Abstract
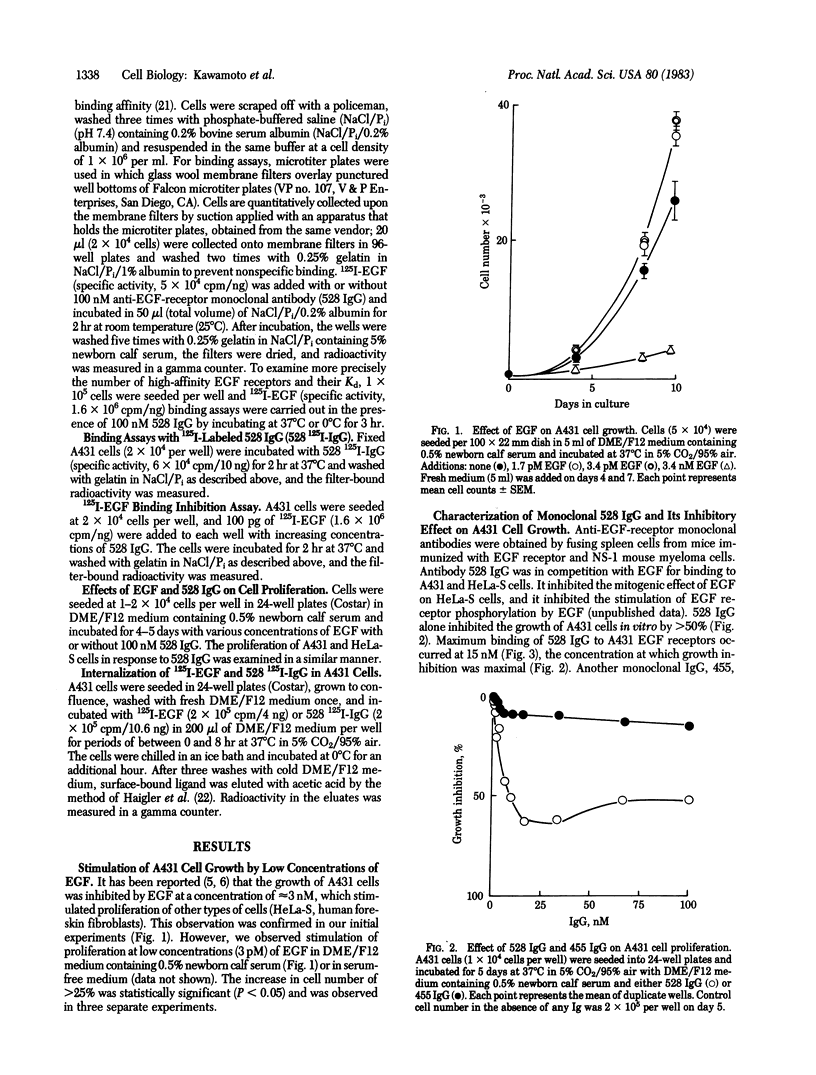
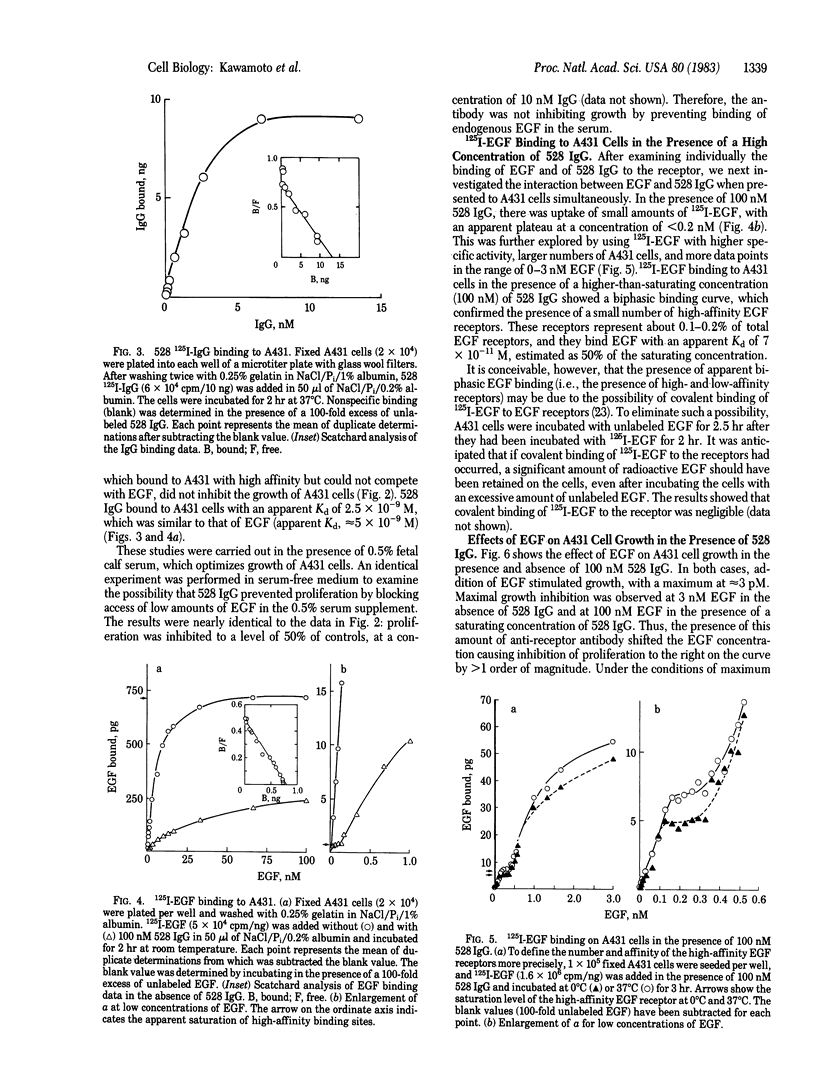
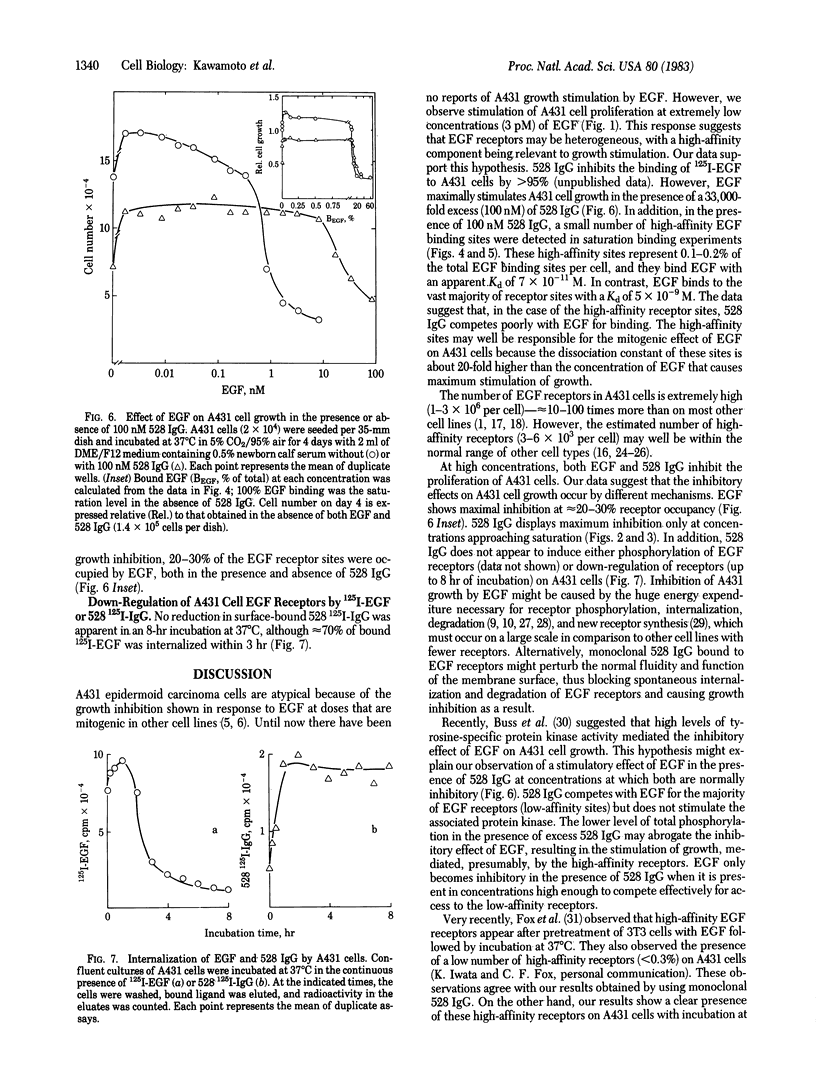
Epidermal growth factor (EGF) at 3 nM maximally inhibits the proliferation of A431 epidermoid carcinoma cells. We show that at lower concentrations, in the range of 3-100 pM, EGF has a mitogenic effect on A431 cells. In the presence of 100 nM anti-EGF-receptor monoclonal IgG (designated 528), which inhibits A431 cell proliferation and blocks greater than 95% of EGF binding, EGF becomes mitogenic for A431 cells at concentrations up to 3 nM. These results suggest that a minor population of high-affinity EGF receptors may be involved in stimulation of A431 cell proliferation. Saturation binding assays with 125I-labeled EGF indicate that approximately equal to 0.1-0.2% of receptors for EGF are high-affinity receptors that bind EGF with an estimated Kd of 7 X 10(-11) M. This affinity is nearly 2 orders of magnitude higher than that of the remaining EGF receptors. Although A431 cell proliferation is maximally inhibited by nonsaturating amounts of EGF (3 nM), maximal inhibition by 528 IgG (approximately equal to 70% of maximal inhibition by EGF) requires saturating concentrations of antibody (approximately equal to 15 nM). Unlike EGF, rapid down-regulation is not observed with 528 IgG. These results indicate different mechanisms of growth inhibition of A431 cells by EGF and 528 IgG.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes D. W. Epidermal growth factor inhibits growth of A431 human epidermoid carcinoma in serum-free cell culture. J Cell Biol. 1982 Apr;93(1):1–4. doi: 10.1083/jcb.93.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes D., Sato G. Methods for growth of cultured cells in serum-free medium. Anal Biochem. 1980 Mar 1;102(2):255–270. doi: 10.1016/0003-2697(80)90151-7. [DOI] [PubMed] [Google Scholar]
- Buss J. E., Kudlow J. E., Lazar C. S., Gill G. N. Altered epidermal growth factor (EGF)-stimulated protein kinase activity in variant A431 cells with altered growth responses to EGF. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2574–2578. doi: 10.1073/pnas.79.8.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Epidermal growth factor. Annu Rev Biochem. 1979;48:193–216. doi: 10.1146/annurev.bi.48.070179.001205. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Human epidermal growth factor and the proliferation of human fibroblasts. J Cell Physiol. 1976 Jun;88(2):227–237. doi: 10.1002/jcp.1040880212. [DOI] [PubMed] [Google Scholar]
- Comens P. G., Simmer R. L., Baker J. B. Direct linkage of 125I-EGF to cell surface receptors. A useful artifact of chloramine-T treatment. J Biol Chem. 1982 Jan 10;257(1):42–45. [PubMed] [Google Scholar]
- Das M., Fox C. F. Molecular mechanism of mitogen action: processing of receptor induced by epidermal growth factor. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2644–2648. doi: 10.1073/pnas.75.6.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicker P., Rozengurt E. Stimulation of DNA synthesis by tumour promoter and pure mitogenic factors. Nature. 1978 Dec 14;276(5689):723–726. doi: 10.1038/276723a0. [DOI] [PubMed] [Google Scholar]
- Fabricant R. N., De Larco J. E., Todaro G. J. Nerve growth factor receptors on human melanoma cells in culture. Proc Natl Acad Sci U S A. 1977 Feb;74(2):565–569. doi: 10.1073/pnas.74.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C. F., Das M. Internalization and processing of the EGF receptor in the induction of DNA synthesis in cultured fibroblasts: the endocytic activation hypothesis. J Supramol Struct. 1979;10(2):199–214. doi: 10.1002/jss.400100210. [DOI] [PubMed] [Google Scholar]
- Gill G. N., Lazar C. S. Increased phosphotyrosine content and inhibition of proliferation in EGF-treated A431 cells. Nature. 1981 Sep 24;293(5830):305–307. doi: 10.1038/293305a0. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Anderson R. G., Brown M. S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979 Jun 21;279(5715):679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Greenburg G., Bialecki H., Zetter B. R. Factors involved in the modulation of cell proliferation in vivo and in vitro: the role of fibroblast and epidermal growth factors in the proliferative response of mammalian cells. In Vitro. 1978 Jan;14(1):85–118. doi: 10.1007/BF02618177. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Haigler H. T., Maxfield F. R., Willingham M. C., Pastan I. Dansylcadaverine inhibits internalization of 125I-epidermal growth factor in BALB 3T3 cells. J Biol Chem. 1980 Feb 25;255(4):1239–1241. [PubMed] [Google Scholar]
- Haigler H. T., McKanna J. A., Cohen S. Direct visualization of the binding and internalization of a ferritin conjugate of epidermal growth factor in human carcinoma cells A-431. J Cell Biol. 1979 May;81(2):382–395. doi: 10.1083/jcb.81.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigler H., Ash J. F., Singer S. J., Cohen S. Visualization by fluorescence of the binding and internalization of epidermal growth factor in human carcinoma cells A-431. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3317–3321. doi: 10.1073/pnas.75.7.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A. C., Cuatrecasas P. Resolution of high and low affinity epidermal growth factor receptors. Inhibition of high affinity component by low temperature, cycloheximide, and phorbol esters. J Biol Chem. 1982 Mar 25;257(6):3053–3060. [PubMed] [Google Scholar]
- King A. C., Willis R. A., Cuatrecasas P. Accumulation of epidermal growth factor within cells does not depend on receptor recycling. Biochem Biophys Res Commun. 1980 Dec 16;97(3):840–845. doi: 10.1016/0006-291x(80)91453-9. [DOI] [PubMed] [Google Scholar]
- Krupp M. N., Livingston J. N. Insulin binding to solubilized material from fat cell membranes: evidence for two binding species. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2593–2597. doi: 10.1073/pnas.75.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landreth G. E., Shooter E. M. Nerve growth factor receptors on PC12 cells: ligand-induced conversion from low- to high-affinity states. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4751–4755. doi: 10.1073/pnas.77.8.4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magun B. E., Matrisian L. M., Bowden G. T. Epidermal growth factor. Ability of tumor promoter to alter its degradation, receptor affinity and receptor number. J Biol Chem. 1980 Jul 10;255(13):6373–6381. [PubMed] [Google Scholar]
- Maturo J. M., 3rd, Hollenberg M. D. Insulin receptor: interaction with nonreceptor glycoprotein from liver cell membranes. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3070–3074. doi: 10.1073/pnas.75.7.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxfield F. R., Davies P. J., Klempner L., Willingham M. C., Pastan I. Epidermal growth factor stimulation of DNA synthesis is potentiated by compounds that inhibit its clustering in coated pits. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5731–5735. doi: 10.1073/pnas.76.11.5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami H., Masui H., Sato G. H., Sueoka N., Chow T. P., Kano-Sueoka T. Growth of hybridoma cells in serum-free medium: ethanolamine is an essential component. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1158–1162. doi: 10.1073/pnas.79.4.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonbrunn A., Krasnoff M., Westendorf J. M., Tashjian A. H., Jr Epidermal growth factor and thyrotropin-releasing hormone act similarly on a clonal pituitary cell strain. Modulation of hormone production and inhbition of cell proliferation. J Cell Biol. 1980 Jun;85(3):786–797. doi: 10.1083/jcb.85.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber A. B., Lax I., Yarden Y., Eshhar Z., Schlessinger J. Monoclonal antibodies against receptor for epidermal growth factor induce early and delayed effects of epidermal growth factor. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7535–7539. doi: 10.1073/pnas.78.12.7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter Y., Hernaez L., Cuatrecasas P. Epidermal growth factor: biological activity requires persistent occupation of high-affinity cell surface receptors. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5788–5791. doi: 10.1073/pnas.75.12.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoyab M., De Larco J. E., Todaro G. J. Biologically active phorbol esters specifically alter affinity of epidermal growth factor membrane receptors. Nature. 1979 May 31;279(5712):387–391. doi: 10.1038/279387a0. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Maxfield F. R., Pastan I. H. alpha 2 Macroglobulin binding to the plasma membrane of cultured fibroblasts. Diffuse binding followed by clustering in coated regions. J Cell Biol. 1979 Sep;82(3):614–625. doi: 10.1083/jcb.82.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]


