Abstract
A significant link between cardiovascular disease and osteoporosis is established in postmenopausal women, but data in men are scarce. We tested the hypothesis that greater severity of abdominal aortic calcification (AAC) was associated with an increased risk of non-spine fracture in 5994 men aged ≥65 years. AAC wasassessed on 5400 baseline lateral thoraco-lumbar radiographs using a validated visual semi-quantitative score. Total hip bone mineral density (BMD) was measured using dual energy X-ray absorptiometry. Incident non-spine fractures were centrally adjudicated. After adjustment for age, BMI, total hip BMD, fall history, prior fracture, smoking status, co-morbidities, race and clinical center, the risk of non-spine fracture (n=805) was increased among men with higher AAC (HR Q4 (AAC score ≥9) vs Q1 (0-1): 1.36, 96%CI: 1.10-1.68). This association was due to an increased risk of hip fracture (n=178) among men with higher AAC (HR Q4 vs Q1: 2.33, 95%CI: 1.41-3.87). By contrast, the association between AAC and the risk of non-spine-non-hip fracture was weaker and not significant (HR Q4 vs Q1: 1.22, 95%CI: 0.96-1.55). The findings regarding higher AAC and increased risk of fracture were not altered in additional analyses accounting for degree of trauma, estimated glomerular filtration rate, presence of lumbar vertebral fractures (which may bias AAC assessment), preexisting cardiovascular disease, ankle brachial index or competing risk of death. Thus, in this large cohort of elderly men, greater AAC was independently associated with an increased risk of hip fracture, but not with other non-spine fractures. These findings suggest that AAC assessment may be a useful method for identification of older men at high risk of hip fracture.
Keywords: abdominal aortic calcification, hip fracture, men
Introduction
Calcification in the aortic media involves genetic factors, transdifferentiation of vascular smooth muscle cells, hormones, cytokines, abnormal mineral metabolism, etc. (1). Severe abdominal aortic calcification (AAC) was associated with higher cardiovascular morbidity and mortality, lower bone mineral density (BMD) and higher bone fragility (2-15). The strength of link between AAC and fracture risk vary in the studies, probably due to population differences, study design, AAC assessment methods, AAC severity and fracture outcomes.
Association between AAC severity and fracture risk has been studied mainly in women (3-5,8,12,14,16) or mixed cohorts (7,11). Few studies have assessed this relationship in men (6,13,15-16). Two studies in men were cross-sectional and assessed radiographic vertebral fractures (13,15). Prospective data in men are limited and discordant. Severe AAC was associated with higher risk of clinical fracture in men aged 50-85 years, but not with hip fracture risk in another cohort of men aged 47-80 years (6,16). Moreover, the investigated associations were not consistently adjusted for potential confounders including fall history, prior fracture, renal function, and peripheral arterial disease. Thus, the link between AAC and hip fracture risk is still questionable especially in men.
Aside from its scientific interest, this question may also have practical implications. BMD is less predictive of fracture in men than in women (17-19). In addition, measurements of bone turnover markers, bone ultrasound parameters and bone architecture by quantitative computed tomography do not improve fracture prediction in men (20-23). Thus, new parameters are needed to improve fracture prediction in men. Therefore, in order to test the hypothesis that severe AAC is associated with higher risk of non-spine fracture, including hip fracture in older men, we assessed baseline AAC in a cohort of men aged ≥65 years and followed them up over an average period of 10.5 years to ascertain incident fractures.
Subjects and Methods
Study population
The Osteoporotic Fractures in Men study (MrOs) enrolled 5994 community-dwelling men from 2000 through 2002 from six U.S. cities (Birmingham, AL; Minneapolis, MN; Palo Alto, CA; Monongahela Valley, PA; Portland, OR; and San Diego, CA) (24). Eligible participants were aged ≥65 years, able to walk without assistance, and had no history of bilateral hip prostheses. The Institutional Review Board at each center approved the study protocol, and a written informed consent was obtained. Among 5991 baseline lateral spine radiographs, 5400 could then be assessed for AAC.
Assessment of the abdominal aortic calcification (AAC) score
AAC was assessed from the digitized baseline lateral radiographs of lumbar spine using a visual semiquantitative method (25). Radiographs could not be assessed for AAC in 591 men (10%). The reasons why the 591 radiographs were deemed unreadable for AAC included unacceptable width or height, rotation, inadequate exposure, or scoliosis. Men with unreadable films were older (74.5 vs 73.5 years p<0.001) and more often self-reported history of non-trauma fracture after the age of 50 (22 vs 17%, p<0.001) than men who had the radiographs assessed, but they had higher total hip BMD (0.98 vs 0.95 g/cm2,p<0.001). Severity of calcific deposits in the anterior and posterior walls of the abdominal aorta adjacent to the first four lumbar vertebrae were assessed in the 8 segments defined, using the midpoint of the intervertebral space above and below the vertebrae as boundaries. Severity scores for these segments (0–3) were added to yield an AAC score (0-24). AAC score was then categorized into quartiles as 0-1, 2-4, 5-8, and 9+. In order to assess intra-observer readability, 20 films were assessed 15 times by PS throughout the reading process. A second expert (JS) read 40 films to assess inter-reader reliability. Agreement was unanimous on the readability of the films (readable/not). The intra- and inter-reader reproducibility for the AAC score were/was high (intraclass correlation coefficient: 0.98 [95%CI 0.96–0.99] and 0.94 [95%CI 0.88–0.97]). For the global AAC score categorized as 0-1, 2-4, 5-8, 9+, the average weighted kappa statistic for intra-reader reproducibility was 0.91 (range 0.75 –1.00). The weighted kappa statistic for inter-reader reproducibility was 0.81 (95%CI 0.68 – 0.93).
Assessment of the incident fractures
Data on the incident fractures were collected as previously described (26-27). Questionnaires were mailed every 4 months to obtain information about recent fractures. The response rate exceeded 99%. Incident fractures were confirmed by centralized physician review of X-ray reports. When a fracture was reported, the clinic staff interviewed the participant about the circumstances. The follow-up time ended at the date of the first fracture of interest in the given model (i.e. of date of the first hip fracture in the analysis of the hip fracture prediction), date of death, date of last contact or termination of the study. For all non-spine fractures the median follow-up time was 10.5 years (interquartile range: 8.5-11.2). The primary outcomes were first hip fracture, non-spine fracture and fragility fracture (non-spine fracture excluding those of skull, face, hand, finger and toe). High and low trauma fractures were included in the primary analysis, because in older adults both types of fracture are associated with low BMD (27). Secondary analyses were performed excluding high trauma fractures. The “non-spine-non-hip fracture” outcome was examined to determine if the association between AAC and non-spine fracture risk was contingent on the association between AAC and hip fracture risk.
Measurement of bone mineral density
Total hip BMD was measured by dual-energy X-ray absorptiometry (QDR 4500W; Hologic Inc., Bedford, MA, USA) using standardized procedures (24). Hip phantom scan results were assessed for quality control. The intra-clinic coefficient of variation (CV) for hip phantoms was 0.37-0.58%. The inter-clinic CV was 0.9%.
Other covariates
At baseline we assessed age, race, smoking, prior fracture, fall history in the past year, self-rated health, and co-morbidities. Self-reported race was categorized as Caucasian or non-Caucasian. Self-reported smoking was categorized as current, former and never-smoker. Participants self-reported falls in the past year as well as prior fractures and the age at which they occurred. Men self-reported physician diagnoses of hypertension, diabetes mellitus, angina pectoris, congestive heart failure, history of myocardial infarction and of stroke. Cardiovascular disaease (CVD) was defined as angina pectoris, congestive heart failure, and history of myocardial infarction or stroke. A co-morbidity score (0–6) was calculated and expressed as 0, 1-2, or ≥3 conditions. Vertebral fractures at baseline were assessed using a semiquantitative method (28). For a subset of 2306 men in this analysis, measurement of 25-hydroxycholecalciferol (25OHD) was performed at the Mayo Clinic using mass spectrometry as previously described (29). Estimated glomerular filtration rate (eGFR) was calculated by the MDRD equation: eGFR (ml/min/1.73m2)= 175*(serum creatinine [mg/dl])−1.154*(Age[yrs])−0.203*1.212 (if African American) (30). The ankle-brachial index (ABI) was calculated as the ratio of the systolic pressure in the ankle to that in the arm as previously described (31).
Statistical analyses
Bivariate comparisons of characteristics of men who did or did not have AAC assessment was determined by a t-test for the continuous variables and a chi2 test for the categorical ones. Comparisons of characteristics of men across the AAC score quartiles were performed using the analysis of variance for continuous variables with normal distributions, a Kruskal-Wallis test for continuous variables with skewed distributions and a chi-square test for categorical variables. The time to first incident fracture of interest in a given model (e.g. time to the first hip fracture which occurred during the follow-up in the hip fracture analyse) was evaluated using Cox proportional hazard models, with results presented as hazard ratio and 95% confidence intervals (HR, 95%CI). The proportionality assumption was verified by examining the Schoenfeld residuals and the interaction between AAC score and log-(time). AAC score was expressed as a continuous variable (1 unit increase) and by quartiles with the quartile 1 (Q1) as reference category. A test for trend across the quartiles was calculated. The models were first adjusted for age and clinical center, because fracture risk and AAC severity increase significantly with age and there could be differences between the men recruited in different centers. Then, other confounders were added (race, BMI, total hip BMD, fall history, prior fracture, smoking, and number of co-morbidities) in order to assess the effect of these variables on the association between AAC severity and fracture risk.
Additional mediation analyses were performed to evaluate the mechanism underlying the association between severe AAC and higher hip fracture risk. As poor renal function is associated with severe AAC and high bone fragility and studies have reported higher fracture risk among older women eGFR<60ml/min/1.73m2 and older men with eGFR<30ml/min/1.73m2 (32-33), analyses were performed further adjusting the models for eGFR, stratifying by level of eGFR and testing for evidence of an interaction between eGFR and AAC score for the prediction of hip fracture risk. As low 25OHD may be associated with severe AAC and higher risk of hip fracture, analyses were performed adjusting the multivariable model for 25OHD. As severe AAC may be associated with poor lower extremity blood flow, the multivariable models were adjusted for ABI expressed as a continuous and dichotomized variable (>0.9 vs ≤0.9) indicating absence of presence of peripheral arterial disease (31). If the association between AAC and fracture risk depended on poor blood flow, such association might be mediated either by the decrease in blood flow in muscles leading to a decrease in muscle strength or by the decrease in blood flow in bone. In the first case, severe AAC might lead to lower gait speed with attendant increase in hip fracture risk. Thus, we adjusted the models for the gait speed. In the second case, severe AAC would be associated with higher risk of fracture of lower extremity, i.e. bones receiving blood from the iliac artery (pelvis, femur, tibia, fibula, ankle, foot). To evaluate this latter possibility, we assessed the association of AAC separately, with fractures of lower extremity and with fractures of the upper body (rib, humerus, etc.).
Finally, sensitivity analyses were performed. As cardiovascular diseases are associated with severe AAC and some studies have reported a higher fracture risk among older adults with cardiovascular diseases (34-35), analyses were performed stratifying by prevalent cardiovascular disease status (yes vs no). Because vertebral fracture is characterized by lower height of vertebral body and the presence of vertebral fractures from levels L1 to L4 may falsely increase the AAC score, we performed analyses excluding 238 men with prevalent radiographic lumbar fractures. Because AAC may be related to unmeasured confounders associated with increased mortality, men with higher AAC may develop fewer fractures because of higher competing risk of mortality. The Fine and Gray model was integrated into multivariable models to calculate HR (95%CI) allowing for competing mortality risks (36).
All analyses were performed using the SAS9.2 software (SAS Institute, Cary, NC, USA) and Stata version 12.1 (StataCorp, College Station, TX, USA).
Results
Characteristics of participants
Figure 1 presents AAC distribution; median AAC was 5 (interquartile range: 2 to 9). Men with higher AAC score were older; more likely to be smokers or to report a history of medical conditions (angina pectoris, stroke, myocardial infarction, heart failure, hypertension, diabetes mellitus and chronic obstructive pulmonary disease); less likely to be Caucasian or to report good to excellent health status; and had lower hip BMD and eGFR (Table 1).
Fig. 1.
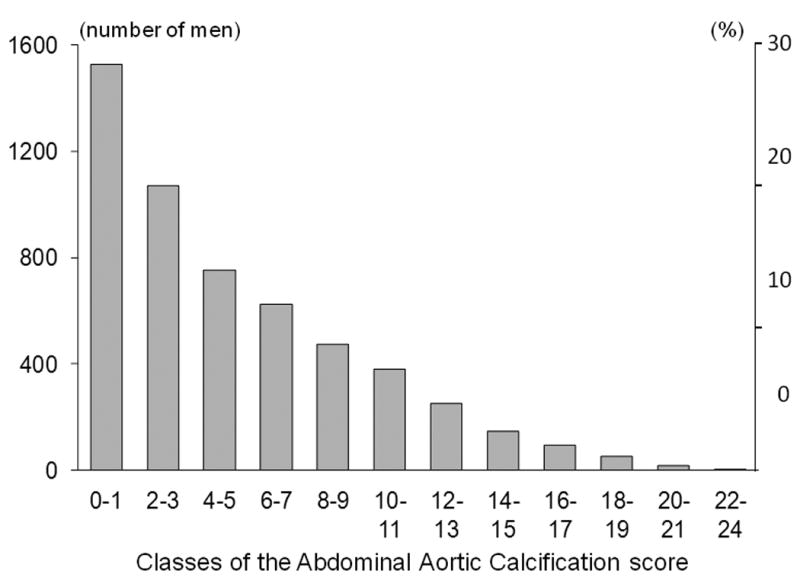
Distribution of the score of the abdominal aortic calcification (AAC) in the MrOS cohort.
Table 1. Descriptive analysis of the 5400 men who had radiographs assessable for the presence of abdominal aortic calcification (AAC) divided according to the quartiles of the AAC score.
| AAC0-1 (n=1528) | AAC2-4 (n=1468) | AAC5-8 (n=1240) | AAC9+ (n=1164) | p | |
|---|---|---|---|---|---|
| Age (yrs) | 71±5 | 73±6 | 74±6 | 76±6 | <0.001 |
| Non-Caucasian, n (%) | 247 (16) | 160 (11) | 110 (9) | 73 (6) | <0.001 |
| BMI (kg/m2) | 27.1±3.9 | 27.2±3.8 | 27.4±3.7 | 27.4±3.9 | 0.07 |
| Health good/excellent, n (%) | 1366 (89) | 1283 (87) | 1049 (85) | 936 (81) | <0.001 |
| Smoking, n (%) | |||||
| never | 747 (49) | 580 (40) | 415 (34) | 283 (24) | <0.001 |
| former | 734 (48) | 842 (57) | 771 (62) | 835 (72) | |
| current | 47 (3) | 46 (3) | 53 (4) | 46 (4) | |
| Angina pectoris, n (%) | 121 (8) | 207 (14) | 195 (16) | 241 (21) | <0.001 |
| Myocardial infarction, n (%) | 89 (6) | 189 (13) | 203 (16) | 253 (22) | <0.001 |
| Congestive heart failure, n (%) | 44 (3) | 63 (4) | 64 (5) | 98 (8) | <0.001 |
| Hypertension, n (%) | 510 (33) | 592 (40) | 573 (46) | 642 (55) | <0.001 |
| Stroke, n (%) | 40 (3) | 88 (6) | 84 (7) | 94 (8) | <0.001 |
| Diabetes mellitus, n (%) | 166 (12) | 193 (14) | 201 (18) | 225 (21) | <0.001 |
| COPD, n (%) | 147 (10) | 137 (9) | 145 (12) | 151 (13) | <0.01 |
| Ankle-brachial index (ABI) | 1.21±0.14 | 1.18±0.15 | 1.15±0.17 | 1.10±0.19 | <.0001 |
| ABI<0.9, n (%) | 36 (2.4) | 56 (3.9) | 84 (7.1) | 147 (13.4) | <.0001 |
| Fall, n (%) | 322 (21) | 279 (19) | 284 (23) | 253 (22) | 0.09 |
| Non-trauma fracture >50 years | 230 (15) | 236 (16) | 211 (17) | 222 (19) | <0.05 |
| 25OHD (ng/ml) | 27.1±9.3 | 26.7±8.2 | 26.2±8.5 | 26.1±8.4 | 0.17 |
| Estimated GFR (ml/min) | 78±17 | 77±18 | 76±19 | 75±20 | <0.001 |
| Total hip BMD (g/cm2) | 0.97±0.14 | 0.95±0.13 | 0.95±0.14 | 0.94±0.14 | <0.001 |
COPD chronic obstructive pulmonary disease, 25OHD – 25-hydroxycholecalciferol, GFR glomerular filtration rate, BMD bone mineral density, ABI minimum of both legs is provided
Association between AAC severity and risk of non-spine fracture
Non-spine fractures occurred in 805 men. After adjustment for confounders, fracture free survival decreased with increasing AAC quartiles (Fig. 2A). The median time to the first fracture was 5.2 years (interquartile range: 2.7; 7.9). Fracture incidence increased across the AAC quartiles (Table 2A). After adjustment for age and clinical center, the risk of non-spine fracture increased with increasing AAC score and was 50% higher in the highest (Q4) vs the lowest (Q1) AAC quartile. The association remained significant in the multivariable model.
Fig. 2.
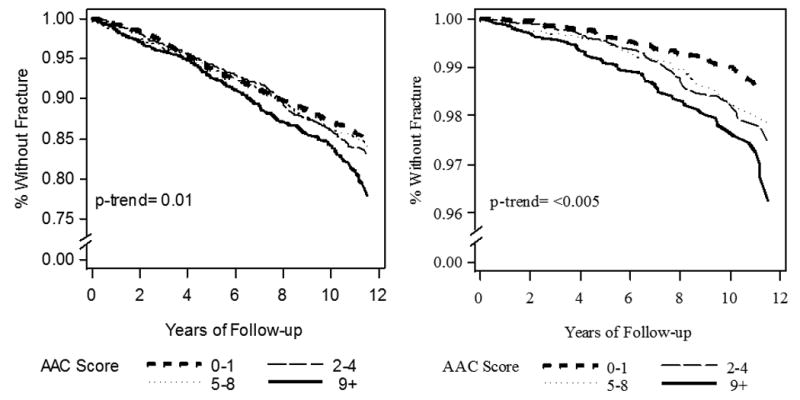
Fracture-free survival in the MrOS cohort according to the quartiles of the abdominal aortic calcification (AAC) score (adjusted for age, BMI, center, race, hip BMD, fall history, prior fracture, smoking and co-morbidities): A) non-spine fractures (n = 805 men), B) hip fractures (n = 178 men)
Table 2. Association between the abdominal aortic calcification (AAC) score and risk of fracture in 5400 men aged ≥65.
| Number of fractures (%) | Adjusted for age and center | Adjusted for age, BMI, center, race, hip BMD, fall history, prior fracture, smoking and co-morbidities | |
|---|---|---|---|
| A) Non-spine fracture (n=805 men) | |||
| AAC per 1 unit | 1.03 (1.02-1.05)† | 1.02 (1.01-1.04)* | |
| Q1 (AAC 0-1) | 186 (12.2) | 1.00 | 1.00 |
| Q2 (AAC 2-4) | 220 (15.0) | 1.17 (0.96-1.42) | 1.11 (0.91-1.36) |
| Q3 (AAC 5-8) | 182 (14.7) | 1.17 (0.95-1.44) | 1.08 (0.87-1.33) |
| Q4 (AAC 9+) | 217 (18.7) | 1.52 (1.24-1.86)# | 1.36 (1.10-1.68)| |
| trend | <0.001 | <0.001 | 0.01 |
| B) Hip fracture (n=178 men) | |||
| AAC per 1 unit | 1.06 (1.03-1.10)† | 1.05 (1.01-1.08)* | |
| Q1 (AAC 0-1) | 23 (1.4) | 1.00 | 1.00 |
| Q2 (AAC 2-4) | 47 (3.1) | 1.78 (1.07-2.97)‡ | 1.68 (1.01-2.80)‡ |
| Q3 (AAC 5-8) | 44 (3.4) | 1.77 (1.05-3.00)‡ | 1.51 (0.89-2.55) |
| Q4 (AAC 9+) | 64 (5.8) | 2.90 (1.77-4.77)# | 2.33 (1.41-3.87)| |
| trend | <0.001 | <0.001 | <0.005 |
| C) Non-spine-non-hip fracture (n=627 men) | |||
| AAC per 1 unit | 1.02 (0.99-1.04) | 1.01 (0.99-1.03) | |
| Q1 (AAC 0-1) | 104 (6.8) | 1.00 | 1.00 |
| Q2 (AAC 2-4) | 131 (9.2) | 1.22 (0.95-1.59) | 1.17 (0.90-1.52) |
| Q3 (AAC 5-8) | 101 (8.4) | 1.17 (0.89-1.55) | 1.06 (0.80-1.41) |
| Q4 (AAC 9+) | 106 (9.6) | 1.34 (1.01-1.78)‡ | 1.19 (0.89-1.59) |
| trend | 0.67 | <0.05 | 0.38 |
p<0.01
p<0.001
p<0.05
p<0.005
p<0.001 – vs Q1
Results were similar in the analyses of the association of AAC with the risk of low trauma non-spine fractures and risk of non-spine fragility fractures. After multivariable adjustment, HR (95%CI) among men in the AAC Q4 vs the AAC Q1 was HR= 1.48, 95%CI: 1.17-1.88 for low trauma non-spine fractures and HR=1.31, 95%CI: 1.05-1.64 for fragility fracture.
Association between AAC severity and risk of hip fracture
Hip fractures occurred in 178 men. After adjustment for confounders, survival without hip fracture decreased with increasing AAC quartiles (Fig. 2B). Median time to the first fracture was 6.3 years (interquartile range: 3.6; 8.4). Hip fracture incidence increased across the AAC quartiles (Table 2B). After adjustment for age and clinical center, hip fracture risk increased with increasing AAC score and was three-fold higher in the AAC Q4 vs Q1. The association between AAC score and hip fracture risk was slightly attenuated after adjustment for all confounders. Results were similar in the analyses restricted to low trauma hip fractures. After multivariable adjustment, the risk of low trauma hip fracture was higher in the AAC Q4 vs Q1: HR= 2.59 (95%CI: 1.52-4.43).
Association between AAC severity and risk of non-spine-non-hip fracture
After excluding men with hip fracture from the analysis, 627 men sustained non-spine-non-hip fractures. Risk of non-spine-non-hip fracture did not increase across the AAC quartiles (Table 2C). After consideration of multiple confounders, severe AAC appeared modestly associated with a higher risk of these fractures, but the association was not significant. Results were similar for the association between AAC severity and risk of low trauma fragility non-spine-non-hip fracture (442 men).
Mediation analyses
Data on eGFR were available in 4958 men. Adjustment for eGFR did not alter the relationship between AAC score and fracture risk. In 4100 men with eGFR>60 ml/min, 628 men sustained non-spine fracture (126 men with hip fracture). The association between AAC and hip fracture risk was similar to that in the overall cohort (HR [Q4 vs Q1]: 2.53, 95%CI: 1.36-4.70). The results were similar in men with eGFR>30 ml/min. Among the 858 men with eGFR<60 ml/min, 118 men sustained non-spine fracture (37 hip fractures). In this group, severe AAC appeared to be related to a higher hip fracture risk but the association failed to reach significance (HR [Q4 vs Q1]: 1.53 95%CI: 0.53-4.36), while associations of hip BMD, age and fall history with fracture risk remained significant. Only 26 men had eGFR<30 ml/min.
In 2306 men who had 25OHD assays, 280 men sustained non-spine fracture (73 men with hip fracture). In models further adjusteed for 25OHD level the relation between AAC and hip fracture risk was similar to that in the overall cohort (HR [Q4 vs Q1]: 3.57, 95%CI: 1.49-8.57; p-trend<0.01).
The association between AAC severity and hip fracture risk persisted in the multivariable models adjusted for ABI (HR [Q4 vs Q1] 2.02, 95%CI: 1.20-3.40; p-trend<0.05). Results were similar for ABI expressed as a continuous or dichotomized variable. The association between AAC severity and hip fracture risk also persisted in the multivariable models adjusted for gait speed (HR [Q4 vs Q1] 2.27 95%CI: 1.37-3.77; p-trend<0.005).
Lower extremity fractures other than hip fracture occurred in 197 men. AAC severity was not associated with their risk (HR [Q4 vs Q1]: 1.18, 95%CI: 0.77-1.80). The association between the AAC severity and risk of non-spine fracture of the upper body was not significant (HR [(Q4 vs Q1] 1.29, 95%CI: 0.84-1.75) either.
Sensitivity analysis
Among the 3950 men without CVD, the risk of non-spine fracture and that of hip fracture were higher among men with greater AAC severity (HR Q4 vs Q1 [95%CI]: 1.35, [1.05-1.75] and 2.35, [1.26-4.39], respectively). Among the 1450 men who self-reported CVD, severe AAC was associated with higher risk of hip fracture (HR= 2.54, 95%CI: 1.02-6.30); the association between AAC and non-spine fracture (HR [Q4 vs Q1]: 1.33, 95%CI: 0.89-2.00) failed to reach significance, but the point estimate was similar to that for the same association among men without CVD. The associations between AAC and fracture outcomes were not altered after excluding 238 men with prevalent lumbar fractures (not shown). Mortality increased across the increasing AAC quartiles (p-trend<0.001). However, when the competing risk of death was accounted for in the model, men with higher AAC remained at increased risk of hip fracture (HR [Q4 vs Q1]: 2.50, 95%CI: 1.52-4.14; p-trend<0.005).
Discussion
In a large cohort of elderly men, severe baseline AAC was associated with a higher risk of incident hip fracture (but not other non-spine fractures) even after adjustment for traditional risk factors for AAC and hip fracture.
Our data expand on the previous findings evaluating the association between AAC and fracture risk. Our results are similar to the cross-sectional and prospective data obtained in older women (3-5). Fracture risk was higher in women with severe AAC vs mild/absent AAC (5-7,13,15). The link was significant for major fracture types (3-5,7,11,13-15) but data were less consistent for various fractures analyzed jointly (6-7,12). Severe AAC was associated with hip fracture risk in 2662 postmenopausal women followed up for 7.5 years (5), but not in 1453 women followed up for up to 36 years (16). Negative results could be related to the long periods to fracture and high mortality in women with severe AAC. Prior studies in men have not been powered to examine the association between AAC and hip fracture. Moreover, the existing studies in men were not controlled for potential confounders such as poor renal function or peripheral artery disease (32,34,37-38).
Severe AAC has been associated with prevalent and incident vertebral fracture in most (3-4,7, 11,13-15), but not all (5), studies. This link is stronger for more vs less severe vertebral fracture and for multiple vs single vertebral fractures (11,13,15). Thus, severe AAC may be associated with major fragility fractures. Inconsistent findings regarding the relation between AAC and fracture risk may be due to the heterogeneity of fracture outcomes (6-7,12).
The link between CVD and fracture risk has been reported previously. Fracture risk increases with CVD severity (34-35,39). Conversely, cardiovascular risk increases with the severity of osteoporosis. In postmenopausal women, cardiovascular risk rises with increasing number and severity of vertebral fractures (40). CVD may be associated with severe AAC. Osteoporosis and CVD share risk factors e.g. smoking, sedentary lifestyle, hormones or cytokines. Thus, the association between AAC and hip fracture may reflect the negative effects of these factors on bone and cardiovascular system. However, in our study, the association between AAC and hip fracture risk remained significant after adjustment for smoking status and co-morbid medical conditions.
The association between AAC severity and fracture risk may be due to poor renal function in elderly individuals with severe AAC. Renal failure is associated with high bone fragility AAC, and CVD (41). However, the relation between AAC severity and hip fracture risk was not altered after adjustment for eGFR. The association persisted in men with eGFR>60 ml/min, but was non-significant in men with eGFR<60 ml/min, likely due to lower power in the latter group. Other factors contributing to the lack of association in this group including heterogeneous bone pathology related to renal failure and higher mortality in men with poorest renal function.
CVD may be associated with a higher risk of loss of consciousness and falls due to a drop in blood pressure or arrhythmias (42-43). These may be related to the disease itself or side effects of medications. However, the association between AAC and hip fracture remained significant after adjustment for fall history.
As severe osteoporosis is associated with increased cardiovascular risk, poor bone status may have contributed to the development of AAC prior to baseline and to the higher fracture risk during the follow-up. Some (3-5-6,10,13-14) studies showed a negative relation between AAC severity and BMD. However, the association between AAC and hip fracture in this study remained significant after adjustment for BMD and prior fracture history. Moreover, low BMD and prior fracture predicted all fractures, including non-spine-non-hip fracture (not shown), whereas severe AAC was associated specifically with higher risk of hip fracture.
The molecular mechanisms underlying the link between AAC and hip fracture are unclear. Low serum 25-hydroxycholecalciferol levels have been associated with higher fracture risk and greater AAC severity (44-45), but the association between severe AAC and higher hip fracture risk in this study was not altered by adjustment for 25OHD levels. A laboratory investigation reported that osteoprotegerin-deficient mice had severe calcification in the large arteries and multiple fractures (46). In addition, high activity of matrix metalloproteinases has been associated with increased bone resorption and severe AAC (47-48). Elevated levels of C-reactive protein and inflammatory cytokines have also been associated with greater AAC and high fracture risk (49). Finally, oxidative stress has been associated with severe arterial calcification and low bone stiffness (50). Any or a combination of these factors might in part explain the link between severe AAC and hip fracture risk.
The asssociation between severe AAC and hip fracture risk remained significant after adjustment for ABI. Moreover, severe AAC was not associated with the risk of other fractures of the lower extremity. These results obtained using different approaches are complementary. They suggest that the investigated association is specific for hip fracture and not mediated by poor blood flow in the lower body. Data on the poor functional performance in patients with peripheral artery disease are inconsistent (51), but adjustment for fall history did not impact the association between AAC and hip fracture risk observed in this study.
The strengths of our study include the large cohort, a high number of fully adjudicated incident fractures, and adjustment for multiple confounders. We also recognize limitations. Although the MrOS cohort is representative of older community-dwelling Caucasian men, the non-Caucasian men and institutionalized men are underrepresented. The self-reported incident fractures were confirmed; however, false negatives are possible. Self-reported falls and fractures prior to baseline could not be confirmed. Co-morbidities were self-reported and dichotomized as yes/no. We did not account for disease duration, severity and treatment. Our study is observational and residual confounding is possible; for example, AAC might be a marker for use of certain medications that might be related to hip fracture risk. Moreover, some potential confounders, such as eating habits were not included in the model.
In conclusion, in a large cohort of older community-dwelling men, men with severe AAC had an increased hip fracture risk after accounting for trauma severity, multiple confounders, impaired blood flow in the lower limbs, gait speed and competing risk of death. By contrast, severe AAC was not associated with the risk of non-spine-non-hip fracture. Our data suggest that severe AAC is specifically associated with the risk of hip fracture in older men; future research should investigate pathophysiological mechanisms underlying this association.
Acknowledgments
The Authors had full access to all the data in the study. PS takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: P.Szulc's personal research-related expenditures sponsored by SERVIER
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute on Aging (NIA), the National Center for Research Resources (NCRR), and NIH Roadmap for Medical Research under the following grant numbers: U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01-AG027810, K24AR048841 and UL1 RR024140.
Footnotes
Disclosures: All the authors declare that they have no conflict of interest concerning this manuscript.
References
- 1.Persy V, D'Haese P. Vascular calcification and bone disease: the calcification paradox. Trends Mol Med. 2009;15:405–416. doi: 10.1016/j.molmed.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Bastos Gonçalves F, Voûte MT, Hoeks SE, Chonchol MB, Boersma EE, Stolker RJ, Verhagen HJ. Calcification of the abdominal aorta as an independent predictor of cardiovascular events: a meta-analysis. Heart. 2012;98:988–994. doi: 10.1136/heartjnl-2011-301464. [DOI] [PubMed] [Google Scholar]
- 3.Schulz E, Arfai K, Liu X, Sayre J, Gilsanz V. Aortic calcification and the risk of osteoporosis and fractures. J Clin Endocrinol Metab. 2004;89:4246–4253. doi: 10.1210/jc.2003-030964. [DOI] [PubMed] [Google Scholar]
- 4.Rajzbaum G, Roger VL, Bézie Y, Chauffert M, Bréville P, Roux F, Safar ME, Blacher J. French women, fractures and aortic calcifications. J Intern Med. 2005;257:117–119. doi: 10.1111/j.1365-2796.2004.01430.x. [DOI] [PubMed] [Google Scholar]
- 5.Bagger YZ, Tankó LB, Alexandersen P, Qin G, Christiansen C. Radiographic measure of aorta calcification is a site-specific predictor of bone loss and fracture risk at the hip. J Intern Med. 2006;259:598–605. doi: 10.1111/j.1365-2796.2006.01640.x. [DOI] [PubMed] [Google Scholar]
- 6.Szulc P, Kiel DP, Delmas PD. Calcifications in the abdominal aorta predict fractures in men: MINOS study. J Bone Miner Res. 2008;23:95–102. doi: 10.1359/jbmr.070903. [DOI] [PubMed] [Google Scholar]
- 7.Naves M, Rodríguez-García M, Díaz-López JB, Gómez-Alonso C, Cannata-Andía JB. Progression of vascular calcifications is associated with greater bone loss and increased bone fractures. Osteoporos Int. 2008;19:1161–1166. doi: 10.1007/s00198-007-0539-1. [DOI] [PubMed] [Google Scholar]
- 8.Wang TK, Bolland MJ, Pelt NC, Horne AM, Mason BH, Ames RW, Grey AB, Ruygrok PN, Gamble GD, Reid IR. Relationships between vascular calcification, calcium metabolism, bone density, and fractures. J Bone Miner Res. 2010;25:2501–2509. doi: 10.1002/jbmr.183. [DOI] [PubMed] [Google Scholar]
- 9.Farhat GN, Cauley JA, Matthews KA, Newman AB, Johnston J, Mackey R, Edmundowicz D, Sutton-Tyrrell K. Volumetric BMD and vascular calcification in middle-aged women: the Study of Women's Health Across the Nation. J Bone Miner Res. 2006;21:1839–1846. doi: 10.1359/jbmr.060903. [DOI] [PubMed] [Google Scholar]
- 10.Hyder JA, Allison MA, Wong N, Papa A, Lang TF, Sirlin C, Gapstur SM, Ouyang P, Carr JJ, Criqui MH. Association of coronary artery and aortic calcium with lumbar bone density: the MESA Abdominal Aortic Calcium Study. Am J Epidemiol. 2009;169:186–194. doi: 10.1093/aje/kwn303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwamoto J, Matsumoto H, Takeda T, Sato Y, Uzawa M. A radiographic study on the associations of age and prevalence of vertebral fractures with abdominal aortic calcification in Japanese postmenopausal women and men. J Osteoporos. 2010;2010:748380. doi: 10.4061/2010/748380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flipon E, Liabeuf S, Fardellone P, Mentaverri R, Ryckelynck T, Grados F, Kamel S, Massy ZA, Dargent-Molina P, Brazier M. Is vascular calcification associated with bone mineral density and osteoporotic fractures in ambulatory, elderly women? Osteoporos Int. 2012;23:1533–1539. doi: 10.1007/s00198-011-1762-3. [DOI] [PubMed] [Google Scholar]
- 13.El Maghraoui A, Rezqi A, Mounach A, Achemlal L, Bezza A, Ghozlani I. Relationship between vertebral fracture prevalence and abdominal aortic calcification in men. Rheumatology (Oxford) 2012;51:1714–1720. doi: 10.1093/rheumatology/kes126. [DOI] [PubMed] [Google Scholar]
- 14.Kim KJ, Kim KM, Park KH, Choi HS, Rhee Y, Lee YH, Cha BS, Kim MJ, Oh SM, Brown JK, Lim SK. Aortic calcification and bone metabolism: the relationship between aortic calcification, BMD, vertebral fracture, 25-hydroxyvitamin D, and osteocalcin. Calcif Tissue Int. 2012;91:370–378. doi: 10.1007/s00223-012-9642-1. [DOI] [PubMed] [Google Scholar]
- 15.Szulc P, Samelson EJ, Sornay-Rendu E, Chapurlat R, Kiel DP. Severity of aortic calcification is positively associated with vertebral fracture in older men-a densitometry study in the STRAMBO cohort. Osteoporos Int. 2013;24:1177–1184. doi: 10.1007/s00198-012-2101-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samelson EJ, Cupples LA, Broe KE, Hannan MT, O'Donnell CJ, Kiel DP. Vascular calcification in middle age and long-term risk of hip fracture: the Framingham Study. J Bone Miner Res. 2007;22:1449–1154. doi: 10.1359/jbmr.070519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schuit SC, van der Klift M, Weel AE, de Laet CE, Burger H, Seeman E, Hofman A, Uitterlinden AG, van Leeuwen JP, Pols HA. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone. 2004;34:195–202. doi: 10.1016/j.bone.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Szulc P, Munoz F, Duboeuf F, Marchand F, Delmas PD. Bone mineral density predicts osteoporotic fractures in elderly men: the MINOS study. Osteoporos Int. 2005;16:1184–1192. doi: 10.1007/s00198-005-1970-9. [DOI] [PubMed] [Google Scholar]
- 19.Sornay-Rendu E, Munoz F, Garnero P, Duboeuf F, Delmas PD. Identification of osteopenic women at high risk of fracture: the OFELY study. J Bone Miner Res. 2005;20:1813–1819. doi: 10.1359/JBMR.050609. [DOI] [PubMed] [Google Scholar]
- 20.Bauer DC, Garnero P, Harrison SL, Cauley JA, Eastell R, Ensrud KE, Orwoll E. Biochemicalmarkers of bone turnover, hip bone loss, and fracture in older men: the MrOS study. J Bone Miner Res. 2009;24:2032–2038. doi: 10.1359/JBMR.090526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szulc P, Montella A, Delmas PD. High bone turnover is associated with accelerated bone loss but not with increased fracture risk in men aged 50 and over: the prospective MINOS study. Ann Rheum Dis. 2008;67:1249–1255. doi: 10.1136/ard.2007.077941. [DOI] [PubMed] [Google Scholar]
- 22.Bauer DC, Ewing SK, Cauley JA, Ensrud KE, Cummings SR, Orwoll ES. Quantitative ultrasound predicts hip and non-spine fracture in men: the MrOS study. Osteoporos Int. 2007;18:771–777. doi: 10.1007/s00198-006-0317-5. [DOI] [PubMed] [Google Scholar]
- 23.Black DM, Bouxsein ML, Marshall LM, Cummings SR, Lang TF, Cauley JA, Ensrud KE, Nielson CM, Orwoll ES. Proximal femoral structure and the prediction of hip fracture in men: a large prospective study using QCT. J Bone Miner Res. 2008;23:1326–1333. doi: 10.1359/JBMR.080316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orwoll E, Blank JB, Barrett-Connor E, Cauley J, Cummings S, Ensrud K, Lewis C, Cawthon PM, Marcus R, Marshall LM, McGowan J, Phipps K, Sherman S, Stefanick ML, Stone K. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study—a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–585. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 25.Kauppila LI, Polak JF, Cupples LA, Hannan MT, Kiel DP, Wilson PW. New indices to classify location, severity and progression of calcific lesions in the abdominal aorta: a 25-year follow-up study. Atherosclerosis. 1997;132:245–250. doi: 10.1016/s0021-9150(97)00106-8. [DOI] [PubMed] [Google Scholar]
- 26.Lewis CE, Ewing SK, Taylor BC, Shikany JM, Fink HA, Ensrud KE, Barrett-Connor E, Cummings SR, Orwoll E. Predictors of non-spine fracture in elderly men: the MrOS study. J Bone Miner Res. 2007;22:211–219. doi: 10.1359/jbmr.061017. [DOI] [PubMed] [Google Scholar]
- 27.Mackey DC, Lui LY, Cawthon PM, Bauer DC, Nevitt MC, Cauley JA, Hillier TA, Lewis CE, Barrett-Connor E, Cummings SR. High-trauma fractures and low bone mineral density in older women and men. JAMA. 2007;298:2381–2388. doi: 10.1001/jama.298.20.2381. [DOI] [PubMed] [Google Scholar]
- 28.Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8:1137–1148. doi: 10.1002/jbmr.5650080915. [DOI] [PubMed] [Google Scholar]
- 29.Ensrud KE, Taylor BC, Paudel ML, Cauley JA, Cawthon PM, Cummings SR, Fink HA, Barrett-Connor E, Zmuda JM, Shikany JM, Orwoll ES. Serum 25-hydroxyvitamin D levels and rate of hip bone loss in older men. J Clin Endocrinol Metab. 2009;94:2773–2780. doi: 10.1210/jc.2008-2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, Van Lente F. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53:766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 31.Collins TC, Ewing SK, Diem SJ, Taylor BC, Orwoll ES, Cummings SR, Strotmeyer ES, Ensrud KE. Peripheral arterial disease is associated with higher rates of hip bone loss and increased fracture risk in older men. Circulation. 2009;119:2305–2312. doi: 10.1161/CIRCULATIONAHA.108.820993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.LaCroix AZ, Lee JS, Wu L, Cauley JA, Shlipak MG, Ott SM, Robbins J, Curb JD, Leboff M, Bauer DC, Jackson RD, Kooperberg CL, Cummings SR. Cystatin-C, renal function, and incidence of hip fracture in postmenopausal women. J Am Geriatr Soc. 2008;56:1434–1441. doi: 10.1111/j.1532-5415.2008.01807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dooley AC, Weiss NS, Kestenbaum B. Increased risk of hip fracture among men with CKD. Am J Kidney Dis. 2008;51:38–44. doi: 10.1053/j.ajkd.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 34.Sennerby U, Melhus H, Gedeborg R, Byberg L, Garmo H, Ahlbom A, Pedersen NL, Michaëlsson K. Cardiovascular diseases and risk of hip fracture. JAMA. 2009;302:1666–1673. doi: 10.1001/jama.2009.1463. [DOI] [PubMed] [Google Scholar]
- 35.Sennerby U, Farahmand B, Ahlbom A, Ljunghall S, Michaëlsson K. Cardiovascular diseases and future risk of hip fracture in women. Osteoporos Int. 2007;18:1355–62. doi: 10.1007/s00198-007-0386-0. [DOI] [PubMed] [Google Scholar]
- 36.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. JASA. 1999;94:496–509. [Google Scholar]
- 37.Jamal SA, Leiter RE, Bauer DC. Hyperhomocysteinaemia and aortic calcification are associated with fractures in patients on haemodialysis. QJM. 2005;98:575–579. doi: 10.1093/qjmed/hci092. [DOI] [PubMed] [Google Scholar]
- 38.von Mühlen D, Allison M, Jassal SK, Barrett-Connor E. Peripheral arterial disease and osteoporosis in older adults: the Rancho Bernardo Study. Osteoporos Int. 2009;20:2071–2078. doi: 10.1007/s00198-009-0912-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gerber Y, Melton LJ, 3rd, Weston SA, Roger VL. Osteoporotic fractures and heart failure in the community. Am J Med. 2011;124:418–425. doi: 10.1016/j.amjmed.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tankó LB, Christiansen C, Cox DA, Geiger MJ, McNabb MA, Cummings SR. Relationship between osteoporosis and cardiovascular disease in postmenopausal women. J Bone Miner Res. 2005;20:1912–1920. doi: 10.1359/JBMR.050711. [DOI] [PubMed] [Google Scholar]
- 41.Henry RM, Kostense PJ, Bos G, Dekker JM, Nijpels G, Heine RJ, Bouter LM, Stehouwer CD. Mild renal insufficiency is associated with increased cardiovascular mortality: The Hoorn Study. Kidney Int. 2002;62:1402–1407. doi: 10.1111/j.1523-1755.2002.kid571.x. [DOI] [PubMed] [Google Scholar]
- 42.Sanders NA, Ganguly JA, Jetter TL, Daccarett M, Wasmund SL, Brignole M, Hamdan MH. Atrial fibrillation: an independent risk factor for nonaccidental falls in older patients. Pacing Clin Electrophysiol. 2012;35:973–979. doi: 10.1111/j.1540-8159.2012.03443.x. [DOI] [PubMed] [Google Scholar]
- 43.Gribbin J, Hubbard R, Gladman JR, Smith C, Lewis S. Risk of falls associated with antihypertensive medication: population-based case-control study. Age Ageing. 2010;39:592–597. doi: 10.1093/ageing/afq092. [DOI] [PubMed] [Google Scholar]
- 44.Naves-Díaz M, Cabezas-Rodríguez I, Barrio-Vázquez S, Fernández E, Díaz-López JB, Cannata-Andía JB. Low calcidiol levels and risk of progression of aortic calcification. Osteoporos Int. 2012;23:1177–1182. doi: 10.1007/s00198-011-1550-0. [DOI] [PubMed] [Google Scholar]
- 45.Cauley JA, Parimi N, Ensrud KE, Bauer DC, Cawthon PM, Cummings SR, Hoffman AR, Shikany JM, Barrett-Connor E, Orwoll E. Serum 25-hydroxyvitamin D and the risk of hip and nonspine fractures in older men. J Bone Miner Res. 2010;25:545–553. doi: 10.1359/jbmr.090826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL, Boyle WJ, Simonet WS. osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260–1268. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qin X, Corriere MA, Matrisian LM, Guzman RJ. Matrix metalloproteinase inhibition attenuates aortic calcification. Arterioscler Thromb Vasc Biol. 2006;26:1510–15126. doi: 10.1161/01.ATV.0000225807.76419.a7. [DOI] [PubMed] [Google Scholar]
- 48.Lynch CC. Matrix metalloproteinases as master regulators of the vicious cycle of bone metastasis. Bone. 2011;48:44–53. doi: 10.1016/j.bone.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 49.Cauley JA, Danielson ME, Boudreau RM, Forrest KY, Zmuda JM, Pahor M, Tylavsky FA, Cummings SR, Harris TB, Newman AB. Inflammatory markers and incident fracture risk in older men and women: the Health Aging and Body Composition Study. J Bone Miner Res. 2007;22:1088–1095. doi: 10.1359/jbmr.070409. [DOI] [PubMed] [Google Scholar]
- 50.Shao JS, Aly ZA, Lai CF, Cheng SL, Cai J, Huang E, Behrmann A, Towler DA. Vascular Bmp Msx2 Wnt signaling and oxidative stress in arterial calcification. Ann N Y Acad Sci. 2007;1117:40–50. doi: 10.1196/annals.1402.075. [DOI] [PubMed] [Google Scholar]
- 51.Arseven A, Guralnik JM, O'Brien Kaleba E, Liu K, Chan C, McDermott MM. Does lower-extremity arterial disease predict future falling among older men and women? Angiology. 2007;58:725–733. doi: 10.1177/0003319707303650. [DOI] [PubMed] [Google Scholar]


