Abstract
Persistent infection by high-risk human papillomavirus (HPV) is associated with cervical carcinoma. Blocking innate immune responses in the cervix is critical for the establishment of persistent infections. Here, we discuss how HPV-16-encoded factors suppress the activity of Toll-like receptor 9, which plays a major role in innate antiviral immune responses.
Keywords: E7, HPV16, immunosuppression, TLR9
HPV Disease and Prevention
More than 500,000 new cases of cervical carcinoma and 275,000 deaths are reported each year worldwide.1 Cervical carcinomas originates upon persistent infection by high-risk (HR) HPV variants, such as HPV-16 and -18. Accumulating evidence indicates that HR HPV types are also responsible for a proportion of oropharyngeal cancers, whose incidence appear to have steadily increased in the last 2 decades in the US and Europe.2 Although a prophylactic vaccine against the HPV-16 and -18 is currently employed, epidemiological data in support of its ability to reduce the incidence of cervical cancer will not be available for another 20 y. In addition, there are other 13 HR HPV types that are not covered by the current formulation of the vaccine, implying that about 30 percent of cervical carcinomas cannot be prevented by prophylactic intervention.
Immunity Against HPV
HR HPV types can alter immunosurveillance and cellular homeostasis by deregulating gene expression in host cells, at least in part via epigenetic mechanisms. Initially, HR HPVs stimulate the proliferation of infected cells as a means to favor viral replication and persistence. This event has been extensively investigated and is mediated by the HPV-encoded oncoproteins E6 and E7. Studying the immunomodulatory activity of HPV is more problematic, for several reasons. Firstly HPV is a non-lytic virus that replicates only in human epidermal keratinocytes. Thus, the ability of the virus to influence the immune system is restricted to the local microenvironment surrounding the infected epidermis. Secondly the tools that are currently available to study the immunobiology of HPV are limited. In particular, HPV cannot replicate in mice, and generating HPV virions in vitro has been challenging. Nonetheless, determining how HPV HR variants are able to block innate immune responses is extremely important to fully understand the initial events involved in the establishment of cervical neoplasms. Cellular innate immune responses are mediated by both hematopoietic and non-hematopoietic cells that express pathogen recognition receptors (PRRs). One such PRRs named Toll-like receptor 9 (TLR9), is able to recognize double-stranded DNA molecules of viral origin and to elicit the production of immunostimulatory and pro-inflammatory cytokines including Type I interferon (IFN).3 In 2007, we demonstrated that E6 and E7 were able to block TLR9-induced cytokine secretion by human keratinocytes.4 Several other reports have shown that HR HPV types have developed various strategies to evade the immune system and persist in the host. For instance, oncoproteins encoded by HPV-16 and -18 are able to inhibit the synthesis of pro-inflammatory cytokines such as chemokine (C-C motif) ligand 5 (CCL5) and interleukin (IL)-1β.5,6 In addition, HPV-16, -18, and -31 are capable of inhibiting the production of (1) IFN-inducible antiviral proteins, such as interferon induced protein with tetratricopeptide repeats 1 (IFIT1) and myxovirus (influenza virus) resistance 1, interferon-inducible protein p78 (MX1), (2) pro-apoptotic factors, such as tumor necrosis factor (ligand) superfamily, member 10 (TNFSF10, best known as TRAIL) and XIAP-associated factor 1 (XAF1), and (3) PRRs, including TLR3, DEAD (Asp-Glu-Ala-Asp) box polypeptide 58 (DDX58, best known as RIG-I) and interferon induced with helicase C domain 1 (IFIH1, best known as MDA5).7 Although informative, most of these studies examined the ability of HPV to deregulate the immune systems in models in which only the viral proteins are expressed.
Mechanism of E7-Dependent TLR9 Downregulation
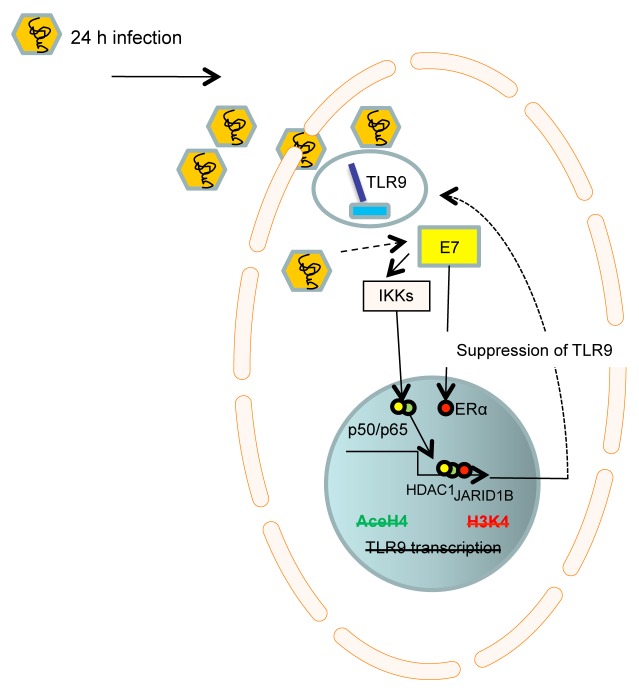
Recent techniques to generate HPV quasivirions have allowed HPV researchers to address questions on viral replication and the interactions between HPV and host cells. We decided to use HPV-16 quasivirions (16QsV) as a tool to determine the mechanism whereby HPV-16 downregulates TLR9 in human primary keratinocytes.8 Infection of human keratinocytes with 16QsV for 24 h and the consequent expression of E7 promoted the formation of a nuclear complex consisting of estrogen receptor 1 (α) (ESR1, best known as ERα) and NF-κB NFKB1/RELA (p50/p65) dimers. This complex bound to DNA on the TLR9 promoter (within a 200-bp region termed site B). In addition, p65 and ERα interacted with histone deacetylase 1 (HDAC1) and lysine-specific demethylase 5B (KDM5B, best known JARID1B), respectively, which promoted histone modifications at site B that contributed to the suppression of TLR9 transcription. The ability of 16QsV to suppress the transcriptional activity of the TLR9 promoter by acting on site B resulted in the loss of type I IFN production upon TLR9 stimulation. The knockdown of E7 in 16QsV-infected human keratinocytes resulted in the loss of the p50/p65/HDAC1:ERα/JARID1B complex at site B and restored histone methylation and acetylation around the TLR9 promoter, allowing for normal TLR9 expression (Fig. 1). Importantly, we corroborated our in vitro findings in samples from cervical cancer patients. Protein proximity ligation experiments on biopsies from HPV-16- infected patients revealed indeed the formation of a p65:ERα complex and the loss of TLR9 expression. This complex was not detected in healthy cervical tissues normally expressing TLR9.
Figure 1. Mechanism whereby HPV-16 promotes TLR9 downregulation. Infection of human epithelial cells with human papillomavirus type 16 (HPV-16) promotes the formation of an inhibitory transcriptional complex which relies the on the E7-dependent, IκB kinase (IKK)-mediated activation of NF-κB p50/p65 dimers as well as of estrogen receptor 1 (α) (ERα). Such an E7-elicited transcriptional complex also recruits histone deacetylase 1 (HDAC1) and lysine-specific demethylase 5B (JARID1B) to a specific region of the Toll-like receptor 9 (TLR9) promoter, resulting in a decreased methylation and acetylation of histones upstream of the TLR9 transcriptional start site.
Concluding Remarks
The ability of TLR9 to inhibit HPV-induced carcinogenesis has recently been highlighted by the fact that specific TLR9 polymorphisms are associated with an increased risk of cervical cancer among women.9 We provided mechanistic details on the capacity of HR HPV16 to interfere with TLR9-mediated immune responses. We can extrapolate from our findings that interfering with the transcriptional repression of TLR9, for example by means of synthetic antagonists of ERα, may provide a novel strategy for the treatment of cervical and perhaps oropharyngeal cancers.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Citation: Hasan U. Human papillomavirus (HPV) deregulation of Toll-like receptor 9. OncoImmunology 2013; 2:e27257; 10.4161/onci.27257
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/27257
References
- 1.Tay SK. Cervical cancer in the human papillomavirus vaccination era. Curr Opin Obstet Gynecol. 2012;24:3–7. doi: 10.1097/GCO.0b013e32834daed9. [DOI] [PubMed] [Google Scholar]
- 2.Marur S, D’Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010;11:781–9. doi: 10.1016/S1470-2045(10)70017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sato A, Linehan MM, Iwasaki A. Dual recognition of herpes simplex viruses by TLR2 and TLR9 in dendritic cells. Proc Natl Acad Sci U S A. 2006;103:17343–8. doi: 10.1073/pnas.0605102103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hasan UA, Bates E, Takeshita F, Biliato A, Accardi R, Bouvard V, Mansour M, Vincent I, Gissmann L, Iftner T, et al. TLR9 expression and function is abolished by the cervical cancer-associated human papillomavirus type 16. J Immunol. 2007;178:3186–97. doi: 10.4049/jimmunol.178.5.3186. [DOI] [PubMed] [Google Scholar]
- 5.Karim R, Meyers C, Backendorf C, Ludigs K, Offringa R, van Ommen GJ, Melief CJ, van der Burg SH, Boer JM. Human papillomavirus deregulates the response of a cellular network comprising of chemotactic and proinflammatory genes. PLoS One. 2011;6:e17848. doi: 10.1371/journal.pone.0017848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niebler M, Qian X, Höfler D, Kogosov V, Kaewprag J, Kaufmann AM, Ly R, Böhmer G, Zawatzky R, Rösl F, et al. Post-translational control of IL-1β via the human papillomavirus type 16 E6 oncoprotein: a novel mechanism of innate immune escape mediated by the E3-ubiquitin ligase E6-AP and p53. PLoS Pathog. 2013;9:e1003536. doi: 10.1371/journal.ppat.1003536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reiser J, Hurst J, Voges M, Krauss P, Münch P, Iftner T, Stubenrauch F. High-risk human papillomaviruses repress constitutive kappa interferon transcription via E6 to prevent pathogen recognition receptor and antiviral-gene expression. J Virol. 2011;85:11372–80. doi: 10.1128/JVI.05279-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hasan UA, Zannetti C, Parroche P, Goutagny N, Malfroy M, Roblot G, Carreira C, Hussain I, Müller M, Taylor-Papadimitriou J, et al. The human papillomavirus type 16 E7 oncoprotein induces a transcriptional repressor complex on the Toll-like receptor 9 promoter. J Exp Med. 2013;210:1369–87. doi: 10.1084/jem.20122394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roszak A, Lianeri M, Sowińska A, Jagodziński PP. Involvement of Toll-like Receptor 9 polymorphism in cervical cancer development. Mol Biol Rep. 2012;39:8425–30. doi: 10.1007/s11033-012-1695-8. [DOI] [PMC free article] [PubMed] [Google Scholar]