Abstract
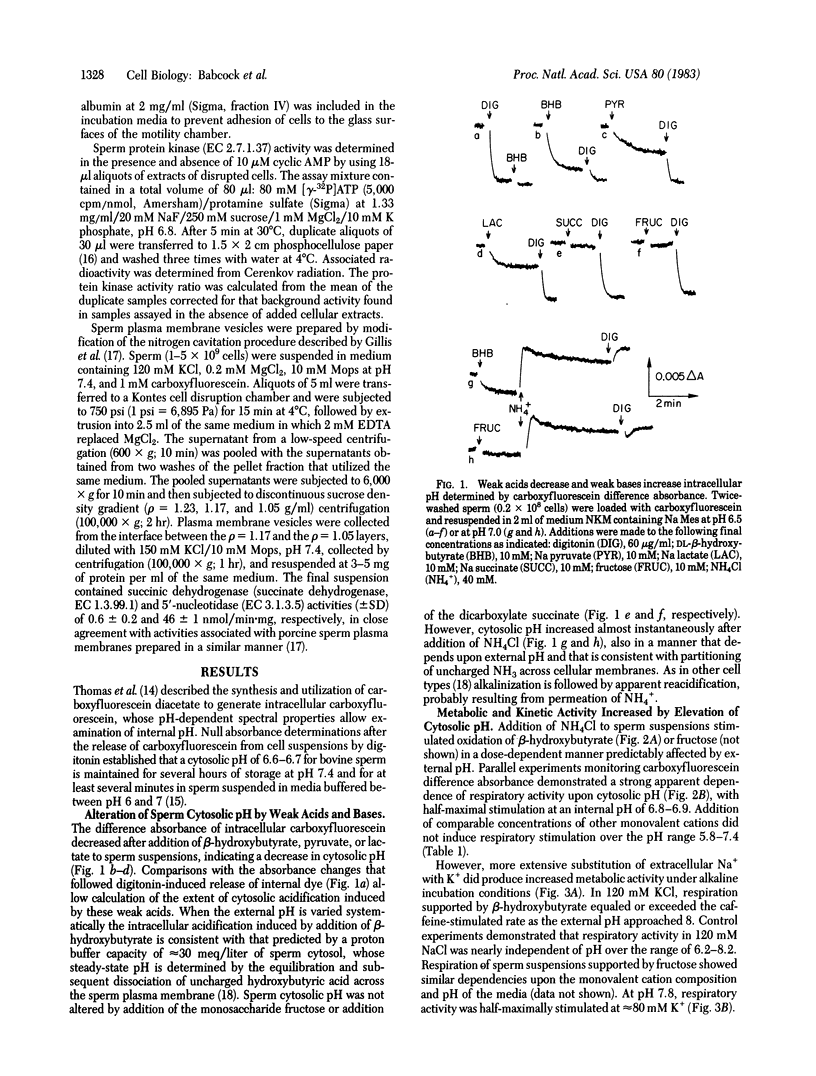
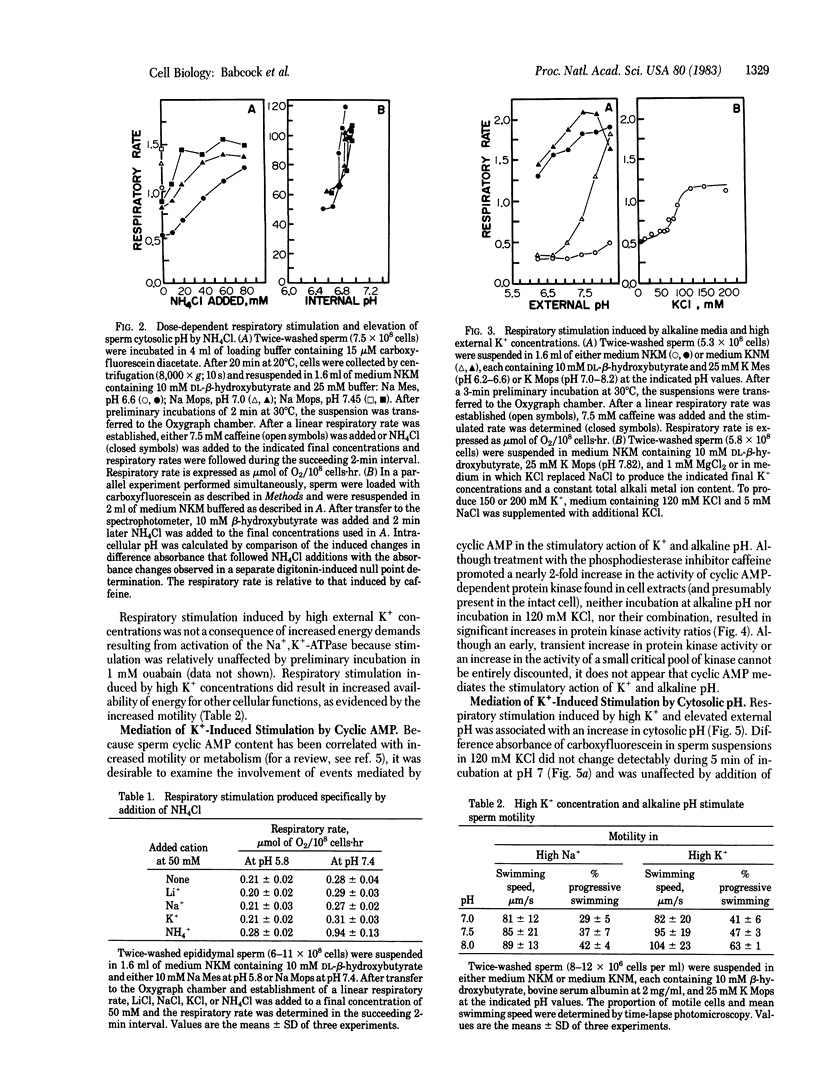
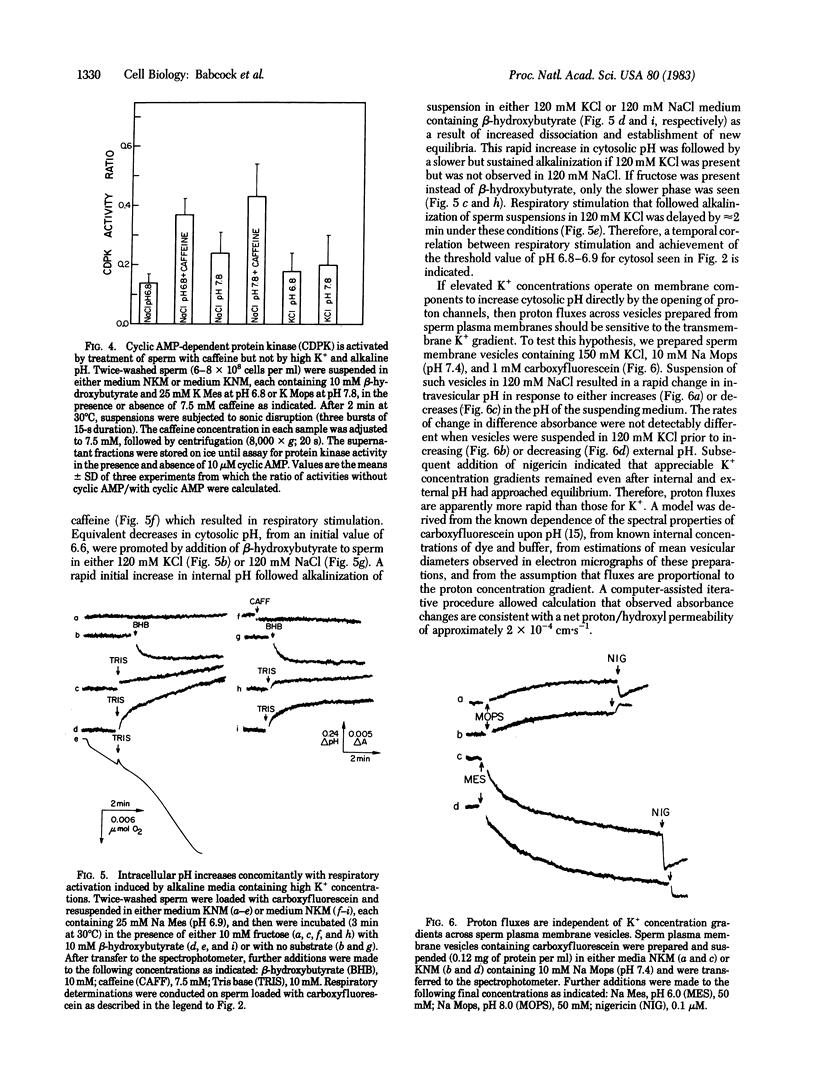
Sperm cytosolic pH, determined by the spectral properties of intracellular carboxyfluorescein, is decreased rapidly by the diffusion and subsequent dissociation of the uncharged weak acids pyruvic, lactic, or hydroxybutyric and is increased by diffusion and subsequent intracellular protonation of the weak base NH3. Metabolic and kinetic activity increases dramatically when intracellular pH is elevated above 6.8-6.9 by addition of 50 mM NH4Cl to sperm suspended in a 120 mM NaCl medium. Respiratory stimulation is not observed upon comparable additions of 50 mM Li+ or K+ or when the pH of the medium is increased from 6.5 to 8.2. However, increases of the external pH to 7.8-8.2 in medium employing 120 mM KCl result in increased metabolic and kinetic activity, comparable to the maximal stimulation induced by the phosphodiesterase inhibitor caffeine. An increase in cytosolic pH from 6.3-6.6 to 6.8 occurs concomitant with the respiratory stimulation induced by KCl in alkaline media. No change in cytosolic pH follows addition of caffeine. Cyclic AMP-dependent protein kinase activity ratios, determined in cellular extracts, are increased by caffeine treatment but are not elevated by 120 mM KCl, by alkaline pH, or by their combination. These observations indicate that cytosolic pH plays a role in the regulation of motility and metabolism of mammalian sperm that is not mediated by cyclic AMP but that may be under control of a plasma membrane voltage-dependent proton channel. However, H+ fluxes across vesicles prepared from sperm membranes are unaffected by variation in the magnitude of the transvesicular K+ concentration gradient.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babcock D. F., First N. L., Lardy H. A. Action of ionophore A23187 at the cellular level. Separation of effects at the plasma and mitochondrial membranes. J Biol Chem. 1976 Jul 10;251(13):3881–3886. [PubMed] [Google Scholar]
- Clement N. R., Gould J. M. Pyranine (8-hydroxy-1,3,6-pyrenetrisulfonate) as a probe of internal aqueous hydrogen ion concentration in phospholipid vesicles. Biochemistry. 1981 Mar 17;20(6):1534–1538. doi: 10.1021/bi00509a019. [DOI] [PubMed] [Google Scholar]
- Garbers D. L., Hardman J. G. Factors released from sea urchin eggs affect cyclic nucleotide metabolism in sperm. Nature. 1975 Oct 23;257(5528):677–678. doi: 10.1038/257677a0. [DOI] [PubMed] [Google Scholar]
- Garbers D. L., Kopf G. S. The regulation of spermatozoa by calcium cyclic nucleotides. Adv Cyclic Nucleotide Res. 1980;13:251–306. [PubMed] [Google Scholar]
- Gillis G., Peterson R., Russell L., Hook L., Freund M. Isolation and characterization of membrane vesicles from human and boar spermatozoa: methods using nitrogen cavitation and ionophore induced vesiculation. Prep Biochem. 1978;8(5):363–378. doi: 10.1080/10826067809412288. [DOI] [PubMed] [Google Scholar]
- Hansbrough J. R., Garbers D. L. Sodium-dependent activation of sea urchin spermatozoa by speract and monensin. J Biol Chem. 1981 Mar 10;256(5):2235–2241. [PubMed] [Google Scholar]
- Meissner G., Young R. C. Proton permeability of sarcoplasmic reticulum vesicles. J Biol Chem. 1980 Jul 25;255(14):6814–6819. [PubMed] [Google Scholar]
- Meizel S., Deamer D. W. The pH of the hamster sperm acrosome. J Histochem Cytochem. 1978 Feb;26(2):98–105. doi: 10.1177/26.2.24069. [DOI] [PubMed] [Google Scholar]
- Mrsny R. J., Meizel S. Potassium ion influx and Na+,K+-ATPase activity are required for the hamster sperm acrosome reaction. J Cell Biol. 1981 Oct;91(1):77–82. [PMC free article] [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Schackmann R. W., Christen R., Shapiro B. M. Membrane potential depolarization and increased intracellular pH accompany the acrosome reaction of sea urchin sperm. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6066–6070. doi: 10.1073/pnas.78.10.6066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. A., Buchsbaum R. N., Zimniak A., Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979 May 29;18(11):2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Tilney L. G., Kiehart D. P., Sardet C., Tilney M. Polymerization of actin. IV. Role of Ca++ and H+ in the assembly of actin and in membrane fusion in the acrosomal reaction of echinoderm sperm. J Cell Biol. 1978 May;77(2):536–550. doi: 10.1083/jcb.77.2.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt J. J., Roskoski R., Jr Rapid protein kinase assay using phosphocellulose-paper absorption. Anal Biochem. 1975 May 26;66(1):253–258. doi: 10.1016/0003-2697(75)90743-5. [DOI] [PubMed] [Google Scholar]
- Wong P. Y., Lee W. M., Tsang A. Y. The effects of extracellular sodium on acid release and motility initiation in rat caudal epididymal spermatozoa in vitro. Exp Cell Res. 1981 Jan;131(1):97–104. doi: 10.1016/0014-4827(81)90410-9. [DOI] [PubMed] [Google Scholar]
- Yanagimachi R. Acceleration of the acrosome reaction and activation of guinea pigs spermatozoa by detergents and other reagents. Biol Reprod. 1975 Dec;13(5):519–526. doi: 10.1095/biolreprod13.5.519. [DOI] [PubMed] [Google Scholar]



