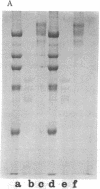
Abstract
Serum spreading factor is a glycoprotein isolated from human serum that promotes spreading of a variety of cell types on culture dishes. We developed mouse hybridoma lines secreting monoclonal antibody to serum spreading factor that markedly inhibited the rate of serum spreading factor-promoted spreading of both fibroblastic and epithelial cells in culture. Fibronectin-promoted cell spreading was unaffected by monoclonal antibody to serum spreading factor, and the factor appeared to be distinct by several criteria from fibronectin or laminin. Human serum-promoted cell spreading was partially inhibited by monoclonal antibody to serum spreading factor. The antibody recognized primarily two forms of serum spreading factor that migrated in NaDodSO4/polyacrylamide gel electrophoresis in a manner consistent with molecular weights of 65,000-70,000 and 75,000-78,000. In addition to being found in plasma, serum spreading factor was also found associated with washed human platelets.
Full text
PDF
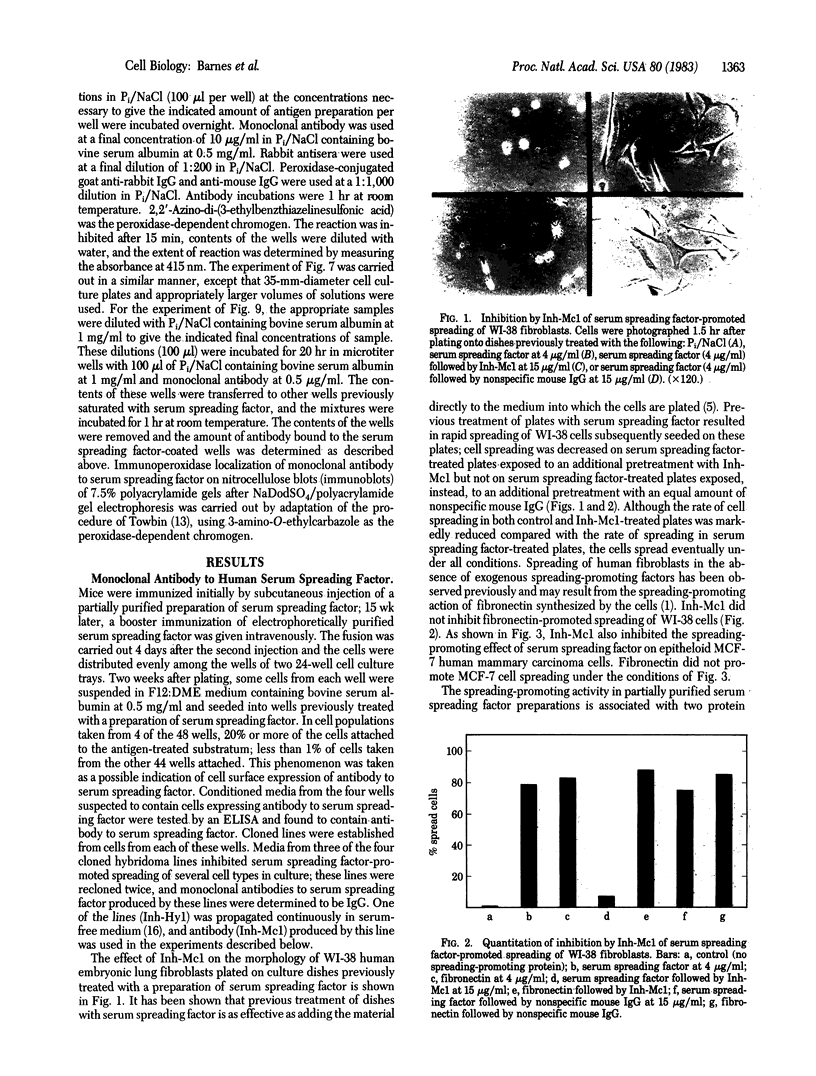
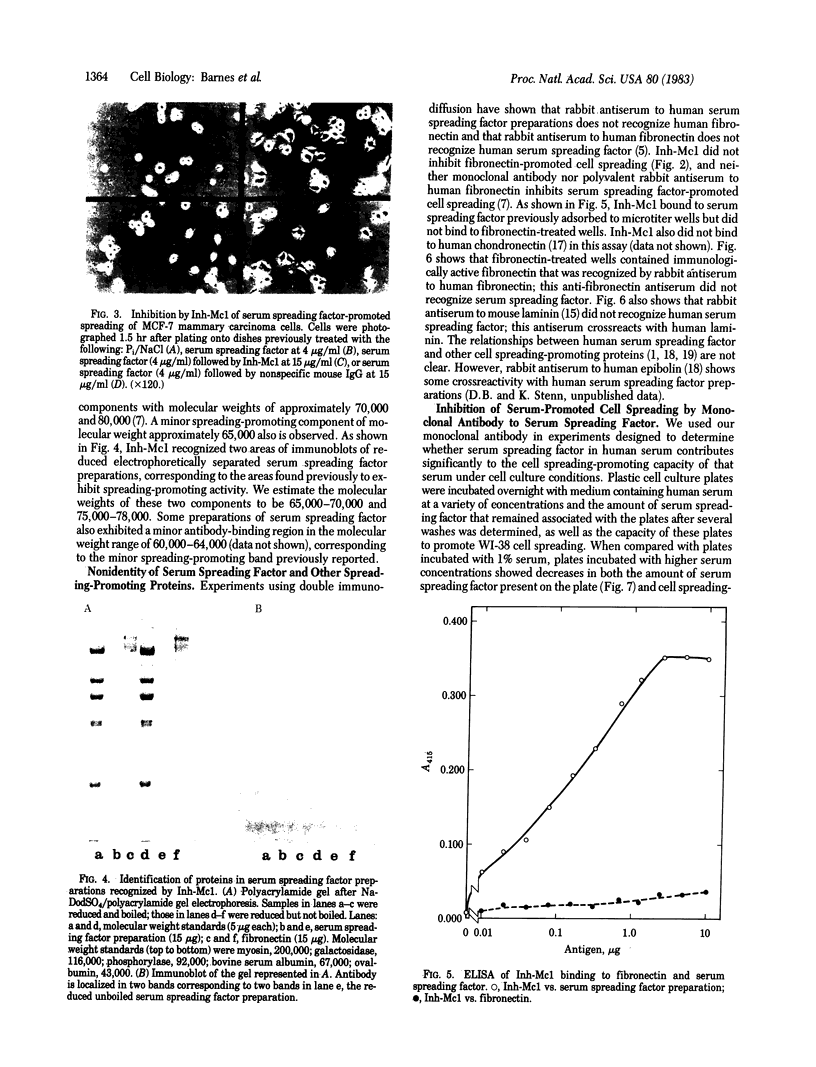
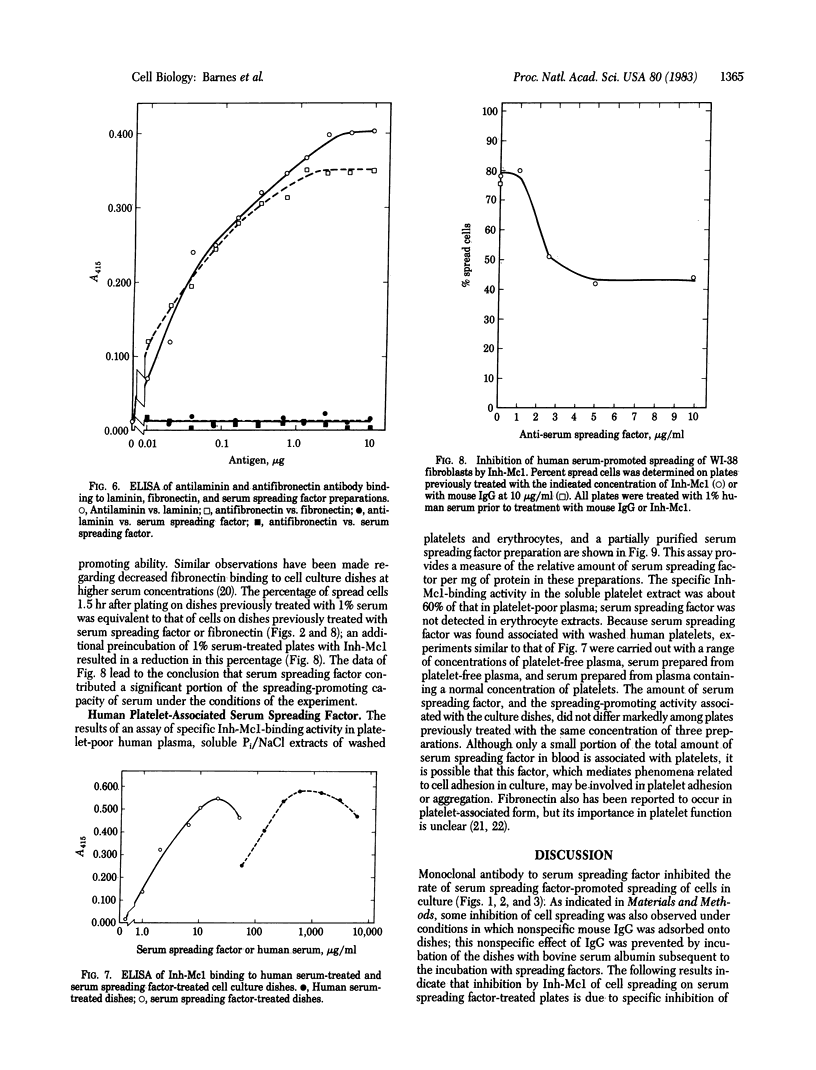
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes D., Sato G. Growth of a human mammary tumour cell line in a serum-free medium. Nature. 1979 Oct 4;281(5730):388–389. doi: 10.1038/281388a0. [DOI] [PubMed] [Google Scholar]
- Barnes D., Wolfe R., Serrero G., McClure D., Sato G. Effects of a serum spreading factor on growth and morphology of cells in serum-free medium. J Supramol Struct. 1980;14(1):47–63. doi: 10.1002/jss.400140106. [DOI] [PubMed] [Google Scholar]
- Barnes D., van der Bosch J., Masui H., Miyazaki K., Sato G. The culture of human tumor cells in serum-free medium. Methods Enzymol. 1981;79(Pt B):368–391. doi: 10.1016/s0076-6879(81)79050-5. [DOI] [PubMed] [Google Scholar]
- Bensusan H. B., Koh T. L., Henry K. G., Murray B. A., Culp L. A. Evidence that fibronectin is the collagen receptor on platelet membranes. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5864–5868. doi: 10.1073/pnas.75.12.5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E. Enzyme immunoassay ELISA and EMIT. Methods Enzymol. 1980;70(A):419–439. doi: 10.1016/s0076-6879(80)70067-8. [DOI] [PubMed] [Google Scholar]
- Grinnell F. Cellular adhesiveness and extracellular substrata. Int Rev Cytol. 1978;53:65–144. doi: 10.1016/s0074-7696(08)62241-x. [DOI] [PubMed] [Google Scholar]
- Grinnell F., Feld M. K. Fibronectin adsorption on hydrophilic and hydrophobic surfaces detected by antibody binding and analyzed during cell adhesion in serum-containing medium. J Biol Chem. 1982 May 10;257(9):4888–4893. [PubMed] [Google Scholar]
- Hayman E. G., Engvall E., A'Hearn E., Barnes D., Pierschbacher M., Ruoslahti E. Cell attachment on replicas of SDS polyacrylamide gels reveals two adhesive plasma proteins. J Cell Biol. 1982 Oct;95(1):20–23. doi: 10.1083/jcb.95.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt A. T., Varner H. H., Silver M. H., Dessau W., Wilkes C. M., Martin G. R. The isolation and partial characterization of chondronectin, an attachment factor for chondrocytes. J Biol Chem. 1982 Mar 10;257(5):2330–2334. [PubMed] [Google Scholar]
- Holmes R. Preparation from human serum of an alpha-one protein which induces the immediate growth of unadapted cells in vitro. J Cell Biol. 1967 Feb;32(2):297–308. doi: 10.1083/jcb.32.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami H., Masui H., Sato G. H., Sueoka N., Chow T. P., Kano-Sueoka T. Growth of hybridoma cells in serum-free medium: ethanolamine is an essential component. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1158–1162. doi: 10.1073/pnas.79.4.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro S. A., Cunningham L. W. Fibronectin and the multiple interaction model for platelet-collagen adhesion. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2644–2648. doi: 10.1073/pnas.76.6.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenn K. S. Epibolin: a protein of human plasma that supports epithelial cell movement. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6907–6911. doi: 10.1073/pnas.78.11.6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl R., Rohde H., Robey P. G., Rennard S. I., Foidart J. M., Martin G. R. Laminin--a glycoprotein from basement membranes. J Biol Chem. 1979 Oct 10;254(19):9933–9937. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whateley J. G., Knox P. Isolation of a serum component that stimulates the spreading of cells in culture. Biochem J. 1980 Feb 1;185(2):349–354. doi: 10.1042/bj1850349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M., Olden K. Fibronectins--adhesive glycoproteins of cell surface and blood. Nature. 1978 Sep 21;275(5677):179–184. doi: 10.1038/275179a0. [DOI] [PubMed] [Google Scholar]