosylceramide (LacCer) in the sediment of receptor-negative, familial hypercholesterolemic (FH) homozygotes has been reported [Chatterjee, S., Sekerke, C.S. & Kwiterovich, P.O., Jr. (1982) J. Lipid Res. 23, 513-522]. We report here the abnormal urinary excretion of significant numbers of renal tubular cells in eight FH homozygotes. The mean activity of gamma-glutamyltransferase, a marker for renal tubular cells, was twice as high in urinary sediment of FH homozygotes as in normals. Membrane-enclosed cytoplasmic vesicles that stained strongly positive with a fluorescein-labeled antibody against LacCer were found in the renal tubular cells of all homozygotes except two who had undergone a portacaval shunt. These two had normal urinary levels of LacCer, and the cytoplasmic vesicles were vacuolated. In the other six, most of the fluorescent antibody label was intracellular and perinuclear. The cytoplasmic vesicles stained strongly with polychromatic Papanicolaou stain, periodic acid/Schiff reagent, and oil red O. Electron microscopy revealed perinuclear membrane-enclosed lipid and free lipid droplets. When two FH homozygotes, who excreted increased LacCer, underwent plasma exchange, the cytoplasmic vesicles became empty, and the urinary LacCer level decreased into the normal range. We conclude that the increased urinary excretion of LacCer in FH homozygotes occurs in renal tubular cells and that the intracellular locatin of LacCer is within cytoplasmic vesicles. The presence of LacCer within these vesicles can be modulated by treatment with plasma exchange.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alving C. R., Richards R. L. Immune reactivities of antibodies against glycolipids--I. Properties of anti-galactocerebroside antibodies purified by a novel technique of affinity binding to liposomes. Immunochemistry. 1977 May;14(5):373–381. [PubMed] [Google Scholar]
- Chatterjee S., Kwiterovich P. O. Glycosphingolipids of human plasma lipoproteins. Lipids. 1976 Jun;11(6):462–466. doi: 10.1007/BF02532836. [DOI] [PubMed] [Google Scholar]
- Chatterjee S., Kwiterovich P. O., Jr, Sekerke C. S. Effects of tunicamycin on the binding and degradation of low density lipoproteins and glycoprotein synthesis in cultured human fibroblasts. J Biol Chem. 1979 May 25;254(10):3704–3707. [PubMed] [Google Scholar]
- Chatterjee S., Sekerke C. S., Kwiterovich P. O., Jr Alterations in cell surface glycosphingolipids and other lipid classes of fibroblasts in familial hypercholesterolemia. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4339–4343. doi: 10.1073/pnas.73.12.4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S., Sekerke C. S., Kwiterovich P. O., Jr Effects of tunicamycin on the cell-surface binding, internalization and degradation of low-density lipoproteins in human fibroblasts. Eur J Biochem. 1981 Dec;120(3):435–441. doi: 10.1111/j.1432-1033.1981.tb05721.x. [DOI] [PubMed] [Google Scholar]
- Chatterjee S., Sekerke C. S., Kwiterovich P. O., Jr Increased urinary excretion of glycosphingolipids in familial hypercholesterolemia. J Lipid Res. 1982 May;23(4):513–522. [PubMed] [Google Scholar]
- Felix J. S., Sun T. T., Littlefield J. W. Human epithelial cells cultured from urine: growth properties and keratin staining. In Vitro. 1980 Oct;16(10):866–874. doi: 10.1007/BF02619424. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem. 1977;46:897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McCluer R. H., Williams M. A., Gross S. K., Meisler M. H. Testosterone effects on the induction and urinary excretion of mouse kidney glycosphingolipids associated with lysosomes. J Biol Chem. 1981 Dec 25;256(24):13112–13120. [PubMed] [Google Scholar]
- Spater H. W., Poruchynsky M. S., Quintana N., Inoue M., Novikoff A. B. Immunocytochemical localization of gamma-glutamyltransferase in rat kidney with protein A-horseradish peroxidase. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3547–3550. doi: 10.1073/pnas.79.11.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffyn A., Stoffyn P., Hauser G. Structure of trihexosylceramide biosynthesized in vitro by rat kidney galactosyltransferase. Biochim Biophys Acta. 1974 Aug 22;360(2):174–178. doi: 10.1016/0005-2760(74)90167-2. [DOI] [PubMed] [Google Scholar]
- Tate S. S., Meister A. Interaction of gamma-glutamyl transpeptidase with amino acids, dipeptides, and derivatives and analogs of glutathione. J Biol Chem. 1974 Dec 10;249(23):7593–7602. [PubMed] [Google Scholar]
- Verdery R. B., 3rd, Theolis R., Jr Regulation of sphingomyelin long chain base synthesis in human fibroblasts in culture. Role of lipoproteins and the low density lipoprotein receptor. J Biol Chem. 1982 Feb 10;257(3):1412–1417. [PubMed] [Google Scholar]