Abstract
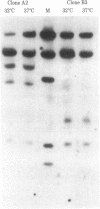
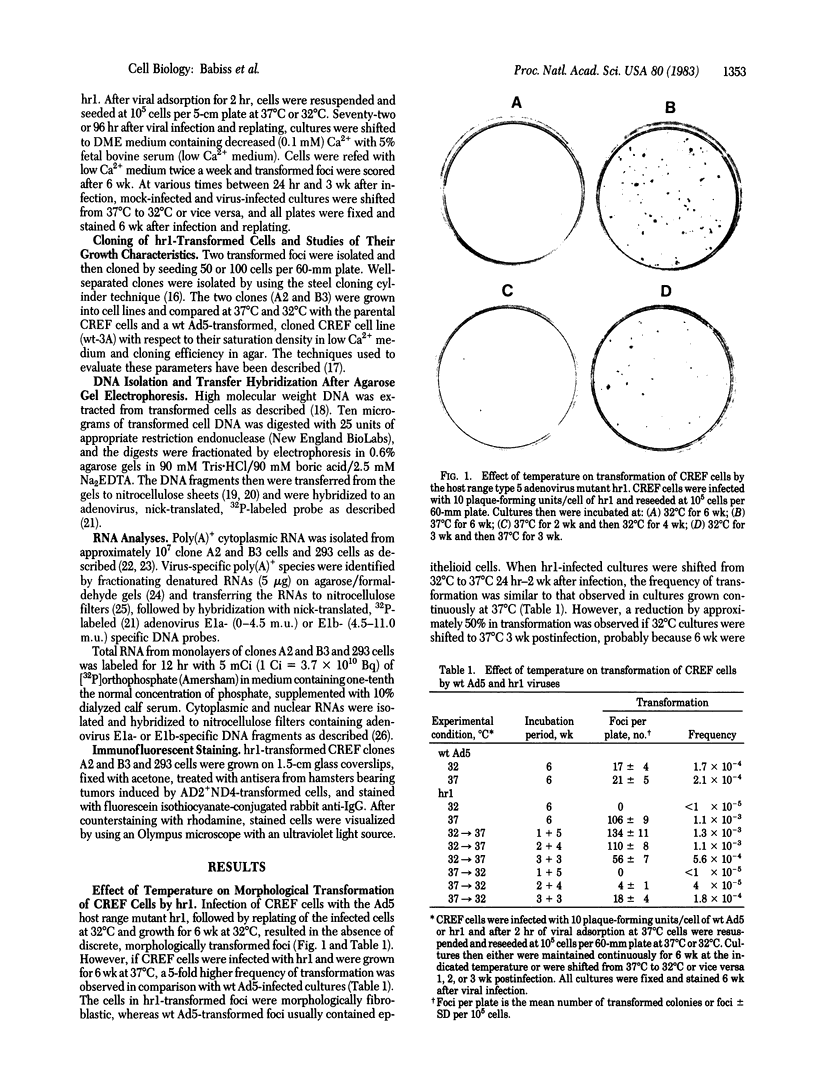
hr1, an E1a (0-4.5 map units) host range mutant of type 5 adenovirus (Ad5), transformed a cloned rat embryo fibroblast (CREF) cell line at about a 5-fold higher frequency than wild-type (wt) Ad5 when cells were cultured at 37 degrees C. However, if the cells were infected with hr1 and maintained at 32 degrees C morphological transformation did not occur. When infected cells were shifted from 32 degrees C to 37 degrees C 2 wk postinfection, the frequency of transformation by 6 wk was identical to that of cells grown continuously at 37 degrees C, whereas cultures shifted from 37 degrees C to 32 degrees C 2 wk postinfection displayed a greater than 96% reduction in morphological transformation. hr1-transformed cells had a fibroblastic morphology as contrasted with the typical epithelioid morphology of wt Ad5-transformed cells, but hr1- and wt Ad5-transformed cells had similar saturation densities, growth rates, and agar cloning efficiencies when assayed at 37 degrees C. However, when cells transformed by hr1 at 37 degrees C were grown at 32 degrees C, they had a saturation density close to that of normal CREF cells and grew at a lower efficiency in agar than wt-transformed cells. DNA transfer/hybridization analysis of two hr1-transformed cloned cell lines, A2 and B3, indicated that A2 cells contained a complete integrated copy of the Ad5 genome, whereas in B3 cells only part of the Ad5 genome was integrated. RNA transfer and RNA/DNA filter hybridization analyses indicated that the types of viral messenger RNAs and the relative amounts of RNA transcribed were similar in the A2 and B3 cell lines when they were grown at 32 degrees C and 37 degrees C. Indirect immunofluorescence, with antisera from hamsters bearing Ad-induced tumors, indicated a temperature dependence in staining--i.e., cells grown at 37 degrees C or shifted from 32 degrees C to 37 degrees C contained intense, particulate staining in the nuclear region, whereas the staining was decreased significantly in cells cultured at 32 degrees C and in cells shifted from 37 degrees C to 32 degrees C. These findings indicate that the gene product affected by the hr1 mutation is cold sensitive and is essential for the expression of the characteristics that identify the transformed cell.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carlock L. R., Jones N. C. Transformation-defective mutant of adenovirus type 5 containing a single altered E1a mRNA species. J Virol. 1981 Dec;40(3):657–664. doi: 10.1128/jvi.40.3.657-664.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derman E., Krauter K., Walling L., Weinberger C., Ray M., Darnell J. E., Jr Transcriptional control in the production of liver-specific mRNAs. Cell. 1981 Mar;23(3):731–739. doi: 10.1016/0092-8674(81)90436-0. [DOI] [PubMed] [Google Scholar]
- Dorsch-Häsler K., Fisher P. B., Weinstein I. B., Ginsberg H. S. Patterns of viral DNA integration in cells transformed by wild type or DNA-binding protein mutants of adenovirus type 5 and effect of chemical carcinogens on integration. J Virol. 1980 May;34(2):305–314. doi: 10.1128/jvi.34.2.305-314.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensinger M. J., Ginsberg H. S. Selection and preliminary characterization of temperature-sensitive mutants of type 5 adenovirus. J Virol. 1972 Sep;10(3):328–339. doi: 10.1128/jvi.10.3.328-339.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esche H., Mathews M. B., Lewis J. B. Proteins and messenger RNAs of the transforming region of wild-type and mutant adenoviruses. J Mol Biol. 1980 Sep 25;142(3):399–417. doi: 10.1016/0022-2836(80)90279-x. [DOI] [PubMed] [Google Scholar]
- Fisher P. B., Babiss L. E., Weinstein I. B., Ginsberg H. S. Analysis of type 5 adenovirus transformation with a cloned rat embryo cell line (CREF). Proc Natl Acad Sci U S A. 1982 Jun;79(11):3527–3531. doi: 10.1073/pnas.79.11.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher P. B., Goldstein N. I., Weinstein I. B. Phenotypic properties and tumor promotor-induced alterations in rat embryo cells transformed by adenovirus. Cancer Res. 1979 Aug;39(8):3051–3057. [PubMed] [Google Scholar]
- Fisher P. B., Mufson R. A., Weinstein I. B., Little J. B. Epidermal growth factor, like tumor promoters, enhances viral and radiation-induced cell transformation. Carcinogenesis. 1981;2(3):183–187. doi: 10.1093/carcin/2.3.183. [DOI] [PubMed] [Google Scholar]
- Fisher P. B., Weinstein I. B., Eisenberg D., Ginsberg H. S. Interactions between adenovirus, a tumor promoter, and chemical carcinogens in transformation of rat embryo cell cultures. Proc Natl Acad Sci U S A. 1978 May;75(5):2311–2314. doi: 10.1073/pnas.75.5.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., Abrahams P. J., Mulder C., Heijneker H. L., Warnaar S. O., De Vries F. A., Fiers W., Van Der Eb A. J. Studies on in vitro transformation by DNA and DNA fragments of human adenoviruses and simian virus 40. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):637–650. doi: 10.1101/sqb.1974.039.01.077. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Harrison T., Williams J. Defective transforming capacity of adenovirus type 5 host-range mutants. Virology. 1978 May 1;86(1):10–21. doi: 10.1016/0042-6822(78)90003-x. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Harrison T., Graham F., Williams J. Host-range mutants of adenovirus type 5 defective for growth in HeLa cells. Virology. 1977 Mar;77(1):319–329. doi: 10.1016/0042-6822(77)90428-7. [DOI] [PubMed] [Google Scholar]
- Ho Y. S., Galos R., Williams J. Isolation of type 5 adenovirus mutants with a cold-sensitive host range phenotype: genetic evidence of an adenovirus transformation maintenance function. Virology. 1982 Oct 15;122(1):109–124. doi: 10.1016/0042-6822(82)90381-6. [DOI] [PubMed] [Google Scholar]
- Houweling A., van den Elsen P. J., van der Eb A. J. Partial transformation of primary rat cells by the leftmost 4.5% fragment of adenovirus 5 DNA. Virology. 1980 Sep;105(2):537–550. doi: 10.1016/0042-6822(80)90054-9. [DOI] [PubMed] [Google Scholar]
- Jones N., Shenk T. Isolation of adenovirus type 5 host range deletion mutants defective for transformation of rat embryo cells. Cell. 1979 Jul;17(3):683–689. doi: 10.1016/0092-8674(79)90275-7. [DOI] [PubMed] [Google Scholar]
- Nevins J. R. Definition and mapping of adenovirus 2 nuclear transcription. Methods Enzymol. 1980;65(1):768–785. doi: 10.1016/s0076-6879(80)65072-1. [DOI] [PubMed] [Google Scholar]
- Ricciardi R. P., Jones R. L., Cepko C. L., Sharp P. A., Roberts B. E. Expression of early adenovirus genes requires a viral encoded acidic polypeptide. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6121–6125. doi: 10.1073/pnas.78.10.6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ruben M., Bacchetti S., Graham F. L. Integration and expression of viral DNA in cells transformed by host range mutants of adenovirus type 5. J Virol. 1982 Feb;41(2):674–685. doi: 10.1128/jvi.41.2.674-685.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiroki K., Maruyama K., Saito I., Fukui Y., Shimojo H. Incomplete transformation of rat cells by a deletion mutant of adenovirus type 5. J Virol. 1981 Jun;38(3):1048–1054. doi: 10.1128/jvi.38.3.1048-1054.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiroki K., Shimojo H., Sawada Y., Uemizu Y., Fujinaga K. Incomplete transformation of rat cells by a small fragment of adenovirus 12 DNA. Virology. 1979 May;95(1):127–136. doi: 10.1016/0042-6822(79)90407-0. [DOI] [PubMed] [Google Scholar]
- Singer R. H., Penman S. Messenger RNA in HeLa cells: kinetics of formation and decay. J Mol Biol. 1973 Aug 5;78(2):321–334. doi: 10.1016/0022-2836(73)90119-8. [DOI] [PubMed] [Google Scholar]
- Solnick D., Anderson M. A. Transformation-deficient adenovirus mutant defective in expression of region 1A but not region 1B. J Virol. 1982 Apr;42(1):106–113. doi: 10.1128/jvi.42.1.106-113.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solnick D., Anderson M. A. Transformation-deficient adenovirus mutant defective in expression of region 1A but not region 1B. J Virol. 1982 Apr;42(1):106–113. doi: 10.1128/jvi.42.1.106-113.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ormondt H., Maat J., De Waard A., Van der Eb A. J. The nucleotide sequence of the transforming HpaI-E fragment of adenovirus type 5 DNA. Gene. 1978 Dec;4(4):309–328. doi: 10.1016/0378-1119(78)90048-3. [DOI] [PubMed] [Google Scholar]
- Van der Eb A. J., Mulder C., Graham F. L., Houweling A. Transformation with specific fragments of adenovirus DNAs. I. Isolation of specific fragments with transforming activity of adenovirus 2 and 5 DNA. Gene. 1977;2(3-4):115–132. doi: 10.1016/0378-1119(77)90012-9. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziff E. B. Transcription and RNA processing by the DNA tumour viruses. Nature. 1980 Oct 9;287(5782):491–499. doi: 10.1038/287491a0. [DOI] [PubMed] [Google Scholar]