Abstract
We report on a procedure for tissue preparation that combines thoroughly controlled physical and chemical treatments: quick-freezing and freeze-drying followed by fixation with OsO4 vapors and embedding by direct resin infiltration. Specimens of frog cutaneous pectoris muscle thus prepared were analyzed for total calcium using electron spectroscopic imaging/electron energy loss spectroscopy (ESI/EELS) approach. The preservation of the ultrastructure was excellent, with positive K/Na ratios revealed in the fibers by x-ray microanalysis. Clear, high-resolution EELS/ESI calcium signals were recorded from the lumen of terminal cisternae of the sarcoplasmic reticulum but not from longitudinal cisternae, as expected from previous studies carried out with different techniques. In many mitochondria, calcium was below detection whereas in others it was appreciable although at variable level. Within the motor nerve terminals, synaptic vesicles as well as some cisternae of the smooth endoplasmic reticulum yielded positive signals at variance with mitochondria, that were most often below detection. Taken as a whole, the present study reveals the potential of our experimental approach to map with high spatial resolution the total calcium within individual intracellular organelles identified by their established ultrastructure, but only where the element is present at high levels.
Full text
PDF


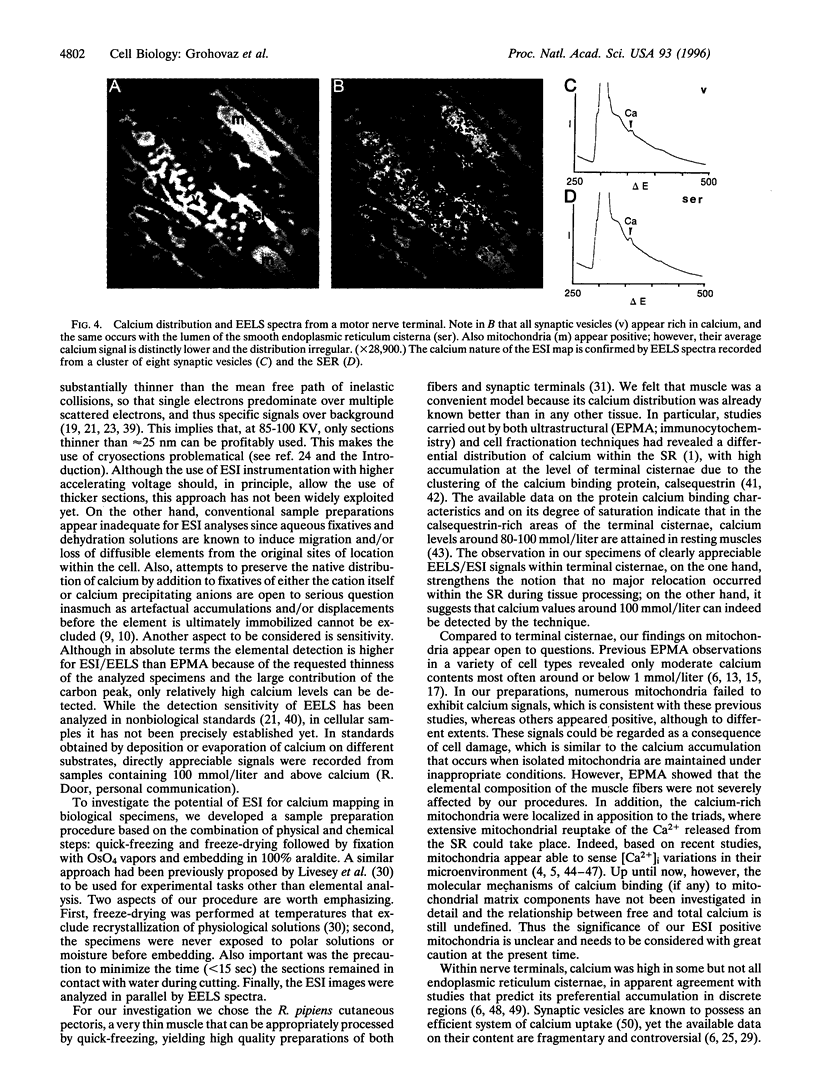

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews S. B., Leapman R. D., Landis D. M., Reese T. S. Activity-dependent accumulation of calcium in Purkinje cell dendritic spines. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1682–1685. doi: 10.1073/pnas.85.5.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews S. B., Reese T. S. Intracellular structure and elemental analysis in rapid-frozen neurons. Ann N Y Acad Sci. 1986;483:284–294. doi: 10.1111/j.1749-6632.1986.tb34534.x. [DOI] [PubMed] [Google Scholar]
- Baumann O., Walz B., Somlyo A. V., Somlyo A. P. Electron probe microanalysis of calcium release and magnesium uptake by endoplasmic reticulum in bee photoreceptors. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):741–744. doi: 10.1073/pnas.88.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond M., Vadasz G., Somlyo A. V., Somlyo A. P. Subcellular calcium and magnesium mobilization in rat liver stimulated in vivo with vasopressin and glucagon. J Biol Chem. 1987 Nov 15;262(32):15630–15636. [PubMed] [Google Scholar]
- Colliex C. Electron energy-loss spectroscopy analysis and imaging of biological specimens. Ann N Y Acad Sci. 1986;483:311–325. doi: 10.1111/j.1749-6632.1986.tb34538.x. [DOI] [PubMed] [Google Scholar]
- Duce I. R., Keen P. Can neuronal smooth endoplasmic reticulum function as a calcium reservoir? Neuroscience. 1978;3(9):837–848. doi: 10.1016/0306-4522(78)90036-2. [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong C., Kenney L. J., Varriano-Marston E. The structure of calsequestrin in triads of vertebrate skeletal muscle: a deep-etch study. J Cell Biol. 1987 Jul;105(1):49–56. doi: 10.1083/jcb.105.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friel D. D., Tsien R. W. An FCCP-sensitive Ca2+ store in bullfrog sympathetic neurons and its participation in stimulus-evoked changes in [Ca2+]i. J Neurosci. 1994 Jul;14(7):4007–4024. doi: 10.1523/JNEUROSCI.14-07-04007.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hall T. A. Capabilities and limitations of probe methods for the microanalysis of chemical elements in biology: a brief introduction. Ultramicroscopy. 1988;24(2-3):181–184. doi: 10.1016/0304-3991(88)90310-5. [DOI] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S. Structural changes after transmitter release at the frog neuromuscular junction. J Cell Biol. 1981 Mar;88(3):564–580. doi: 10.1083/jcb.88.3.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen A. O., Shen A. C., Campbell K. P., MacLennan D. H. Ultrastructural localization of calsequestrin in rat skeletal muscle by immunoferritin labeling of ultrathin frozen sections. J Cell Biol. 1983 Nov;97(5 Pt 1):1573–1581. doi: 10.1083/jcb.97.5.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körtje K. H., Körtje D. The application of electron spectroscopic imaging for quantification of the area fractions of calcium-containing precipitates in nervous tissue. J Microsc. 1992 Jun;166(Pt 3):343–358. doi: 10.1111/j.1365-2818.1992.tb01533.x. [DOI] [PubMed] [Google Scholar]
- LeFurgey A., Bond M., Ingram P. Frontiers in electron probe microanalysis: application to cell physiology. Ultramicroscopy. 1988;24(2-3):185–219. doi: 10.1016/0304-3991(88)90311-7. [DOI] [PubMed] [Google Scholar]
- Leapman R. D., Hunt J. A., Buchanan R. A., Andrews S. B. Measurement of low calcium concentrations in cryosectioned cells by parallel-EELS mapping. Ultramicroscopy. 1993 Feb;49(1-4):225–234. doi: 10.1016/0304-3991(93)90229-q. [DOI] [PubMed] [Google Scholar]
- Leapman R. D., Ornberg R. L. Quantitative electron energy loss spectroscopy in biology. Ultramicroscopy. 1988;24(2-3):251–268. doi: 10.1016/0304-3991(88)90314-2. [DOI] [PubMed] [Google Scholar]
- Livesey S. A., del Campo A. A., McDowall A. W., Stasny J. T. Cryofixation and ultra-low-temperature freeze-drying as a preparative technique for TEM. J Microsc. 1991 Feb;161(Pt 2):205–215. doi: 10.1111/j.1365-2818.1991.tb03084.x. [DOI] [PubMed] [Google Scholar]
- Martínez-Serrano A., Satrústegui J. Regulation of cytosolic free calcium concentration by intrasynaptic mitochondria. Mol Biol Cell. 1992 Feb;3(2):235–248. doi: 10.1091/mbc.3.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw C. F., Somlyo A. V., Blaustein M. P. Localization of calcium in presynaptic nerve terminals. An ultrastructural and electron microprobe analysis. J Cell Biol. 1980 May;85(2):228–241. doi: 10.1083/jcb.85.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentré P., Halpern S. Localization of cations by pyroantimonate. II. Electron probe microanalysis of calcium and sodium in skeletal muscle of mouse. J Histochem Cytochem. 1988 Jan;36(1):55–64. doi: 10.1177/36.1.3335770. [DOI] [PubMed] [Google Scholar]
- Miller R. J. The control of neuronal Ca2+ homeostasis. Prog Neurobiol. 1991;37(3):255–285. doi: 10.1016/0301-0082(91)90028-y. [DOI] [PubMed] [Google Scholar]
- Nicaise G., Gillot I., Julliard A. K., Keicher E., Blaineau S., Amsellem J., Meyran J. C., Hernandez-Nicaise M. L., Ciapa B., Gleyzal C. X-ray microanalysis of calcium containing organelles in resin embedded tissue. Scanning Microsc. 1989 Mar;3(1):199–220. [PubMed] [Google Scholar]
- Nicaise G., Maggio K., Thirion S., Horoyan M., Keicher E. The calcium loading of secretory granules. A possible key event in stimulus-secretion coupling. Biol Cell. 1992;75(2):89–99. doi: 10.1016/0248-4900(92)90128-n. [DOI] [PubMed] [Google Scholar]
- Ottensmeyer F. P., Andrew J. W. High-resolution microanalysis of biological specimens by electron energy loss spectroscopy and by electron spectroscopic imaging. J Ultrastruct Res. 1980 Sep;72(3):336–348. doi: 10.1016/s0022-5320(80)90069-6. [DOI] [PubMed] [Google Scholar]
- PORTER K. R., PALADE G. E. Studies on the endoplasmic reticulum. III. Its form and distribution in striated muscle cells. J Biophys Biochem Cytol. 1957 Mar 25;3(2):269–300. doi: 10.1083/jcb.3.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parducz A., Dunant Y. Transient increase of calcium in synaptic vesicles after stimulation. Neuroscience. 1993 Jan;52(1):27–33. doi: 10.1016/0306-4522(93)90178-i. [DOI] [PubMed] [Google Scholar]
- Peachey L. D. The sarcoplasmic reticulum and transverse tubules of the frog's sartorius. J Cell Biol. 1965 Jun;25(3 Suppl):209–231. doi: 10.1083/jcb.25.3.209. [DOI] [PubMed] [Google Scholar]
- Plattner H., Bachmann L. Cryofixation: a tool in biological ultrastructural research. Int Rev Cytol. 1982;79:237–304. doi: 10.1016/s0074-7696(08)61676-9. [DOI] [PubMed] [Google Scholar]
- Poenie M., Epel D. Ultrastructural localization of intracellular calcium stores by a new cytochemical method. J Histochem Cytochem. 1987 Sep;35(9):939–956. doi: 10.1177/35.9.3611737. [DOI] [PubMed] [Google Scholar]
- Pozzan T., Rizzuto R., Volpe P., Meldolesi J. Molecular and cellular physiology of intracellular calcium stores. Physiol Rev. 1994 Jul;74(3):595–636. doi: 10.1152/physrev.1994.74.3.595. [DOI] [PubMed] [Google Scholar]
- Probst W. Ultrastructural localization of calcium in the CNS of vertebrates. Histochemistry. 1986;85(3):231–239. doi: 10.1007/BF00494809. [DOI] [PubMed] [Google Scholar]
- Rizzuto R., Bastianutto C., Brini M., Murgia M., Pozzan T. Mitochondrial Ca2+ homeostasis in intact cells. J Cell Biol. 1994 Sep;126(5):1183–1194. doi: 10.1083/jcb.126.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R., Brini M., Murgia M., Pozzan T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science. 1993 Oct 29;262(5134):744–747. doi: 10.1126/science.8235595. [DOI] [PubMed] [Google Scholar]
- Rizzuto R., Simpson A. W., Brini M., Pozzan T. Rapid changes of mitochondrial Ca2+ revealed by specifically targeted recombinant aequorin. Nature. 1992 Jul 23;358(6384):325–327. doi: 10.1038/358325a0. [DOI] [PubMed] [Google Scholar]
- Roos N., Barnard T. A comparison of subcellular element concentrations in frozen-dried, plastic-embedded, dry-cut sections and frozen-dried cryosections. Ultramicroscopy. 1985;17(4):335–343. doi: 10.1016/0304-3991(85)90200-1. [DOI] [PubMed] [Google Scholar]
- Shuman H., Somlyo A. P. Electron energy loss analysis of near-trace-element concentrations of calcium. Ultramicroscopy. 1987;21(1):23–32. doi: 10.1016/0304-3991(87)90004-0. [DOI] [PubMed] [Google Scholar]
- Shuman H., Somlyo A. V., Somlyo A. P. Quantitative electron probe microanalysis of biological thin sections: methods and validity. Ultramicroscopy. 1976 Sep-Oct;1(4):317–339. doi: 10.1016/0304-3991(76)90049-8. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Bond M., Somlyo A. V. Calcium content of mitochondria and endoplasmic reticulum in liver frozen rapidly in vivo. Nature. 1985 Apr 18;314(6012):622–625. doi: 10.1038/314622a0. [DOI] [PubMed] [Google Scholar]
- Somlyo A. V., Gonzalez-Serratos H. G., Shuman H., McClellan G., Somlyo A. P. Calcium release and ionic changes in the sarcoplasmic reticulum of tetanized muscle: an electron-probe study. J Cell Biol. 1981 Sep;90(3):577–594. doi: 10.1083/jcb.90.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo A. V., McClellan G., Gonzalez-Serratos H., Somlyo A. P. Electron probe X-ray microanalysis of post-tetanic Ca2+ and Mg2+ movements across the sarcoplasmic reticulum in situ. J Biol Chem. 1985 Jun 10;260(11):6801–6807. [PubMed] [Google Scholar]
- Stelly N., Halpern S., Nicolas G., Fragu P., Adoutte A. Direct visualization of a vast cortical calcium compartment in Paramecium by secondary ion mass spectrometry (SIMS) microscopy: possible involvement in exocytosis. J Cell Sci. 1995 May;108(Pt 5):1895–1909. doi: 10.1242/jcs.108.5.1895. [DOI] [PubMed] [Google Scholar]
- Torri-Tarelli F., Grohovaz F., Fesce R., Ceccarelli B. Temporal coincidence between synaptic vesicle fusion and quantal secretion of acetylcholine. J Cell Biol. 1985 Oct;101(4):1386–1399. doi: 10.1083/jcb.101.4.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y. Fluorescent indicators of ion concentrations. Methods Cell Biol. 1989;30:127–156. doi: 10.1016/s0091-679x(08)60978-4. [DOI] [PubMed] [Google Scholar]
- Volpe P., Simon B. J. The bulk of Ca2+ released to the myoplasm is free in the sarcoplasmic reticulum and does not unbind from calsequestrin. FEBS Lett. 1991 Jan 28;278(2):274–278. doi: 10.1016/0014-5793(91)80134-o. [DOI] [PubMed] [Google Scholar]
- Wang Y. Y., Ho R., Shao Z., Somlyo A. P. Optimization of quantitative electron energy loss spectroscopy in the low loss region: phosphorus L-edge. Ultramicroscopy. 1992 Apr-May;41(1-3):11–31. doi: 10.1016/0304-3991(92)90091-w. [DOI] [PubMed] [Google Scholar]
- Weibel E. R., Paumgartner D. Integrated stereological and biochemical studies on hepatocytic membranes. II. Correction of section thickness effect on volume and surface density estimates. J Cell Biol. 1978 May;77(2):584–597. doi: 10.1083/jcb.77.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zierold K. Cryofixation methods for ion localization in cells by electron probe microanalysis: a review. J Microsc. 1991 Feb;161(Pt 2):357–366. doi: 10.1111/j.1365-2818.1991.tb03096.x. [DOI] [PubMed] [Google Scholar]
- Zierold K., Schäfer D. Preparation of cultured and isolated cells for X-ray microanalysis. Scanning Microsc. 1988 Sep;2(3):1775–1790. [PubMed] [Google Scholar]