Abstract
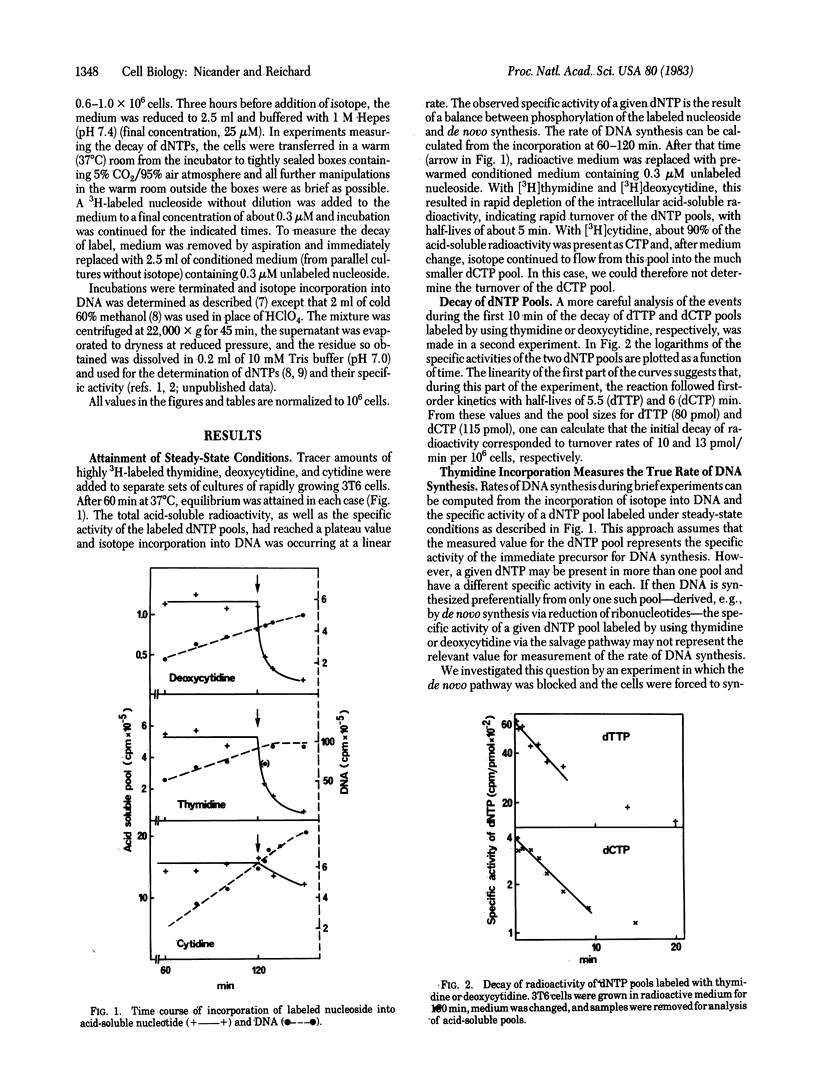
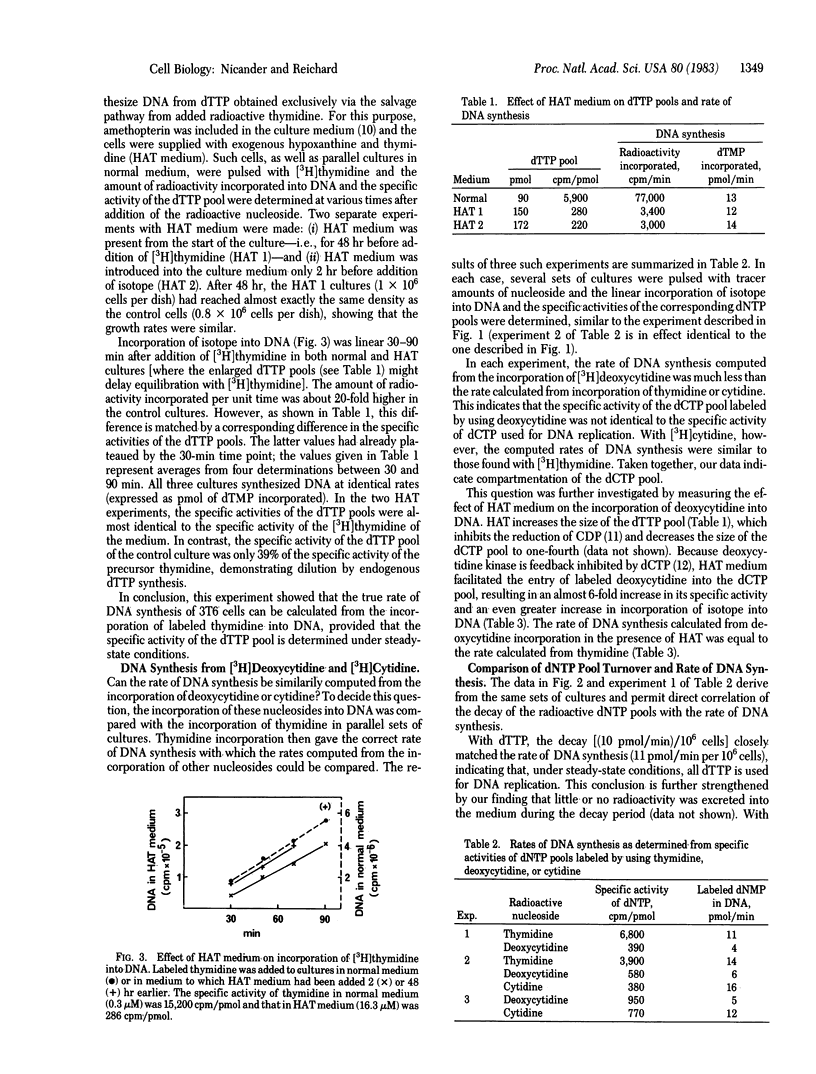
The 3H-labeled nucleosides cytidine, deoxycytidine, and thymidine are rapidly incorporated into DNA via dCTP or dTTP pools. Between 30 and 60 min after addition of tracer amounts of a labeled nucleoside to the medium of rapidly growing 3T6 cells, dNTP pools attained a constant specific activity resulting from a steady-state equilibrium between incorporation of nucleoside, de novo synthesis, and linear incorporation of isotope into DNA. Removal of labeled deoxycytidine or thymidine depleted the dNTP pools of isotope within a few minutes and incorporation into DNA stopped. When de novo synthesis of dTTP was blocked with amethopterin, the intracellular dTTP pool rapidly reached the specific activity of thymidine of the medium and isotope incorporation into DNA then measured absolute rates of DNA synthesis. In experiments with and without amethopterin, we found no kinetic evidence for the existence of more than one dTTP pool and the decay of the pool suggested that all dTTP served as precursor of DNA. In contrast, experiments with deoxycytidine and cytidine suggested the presence of separate dCTP pools with preferential DNA synthesis from the pool labeled from cytidine.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerblom L., Pontis E., Reichard P. Effects of azidocytidine on DNA synthesis and deoxynucleotide pools of mouse fibroblast cell lines. J Biol Chem. 1982 Jun 25;257(12):6776–6782. [PubMed] [Google Scholar]
- Bestwick R. K., Mathews C. K. Unusual compartmentation of precursors for nuclear and mitochondrial DNA in mouse L cells. J Biol Chem. 1982 Aug 25;257(16):9305–9308. [PubMed] [Google Scholar]
- Bjursell G., Reichard P. Effects of thymidine on deoxyribonucleoside triphosphate pools and deoxyribonucleic acid synthesis in Chinese hamster ovary cells. J Biol Chem. 1973 Jun 10;248(11):3904–3909. [PubMed] [Google Scholar]
- Hellgren D., Nilsson S., Reichard P. Effects of arabinosyl-cytosine on thymidine triphosphate pools and polyoma DNA replication. Biochem Biophys Res Commun. 1979 May 14;88(1):16–22. doi: 10.1016/0006-291x(79)91690-5. [DOI] [PubMed] [Google Scholar]
- Kuebbing D., Werner R. A model for compartmentation of de novo and salvage thymidine nucleotide pools in mammalian cells. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3333–3336. doi: 10.1073/pnas.72.9.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- Lindberg U., Skoog L. A method for the determination of dATP and dTTP in picomole amounts. Anal Biochem. 1970 Mar;34:152–160. doi: 10.1016/0003-2697(70)90096-5. [DOI] [PubMed] [Google Scholar]
- Mathews C. K., North T. W., Prem veer Reddy G. Multienzyme complexes in DNA precursor biosynthesis. Adv Enzyme Regul. 1978;17:133–156. doi: 10.1016/0065-2571(79)90011-6. [DOI] [PubMed] [Google Scholar]
- Momparler R. L., Fischer G. A. Mammalian deoxynucleoside kinase. I. Deoxycytidine kinase: purification, properties, and kinetic studies with cytosine arabinoside. J Biol Chem. 1968 Aug 25;243(16):4298–4304. [PubMed] [Google Scholar]
- Nordenskjöld B. A., Skoog L., Brown N. C., Reichard P. Deoxyribonucleotide pools and deoxyribonucleic acid synthesis in cultured mouse embryo cells. J Biol Chem. 1970 Oct 25;245(20):5360–5368. [PubMed] [Google Scholar]
- Plagemann P. G., Marz R., Wohlhueter R. M. Transport and metabolism of deoxycytidine and 1-beta-D-arabinofuranosylcytosine into cultured Novikoff rat hepatoma cells, relationship to phosphorylation, and regulation of triphosphate synthesis. Cancer Res. 1978 Apr;38(4):978–989. [PubMed] [Google Scholar]
- Prem veer Reddy G., Pardee A. B. Multienzyme complex for metabolic channeling in mammalian DNA replication. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3312–3316. doi: 10.1073/pnas.77.6.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichard P. From deoxynucleotides to DNA synthesis. Fed Proc. 1978 Jan;37(1):9–14. [PubMed] [Google Scholar]
- Skoog K. L., Nordenskjöld B. A., Bjursell K. G. Deoxyribonucleoside-triphosphate pools and DNA synthesis in synchronized hamster cells. Eur J Biochem. 1973 Mar 15;33(3):428–432. doi: 10.1111/j.1432-1033.1973.tb02699.x. [DOI] [PubMed] [Google Scholar]
- Skoog L. An enzymatic method for the determination of dCTP and dGTP in picomole amounts. Eur J Biochem. 1970 Dec;17(2):202–208. doi: 10.1111/j.1432-1033.1970.tb01154.x. [DOI] [PubMed] [Google Scholar]
- Skoog L., Bjursell G. Nuclear and cytoplasmic pools of deoxyribonucleoside triphosphates in Chinese hamster ovary cells. J Biol Chem. 1974 Oct 25;249(20):6434–6438. [PubMed] [Google Scholar]
- Stimac E., Housman D., Huberman J. A. Effects of inhibition of protein synthesis on DNA replication in cultured mammalian cells. J Mol Biol. 1977 Sep 25;115(3):485–511. doi: 10.1016/0022-2836(77)90167-x. [DOI] [PubMed] [Google Scholar]


