Abstract
Background and Objectives
Studies of the association between excess body weight and risk of meningioma have produced inconsistent results. Therefore, a meta-analysis of published studies was performed to better assess the association between meningioma and excess body weight.
Methods
A literature search was conducted in the PubMed and EMBASE databases without any limitations. The reference lists of identified articles were also screened for additional studies. The summary relative risks (RRs) and 95% confidence intervals (CI) were calculated using fixed- or random-effects models.
Results
A total of 6 studies provided risk estimates for overweight or obesity. Overall, the combined RRs were 1.12 (95% CI = 0.98–1.28) for overweight and 1.45 (95% CI = 1.26–1.67) for obesity. After stratification by gender, no significant association was observed for obese men (RR = 1.30, 95% CI = 0.64–2.62), while significant association was detected for obese women (RR = 1.46, 95% CI = 1.26–1.69). No substantial differences emerged across strata of study design and geographic areas.
Conclusion
The results of this meta-analysis suggest that obesity but not overweight is associated with an increased risk of meningioma. Due to the limited number of studies, further research is needed to confirm the association.
Introduction
Meningiomas are the second most common brain neoplasms, representing approximately 20% of all intracranial tumors [1]. Most meningiomas are benign and rarely display biologically aggressive behavior [1], [2]. Despite decades of research, the aetiology of meningioma is poorly understood. Aside from certain rare genetic conditions (neurofibromatosis type I, Li Fraumeni syndrome), the only confirmed risk factor is exposure to high doses of ionizing radiation [3]–[5]. However, as the 2 types of exposures are uncommon, they can explain only a small number of the total cases. Furthermore, the incidence of meningioma has clearly risen in many Western countries [3]. Therefore, early intervention on modifiable risk factors of meningioma is very important.
Over the past several decades, obesity has emerged as a leading public health concern in the developed countries [6], [7]. Previous studies have shown that obesity contributes to increase the incidence or death of colorectal adenomas, postmenopausal breast cancer, gallbladder cancer, endometrial cancer, pancreatic cancer, renal cancer, and liver cancer [8], [9]. However, the relationship between meningioma and obesity is still unclear. In recent years, a number of studies have explored the association between the risk of meningioma and excess body weight, but the results were conflicting [10]–[21]. Several studies indicated that excess body weight was associated with a higher risk of meningioma [10]–[12], [14], [16], whereas no significant association was reported in other studies [13], [15], [17]–[21]. This discrepancy in the results may result from different characteristics of subjects or study methodologies. Moreover, no quantitative summary of the evidence has ever been reported. Therefore, a meta-analysis of published cohort and case-control studies was conducted to quantify the effect of obesity and overweight on the occurrence of meningioma.
Materials and Methods
Search Strategy
Two reviewers (CS and ZYQ) independently performed a literature search of the PubMed and EMBASE databases without any limitations on language and publication date. The following search terms were used: “body mass index”, “overweight”, “obesity”, “body weight”, “body size”, “anthropometry”, and “adiposity” combined with “meningioma”, “brain cancer”, “brain tumor”, and “brain neoplasm”. We also reviewed the reference lists of included articles for additional studies. The last updated search was performed on August 23, 2013.
Study Selection
Studies were identified for this meta-analysis if they fulfilled all the following inclusion criteria: (1) used a case-control or cohort design; (2) clear description of overweight or obesity defined by body mass index (BMI) in kg/m2; (3) assessed the relationship between risk of meningioma and overweight or obesity; (4) reported estimates of relative risk [odds ratio (OR), hazard ratio (HR)] with corresponding 95% CIs or sufficient data to estimate them; (5) in the case of multiple reports of the same study population, only the most recent and informative one was included; (6) we excluded those studies in which non-obese people were reference subjects because non-obese people include a number of overweight people; and (7) we also excluded those studies that involved total brain tumors because brain tumors are a heterogeneous group of tumors that vary in tissue origins, invasive potential and prognosis.
Data Extraction
Two authors (CS and ZYQ) independently abstracted the following data in a standard format: the first author, publication year, country in which performed, study period, age range of participants, sex, number of subjects (cases, controls or cohort size), meningioma diagnosis method, measure of exposure, risk estimates and corresponding 95% CI, and matching and adjustments. Any disagreements were resolved by discussion.
Assessment of Methodological Quality
The Newcastle-Ottawa Scale (NOS) for assessing the quality of observational studies was used to assess the quality of included studies [22]. The NOS is based on three major components: selection of the study groups (0–4 stars), comparability of cases and controls (0–2 stars), or cohorts, and ascertainment of exposure/outcome (0–3 stars). A study awarded 6 stars or more is considered a high-quality study.
Statistical Analysis
The RR was used as the measure of the relationship between meningioma and overweight or obesity. Because meningioma is rare, ORs and HRs were accurate approximations of RRs [23]. In this meta-analysis, the most fully adjusted risk estimates were used; however, if such estimates were unavailable, crude effect estimates with 95% CIs were included. In this meta-analysis, we only reported the risk estimates based on the baseline data. Heterogeneity among studies was assessed by Cochran’s Q and I2 statistics [24], [25]. For Cochran’s Q statistic, substantial heterogeneity was defined as P<0.1 [24]. The I2 statistic ranges in value from 0 to 100% (I2<25%, low heterogeneity; I2 = 25%–50%, moderate heterogeneity; and I2>50%, high heterogeneity) [25]. Both the fixed- and random-effects models were used to calculate the pooled RR [26]. If substantial heterogeneity was found, we presented the results from random-effects models. Subgroup analyses were conducted according to study design (case-control and cohort), gender (male and female), and geographic regions (Europe and North America). A sensitivity analysis was performed to assess the influence of the individual studies on the overall results by omitting one study at a time. Publication bias was assessed by Egger’s test (P<0.05 was considered significant) [27].
We defined body mass categories according to the World Health Organization (WHO) guidelines: underweight (BMI<18.5 kg/m2), normal weight (BMI≥18.5 and <25 kg/m2), overweight (BMI≥25 and <30 kg/m2), and obesity (BMI≥30 kg/m2). In this meta-analysis, normal weight was used as the reference category. When non-standard categories of BMI were reported, we selected the category that most closely approximated those defined by the WHO guidelines. When more than one estimate in a study fell into the range representing overweight or obesity, we calculated a combined risk estimate using the method proposed by Hamling et al [28]. All statistical analyses were performed using STATA, version 11.0 (STATA, College Station, TX, USA).
Results
Literature Search and Study Characteristics
Fig. S1 shows a flow diagram for the selection process. A total of 2607 potentially relevant studies were identified from the initial search. After a careful review, the remaining 26 articles were considered of interest and their full-text was assessed for eligibility. Of 26 studies, 20 were excluded after reading the full-text [10], [14], [15], [17], [18], [20], [29]–[42]. The major reasons for excluding these studies were as follows: evaluating overweight and obesity together (n = 2) [15], [18], no available data [20], obesity measured by Quetelet index, Cohen’s Kappa index or weight (n = 2) [10], [17], non-obese people as the reference (n = 1) [14], and involving total brain tumor in their subjects (n = 14) [29]–[42]. Thus, a final total of 6 studies (4 cohort studies and 2 case-control studies) were included in this meta-analysis [11]–[13], [16], [19], [21]. The range of publication periods for the included studies was 2006–2013. All studies were published in English. Of 6 studies, 3 were performed in North America [12], [13], [16] and 3 in Europe [11], [19], [21]. Two studies included women and men [19], [21] and 4 studies included women only as subjects [11]–[13], [16]. The data on weight and height were collected through self-reporting [11]–[13], [16], measurement [21], or both of the 2 methods [19]. The definition of cases was based on the radiological criteria or pathology reports. Additional characteristics of the included studies are shown in Table 1. The quality of the included studies was evaluated by NOS. Table S1 shows the results of the assessment of methodological quality. All included studies obtained more than six stars, suggesting that the overall quality of the studies is good.
Table 1. Characteristic of the included studies in this meta-analysis.
| First author, Publication year | Countrya | Study period | Age | Sex | Cases/Cohort | Case diagnosis | measurement method | Matching or adjustment |
| Cohort studies | ||||||||
| Benson, 2008 | 5 | 1996–2001/6.2 | 50–65 | F | 390/1,249,670 | Cancer registry | Self-reported | Age, height, strenuous exercise, socioeconomic level, smoking, alcohol intake, parity, age at first birth, OC |
| Johnson,2011 | 1 | 1986–2004/10.5 | 55–85.7 | F | 125/291,021 | Medicare data | Self-reported | Age. |
| Michaud, 2011 | 2–11 | 1991–2004/8.4 | 35–70 | M/F | 203/380,775 | Cancer registry | Self-reported, Measured | Age, country, sex, education. |
| Wiedmann,2013 | 4 | 1984–1986/23.5 | ≥20 | M/F | 81/74,242 | Cancer registry | Measured | Age, sex |
| Case-control studies | ||||||||
| Claus,2013 | 1 | 2006–2011 | 29–79 | F | 1,127/1,092 | Cancer registry | Self-reported | Age, sex, residence, race, education, menopause status, age at menopause, age at menarche, smoking, alcohol use, breastfeeding, OC, HRT, number of FLB, age at FTP. |
| Custer,2006 | 1 | 1995–1998 | ≥18 | F | 143/286 | Pathology reports | Self-reported | Age, race, marital status |
M, male; F, female; OC, oral contraceptive; HRT, hormone replacement therapy; FLB, first live birth; FTP, full-term pregnancy.
Studies were conducted in: (1) USA, (2) Sweden, (3) Denmark, (4) Norway, (5) United Kingdom, (6) France, (7) Netherlands, (8) Spain, (9) Italy, (10) Germany, (11) Greece.
Meta-analysis Results
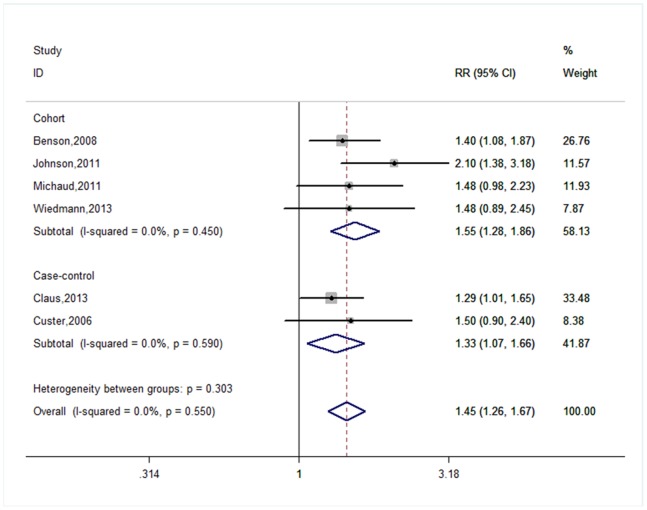
Figure 1 shows the forest plots for obesity versus normal weight. The summary RRs for case-control, cohort studies, and all studies were 1.33 (95% = 1.07–1.66, PHeterogeneity = 0.590, I2 = 0.0%), 1.55 (95% = 1.28–1.86, PHeterogeneity = 0.450, I2 = 0.0%), and 1.45 (95% CI = 1.26–1.67, PHeterogeneity = 0.550, I2 = 0.0%), respectively. In subgroup analyses by gender, a statistically significant link between the risk of meningioma and obesity was observed for females (RR = 1.46, 95% CI = 1.26–1.69, PHeterogeneity = 0.515, I2 = 0.0%), but not for males (RR = 1.30, 95% CI = 0.64–2.62, PHeterogeneity = 0.427, I2 = 0.0%). In subgroup analyses by geographic regions, the pooled results were significant in both North American studies (RR = 1.47, 95% CI = 1.21–1.78, PHeterogeneity = 0.142, I2 = 48.7%) and European studies (RR = 1.43, 95% CI = 1.16–1.77, PHeterogeneity = 0.967, I2 = 0.0%).
Figure 1. Forest plot for obesity versus normal weight.
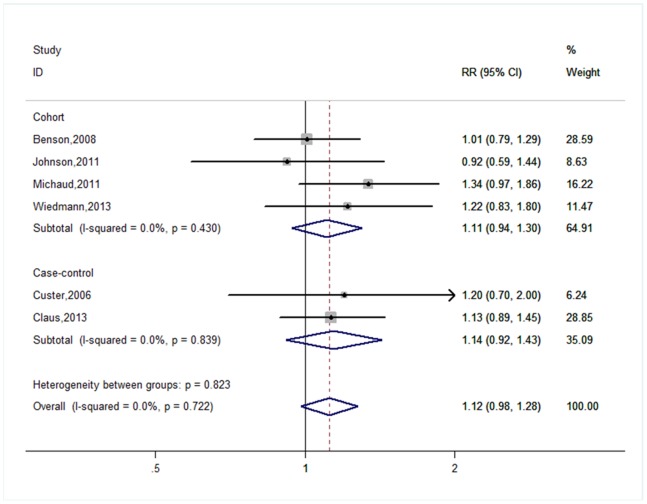
Figure 2 shows the forest plots for overweight versus normal weight. The pooled results based on all studies suggested there was no significant association between risk of brain tumor and overweight (RR = 1.12, 95% CI = 0.98–1.28, PHeterogeneity = 0.722, I2 = 0.0%). In subgroup analyses, we found that the associations between overweight and risk of meningioma were not significantly modified by gender, geographic regions, or study design (Table 2).
Figure 2. Forest plot for overweight versus normal weight.
Table 2. Summary risk estimates of the association between BMI and meningioma risk.
| Group | Overweight (25≤BMI≤29.9 kg/m2) | Obesity (BMI≥30 kg/m2) | ||||||
| Heterogeneity | Heterogeneity | |||||||
| Number ofstudies | RR(95% CI) | I2 | P | Number ofstudies | RR(95% CI) | I2 | P | |
| All studies | 6 | 1.12(0.98–1.28) | 0.0% | 0.722 | 6 | 1.45(1.26–1.67) | 0.0% | 0.550 |
| Study design | ||||||||
| Case-control | 2 | 1.14(0.92–1.43) | 0.0% | 0.839 | 2 | 1.33(1.07–1.66) | 0.0% | 0.590 |
| Cohort | 4 | 1.11(0.94–1.30) | 0.0% | 0.430 | 4 | 1.55(1.28–1.86) | 0.0% | 0.450 |
| Gender | ||||||||
| Male | 2 | 1.03(0.64–1.66) | 0.0% | 0.603 | 2 | 1.30(0.64–2.62) | 0.0% | 0.427 |
| Female | 6 | 1.13(0.98–1.29) | 0.0% | 0.573 | 6 | 1.46(1.26–1.69) | 0.0% | 0.515 |
| Geographic area | ||||||||
| Europe | 3 | 1.14(0.96–1.36) | 0.1% | 0.368 | 3 | 1.43(1.16–1.77) | 0.0% | 0.967 |
| North America | 3 | 1.09(0.90–1.33) | 0.0% | 0.682 | 3 | 1.47(1.21–1.78) | 48.7% | 0.142 |
Sensitivity Analysis
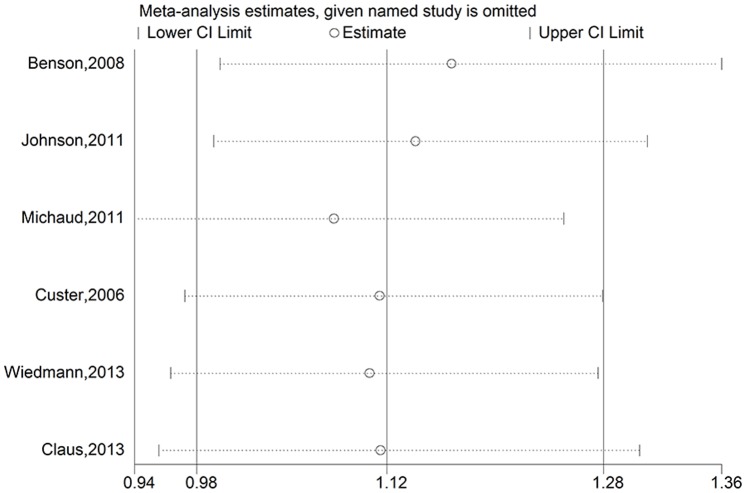
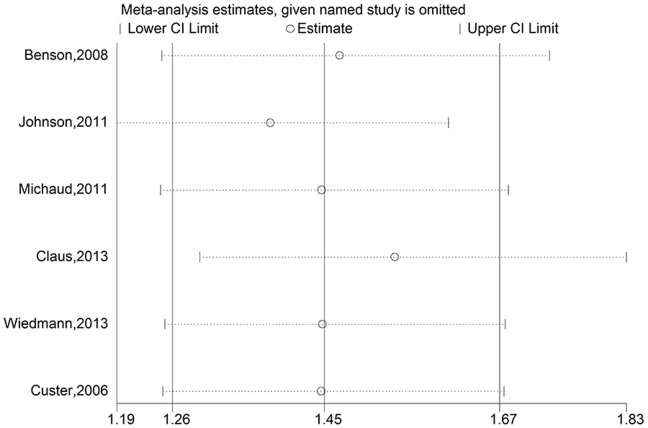
To assess the stability of the results of the meta-analysis, sensitivity analyses were conducted by excluding one study at a time. For overweight, a borderline significant association was found after omitting the Million Women Study [11] and the Iowa Women’s Health Study [16]. The pooled RRs were 1.17 (95% CI = 1.00–1.36, PHeterogeneity = 0.752, I2 = 0.0%) for excluding the Million Women Study and 1.14 (95% CI = 0.99–1.31, PHeterogeneity = 0.728, I2 = 0.0%) for excluding the Iowa Women’s Health Study (Figure 3). The other results of sensitivity analyses for overweight were not significantly altered (data not shown). For obesity, none of the results was significantly altered, indicating that our results were robust (Figure 4).
Figure 3. Sensitivity analyses for overweight versus normal weight.
Figure 4. Sensitivity analyses for obesity versus normal weight.
Publication Bias
The results of Egger’s test suggest that no evidence of publication bias was observed (P = 0.204 for obesity and P = 0.764 for overweight).
Discussion
This meta-analysis of 4 cohort studies and 2 case-control studies assessed the association of meningioma with obesity or overweight. Our analysis identified an association between an increased risk of meningioma and obesity. However, no significant correlation with overweight was observed. In further analyses by gender and geographic area, similar trends were observed.
Several potential mechanisms have been proposed to explain how obesity can contribute to the development of meningioma, although the exact biological mechanisms are unclear. Currently, the most well-known mechanism is the insulin-like growth factor (IGF) hypothesis of obesity-related cancer [8], [43]–[45]. Obesity is associated with insulin resistance and hyperinsulinemia, which reduce the levels of insulin-like growth factor binding protein 1 (IGFBP-1) and insulin-like growth factor binding protein 2 (IGFBP-2). The decrease in these proteins leads to higher circulating concentrations of free or bioactive insulin-like growth factor 1 (IGF-1) and a change in cell environment that stimulates tumor growth and inhibits apoptosis. Furthermore, the involvement of the IGF system in brain development has been demonstrated by in vitro and in vivo studies [46], [47]. Finally, laboratory studies have confirmed that IGF1, IGF2, and IGF1R genes are overexpressed in meningioma [46]. Other possible mechanisms include chronic inflammation, alterations in adipokine concentrations and sex hormones, sharing genetic susceptibility, obesity-related hypoxia, and migrating adipose stromal cells [44], [48].
In this meta-analysis, we further investigated the correlation with obesity separately for females and males. The results of subgroup analyses show that obesity was associated with a significantly elevated risk of meningioma in females, but not in males. The potential explanations for the sex difference might be related to the effect of sex hormones. Obesity is positively associated with circulating concentrations of testosterone in females [49], [50], but inversely associated with testosterone concentrations in males [51], [52]. There is evidence that testosterone promotes cell proliferation and local production of IGF-I and IGF-I-R [53]. Moreover, estrogens also interact with IGF, which stimulates tumor growth and prohibits cell apoptosis [44]. Recently, a meta-analysis of 11 studies has suggested that the use of hormone replacement therapy is correlated with an increased risk of meningioma in women [54]. In our meta-analysis, many subjects have implied that they used female hormone when their menstrual cycle ended [12], [13], [16]. Thus, it is conceivable that obese females bear a larger risk of meningioma than obsess males. An alternative explanation for observed gender differences is that these findings may have occurred by chance because a limited number of studies were involved in subgroup analyses. Therefore, further evaluation of obesity relative to risk of meningioma is needed with more attention to the influence of gender.
Two cohort studies have examined the association between waist-hip ratio (WHR) and risk of meningioma: the Iowa Women’s Health Study (IWHS) [16] and the European Prospective Investigation into Cancer and Nutrition (EPIC) [19]. Michaud and colleagues in the EPIC found that abdominal obesity (defined as WHR) was associated with an increased risk of meningioma, although these correlations were not statistically significant [19]. In the latter study, a similar trend was detected for meningioma [16]. Compared with BMI, WHR is considered to be a more accurate index of obesity because the WHR takes the anatomic distribution of body fat in account and distinguishes lean muscle mass from fat mass [55]–[57]. Therefore, both BMI and WHR should be considered in future studies.
When obesity was found to be closely related to a higher risk of meningioma, several researchers proposed the hypothesis that underweight is related to a low risk of meningioma. To our knowledge, only 3 studies to date have analyzed the relationship between the risk of meningioma and underweight [18], [19], [21]. A hospital-based case-control study with 479 participants found no significant positive association (OR = 1.3, 95% CI = 0.6–3.0) between meningioma and underweight (defined by BMI<19 kg/m2) [18]. However, an inverse result was observed in the Nord-Trøndelag Health Study [21]. This prospective study showed that underweight (defined by BMI<20 kg/m2) was not meaningfully correlated with a lower risk of meningioma (RR = 0.67, 95% CI = 0.29–1.56) [21]. In EPIC, no significant association was detected (RR = 1.00, 95% CI = 0.46–2.19) [19]. These findings may be chance results due to the limited number of subjects, various study designs, and non-standard definitions of underweight used. Hence, additional well-designed studies are warranted to better understand the association between underweight and the risk of meningioma.
Several potential limitations of this meta-analysis should be noted. First, our meta-analysis was based on the small number of studies. Indeed, a great number of studies have evaluated the relationship between obesity and the risk of brain tumors [29]–[42]. However, brain tumors are a heterogeneous group of tumors that vary in tissue origins, invasive potential and prognosis. Thus, these studies cannot be included in this meta-analysis and further evaluation of obesity with risk of brain tumors is needed with particular attention to stratification by the type of tumor. Second, as all included studies were observational, we cannot exclude the possibility that our findings could be due to unmeasured or residual variables. Third, the estimation of weight and height in most of included studies was based on subjects’ self-reporting. It is possible that the weight has been underreported, particularly by overweight or obese individuals, and that height has been overestimated. Thus, this factor might have resulted in a degree of underestimation of the true associations. Fourth, because no studies involved Chinese/Asian populations, additional investigations in non-Western countries are warranted to extend the current findings [58]. Fifth, obesity may not be the main causative factor because obesity could be a consequence of other causative factors, for example, sex hormones and unhealthy lifestyles (i.e., smoking, heavy alcohol consumption and less exercise). The involvement of female hormones in meningioma carcinogenesis has been demonstrated in experimental and histopathologic studies as well as observational studies [59]–[64]. Additionally, the unhealthy lifestyles listed above have generally been considered to increase the risk of cancer. Finally, publication bias is often a concern in a meta-analysis because null results tend to be unpublished.
In summary, the results of this meta-analysis show that obesity is positively associated with the risk of meningioma. These findings also indicate that maintaining a healthy body weight may, in part, prevent the occurrence of meningioma.
Supporting Information
Flow diagram of study selection.
(TIF)
Methodological quality of included studies based on the Newcastle–Ottawa Scale.
(DOCX)
(DOC)
Funding Statement
The authors have no support or funding to report.
References
- 1. Claus EB, Bondy ML, Schildkraut JM, Wiemels JL, Wrensch M, et al. (2005) Epidemiology of intracranial meningioma. Neurosurgery 57: 1088–95. [DOI] [PubMed] [Google Scholar]
- 2. Ragel BT, Jensen RL (2005) Molecular genetics of meningiomas. Neurosurg Focus 19: E9. [DOI] [PubMed] [Google Scholar]
- 3. Wrensch M, Minn Y, Chew T, Bondy M, Berger MS (2002) Epidemiology of primary brain tumors: current concepts and review of the literature. Neuro Oncol 4: 278–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cowppli-Bony A, Bouvier G, Rué M, Loiseau H, Vital A, et al. (2011) Brain tumors and hormonal factors: review of the epidemiological literature. Cancer Causes Control 22: 697–714. [DOI] [PubMed] [Google Scholar]
- 5. Wiemels J, Wrensch M, Claus EB (2010) Epidemiology and etiology of meningioma. J Neurooncol 99: 307–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, et al. (2006) Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 295: 1549–55. [DOI] [PubMed] [Google Scholar]
- 7. Rennie KL, Jebb SA (2005) Prevalence of obesity in Great Britain. Obes Rev 6: 11–2. [DOI] [PubMed] [Google Scholar]
- 8. Calle EE, Kaaks R (2004) Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer 4: 579–91. [DOI] [PubMed] [Google Scholar]
- 9. Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M (2008) Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 371: 569–78. [DOI] [PubMed] [Google Scholar]
- 10. Bellur SN, Chandra V, Anderson RJ (1983) Association of meningiomas with obesity. Ann Neurol 13: 346–7. [DOI] [PubMed] [Google Scholar]
- 11. Benson VS, Pirie K, Green J, Casabonne D, Beral V, et al. (2008) Lifestyle factors and primary glioma and meningioma tumours in the Million Women Study cohort. Br J Cancer 99: 185–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Claus EB, Calvocoressi L, Bondy ML, Wrensch M, Wiemels JL, et al. (2013) Exogenous hormone use, reproductive factors, and risk of intracranial meningioma in females. J Neurosurg 118: 649–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Custer B, Longstreth WT Jr, Phillips LE, Koepsell TD, Van Belle G (2006) Hormonal exposures and the risk of intracranial meningioma in women: a population-based case-control study. BMC Cancer 6: 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hemminki K, Li X, Sundquist J, Sundquist K (2011) Obesity and familial obesity and risk of cancer. Eur J Cancer Prev 20: 438–43. [DOI] [PubMed] [Google Scholar]
- 15. Jhawar BS, Fuchs CS, Colditz GA, Stampfer MJ (2003) Sex steroid hormone exposures and risk for meningioma. J Neurosurg 99: 848–53. [DOI] [PubMed] [Google Scholar]
- 16. Johnson DR, Olson JE, Vierkant RA, Hammack JE, Wang AH, et al. (2011) Risk factors for meningioma in postmenopausal women: results from the Iowa Women’s Health Study. Neuro Oncol 13: 1011–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jacobs DH, McFarlane MJ, Holmes FF (1986) Meningiomas and obesity reconsidered. Ann Neurol 20: 376. [DOI] [PubMed] [Google Scholar]
- 18. Lee E, Grutsch J, Persky V, Glick R, Mendes J, et al. (2006) Association of meningioma with reproductive factors. Int J Cancer 119: 1152–7. [DOI] [PubMed] [Google Scholar]
- 19. Michaud DS, Bové G, Gallo V, Schlehofer B, Tjønneland A, et al. (2011) Anthropometric measures, physical activity, and risk of glioma and meningioma in a large prospective cohort study. Cancer Prev Res (Phila) 4: 1385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schneider B, Pülhorn H, Röhrig B, Rainov NG (2005) Predisposing conditions and risk factors for development of symptomatic meningioma in adults. Cancer Detect Prev 29: 440–7. [DOI] [PubMed] [Google Scholar]
- 21. Wiedmann M, Brunborg C, Lindemann K, Johannesen TB, Vatten L, et al. (2013) Body mass index and the risk of meningioma, glioma and schwannoma in a large prospective cohort study (The HUNT Study). Br J Cancer 109: 289–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, et al. (2009) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed 1 May 2013.
- 23. Greenland S (1987) Quantitative methods in the review of epidemiologic literature. Epidemiol Rev 9: 1–30. [DOI] [PubMed] [Google Scholar]
- 24. Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21: 1539–58. [DOI] [PubMed] [Google Scholar]
- 25. Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, et al. (2011) GRADE Working Group. GRADE guidelines: 7. Rating the quality of evidence–inconsistency. J Clin Epidemiol 64: 1294–302. [DOI] [PubMed] [Google Scholar]
- 26. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials. 1986 7: 177–88. [DOI] [PubMed] [Google Scholar]
- 27. Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hamling J, Lee P, Weitkunat R, Ambühl M (2008) Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med 27: 954–70. [DOI] [PubMed] [Google Scholar]
- 29. Albanes D, Taylor PR (1990) International differences in body height and weight and their relationship to cancer incidence. Nutr Cancer 14: 69–77. [DOI] [PubMed] [Google Scholar]
- 30. Attner B, Landin-Olsson M, Lithman T, Noreen D, Olsson H (2012) Cancer among patients with diabetes, obesity and abnormal blood lipids: a population-based register study in Sweden. Cancer Causes Control 23: 769–77. [DOI] [PubMed] [Google Scholar]
- 31. Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ (2003) Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 348: 1625–38. [DOI] [PubMed] [Google Scholar]
- 32. Cabaniols C, Giorgi R, Chinot O, Ferahta N, Spinelli V, et al. (2011) Links between private habits, psychological stress and brain cancer: a case-control pilot study in France. J Neurooncol 103: 307–16. [DOI] [PubMed] [Google Scholar]
- 33. Helseth A, Tretli S (1989) Pre-morbid height and weight as risk factors for development of central nervous system neoplasms. Neuroepidemiology 8: 277–82. [DOI] [PubMed] [Google Scholar]
- 34. Møller H, Mellemgaard A, Lindvig K, Olsen JH (1994) Obesity and cancer risk: a Danish record-linkage study. Eur J Cancer 30: 344–50. [DOI] [PubMed] [Google Scholar]
- 35. Oh SW, Yoon YS, Shin SA (2005) Effects of excess weight on cancer incidences depending on cancer sites and histologic findings among men: Korea National Health Insurance Corporation Study. J Clin Oncol 23: 4742–54. [DOI] [PubMed] [Google Scholar]
- 36. Pan SY, Johnson KC, Ugnat AM, Wen SW, Mao Y, et al. (2004) Association of obesity and cancer risk in Canada. Am J Epidemiol 159: 259–68. [DOI] [PubMed] [Google Scholar]
- 37. Parr CL, Batty GD, Lam TH, Barzi F, Fang X, et al. (2010) Body-mass index and cancer mortality in the Asia-Pacific Cohort Studies Collaboration: pooled analyses of 424,519 participants. Lancet Oncol 11: 741–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Reeves GK, Pirie K, Beral V, Green J, Spencer E, et al. (2007) Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ 335: 1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Samanic C, Gridley G, Chow WH, Lubin J, Hoover RN, et al. (2004) Obesity and cancer risk among white and black United States veterans. Cancer Causes Control 15: 35–43. [DOI] [PubMed] [Google Scholar]
- 40. Samanic C, Chow WH, Gridley G, Jarvholm B, Fraumeni JF Jr (2006) Relation of body mass index to cancer risk in 362,552 Swedish men. Cancer Causes Control 17: 901–9. [DOI] [PubMed] [Google Scholar]
- 41. Tulinius H, Sigfússon N, Sigvaldason H, Bjarnadóttir K, Tryggvadóttir L (1997) Risk factors for malignant diseases: a cohort study on a population of 22,946 Icelanders. Cancer Epidemiol Biomarkers Prev 6: 863–73. [PubMed] [Google Scholar]
- 42. Wolk A, Gridley G, Svensson M, Nyrén O, McLaughlin JK, et al. (2001) A prospective study of obesity and cancer risk (Sweden). Cancer Causes Control 12: 13–21. [DOI] [PubMed] [Google Scholar]
- 43. Renehan AG, Frystyk J, Flyvbjerg A (2006) Obesity and cancer risk: the role of the insulin-IGF axis. Trends Endocrinol Metab 17: 328–36. [DOI] [PubMed] [Google Scholar]
- 44. Basen-Engquist K, Chang M (2011) Obesity and cancer risk: recent review and evidence. Curr Oncol Rep 13: 71–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Osório-Costa F, Rocha GZ, Dias MM, Carvalheira JB (2009) Epidemiological and molecular mechanisms aspects linking obesity and cancer. Arq Bras Endocrinol Metabol 53: 213–26. [DOI] [PubMed] [Google Scholar]
- 46. Russo VC, Gluckman PD, Feldman EL, Werther GA (2005) The insulin-like growth factor system and its pleiotropic functions in brain. Endocr Rev 26: 916–43. [DOI] [PubMed] [Google Scholar]
- 47. Joseph D’Ercole A, Ye P (2008) Expanding the mind: insulin-like growth factor I and brain development. Endocrinology 149: 5958–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Roberts DL, Dive C, Renehan AG (2010) Biological mechanisms linking obesity and cancer risk: new perspectives. Annu Rev Med 61: 301–16. [DOI] [PubMed] [Google Scholar]
- 49. Bezemer ID, Rinaldi S, Dossus L, Gils CH, Peeters PH, et al. (2005) C-peptide, IGF-I, sex-steroid hormones and adiposity: a cross-sectional study in healthy women within the European Prospective Investigation into Cancer and Nutrition (EPIC). Cancer Causes Control 16: 561–72. [DOI] [PubMed] [Google Scholar]
- 50. Key TJ, Appleby PN, Reeves GK, Roddam A, Dorgan JF, et al. (2003) Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst 95: 1218–26. [DOI] [PubMed] [Google Scholar]
- 51. Derby CA, Zilber S, Brambilla D, Morales KH, McKinlay JB (2006) Body mass index, waist circumference and waist to hip ratio and change in sex steroid hormones: the Massachusetts Male Ageing Study. Clin Endocrinol (Oxf) 65: 125–31. [DOI] [PubMed] [Google Scholar]
- 52. Oh JY, Barrett-Connor E, Wedick NM, Wingard DL, Rancho Bernardo Study (2002) Endogenous sex hormones and the development of type 2 diabetes in older men and women: the Rancho Bernardo study. Diabetes Care 25: 55–60. [DOI] [PubMed] [Google Scholar]
- 53. Maor G, Segev Y, Phillip M (1999) Testosterone stimulates insulin-like growth factor-I and insulin-like growth factor-I-receptor gene expression in the mandibular condyle–a model of endochondral ossification. Endocrinology 140: 1901–10. [DOI] [PubMed] [Google Scholar]
- 54. Fan ZX, Shen J, Wu YY, Yu H, Zhu Y, et al. (2013) Hormone replacement therapy and risk of meningioma in women: a meta-analysis. Cancer Causes Control 24: 1517–25. [DOI] [PubMed] [Google Scholar]
- 55. Okorodudu DO, Jumean MF, Montori VM, Romero-Corral A, Somers VK, et al. (2010) Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and meta-analysis. Int J Obes (Lond) 34: 791–9. [DOI] [PubMed] [Google Scholar]
- 56. Smalley KJ, Knerr AN, Kendrick ZV, Colliver JA, Owen OE (1990) Reassessment of body mass indices. Am J Clin Nutr 52: 405–8. [DOI] [PubMed] [Google Scholar]
- 57. Wellens RI, Roche AF, Khamis HJ, Jackson AS, Pollock ML, et al. (1990) Relationships between the Body Mass Index and body composition. Obes Res 4: 35–44. [DOI] [PubMed] [Google Scholar]
- 58. Qi ZY, Shao C, Zhang X, Hui GZ, Wang Z (2013) Exogenous and Endogenous Hormones in Relation to Glioma in Women: A Meta-analysis of 11 Case-Control Studies. PLoS ONE 8: e68695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hsu DW, Efird JT, Hedley-Whyte ET (1997) Progesterone and estrogen receptors in meningiomas: prognostic considerations. J Neurosurg 86: 113–120. [DOI] [PubMed] [Google Scholar]
- 60. Rubinstein AB, Loven D, Geier A, Reichenthal E, Gadoth N (1994) Hormone receptors in initially excised versus recurrent intracranial meningiomas. J Neurosurg 81: 184–187. [DOI] [PubMed] [Google Scholar]
- 61. Jay JR, MacLaughlin DT, Riley KR, Martuza RL (1985) Modulation of meningioma cell growth by sex steroid hormones in vitro. J Neurosurg 62: 757–62. [DOI] [PubMed] [Google Scholar]
- 62. Hatiboglu MA, Cosar M, Iplikcioglu AC, Ozcan D (2008) Sex steroid and epidermal growth factor profile of giant meningiomas associated with pregnancy. Surg Neurol 69: 356–362. [DOI] [PubMed] [Google Scholar]
- 63. Rao G, Giordano SH, Liu J, McCutcheon IE (2009) The association of breast cancer and meningioma in men and women. Neurosurgery 65: 483–489. [DOI] [PubMed] [Google Scholar]
- 64. Qi ZY, Shao C, Huang YL, Hui GZ, Zhou YX, et al. (2013) Reproductive and exogenous hormone factors in relation to risk of meningioma in women: a meta-analysis. PLoS One 8: e83261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flow diagram of study selection.
(TIF)
Methodological quality of included studies based on the Newcastle–Ottawa Scale.
(DOCX)
(DOC)