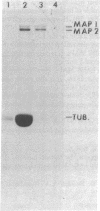
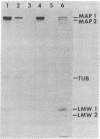
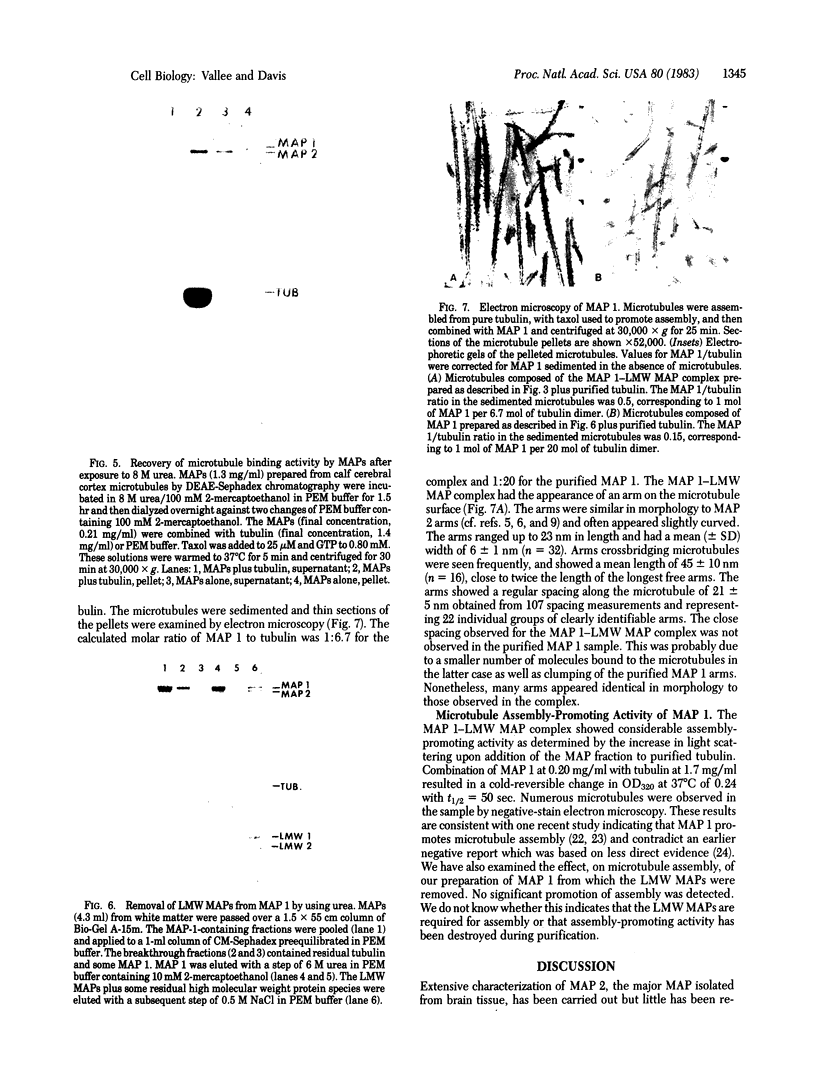
Abstract
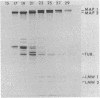
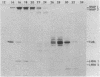
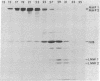
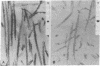
Microtubule-associated protein 1 (MAP 1; Mr = 350,000) was analyzed by column chromatography of microtubule protein obtained from calf brain gray and white matter. Two low molecular weight proteins (LMW MAPs; Mr 28,000 and 30,000) were found to cochromatograph with MAP 1 under all conditions examined. MAP 1 and the LMW MAPs were purified from calf brain white matter as a complex containing approximately equimolar amounts of the three species. Urea (6 M) was used to remove the LMW MAPs from MAP 1. Binding of MAP 1 to microtubules was unaffected by urea and occurred with or without the LMW species. Electron microscopy of microtubules composed of purified tubulin and either MAP 1 preparation revealed that, like MAP 2, MAP 1 has the appearance of a filamentous arm on the microtubule surface.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell C. W., Fronk E., Gibbons I. R. Polypeptide subunits of dynein 1 from sea urchin sperm flagella. J Supramol Struct. 1979;11(3):311–317. doi: 10.1002/jss.400110305. [DOI] [PubMed] [Google Scholar]
- Berkowitz S. A., Katagiri J., Binder H. K., Williams R. C., Jr Separation and characterization of microtubule proteins from calf brain. Biochemistry. 1977 Dec 13;16(25):5610–5617. doi: 10.1021/bi00644a035. [DOI] [PubMed] [Google Scholar]
- Borisy G. G., Marcum J. M., Olmsted J. B., Murphy D. B., Johnson K. A. Purification of tubulin and associated high molecular weight proteins from porcine brain and characterization of microtubule assembly in vitro. Ann N Y Acad Sci. 1975 Jun 30;253:107–132. doi: 10.1111/j.1749-6632.1975.tb19196.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Hwo S. Y., Kirschner M. W. Purification of tau, a microtubule-associated protein that induces assembly of microtubules from purified tubulin. J Mol Biol. 1977 Oct 25;116(2):207–225. doi: 10.1016/0022-2836(77)90213-3. [DOI] [PubMed] [Google Scholar]
- Ellisman M. H., Porter K. R. Microtrabecular structure of the axoplasmic matrix: visualization of cross-linking structures and their distribution. J Cell Biol. 1980 Nov;87(2 Pt 1):464–479. doi: 10.1083/jcb.87.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fellous A., Francon J., Lennon A. M., Nunez J. Microtubule assembly in vitro. Purification of assembly-promoting factors. Eur J Biochem. 1977 Aug 15;78(1):167–174. doi: 10.1111/j.1432-1033.1977.tb11726.x. [DOI] [PubMed] [Google Scholar]
- Gaskin F., Cantor C. R., Shelanski M. L. Turbidimetric studies of the in vitro assembly and disassembly of porcine neurotubules. J Mol Biol. 1974 Nov 15;89(4):737–755. doi: 10.1016/0022-2836(74)90048-5. [DOI] [PubMed] [Google Scholar]
- Herzog W., Weber K. Fractionation of brain microtubule-associated proteins. Isolation of two different proteins which stimulate tubulin polymerization in vitro. Eur J Biochem. 1978 Dec 1;92(1):1–8. doi: 10.1111/j.1432-1033.1978.tb12716.x. [DOI] [PubMed] [Google Scholar]
- Hirokawa N. Cross-linker system between neurofilaments, microtubules, and membranous organelles in frog axons revealed by the quick-freeze, deep-etching method. J Cell Biol. 1982 Jul;94(1):129–142. doi: 10.1083/jcb.94.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Binder L. I., Rosenbaum J. L. The periodic association of MAP2 with brain microtubules in vitro. J Cell Biol. 1979 Feb;80(2):266–276. doi: 10.1083/jcb.80.2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhausen T., Harrison S. C. Protein organization in clathrin trimers. Cell. 1981 Mar;23(3):755–761. doi: 10.1016/0092-8674(81)90439-6. [DOI] [PubMed] [Google Scholar]
- Kuznetsov S. A., Rodionov V. I., Gelfand V. I., Rosenblat V. A. Microtubule-associated protein MAP1 promotes microtubule assembly in vitro. FEBS Lett. 1981 Dec 7;135(2):241–244. doi: 10.1016/0014-5793(81)80791-0. [DOI] [PubMed] [Google Scholar]
- Kuznetsov S. A., Rodionov V. I., Gelfand V. I., Rosenblat V. A. Purification of high-Mr microtubule proteins MAP1 and MAP2. FEBS Lett. 1981 Dec 7;135(2):237–240. doi: 10.1016/0014-5793(81)80790-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matus A., Bernhardt R., Hugh-Jones T. High molecular weight microtubule-associated proteins are preferentially associated with dendritic microtubules in brain. Proc Natl Acad Sci U S A. 1981 May;78(5):3010–3014. doi: 10.1073/pnas.78.5.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P., Walter U., Theurkauf W. E., Vallee R. B., De Camilli P. Frozen tissue sections as an experimental system to reveal specific binding sites for the regulatory subunit of type II cAMP-dependent protein kinase in neurons. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5562–5566. doi: 10.1073/pnas.79.18.5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D. B., Vallee R. B., Borisy G. G. Identity and polymerization-stimulatory activity of the nontubulin proteins associated with microtubules. Biochemistry. 1977 Jun 14;16(12):2598–2605. doi: 10.1021/bi00631a004. [DOI] [PubMed] [Google Scholar]
- Pearse B. M. On the structural and functional components of coated vesicles. J Mol Biol. 1978 Dec 25;126(4):803–812. doi: 10.1016/0022-2836(78)90021-9. [DOI] [PubMed] [Google Scholar]
- Sloboda R. D., Rudolph S. A., Rosenbaum J. L., Greengard P. Cyclic AMP-dependent endogenous phosphorylation of a microtubule-associated protein. Proc Natl Acad Sci U S A. 1975 Jan;72(1):177–181. doi: 10.1073/pnas.72.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. S., Järlfors U., Cameron B. F. Morphological evidence for the participation of microtubules in axonal transport. Ann N Y Acad Sci. 1975 Jun 30;253:472–506. doi: 10.1111/j.1749-6632.1975.tb19223.x. [DOI] [PubMed] [Google Scholar]
- Theurkauf W. E., Vallee R. B. Molecular characterization of the cAMP-dependent protein kinase bound to microtubule-associated protein 2. J Biol Chem. 1982 Mar 25;257(6):3284–3290. [PubMed] [Google Scholar]
- Vallee R. B. A taxol-dependent procedure for the isolation of microtubules and microtubule-associated proteins (MAPs). J Cell Biol. 1982 Feb;92(2):435–442. doi: 10.1083/jcb.92.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee R. B., Borisy G. G. The non-tubulin component of microtubule protein oligomers. Effect on self-association and hydrodynamic properties. J Biol Chem. 1978 Apr 25;253(8):2834–2845. [PubMed] [Google Scholar]
- Vallee R. B., DiBartolomeis M. J., Theurkauf W. E. A protein kinase bound to the projection portion of MAP 2 (microtubule-associated protein 2). J Cell Biol. 1981 Sep;90(3):568–576. doi: 10.1083/jcb.90.3.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee R. Structure and phosphorylation of microtubule-associated protein 2 (MAP 2). Proc Natl Acad Sci U S A. 1980 Jun;77(6):3206–3210. doi: 10.1073/pnas.77.6.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villasante A., de la Torre J., Manso-Martínez R., Avila J. Microtubule-associated-protein MAP1 is not implicated in the polymerization of microtubules. Eur J Biochem. 1980 Dec;112(3):611–616. doi: 10.1111/j.1432-1033.1980.tb06126.x. [DOI] [PubMed] [Google Scholar]
- Weatherbee J. A., Sherline P., Mascardo R. N., Izant J. G., Luftig R. B., Weihing R. R. Microtubule-associated proteins of HeLa cells: heat stability of the 200,000 mol wt HeLa MAPs and detection of the presence of MAP-2 in HeLa cell extracts and cycled microtubules. J Cell Biol. 1982 Jan;92(1):155–163. doi: 10.1083/jcb.92.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingarten M. D., Lockwood A. H., Hwo S. Y., Kirschner M. W. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975 May;72(5):1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingsheim H. P., Herzog W., Weber K. Differences in surface morphology of microtubules reconstituted from pure brain tubulin using two different microtubule-associated proteins: the high molecular weight MAP 2 proteins and tau proteins. Eur J Cell Biol. 1979 Jun;19(2):175–183. [PubMed] [Google Scholar]