Abstract
Although it is now accepted that chronic inflammation plays an essential role in tumorigenesis, the underlying molecular mechanisms linking inflammation and cancer remain to be fully explored. Inflammatory mediators present in the tumor microenvironment, including cytokines and growth factors, as well as reactive oxygen species (ROS) and reactive nitrogen species (RNS), have been implicated in the etiology of inflammation-associated cancers. Epithelial NADPH oxidase (Nox) family proteins, which generate ROS regulated by cytokines, are upregulated during chronic inflammation and cancer. ROS serve as effector molecules participating in host defense or as chemo-attractants recruiting leukocytes to wounds, thereby influencing the inflammatory reaction in damaged tissues. ROS can alter chromosomal DNA, leading to genomic instability, and may serve as signaling molecules that affect tumor cell proliferation, survival, metabolism, angiogenesis, and metastasis. Targeting Noxs and their downstream signaling components may be a promising approach to pre-empting inflammation-related malignancies.
Keywords: NADPH oxidase, Inflammation, Reactive oxygen, Cancer
1. Introduction
As early as 1863, Rudolf Virchow noted leukocyte infiltrates in tumor tissue, suggesting that cancer may arise from chronic inflammation [1]. Epidemiological data indicate that over 25% of all cancers are related to chronic infection and other types of unresolved inflammation [2,3]. Mounting evidence supports the hypothesis that chronic inflammation is an important risk factor for the development of cancer. Table 1 illustrates some examples of infection- and inflammation-associated cancers.
Table 1.
Cancers associated with infection and inflammation.
| Infection/inflammation | Cancer | Reference |
|---|---|---|
| Helicobacter pylori | Gastric cancer | [128] |
| Inflammatory bowel disease | Colorectal cancer | [129] |
| Hepatitis B/C virus | Hepatocellular carcinoma | [130] |
| Prostatitis | Prostate cancer | [131] |
| Pancreatitis | Pancreatic cancer | [132] |
| Human papillomavirus | Cervical cancer | [133] |
| Chronic obstructive pulmonary disease | Lung cancer | [134] |
| Schistosoma haematobium | Bladder cancer | [116] |
Inflammation is a normal host response to tissue damage inflicted by infections or other stimuli. Whereas most pathogens provoke an acute inflammatory response that results in complete clearance of the irritants in a suitable host, inadequate resolution of inflammation and an unchecked inflammatory reaction can evoke chronic inflammation, predisposing the host to various diseases, including cancer. Inflammation contributes to tumor initiation by inducing DNA damage and chromosomal instability. It promotes tumor development by enhancing tumor cell proliferation and resistance to apoptosis. Inflammation also stimulates angiogenesis and tissue remodeling, both of which contribute to tumor cell invasion and metastasis [4–6]. All of these altered biochemical processes are effected by chemical mediators of inflammation present in the tumor microenvironment. Tumors are heterogeneous, complex structural entities in which cancer cells are embedded in an extracellular matrix and vascular network, surrounded by a wide variety of innate and adaptive immune cells, and stromal cells [4,5]. This diverse cell network communicates by means of direct contact or cytokine and chemokine production, and acts in both autocrine and paracrine fashions to govern tumor growth and progression [3,4].
During chronic inflammation, a wide array of intracellular signaling pathways, comprising cell surface receptors, kinases, and transcription factors, are often dysregulated, leading to abnormal expression of pro-inflammatory genes involved in malignant transformation. Inflammation activates a variety of protein kinases, including members of the Janus-activated kinase (JAK), phosphati-dylinosito-3-kinase (PI3K/AKT), and mitogen-activated protein kinase (MAPK) families to alter cellular proliferation. Inflammation-induced aberrant activation of certain transcription factors, such as signal transducer and activator of transcription (STAT) family members, nuclear–factor kappa B (NF-κB), activation protein-1(AP-1), and hypoxia inducible factor-1α (HIF-1α), has also been implicated in tumor growth, angiogenesis, and metastasis [3–5]. This manuscript discusses the link between inflammation and carcinogenesis focusing on mediators involved in oxidative stress.
2. Role of redox-regulated transcription factors in inflammation-associated carcinogenesis
The expression of pro-inflammatory mediators is transcriptionally regulated by a variety of redox-sensitive transcription factors (TFs), including NF-κB, AP-1, STAT1/STAT3, HIFs, and Nrf2. The following section briefly reviews the involvement of these TFs in linking inflammation with cancer.
2.1. NF-κB
NF-κB is a heterodimeric protein mainly composed of p65 and p50 subunits. It is a ubiquitous redox-regulated TF that is retained in the cytoplasm by forming an inactive complex with its cytosolic repressor IkB [7,8]. Oxidative and pro-inflammatory stimuli activate NF-κB through phosphorylation-dependent proteasomal degradation of IkBα, thereby facilitating nuclear accumulation of NF-κB. Upon nuclear localization, NF-κB binds to the kB elements located in the proximal promoter region of genes encoding pro-inflammatory mediators, such as cytokines [9], INOS [10], and COX-2 [11], that are involved in inflammation-associated carcinogenesis. The NF-κB signaling pathway can be activated by pro-inflammatory stimuli (IL-1, TNF-α), viruses, genotoxic stress, the toll-like receptor (TLR)-MyD88 complex, oncogenes in tumor cells, growth factors, and hypoxia and acidic conditions in solid tumors [3,12,13]. NF-κB is constitutively active in most tumors and in chronic inflammatory conditions such as inflammatory bowel disease (IBD) and gastritis [14,15]. NF-κB-targeted gene products include: anti-apoptotic proteins (BCL-2, BCL-XL), inflammatory mediators (TNF-α, IL-6, IL-8, COX-2), effectors of invasion and metastasis (adhesion molecules, matrix metalloproteinases [MMPs]), promoters of DNA damage (reactive oxygen species [ROS], reactive nitrogen species [RNS]), inducers of cell proliferation (c-MYC, cyclin D1), and angiogenic factors (VEGF, angiopoietin) [3,4]. In a colitis-associated cancer model and Mdr2-knockout mice, NF-κB has been shown to play a pivotal role linking inflammation to cancer as well as to cholestatic hepatitis and hepatocellular carcinoma [16,17]. The activation of NF-κB has also been shown to be a critical event in the development of gastric mucosa-associated lymphoid tissue (MALT) lymphoma that is associated with chronic inflammation by Helicobacter pylori [18]. NF-κB-regulated genes play a major role in modulating the level of intracellular ROS; in monocyte and microglial cells NF-κB mediates LPS/IFN-α-induced NADPH oxidase 2 (Nox2) expression [19]. LPS also induces Nox1 expression in mouse macrophages and guinea pig gastric mucosal cells in an NF-κB-dependent manner [20]. Our laboratory found that NF-κB is involved in LPS/IFN-α-induced Dual oxidase (Duox)/DuoxA2 expression in human pancreatic cancer cells [21]. NF-κB also regulates many detoxifying enzymes, such as MnSOD [22], catalase [23], and thioredoxin-1 and thioredoxin-2 [24]. Conversely, ROS can affect the activity of NF-κB in many ways. For example, ROS have been shown to activate NF-κB through alternative IkBα phosphorylation or by direct oxidation of NF-κB, inhibiting DNA binding [23].
2.2. Stat3
STAT3 is a redox-sensitive TF that serves as a molecular switch between inflammation and cancer [25,26]. STAT3 can be activated by phosphorylation of Tyr705 in response to various stimuli, followed by the formation of a STAT3 dimer that translocates to the nucleus and binds to the promoter regions of genes encoding inflammatory and cell cycle regulatory proteins [27,28]. STAT3 supports oncogenesis through mechanisms ranging from activation of genes crucial for proliferation and survival to enhancement of angiogenesis and metastasis [29]. Many cytokines and growth factors activate STAT3, including the IL-6 family, EGF family members, VEGF, IL-23, IL-21, PDGF as well as oncogenic proteins, such as Src and Ras. Activated nuclear STAT3 has been detected in many forms of malignancy, including breast, colon, gastric, lung, head and neck, skin, and prostate cancer [26,29]. Myeloid cell-derived IL-6 can enhance the proliferation of tumor-initiating cells and can protect normal and premalignant intestinal epithelial cells from apoptosis in STAT3-dependent manner, promoting colitis-associated cancer progression [30]. Obesity can increase IL-6 and TNF-α production, which causes hepatic inflammation and activates STAT3 to promote hepatocellular carcinoma [31]. STAT3 is redox-sensitive, and ROS scavengers and inhibitors of Nox generally inhibit STAT3 activity. Oxidation of a conserved cysteine in the DNA-binding domain as well as C-terminal transactivation domains of STAT3 by H2O2 blocks binding to consensus serum-inducible elements [32,33].
2.3. HIFs
Hypoxia inducible factors are members of the bHLH-PAS family of proteins that bind to canonical DNA sequences (hypoxia responsive elements, or HREs) in the promoter or enhancer of target genes [34]. They consist of an O2-labile α subunit and a constitutively-expressed α subunit. Hydroxylation of two conserved proline residues within the O2-dependent degradation domain of the α subunit catalyzed by proline hydroxylases (PHDs) under normal conditions mediates HIF-1a degradation via the 26S proteasome [35]. When stabilized under hypoxia, HIF-1α translocates to the nucleus, dimerizes with HIF-1α, recruits co-activators CBP/P300, and binds to the HREs, driving gene transcription involved in adaptation to hypoxic stress [36]. At least 150 genes encoding proteins that regulate metabolism, survival, motility, angiogenesis, hematopoiesis, and other functions have been identified as being HIF-regulated [37,38]. Elevated expression of HIF-1α was detected in the colonic epithelium of patients with IBD as well as colorectal tumors [39]. Overexpression of HIF-1α (59.2%) is frequently detected in human pancreatic carcinoma, whereas it is almost absent in normal pancreatic tissue. Moreover, HIF-1α expression is significantly associated with tumor size and microvessel density [40]. HIF-1α is regulated by various inflammatory mediators (TNF-α, IL-1α, TGF-α) and growth factors (EGF) in addition to oxygen [41–44]. Nox-derived ROS have been shown to regulate HIF-1α stability through inhibition of PHD activity by oxidation of Fe2+ to Fe3+, decreasing the availability of Fe2+, the cofactor for PHDs [45]. ROS can also activate ERK, p38, and PI3K/AKT pathways increasing HIF-1α synthesis under both normoxic or hypoxic conditions [46]. HIF-1α also modulates macrophage inflammatory responses by regulating important inflammatory cytokines, including CXCR4, VEGF, and IL-8 [37].
3. Role of ROS and NADPH oxidases in inflammation, tumor initiation, promotion, and progression
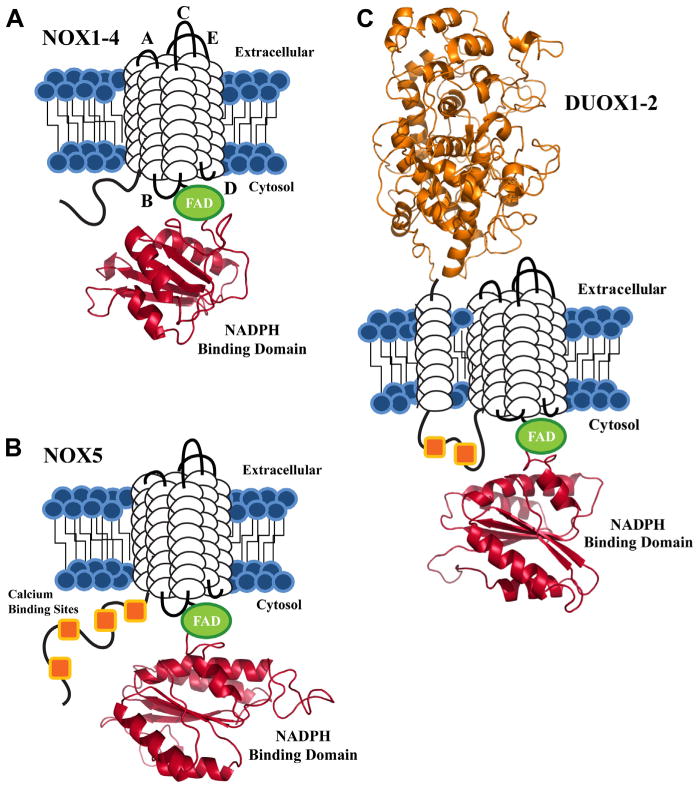
ROS, including superoxide ( ), H2O2, and the hydroxyl radical, are produced by the partial reduction of oxygen. Under physiological conditions, formation of ROS is counterbalanced by endogenous antioxidant defense systems including superoxide dismutase, glutathione peroxidase, catalase, peroxiredoxins, and thioredoxin. When ROS production exceeds cellular antioxidant capacity, oxidative stress can damage DNA, proteins, and lipids [47–49]. The phagocyte NADPH oxidase, Nox2, was the first identified enzyme that generates ROS as its primary function [50]. NADPH oxidase (Nox)/dual oxidase (Duox) membrane proteins catalyze the purposeful generation of ROS in non-phagocytic cells, including vascular endothelium and tumor cells [51]. The Nox family, comprised of seven enzymatic isoforms, produces ROS by the NADPH-dependent, one-electron reduction of oxygen to superoxide. Isoform-specific conversion of superoxide to hydrogen peroxide is unique to Nox4 and the Duoxs. Structurally, each Nox/Duox protein associates with the plasma membrane through 6 transmembrane (TM) helices and allows for NADPH oxidation through a C-terminal FAD/NADPH oxidase domain (Fig. 1). The Nox5 isoform varies from its Nox counterparts by an amino-terminal EF-calcium binding region. A Duox-specific extracellular peroxidase-like domain and two cytosolic calcium binding sites define the structural characteristics unique to the two Duox family members [52–55].
Fig. 1.
Schematic view of the conserved structural features of the NADPH oxidase proteins. Each isoform contains 6 putative TM domains (white cylindrical loops), with C-terminal FAD (green) and NADPH binding domains (red). The NADPH binding domain structural models were created by the SWISS-MODEL program server with hNOX2 (PDB: 3A1F) as the template, visualized by Pymol software. (A) NOX1-4 are depicted with loop regions labeled based on established designations: extracellular loops A, C, E and intracellular loops B and D. (B) The NOX5 isoform shares the same structural motif as NOX1-4, with a novel N-terminal calcium binding region, composed of 4 EF-hand calcium binding sites (orange squares). (C) DUOX1-2 are unique to the NADPH oxidase family, as both isoforms contain an extracellular N-terminal peroxidase homology domain (orange) and two cytosolic calcium binding sites.
3.1. Noxs and inflammation
Oxidative stress plays a critical role in modulating the immune response to inflammatory stimuli. Recent evidence suggests that the source of at least some of the ROS that accompany acute and chronic inflammation in many organs is one or more members of the Nox family [56,57]. Nox2 has been identified as a primary component of the microbicidal oxidase system of phagocytes; its deficiency is associated with chronic granulomatous disease [50]. Nox1 is expressed in both normal and malignant colonic tissue [58]. Nox1-induced O2•− at the luminal surface of the colon has been suggested to enhance host defense [59]. Duox2 is found in bronchial epithelium and throughout the gastrointestinal tract [60,61]. In airway mucosal cells, Duox2 plays an important role in the generation of H2O2 for host defense against a variety of pathogens [62,63]. Duox2-induced ROS are indispensible for gut antimicrobial activities in drosophila [64]. An epithelial cell Duox2-derived H2O2 gradient at the site of wounds mediates leukocyte recruitment [65]. Duox2 expression is significantly increased in patients with IBD compared to healthy control subjects [66]. Many pro-inflammatory mediators, such as cytokines and growth factors, can induce the expression or regulate the activity of Nox isoforms [67–70], as outlined in Table 2. Inflammatory response-related TFs, such as STAT1, IRF1, GATA-1 (for Nox2) [71,72], GATA-6 (for Nox1) [73,74], NF-κB (for Nox1, Nox2, and Duox2) [21,67,75], are involved in regulating Nox family member expression. Hypoxia, common both in inflammation and solid tumors, can also induce Nox expression. The human Nox4 and Nox2 promoters have putative HREs to which HIFs bind under hypoxic conditions. Both Nox4 and Nox2 mRNAs are enhanced in vitro and in vivo in response to HIF stabilization [76–78]. We also found that Nox1 expression across a panel of 60 human tumor cell lines correlates significantly with certain inflammatory and immune response pathways, suggesting that Nox1-mediated ROS production is involved in inflammation [79]. Hepatitis C virus induces Nox1 and Nox4 expression in hepatocytes in vitro and in the human liver. These Nox proteins have been implicated as persistent, endogenous ROS generators that might contribute to hepatitis C virus-related pathologies [80].
Table 2.
Cytokines and growth factors involved in Nox expression and regulation of activity.
| Nox | Cytokines | Growth factors | References |
|---|---|---|---|
| Nox1 | IL-13, TGF-β, IFN-γ, TNF-α | EGF, PDGF, phorbol myristate acetate (PMA) | [135–139] |
| Nox2 | IL-1β, IFN-γ, TGF-β, TGF-α | EGF, PDGF, PMA | [111,135,140,141] |
| Nox3 | |||
| Nox4 | TGF-β, TNF-α, IL-13 | EGF, IGF-1, PDGF, PMA | [101,135,136,142,143] |
| Nox5 | PMA | [144] | |
| Duox1 | IL-4, IL-13 | PMA | [145–148] |
| Duox2 | IL-1α, IL-4, IL-13, IFN-γ | PMA | [21,149–151] |
3.2. Nox-mediated oxidative DNA damage in inflammation and tumorigenesis
Recent studies implicate Nox-mediated ROS as a modulator of the inflammatory response and DNA damage in the context of tumorigenesis. A direct link between enhanced ROS production by Nox homologues leading to oxidative DNA damage and genetic instability was demonstrated when overexpression of the human Nox1 complex increased steady-state levels of DNA 8-oxo-7,8-dihydroguanine and caused a threefold increase in the HPRT mutation rate in HeLa cells [81]. Nox-mediated tumorigenesis was observed when rats administered testosterone and 17β-estradiol developed dysplasia and stromal inflammation of the lateral lobe of the prostate [82]. The inflammatory stroma showed an increased expression of Nox1, 2, and 4, and an accumulation of 8-hydroxy-2′-deoxyguanosine, and 4-hydroxy-2-nonenal (HNE)-modified protein adducts [82]. These oxidative by-products are indicative of hormone-induced, oxidative stress-related inflammation and DNA damage potentially underlying the dysplastic transformation of prostate epithelial cells. Our laboratory found that both IFN-γ and LPS can synergistically induce Duox2/DuoxA2 expression in human pancreatic cancer cell lines, which leads to increased ROS production, and DNA damage [21]. In patients with chronic pancreatitis and pancreatic cancer, Duox2 expression is significantly increased, suggesting that Duox2- mediated ROS may play an important role both for host defense and in the development of cancer [83]. Nox1- and Nox4-mediated, ROS-induced genomic instability has been reported for Ras oncogene-related DNA damage and senescence [84–86]. Up-regulation of Nox expression at sites of inflammation and DNA damage and recent reports of DNA damage activating the Nox1-Rac1 pathway [87], strongly suggest that Nox-mediated oxidative stress may be involved in nuclear signaling, DNA damage, and subsequently, the fate of the cell [88]. The detection of a functional and nuclear-localized splice variant of Nox4 (Nox4D) whose overexpression induced DNA damage further supports this concept. By modulating genomic integrity, Nox-dependent ROS formation increases the likelihood of developing tumor-initiating cells with the potential to give rise to pre-neoplastic lesions, and eventually to neoplasia.
3.3. Nox-derived ROS in tumor promotion
Tumor cells produce significant amounts of ROS [51], derived in part from Nox isoforms. Although high concentrations of ROS are toxic, optimal ROS levels promote cell proliferation [89,90]. Nox-derived ROS increase growth factor-mediated tyrosine auto-phosphorylation by inactivating protein tyrosine phosphatases (PTPs), leading to hyper-phosphorylation and activation of receptor tyrosine kinases (RTKs) and other downstream kinases. The cysteine residues within the catalytic domain of PTPs are highly susceptible to H2O2-induced oxidation and inactivation [91–93]. In HepG2 and A431 human cancer cells, high levels of intrinsic ROS can inactivate PTP1B and regulate cell proliferation, contributing to the transformed phenotype [94]. Nox1 is highly expressed in colon cancer; stable knockdown of Nox1 expression in HT-29 human colon cancer cells results in decreased ROS levels, and a corresponding increase in phosphatase activity, which in turn inhibits MAPK signaling. Decreased MAPK activity is associated with significant inhibition of cyclin D1 and a G1/S block in the cell cycle. Decreased Nox1 expression also contributes to a significantly diminished growth rate and blood vessel density in HT-29 xenografts [152]. We found that diphenylene iodonium (DPI) also decreases ROS production and inhibits the growth of Caco2 and HT-29 cells at concentrations of 10–250 nM. The decreased tumor cell proliferation was caused by a profound block in cell cycle progression at the G1/S interface, accompanying p27 up-regulation and deceases in cyclin D1, A, and E expression. DPI also significantly decreased the growth of both HT-29 and LS-174T human tumor xenografts [95]. Nox1 has additionally been reported to mediate cell survival through NAD-dependent histone deacetylase sirtuin 1 activation, which inactivates p53, ultimately impairing p53-mediated transcriptional induction of pro-apoptotic proteins, such as Bax [96,97]. Further, activation of the Wnt-β-catenin signaling pathway by Nox1-derived ROS promotes cell proliferation by oxidizing and inactivating nucleoredoxin (NRX), a redox-sensitive, regulatory protein modulating Wnt-β-catenin signaling [98].
3.4. Role of Nox-derived ROS in angiogenesis
Angiogenesis is necessary for solid tumor growth past a diameter of ~1–2 mm. Compartmentalized, Nox-derived ROS appear to coordinate the angiogenic switch by increasing VEGF production by tumor cells. These processes are initiated when a tumor experiences hypoxia [48]. However, under normoxic conditions, both Nox4 and mitochondrial-derived ROS are involved in enhancing HIF-1α and VEGF expression in ovarian cancer cells [99]. In Ras-transformed colon cancer cells, Nox1-derived ROS increase ERK-dependent phosphorylation of the transcription factor SP1, which is responsible for the up-regulation of VEGF mRNA and neo-vascularization in nude mice [100]. Our laboratory has found that Nox1-derived ROS are essential for angiogenesis in vivo; stable knockdown of Nox1 expression in HT-29 colon cancer cells significantly decreases VEGF expression and blood vessel density in HT-29 xenografts [152].
3.5. A role for Noxs in tumor cell invasion and metastasis
Metastasis is a crucial aspect of tumorigenesis; 90% of cancer mortality is caused by metastasis [4]. It is a multistep process that requires intracellular changes such as epithelial to mesenchymal transition (EMT), extracellular matrix degradation, reduced cell adhesion, and increased migration [48]. Noxs can influence tumor cell migration and invasion in response to stimulation by soluble mediators present in the tumor microenvironment. TGF-β induces Nox4 expression and ROS generation in human breast epithelial cells; Nox4 derived ROS may be critical for the progression of the EMT in the breast epithelium [101]. Protein localization studies showed that Nox1 and Nox4 often co-localize with Src and the Src family of kinases in invasive microdomains, such as invadipodia and focal adhesions, where they form an active redox signaling platform to coordinate cell adhesion, migration, and invasion [102–106]. LPS induces ROS production in colon cancer cells via NF-κB dependent up-regulation of Nox1 and Nox2 protein expression. The LPS-Nox1 redox signaling axis also plays a crucial role in facilitation of colon cancer cell adhesion and metastasis [107].
4. Involvement of epithelial and stromal cells in oxidative stress-driven tumor progression
Many solid tumors exist in an oxidative milieu, resulting from an increased basal metabolic activity of the tumor cells, mitochondrial dysfunction due to a hypoxic tumor microenvironment, uncontrolled oncogene-dependent growth factor and/or cytokine signaling, as well as enhanced ROS generation as a consequence of upregulated expression of Noxs, COXs, and lipoxygenases (LOXs) [108]. Decreased expression of certain antioxidant enzymes (catalase, glutathione peroxidase, and/or superoxide dismutase) in cancer cells also may contribute to oxidative stress [109]. Oxidative stress can influence both cancer and stromal cells. For tumor cells, ROS can: (1) result in DNA damage and genomic instability that promotes tumor initiation and progression, (2) modulate intracellular signaling pathways controlling cell proliferation and survival, (3) influence cell motility, invasiveness, angiogenesis, and metastasis, and (4) mediate tumor cell resistance to many anticancer drugs [4,108].
Besides acting directly on tumor cells, ROS have also been shown to have profound effects on stromal cells in the tumor microenvironment, particularly on cancer-associated fibroblasts (CAFs). CAFs originate either from resident tissue fibroblasts which infiltrate growing tumors, or from bone marrow mesenchymal stem cells. These fibroblasts need to be activated through a process called mesenchymal–mesenchymal transition (MMT), converting them into myofibroblasts or CAFs, contractile cells able to affect tumor progression through the secretion of cytokines and the deposition of extracellular matrix [108].
Pro-inflammatory cells of the tumor microenvironment play important roles in the development of several different tumor histologies. Sustained activation of pancreatic stellate cells (PaSCs) is responsible for the fibrotic process associated with both chronic pancreatitis and pancreatic cancer [110]. Both exogenous ROS released by damaged pancreatic acinar cells or leukocytes recruited in response to pancreatic injury, and endogenous ROS derived from PaSCs and Nox activity in pancreatic duct cells have been implicated in the regulation of PaSC proliferation, chemokine production, and expression of α-smooth muscle actin (α-SMA) and collagen [110,111]. In a tumor-stroma model of skin carcinogenesis, TGF-α1 initiates ROS-dependent, myofibroblast transdifferentiation. ROS modulate protein kinase C activity and the secretion of HGF, IL-6, and VEGF, ultimately enhancing the invasive capacity of the skin tumor [112].
Mutations in the BRCA1 (breast cancer type 1 susceptibility) gene strongly predispose toward the development of breast and ovarian cancers. Inactivation of BRCA1 induces high levels of oxidative stress. BRCA1-deficient epithelial cancer cells produce large amounts of H2O2, which induces oxidative stress and glycolysis in CAFs. These CAFs then provide lactate to epithelial cancer cells for oxidative mitochondrial metabolism. Importantly, this metabolic symbiosis phenotype is reversed by genetic replacement of the wild type BRCA1 gene in epithelial cancer cells, or by the administration of the antioxidant n-acetyl cysteine (NAC) [113]. In another oxidative stress-based model of tumor-stromal co-evolution, breast cancer cells induce a loss of caveolin-1 in adjacent fibroblasts, which triggers nitric oxide production, mitochondrial dysfunction, and oxidative stress in fibroblasts. Oxidative stress in fibroblasts then promotes further DNA damage and genetic instability in cancer cells, by way of a bystander effect. Thus, oxidative interactions between breast cancer cells and fibroblasts lead to a more aggressive phenotype (mutagenic evolution) [114].
5. Strategies to overcome chronic inflammation-associated cancer
The prevalence of cancer related to chronic infection has been estimated to be 1.9 million cases per year, or 17.8% of the global cancer burden [115]. Hence, development of effective therapies to prevent the adverse effects of cancer-causing pathogens, such as the successful vaccines against hepatitis B virus (HBV) and human papilloma virus (HPV), will contribute significantly to decreasing morbidity from hepatocellular and cervical carcinoma, respectively [116]. In like manner, eradication of chronic H. pylori infection with antimicrobials is likely to reduce the incidence of gastric cancer [18,116]. For each of these infectious diseases, chronic inflammation plays a role in the pathological basis for tumor development; accordingly, smoldering inflammation has been proposed as the 7th hallmark of cancer.
Recent preclinical studies have suggested that early detection and timely treatment of inflammatory stress might be a useful strategy to interdict the carcinogenic process in patients suffering from chronic inflammation of the prostate or pancreas that is not clearly related to an infectious pathogen [117]. Well known anti-inflammatory drugs, such as aspirin, non-steroidal anti-inflammatory agents (NSAIDs), cyclo-oxygenase-2 (COX-2) inhibitors (e. g. celecoxib), and glucocorticoids (e.g. dexamethasone) have all been demonstrated to reduce tumor incidence or progression and reduce mortality when employed in a variety of preclinical model systems, as well as in man [4,6,116].
5.1. Tumor-promoting inflammation: therapeutic targets
Chemical mediators of inflammation, including a wide variety of cytokines, are present in the tumor microenvironment. Chemokines, growth factors, COX-2, and prostaglandins are the critical mediators of chronic inflammation-associated cancer. Strategies to specifically inhibit the production and/or function of inflammatory mediators are actively being investigated for their therapeutic activity. For example, lenalidomide, a structural analogue of thalidomide, which suppresses the production of several inflammatory cytokines without exhibiting marked cytotoxic activity against tumor cells in vitro, has substantial therapeutic efficacy in patients with advanced multiple myeloma when combined with dexamethasone [118]. Phase I/II trials of antagonists of IL-6, IL-6 receptor, CCL2, CCR4, and CXCR4 for several epithelial and hematopoietic malignancies are ongoing [6]. Clinical trials of TNF-α antagonists in patients with advanced cancer have also demonstrated therapeutic benefit [119,120], as have agents blocking IL1α and its receptor [121].
Constitutively-activated transcription factors, such as NF-κB, STAT3, HIF-1α, and AP-1, induce the expression of genes that stimulate tumor cell proliferation and survival. Among these, STAT3 has emerged as a critical regulator of tumor-associated inflammation, and it is a promising target for inflammation-associated cancer therapy. In addition to the well-known promotion of tumor cell proliferation and survival, angiogenesis, and invasion by STAT3, activated STAT3 can negatively regulate Th1-type immune responses and promote expansion of myeloid-derived suppressor cells (MDSCs) and regulatory T-cell functions in the tumor micro-environment, restraining anti-tumor immune responses. Thus, targeting STAT3 has the potential to not only inhibit tumor growth directly, but also to alter the immunological environment so as to favor control of tumor proliferation. Approaches currently under study to inhibit STAT3 activation include targeted delivery of STAT3 siRNAs into immune cells, the systemic delivery of STAT3 antisense molecules targeting tumor cells, as well as the development of small molecule inhibitors directed against both upstream and downstream mediators of the STAT3 pathway [29,122].
Oxidative stress plays an important role in both chronic inflammation and carcinogenesis. Considerable effort has been directed toward the development of therapeutics for reactive oxygen species (ROS)-related diseases [123]; these efforts have focused on suppressing ROS generation using small molecule antioxidants, as well enzyme mimetics of the antioxidant proteins superoxide dismutase and catalase [124]. The ROS scavenger NAC has been shown to slow tumor progression in a p53-dependent mouse lymphomagenesis model by reducing ROS-mediated genomic instability [125]. HIF-1α-dependent antitumor effects of NAC and vitamin C in a Myc-dependent mouse tumor model have also been reported [126]. The Nox inhibitors diphenylene iodonium and 2-di-thienyl iodonium have been shown to decrease the growth of both HT-29 and LS-174T human colon cancer xenografts in vivo [95]. The Nox4 inhibitor fulvene-5 potently inhibits Nox4 and blocks the growth of endothelial tumors in mice [127]. Thus, there is considerable preclinical evidence that modulation of oxidant stress levels in tumor cells can alter their proliferative potential; verification of these approaches in human malignancies awaits future clinical investigation.
6. Summary
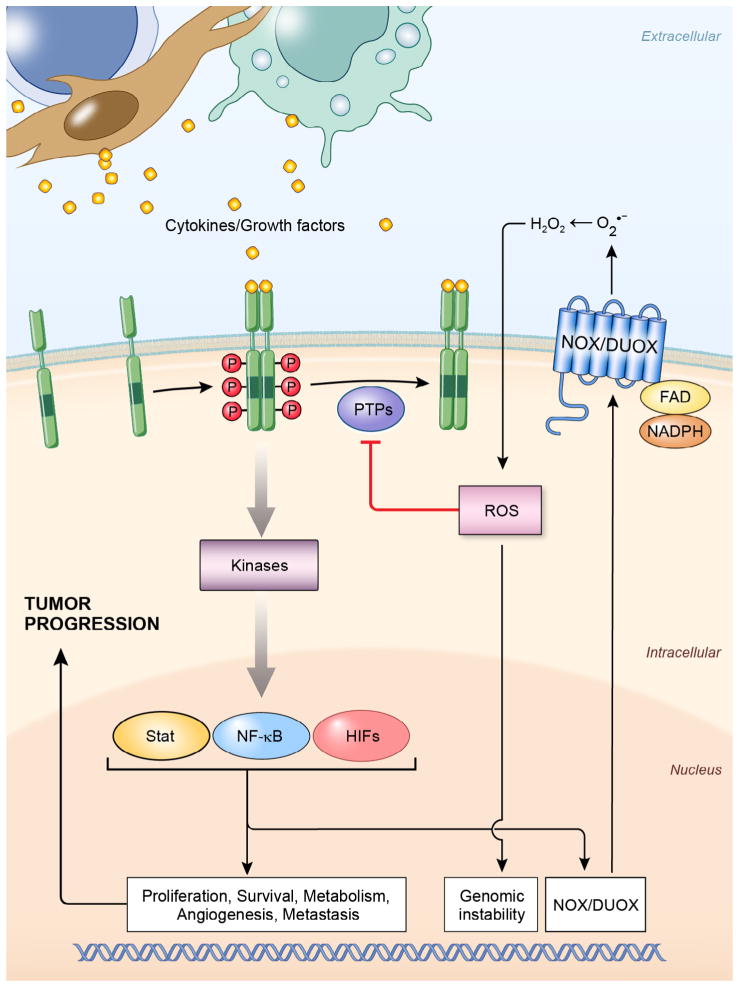
Chronic inflammation creates a microenvironment in which cancer cells, various immune cells, and stromal cells coexist. These diverse cellular species communicate by secreting inflammatory mediators such as cytokines and growth factors. As illustrated in Fig. 2, cytokines or growth factors can exert their effect on premalignant cells directly, by engaging their cognate receptors, thereby activating downstream kinases and TFs to regulate an array of genes involved in cell proliferation, survival, metabolism, angiogenesis, and metastasis. All of these events lead to enhanced tumor growth. Cytokines and growth factors can also upregulate the expression and enhance the activity of Nox family members in epithelial cells, increasing ROS production in the tumor microenvironment, which damages genomic DNA and may produce mutational hits that can initiate tumor formation in a chronic inflammatory background. ROS additionally can propagate cytokine and growth factor signals via oxidation of redox-sensitive cysteine residues located in the catalytic domains of PTPs, or by directly targeting cysteine residues in transcription factors. Considering the essential role of cytokines, growth factors, and ROS generating Nox enzymes in tumor initiation, promotion, and progression, targeting each of these components may provide a novel strategy to fight both chronic inflammation and cancer.
Fig. 2.
Role of cytokines, growth factors, and Noxs in tumor initiation, promotion, and progression. Schematic representation showing cytokines and growth factors are secreted by inflammatory cells and stromal cells in the tumor microenvironment during chronic inflammation; they engage their cognate receptors to activate downstream kinases and transcription factors such as NF-κB, STAT, and HIFs. These TFs can up-regulate the expression, and enhance the activity of Noxs. They can also regulate target genes involved in cell cycle control, apoptosis, metabolism, angiogenesis, and metastasis. On the other hand, Nox-derived ROS can oxidize redox-sensitive cysteine residues in the catalytic domain of PTPs and inactivate their ability to limit the propagation of cytokine-derived signals. ROS can also directly oxidize the cysteine residues in some TFs and regulate their transcriptional activity. [PTP, protein tyrosine phosphatase; ROS, reactive oxygen species; HIFs, hypoxia-inducible factors; Noxs, NADPH oxidases].
Acknowledgments
This work was supported by federal funds from the Center for Cancer Research and the Division of Cancer Treatment and Diagnosis, National Cancer Institute, National Institutes of Health. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services.
Abbreviations
- ROS
reactive oxygen species
- Nox
NADPH oxidase
- Duox
dual oxidase
- RNS
reactive nitrogen species
- MMP
matrix metalloproteinase
- VEGF
vascular endothelial growth factor
- COX-2
Cyclooxygenase 2
- IBD
inflammatory bowel disease
- SOCS1
suppressor of cytokine signaling-1
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- STAT1
signal transducers and activators of transcription
- HIF1
hypoxia-inducible factors
- Nrf2
nuclear factor erthroid-2 related factor
- INOS
Inducible Nitric Oxide Synthase
- LPS
lipopolysaccharide
- PHD
proline hydroxylase
- PTP
protein tyrosine phosphatase
- RTK
receptor tyrosine kinase
- DPI
diphenylene iodonium
- DTI
di-2-thienyliodonium chloride
- EMT
epithelial to mesenchymal transition
- PMA
phorbol 12-myristate 13-acetate
- Ils
interleukins
- EGF
epidermal growth factor
- PDGF
platelet-derived growth factor
- IGF-1
insulin-like growth factor 1
- TGF-β
transforming growth factor beta
Footnotes
7. Conflict of Interest
None.
References
- 1.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 2.Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer. 2007;121:2373–2380. doi: 10.1002/ijc.23173. [DOI] [PubMed] [Google Scholar]
- 3.Vendramini-Costa DB, Carvalho JE. Molecular link mechanisms between inflammation and cancer. Curr Pharm Des. 2012;18:3831–3852. doi: 10.2174/138161212802083707. [DOI] [PubMed] [Google Scholar]
- 4.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kundu JK, Surh YJ. Emerging avenues linking inflammation and cancer. Free Rad Biol Med. 2012;52:2013–2037. doi: 10.1016/j.freeradbiomed.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 6.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 7.Gilmore TD. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene. 2006;25:6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- 8.Karin M. How NF-kappaB is activated: the role of the IkappaB kinase (IKK) complex. Oncogene. 1999;18:6867–6874. doi: 10.1038/sj.onc.1203219. [DOI] [PubMed] [Google Scholar]
- 9.Gupta SC, Kim JH, Kannappan R, Reuter S, Dougherty PM, Aggarwal BB. Role of nuclear factor kappaB-mediated inflammatory pathways in cancer-related symptoms and their regulation by nutritional agents. Exp Biol Med (Maywood) 2011;236:658–671. doi: 10.1258/ebm.2011.011028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie QW, Kashiwabara Y, Nathan C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J Biol Chem. 1994;269:4705–4708. [PubMed] [Google Scholar]
- 11.Newton R, Kuitert LM, Bergmann M, Adcock IM, Barnes PJ. Evidence for involvement of NF-kappaB in the transcriptional control of COX-2 gene expression by IL-1beta. Biochem Biophys Res Commun. 1997;237:28–32. doi: 10.1006/bbrc.1997.7064. [DOI] [PubMed] [Google Scholar]
- 12.Aggarwal BB, Vijayalekshmi RV, Sung B. Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin Cancer Res. 2009;15:425–430. doi: 10.1158/1078-0432.CCR-08-0149. [DOI] [PubMed] [Google Scholar]
- 13.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 14.Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-kappaB as the matchmaker. Nat Immunol. 2011;12:715–723. doi: 10.1038/ni.2060. [DOI] [PubMed] [Google Scholar]
- 15.Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 17.Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 18.Sagaert X, van CE, De HG, Geboes K, Tousseyn T. Gastric MALT lymphoma: a model of chronic inflammation-induced tumor development. Nat Rev Gastroenterol Hepatol. 2010;7:336–346. doi: 10.1038/nrgastro.2010.58. [DOI] [PubMed] [Google Scholar]
- 19.Cassatella MA, Bazzoni F, Flynn RM, Dusi S, Trinchieri G, Rossi F. Molecular basis of interferon-gamma and lipopolysaccharide enhancement of phagocyte respiratory burst capability. Studies on the gene expression of several NADPH oxidase components. J Biol Chem. 1990;265:20241–20246. [PubMed] [Google Scholar]
- 20.Kawahara T, Kohjima M, Kuwano Y, Mino H, Teshima-Kondo S, Takeya R, Tsunawaki S, Wada A, Sumimoto H, Rokutan K. Helicobacter pylori lipopolysaccharide activates Rac1 and transcription of NADPH oxidase Nox1 and its organizer NOXO1 in guinea pig gastric mucosal cells. Am J Physiol Cell Physiol. 2005;288:C450–C457. doi: 10.1152/ajpcell.00319.2004. [DOI] [PubMed] [Google Scholar]
- 21.Wu Y, Lu J, Antony S, Juhasz A, Liu H, Jiang G, Meitzler JL, Hollingshead M, Haines DC, Butcher D, Roy K, Doroshow JH. Activation of TLR4 is required for the synergistic induction of dual oxidase 2 and dual oxidase A2 by IFN-gamma and lipopolysaccharide in human pancreatic cancer cell lines. J Immunol. 2013;190:1859–1872. doi: 10.4049/jimmunol.1201725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rojo AI, Salinas M, Martin D, Perona R, Cuadrado A. Regulation of Cu/Zn-superoxide dismutase expression via the phosphatidylinositol 3 kinase/Akt pathway and nuclear factor-kappaB. J Neurosci. 2004;24:7324–7334. doi: 10.1523/JNEUROSCI.2111-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 2011;21:103–115. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schieven GL, Kirihara JM, Myers DE, Ledbetter JA, Uckun FM. Reactive oxygen intermediates activate NF-kappa B in a tyrosine kinase-dependent mechanism and in combination with vanadate activate the p56lck and p59fyn tyrosine kinases in human lymphocytes. Blood. 1993;82:1212–1220. [PubMed] [Google Scholar]
- 25.Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 26.Grivennikov SI, Karin M. Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 2010;21:11–19. doi: 10.1016/j.cytogfr.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhong Z, Wen Z, Darnell JE., Jr Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264:95–98. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
- 29.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 30.Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, Karin M. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, Osterreicher CH, Takahashi H, Karin M. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li L, Cheung SH, Evans EL, Shaw PE. Modulation of gene expression and tumor cell growth by redox modification of STAT3. Cancer Res. 2010;70:8222–8232. doi: 10.1158/0008-5472.CAN-10-0894. [DOI] [PubMed] [Google Scholar]
- 33.Simon AR, Rai U, Fanburg BL, Cochran BH. Activation of the JAK-STAT pathway by reactive oxygen species. Am J Physiol. 1998;275:C1640–C1652. doi: 10.1152/ajpcell.1998.275.6.C1640. [DOI] [PubMed] [Google Scholar]
- 34.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG., Jr HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 36.Ema M, Hirota K, Mimura J, Abe H, Yodoi J, Sogawa K, Poellinger L, Fujii-Kuriyama Y. Molecular mechanisms of transcription activation by HLF and HIF1alpha in response to hypoxia: their stabilization and redox signal-induced interaction with CBP/p300. EMBO J. 1999;18:1905–1914. doi: 10.1093/emboj/18.7.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Imtiyaz HZ, Simon MC. Hypoxia-inducible factors as essential regulators of inflammation. Curr Top Microbiol Immunol. 2010;345:105–120. doi: 10.1007/82_2010_74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell. 2007;129:465–472. doi: 10.1016/j.cell.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mariani F, Sena P, Marzona L, Riccio M, Fano R, Manni P, Gregorio CD, Pezzi A, Leon MP, Monni S, Pol AD, Roncucci L. Cyclooxygenase-2 and hypoxia-inducible factor-1alpha protein expression is related to inflammation, and up-regulated since the early steps of colorectal carcinogenesis. Cancer Lett. 2009;279:221–229. doi: 10.1016/j.canlet.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 40.Kitada T, Seki S, Sakaguchi H, Sawada T, Hirakawa K, Wakasa K. Clinicopathological significance of hypoxia-inducible factor-1alpha expression in human pancreatic carcinoma. Histopathology. 2003;43:550–555. doi: 10.1111/j.1365-2559.2003.01733.x. [DOI] [PubMed] [Google Scholar]
- 41.Hellwig-Burgel T, Rutkowski K, Metzen E, Fandrey J, Jelkmann W. Interleukin-1beta and tumor necrosis factor-alpha stimulate DNA binding of hypoxia-inducible factor-1. Blood. 1999;94:1561–1567. [PubMed] [Google Scholar]
- 42.Kuo HP, Lee DF, Xia W, Wei Y, Hung MC. TNFalpha induces HIF-1alpha expression through activation of IKKbeta. Biochem Biophys Res Commun. 2009;389:640–644. doi: 10.1016/j.bbrc.2009.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sodhi A, Montaner S, Miyazaki H, Gutkind JS. MAPK and Akt act cooperatively but independently on hypoxia inducible factor-1alpha in rasV12 upregulation of VEGF. Biochem Biophys Res Commun. 2001;287:292–300. doi: 10.1006/bbrc.2001.5532. [DOI] [PubMed] [Google Scholar]
- 44.Zhong H, Chiles K, Feldser D, Laughner E, Hanrahan C, Georgescu MM, Simons JW, Semenza GL. Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 2000;60:1541–1545. [PubMed] [Google Scholar]
- 45.Gerald D, Berra E, Frapart YM, Chan DA, Giaccia AJ, Mansuy D, Pouyssegur J, Yaniv M, Mechta-Grigoriou F. Jun D reduces tumor angiogenesis by protecting cells from oxidative stress. Cell. 2004;118:781–794. doi: 10.1016/j.cell.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 46.Kietzmann T, Gorlach A. Reactive oxygen species in the control of hypoxia-inducible factor-mediated gene expression. Semin Cell Dev Biol. 2005;16:474–486. doi: 10.1016/j.semcdb.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 47.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 48.Block K, Gorin Y. Aiding and abetting roles of NOX oxidases in cellular transformation. Nat Rev Cancer. 2012;12:627–637. doi: 10.1038/nrc3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weyemi U, Redon CE, Parekh PR, Dupuy C, Bonner WM. NADPH oxidases NOXs and DUOXs as putative targets for cancer therapy. Anticancer Agents Med Chem. 2013;13:502–514. [PMC free article] [PubMed] [Google Scholar]
- 50.Royer-Pokora B, Kunkel LM, Monaco AP, Goff SC, Newburger PE, Baehner RL, Cole FS, Curnutte JT, Orkin SH. Cloning the gene for an inherited human disorder – chronic granulomatous disease – on the basis of its chromosomal location. Nature. 1986;322:32–38. doi: 10.1038/322032a0. [DOI] [PubMed] [Google Scholar]
- 51.Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794–798. [PubMed] [Google Scholar]
- 52.Dupuy C, Ohayon R, Valent A, Noel-Hudson MS, Deme D, Virion A. Purification of a novel flavoprotein involved in the thyroid NADPH oxidase. Cloning of the porcine and human cdnas. J Biol Chem. 1999;274:37265–37269. doi: 10.1074/jbc.274.52.37265. [DOI] [PubMed] [Google Scholar]
- 53.Krause KH. Tissue distribution and putative physiological function of NOX family NADPH oxidases. Jpn J Infect Dis. 2004;57:S28–S29. [PubMed] [Google Scholar]
- 54.Leto TL, Morand S, Hurt D, Ueyama T. Targeting and regulation of reactive oxygen species generation by Nox family NADPH oxidases. Antioxid Redox Signal. 2009;11:2607–2619. doi: 10.1089/ars.2009.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martyn KD, Frederick LM, von LK, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 56.Kolls JK. Oxidative stress in sepsis: a redox redux. J Clin Invest. 2006;116:860–863. doi: 10.1172/JCI28111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu M, Lam J, Rada B, Leto TL, Levine SJ. Double-stranded RNA induces shedding of the 34-kDa soluble TNFR1 from human airway epithelial cells via TLR3-TRIF-RIP1-dependent signaling: roles for dual oxidase 2- and caspase-dependent pathways. J Immunol. 2011;186:1180–1188. doi: 10.4049/jimmunol.1001499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Juhasz A, Ge Y, Markel S, Chiu A, Matsumoto L, van BJ, Roy K, Doroshow JH. Expression of NADPH oxidase homologues and accessory genes in human cancer cell lines, tumours and adjacent normal tissues. Free Rad Res. 2009;43:523–532. doi: 10.1080/10715760902918683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Geiszt M, Lekstrom K, Brenner S, Hewitt SM, Dana R, Malech HL, Leto TL. NAD(P)H oxidase 1, a product of differentiated colon epithelial cells, can partially replace glycoprotein 91phox in the regulated production of superoxide by phagocytes. J Immunol. 2003;171:299–306. doi: 10.4049/jimmunol.171.1.299. [DOI] [PubMed] [Google Scholar]
- 60.Ameziane-El-Hassani R, Morand S, Boucher JL, Frapart YM, Apostolou D, Agnandji D, Gnidehou S, Ohayon R, Noel-Hudson MS, Francon J, Lalaoui K, Virion A, Dupuy C. Dual oxidase-2 has an intrinsic Ca2+-dependent H2O2-generating activity. J Biol Chem. 2005;280:30046–30054. doi: 10.1074/jbc.M500516200. [DOI] [PubMed] [Google Scholar]
- 61.Rada B, Leto TL. Characterization of hydrogen peroxide production by Duox in bronchial epithelial cells exposed to Pseudomonas aeruginosa. FEBS Lett. 2010;584:917–922. doi: 10.1016/j.febslet.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Geiszt M, Witta J, Baffi J, Lekstrom K, Leto TL. Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. FASEB J. 2003;17:1502–1504. doi: 10.1096/fj.02-1104fje. [DOI] [PubMed] [Google Scholar]
- 63.Leto TL, Geiszt M. Role of Nox family NADPH oxidases in host defense. Antioxid Redox Signal. 2006;8:1549–1561. doi: 10.1089/ars.2006.8.1549. [DOI] [PubMed] [Google Scholar]
- 64.Ha EM, Oh CT, Bae YS, Lee WJ. A direct role for dual oxidase in Drosophila gut immunity. Science. 2005;310:847–850. doi: 10.1126/science.1117311. [DOI] [PubMed] [Google Scholar]
- 65.Niethammer P, Grabher C, Look AT, Mitchison TJ. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature. 2009;459:996–999. doi: 10.1038/nature08119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lipinski S, Till A, Sina C, Arlt A, Grasberger H, Schreiber S, Rosenstiel P. DUOX2-derived reactive oxygen species are effectors of NOD2-mediated antibacterial responses. J Cell Sci. 2009;122:3522–3530. doi: 10.1242/jcs.050690. [DOI] [PubMed] [Google Scholar]
- 67.Anrather J, Racchumi G, Iadecola C. NF-kappaB regulates phagocytic NADPH oxidase by inducing the expression of gp91phox. J Biol Chem. 2006;281:5657–5667. doi: 10.1074/jbc.M506172200. [DOI] [PubMed] [Google Scholar]
- 68.Katsuyama M. NOX/NADPH oxidase, the superoxide-generating enzyme: its transcriptional regulation and physiological roles. J Pharmacol Sci. 2010;114:134–146. doi: 10.1254/jphs.10r01cr. [DOI] [PubMed] [Google Scholar]
- 69.Lambeth JD, Kawahara T, Diebold B. Regulation of Nox and Duox enzymatic activity and expression. Free Radic Biol Med. 2007;43:319–331. doi: 10.1016/j.freeradbiomed.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Woolley JF, Corcoran A, Groeger G, Landry WD, Cotter TG. Redox-regulated growth factor survival signaling. Antioxid Redox Signal. doi: 10.1089/ars.2012.5028. [DOI] [PubMed] [Google Scholar]
- 71.Kumatori A, Yang D, Suzuki S, Nakamura M. Cooperation of STAT-1 and IRF-1 in interferon-gamma-induced transcription of the gp91(phox) gene. J Biol Chem. 2002;277:9103–9111. doi: 10.1074/jbc.M109803200. [DOI] [PubMed] [Google Scholar]
- 72.Yang D, Suzuki S, Hao LJ, Fujii Y, Yamauchi A, Yamamoto M, Nakamura M, Kumatori A. Eosinophil-specific regulation of gp91(phox) gene expression by transcription factors GATA-1 and GATA-2. J Biol Chem. 2000;275:9425–9432. doi: 10.1074/jbc.275.13.9425. [DOI] [PubMed] [Google Scholar]
- 73.Valencia A, Sapp E, Kimm JS, McClory H, Reeves PB, Alexander J, Ansong KA, Masso N, Frosch MP, Kegel KB, Li X, Difiglia M. Elevated NADPH oxidase activity contributes to oxidative stress and cell death in Huntington’s disease. Hum Mol Genet. 2013;22:1112–1131. doi: 10.1093/hmg/dds516. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 74.Valente AJ, Zhou Q, Lu Z, He W, Qiang M, Ma W, Li G, Wang L, Banfi B, Steger K, Krause KH, Clark RA, Li S. Regulation of NOX1 expression by GATA, HNF-1alpha, and Cdx transcription factors. Free Radic Biol Med. 2008;44:430–443. doi: 10.1016/j.freeradbiomed.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 75.Lee SH, Park DW, Park SC, Park YK, Hong SY, Kim JR, Lee CH, Baek SH. Calcium-independent phospholipase A2beta-Akt signaling is involved in lipopolysaccharide-induced NADPH oxidase 1 expression and foam cell formation. J Immunol. 2009;183:7497–7504. doi: 10.4049/jimmunol.0900503. [DOI] [PubMed] [Google Scholar]
- 76.Diebold I, Petry A, Hess J, Gorlach A. The NADPH oxidase subunit NOX4 is a new target gene of the hypoxia-inducible factor-1. Mol Biol Cell. 2010;21:2087–2096. doi: 10.1091/mbc.E09-12-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Diebold I, Petry A, Sabrane K, Djordjevic T, Hess J, Gorlach A. The HIF1 target gene NOX2 promotes angiogenesis through urotensin-II. J Cell Sci. 2012;125:956–964. doi: 10.1242/jcs.094060. [DOI] [PubMed] [Google Scholar]
- 78.Maranchie JK, Zhan Y. Nox4 is critical for hypoxia-inducible factor 2-alpha transcriptional activity in von Hippel-Lindau-deficient renal cell carcinoma. Cancer Res. 2005;65:9190–9193. doi: 10.1158/0008-5472.CAN-05-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Doroshow JH, Juhasz A, Ge Y, Holbeck S, Lu J, Antony S, Wu Y, Jiang G, Roy K. Antiproliferative mechanisms of action of the flavin dehydrogenase inhibitors diphenylene iodonium and di-2-thienyliodonium based on molecular profiling of the NCI-60 human tumor cell panel. Biochem Pharmacol. 2012;83:1195–1207. doi: 10.1016/j.bcp.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.de Mochel NS, Seronello S, Wang SH, Ito C, Zheng JX, Liang TJ, Lambeth JD, Choi J. Hepatocyte NAD(P)H oxidases as an endogenous source of reactive oxygen species during hepatitis C virus infection. Hepatology. 2010;52:47–59. doi: 10.1002/hep.23671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chiera F, Meccia E, Degan P, Aquilina G, Pietraforte D, Minetti M, Lambeth D, Bignami M. Overexpression of human NOX1 complex induces genome instability in mammalian cells. Free Radic Biol Med. 2008;44:332–342. doi: 10.1016/j.freeradbiomed.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 82.Tam NN, Leav I, Ho SM. Sex hormones induce direct epithelial and inflammation-mediated oxidative/nitrosative stress that favors prostatic carcinogenesis in the noble rat. Am J Pathol. 2007;171:1334–1341. doi: 10.2353/ajpath.2007.070199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu Y, Antony S, Hewitt SM, Jiang G, Yang SX, Meitzler JL, Juhasz A, Lu J, Liu H, Doroshow JH, Roy K. Functional activity and tumor-specific expression of dual oxidase 2 in pancreatic cancer cells and human malignancies characterized with a novel monoclonal antibody. Int J Oncol. 2013;42:1229–1238. doi: 10.3892/ijo.2013.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kodama R, Kato M, Furuta S, Ueno S, Zhang Y, Matsuno K, Yabe-Nishimura C, Tanaka E, Kamata T. ROS-generating oxidases Nox1 and Nox4 contribute to oncogenic Ras-induced premature senescence. Genes Cells. 2013;18:32–41. doi: 10.1111/gtc.12015. [DOI] [PubMed] [Google Scholar]
- 85.Weyemi U, Dupuy C. The emerging role of ROS-generating NADPH oxidase NOX4 in DNA-damage responses. Mutat Res. 2012;751:77–81. doi: 10.1016/j.mrrev.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 86.Weyemi U, Lagente-Chevallier O, Boufraqech M, Prenois F, Courtin F, Caillou B, Talbot M, Dardalhon M, Al GA, Bidart JM, Schlumberger M, Dupuy C. ROS-generating NADPH oxidase NOX4 is a critical mediator in oncogenic H-Ras-induced DNA damage and subsequent senescence. Oncogene. 2012;31:1117–1129. doi: 10.1038/onc.2011.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kang MA, So EY, Simons AL, Spitz DR, Ouchi T. DNA damage induces reactive oxygen species generation through the H2AX-Nox1/Rac1 pathway. Cell Death Dis. 2012;3:e249. doi: 10.1038/cddis.2011.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Anilkumar N, Jose GS, Sawyer I, Santos CX, Sand C, Brewer AC, Warren D, Shah AM. A 28-kDa splice variant of NADPH Oxidase-4 is nuclear-localized and involved in redox signaling in vascular cells. Arterioscler Thromb Vasc Biol. 2013;33:e104–e112. doi: 10.1161/ATVBAHA.112.300956. [DOI] [PubMed] [Google Scholar]
- 89.Burdon RH, Gill V, Rice-Evans C. Oxidative stress and tumour cell proliferation. Free Radic Res Commun. 1990;11:65–76. doi: 10.3109/10715769009109669. [DOI] [PubMed] [Google Scholar]
- 90.Burdon RH. Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Radic Biol Med. 1995;18:775–794. doi: 10.1016/0891-5849(94)00198-s. [DOI] [PubMed] [Google Scholar]
- 91.Chiarugi P, Cirri P. Redox regulation of protein tyrosine phosphatases during receptor tyrosine kinase signal transduction. Trends Biochem Sci. 2003;28:509–514. doi: 10.1016/S0968-0004(03)00174-9. [DOI] [PubMed] [Google Scholar]
- 92.Sastry SK, Elferink LA. Checks and balances: interplay of RTKs and PTPs in cancer progression. Biochem Pharmacol. 2011;82:435–440. doi: 10.1016/j.bcp.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 93.Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- 94.Lou YW, Chen YY, Hsu SF, Chen RK, Lee CL, Khoo KH, Tonks NK, Meng TC. Redox regulation of the protein tyrosine phosphatase PTP1B in cancer cells. FEBS J. 2008;275:69–88. doi: 10.1111/j.1742-4658.2007.06173.x. [DOI] [PubMed] [Google Scholar]
- 95.Doroshow JH, Gaur S, Markel S, Lu J, van BJ, Synold TW, Xi B, Wu X, Juhasz A. Effects of iodonium-class flavin dehydrogenase inhibitors on growth, reactive oxygen production cell cycle progression NADPH oxidase 1 levels, and gene expression in human colon cancer cells and xenografts. Free Radic Biol Med. 2013;57:162–175. doi: 10.1016/j.freeradbiomed.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A, Guarente L, Gu W. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 2001;107:137–148. doi: 10.1016/s0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- 97.Puca R, Nardinocchi L, Starace G, Rechavi G, Sacchi A, Givol D, D’Orazi G. Nox1 is involved in p53 deacetylation and suppression of its transcriptional activity and apoptosis. Free Radic Biol Med. 2010;48:1338–1346. doi: 10.1016/j.freeradbiomed.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 98.Kajla S, Mondol AS, Nagasawa A, Zhang Y, Kato M, Matsuno K, Yabe-Nishimura C, Kamata T. A crucial role for Nox 1 in redox-dependent regulation of Wnt-beta-catenin signaling. FASEB J. 2012;26:2049–2059. doi: 10.1096/fj.11-196360. [DOI] [PubMed] [Google Scholar]
- 99.Xia C, Meng Q, Liu LZ, Rojanasakul Y, Wang XR, Jiang BH. Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer Res. 2007;67:10823–10830. doi: 10.1158/0008-5472.CAN-07-0783. [DOI] [PubMed] [Google Scholar]
- 100.Komatsu D, Kato M, Nakayama J, Miyagawa S, Kamata T. NADPH oxidase 1 plays a critical mediating role in oncogenic Ras-induced vascular endothelial growth factor expression. Oncogene. 2008;27:4724–4732. doi: 10.1038/onc.2008.102. [DOI] [PubMed] [Google Scholar]
- 101.Boudreau HE, Casterline BW, Rada B, Korzeniowska A, Leto TL. Nox4 involvement in TGF-beta and SMAD3-driven induction of the epithelial-to-mesenchymal transition and migration of breast epithelial cells. Free Radic Biol Med. 2012;53:1489–1499. doi: 10.1016/j.freeradbiomed.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Diaz B, Courtneidge SA. Redox signaling at invasive microdomains in cancer cells. Free Radic Biol Med. 2012;52:247–256. doi: 10.1016/j.freeradbiomed.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gianni D, Bohl B, Courtneidge SA, Bokoch GM. The involvement of the tyrosine kinase c-Src in the regulation of reactive oxygen species generation mediated by NADPH oxidase-1. Mol Biol Cell. 2008;19:2984–2994. doi: 10.1091/mbc.E08-02-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Giannoni E, Taddei ML, Chiarugi P. Src redox regulation: again in the front line. Free Radic Biol Med. 2010;49:516–527. doi: 10.1016/j.freeradbiomed.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 105.Ushio-Fukai M. Localizing NADPH oxidase-derived ROS. Sci STKE. 2006:re8. doi: 10.1126/stke.3492006re8. [DOI] [PubMed] [Google Scholar]
- 106.Ushio-Fukai M. Redox signaling in angiogenesis: role of NADPH oxidase. Cardiovasc Res. 2006;71:226–235. doi: 10.1016/j.cardiores.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 107.O’Leary DP, Bhatt L, Woolley JF, Gough DR, Wang JH, Cotter TG, Redmond HP. TLR-4 signalling accelerates colon cancer cell adhesion via NF-kappaB mediated transcriptional up-regulation of Nox-1. PLoS One. 2012;7:e44176. doi: 10.1371/journal.pone.0044176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Giannoni E, Parri M, Chiarugi P. EMT and oxidative stress: a bidirectional interplay affecting tumor malignancy. Antioxid Redox Signal. 2012;16:1248–1263. doi: 10.1089/ars.2011.4280. [DOI] [PubMed] [Google Scholar]
- 109.Kodydkova J, Vavrova L, Stankova B, Macasek J, Krechler T, Zak A. Antioxidant status and oxidative stress markers in pancreatic cancer and chronic pancreatitis. Pancreas. 2013;42:614–621. doi: 10.1097/MPA.0b013e318288360a. [DOI] [PubMed] [Google Scholar]
- 110.Omary MB, Lugea A, Lowe AW, Pandol SJ. The pancreatic stellate cell: a star on the rise in pancreatic diseases. J Clin Invest. 2007;117:50–59. doi: 10.1172/JCI30082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Masamune A, Watanabe T, Kikuta K, Satoh K, Shimosegawa T. NADPH oxidase plays a crucial role in the activation of pancreatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2008;294:G99–G108. doi: 10.1152/ajpgi.00272.2007. [DOI] [PubMed] [Google Scholar]
- 112.Cat B, Stuhlmann D, Steinbrenner H, Alili L, Holtkotter O, Sies H, Brenneisen P. Enhancement of tumor invasion depends on transdifferentiation of skin fibroblasts mediated by reactive oxygen species. J Cell Sci. 2006;119:2727–2738. doi: 10.1242/jcs.03011. [DOI] [PubMed] [Google Scholar]
- 113.Martinez-Outschoorn UE, Balliet R, Lin Z, Whitaker-Menezes D, Birbe RC, Bombonati A, Pavlides S, Lamb R, Sneddon S, Howell A, Sotgia F, Lisanti MP. BRCA1 mutations drive oxidative stress and glycolysis in the tumor microenvironment: implications for breast cancer prevention with antioxidant therapies. Cell Cycle. 2012;11:4402–4413. doi: 10.4161/cc.22776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Martinez-Outschoorn UE, Balliet RM, Rivadeneira DB, Chiavarina B, Pavlides S, Wang C, Whitaker-Menezes D, Daumer KM, Lin Z, Witkiewicz AK, Flomenberg N, Howell A, Pestell RG, Knudsen ES, Sotgia F, Lisanti MP. Oxidative stress in cancer associated fibroblasts drives tumor-stroma co-evolution: a new paradigm for understanding tumor metabolism, the field effect and genomic instability in cancer cells. Cell Cycle. 2010;9:3256–3276. doi: 10.4161/cc.9.16.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Trinchieri G. Cancer and inflammation: an old intuition with rapidly evolving new concepts. Annu Rev Immunol. 2012;30:677–706. doi: 10.1146/annurev-immunol-020711-075008. [DOI] [PubMed] [Google Scholar]
- 116.Porta C, Riboldi E, Sica A. Mechanisms linking pathogen-associated inflammation and cancer. Cancer Lett. 2011;305:250–262. doi: 10.1016/j.canlet.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 117.Marnett LJ. Inflammation and cancer: chemical approaches to mechanisms, imaging, and treatment. J Org Chem. 2012;77:5224–5238. doi: 10.1021/jo300214d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Weber DM, Chen C, Niesvizky R, Wang M, Belch A, Stadtmauer EA, Siegel D, Borrello I, Rajkumar SV, Chanan-Khan AA, Lonial S, Yu Z, Patin J, Olesnyckyj M, Zeldis JB, Knight RD. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. New Engl J Med. 2007;357:2133–2142. doi: 10.1056/NEJMoa070596. [DOI] [PubMed] [Google Scholar]
- 119.Harrison ML, Obermueller E, Maisey NR, Hoare S, Edmonds K, Li NF, Chao D, Hall K, Lee C, Timotheadou E, Charles K, Ahern R, King DM, Eisen T, Corringham R, DeWitte M, Balkwill F, Gore M. Tumor necrosis factor alpha as a new target for renal cell carcinoma: two sequential phase II trials of infliximab at standard and high dose. J Clin Oncol. 2007;25:4542–4549. doi: 10.1200/JCO.2007.11.2136. [DOI] [PubMed] [Google Scholar]
- 120.Madhusudan S, Muthuramalingam SR, Braybrooke JP, Wilner S, Kaur K, Han C, Hoare S, Balkwill F, Ganesan TS. Study of etanercept, a tumor necrosis factor-alpha inhibitor, in recurrent ovarian cancer. J Clin Oncol. 2005;23:5950–5959. doi: 10.1200/JCO.2005.04.127. [DOI] [PubMed] [Google Scholar]
- 121.Dunn JH, Ellis LZ, Fujita M. Inflammasomes as molecular mediators of inflammation and cancer: potential role in melanoma. Cancer Lett. 2012;314:24–33. doi: 10.1016/j.canlet.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 122.Lee H, Pal SK, Reckamp K, Figlin RA, Yu H. STAT3: a target to enhance antitumor immune response. Curr Top Microbiol Immunol. 2011;344:41–59. doi: 10.1007/82_2010_51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bonner MY, Arbiser JL. Targeting NADPH oxidases for the treatment of cancer and inflammation. Cell Mol Life Sci. 2012;69:2435–2442. doi: 10.1007/s00018-012-1017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fang J, Seki T, Maeda H. Therapeutic strategies by modulating oxygen stress in cancer and inflammation. Adv Drug Deliv Rev. 2009;61:290–302. doi: 10.1016/j.addr.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 125.Sablina AA, Budanov AV, Ilyinskaya GV, Agapova LS, Kravchenko JE, Chumakov PM. The antioxidant function of the p53 tumor suppressor. Nat Med. 2005;11:1306–1313. doi: 10.1038/nm1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gao P, Zhang H, Dinavahi R, Li F, Xiang Y, Raman V, Bhujwalla ZM, Felsher DW, Cheng L, Pevsner J, Lee LA, Semenza GL, Dang CV. HIF-dependent antitumorigenic effect of antioxidants in vivo. Cancer Cell. 2007;12:230–238. doi: 10.1016/j.ccr.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bhandarkar SS, Jaconi M, Fried LE, Bonner MY, Lefkove B, Govindarajan B, Perry BN, Parhar R, Mackelfresh J, Sohn A, Stouffs M, Knaus U, Yancopoulos G, Reiss Y, Benest AV, Augustin HG, Arbiser JL. Fulvene-5 potently inhibits NADPH oxidase 4 and blocks the growth of endothelial tumors in mice. J Clin Invest. 2009;119:2359–2365. doi: 10.1172/JCI33877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.McLean MH, El-Omar EM. Genetics of inflammation in the gastrointestinal tract and how it can cause cancer. Recent Results Cancer Res. 2011;185:173–183. doi: 10.1007/978-3-642-03503-6_11. [DOI] [PubMed] [Google Scholar]
- 129.O’Connor PM, Lapointe TK, Beck PL, Buret AG. Mechanisms by which inflammation may increase intestinal cancer risk in inflammatory bowel disease. Inflamm Bowel Dis. 2010;16:1411–1420. doi: 10.1002/ibd.21217. [DOI] [PubMed] [Google Scholar]
- 130.Alison MR, Nicholson LJ, Lin WR. Chronic inflammation and hepatocellular carcinoma. Recent Results Cancer Res. 2011;185:135–148. doi: 10.1007/978-3-642-03503-6_8. [DOI] [PubMed] [Google Scholar]
- 131.Dennis LK, Lynch CF, Torner JC. Epidemiologic association between prostatitis and prostate cancer. Urology. 2002;60:78–83. doi: 10.1016/s0090-4295(02)01637-0. [DOI] [PubMed] [Google Scholar]
- 132.Garcea G, Dennison AR, Steward WP, Berry DP. Role of inflammation in pancreatic carcinogenesis and the implications for future therapy. Pancreatology. 2005;5:514–529. doi: 10.1159/000087493. [DOI] [PubMed] [Google Scholar]
- 133.Franco EL, Rohan TE, Villa LL. Epidemiologic evidence and human papillomavirus infection as a necessary cause of cervical cancer. J Natl Cancer Inst. 1999;91:506–511. doi: 10.1093/jnci/91.6.506. [DOI] [PubMed] [Google Scholar]
- 134.Punturieri A, Szabo E, Croxton TL, Shapiro SD, Dubinett SM. Lung cancer and chronic obstructive pulmonary disease: needs and opportunities for integrated research. J Natl Cancer Inst. 2009;101:554–559. doi: 10.1093/jnci/djp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Carmona-Cuenca I, Herrera B, Ventura JJ, Roncero C, Fernandez M, Fabregat I. EGF blocks NADPH oxidase activation by TGF-beta in fetal rat hepatocytes, impairing oxidative stress, and cell death. J Cell Physiol. 2006;207:322–330. doi: 10.1002/jcp.20568. [DOI] [PubMed] [Google Scholar]
- 136.Hu R, Wang YL, Edderkaoui M, Lugea A, Apte MV, Pandol SJ. Ethanol augments PDGF-induced NADPH oxidase activity and proliferation in rat pancreatic stellate cells. Pancreatology. 2007;7:332–340. doi: 10.1159/000105499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kamizato M, Nishida K, Masuda K, Takeo K, Yamamoto Y, Kawai T, Teshima-Kondo S, Tanahashi T, Rokutan K. Interleukin 10 inhibits interferon gamma- and tumor necrosis factor alpha-stimulated activation of NADPH oxidase 1 in human colonic epithelial cells and the mouse colon. J Gastroenterol. 2009;44:1172–1184. doi: 10.1007/s00535-009-0119-6. [DOI] [PubMed] [Google Scholar]
- 138.Mandal D, Fu P, Levine AD. REDOX regulation of IL-13 signaling in intestinal epithelial cells: usage of alternate pathways mediates distinct gene expression patterns. Cell Signal. 2010;22:1485–1494. doi: 10.1016/j.cellsig.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 139.Nisimoto Y, Tsubouchi R, Diebold BA, Qiao S, Ogawa H, Ohara T, Tamura M. Activation of NADPH oxidase 1 in tumour colon epithelial cells. Biochem J. 2008;415:57–65. doi: 10.1042/BJ20080300. [DOI] [PubMed] [Google Scholar]
- 140.Belambri SA, Hurtado-Nedelec M, Senator A, Makni-Maalej K, Fay M, Gougerot-Pocidalo MA, Marie JC, Dang PM, El-Benna J. Phosphorylation of p47phox is required for receptor-mediated NADPH oxidase/NOX2 activation in Epstein-Barr virus-transformed human B lymphocytes. Am J Blood Res. 2012;2:187–193. [PMC free article] [PubMed] [Google Scholar]
- 141.Mohammed AM, Syeda K, Hadden T, Kowluru A. Upregulation of phagocyte-like NADPH oxidase by cytokines in pancreatic beta-cells: attenuation of oxidative and nitrosative stress by 2-bromopalmitate. Biochem Pharmacol. 2013;85:109–114. doi: 10.1016/j.bcp.2012.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kim HJ, Kim CH, Ryu JH, Joo JH, Lee SN, Kim MJ, Lee JG, Bae YS, Yoon JH. Crosstalk between platelet-derived growth factor-induced Nox4 activation and MUC8 gene overexpression in human airway epithelial cells. Free Radic Biol Med. 2011;50:1039–1052. doi: 10.1016/j.freeradbiomed.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 143.Lee JK, Edderkaoui M, Truong P, Ohno I, Jang KT, Berti A, Pandol SJ, Gukovskaya AS. NADPH oxidase promotes pancreatic cancer cell survival via inhibiting JAK2 dephosphorylation by tyrosine phosphatases. Gastroenterology. 2007;133:1637–1648. doi: 10.1053/j.gastro.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 144.Serrander L, Jaquet V, Bedard K, Plastre O, Hartley O, Arnaudeau S, Demaurex N, Schlegel W, Krause KH. NOX5 is expressed at the plasma membrane and generates superoxide in response to protein kinase C activation. Biochimie. 2007;89:1159–1167. doi: 10.1016/j.biochi.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 145.Harper RW, Xu C, Eiserich JP, Chen Y, Kao CY, Thai P, Setiadi H, Wu R. Differential regulation of dual NADPH oxidases/peroxidases, Duox1 and Duox2, by Th1 and Th2 cytokines in respiratory tract epithelium. FEBS Lett. 2005;579:4911–4917. doi: 10.1016/j.febslet.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 146.Hirakawa S, Saito R, Ohara H, Okuyama R, Aiba S. Dual oxidase 1 induced by Th2 cytokines promotes STAT6 phosphorylation via oxidative inactivation of protein tyrosine phosphatase 1B in human epidermal keratinocytes. J Immunol. 2011;186:4762–4770. doi: 10.4049/jimmunol.1000791. [DOI] [PubMed] [Google Scholar]
- 147.Li Q, Lei RX, Zhou XD, Kolosov VP, Perelman JM. Regulation of PMA-induced MUC5AC expression by heparin in human bronchial epithelial cells. Mol Cell Biochem. 2012;360:383–391. doi: 10.1007/s11010-011-1078-9. [DOI] [PubMed] [Google Scholar]
- 148.Shao MX, Nadel JA. Dual oxidase 1-dependent MUC5AC mucin expression in cultured human airway epithelial cells. Proc Natl Acad Sci U S A. 2005;102:767–772. doi: 10.1073/pnas.0408932102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.De D, Wang XD, Dumont JE, Miot F. Characterization of ThOX proteins as components of the thyroid H(2)O(2)-generating system. Exp Cell Res. 2002;273:187–196. doi: 10.1006/excr.2001.5444. [DOI] [PubMed] [Google Scholar]
- 150.Gerard AC, Boucquey M, van den Hove MF, Colin IM. Expression of TPO and ThOXs in human thyrocytes is downregulated by IL-1alpha/IFN-gamma, an effect partially mediated by nitric oxide. Am J Physiol Endocrinol Metab. 2006;291:E242–E253. doi: 10.1152/ajpendo.00439.2005. [DOI] [PubMed] [Google Scholar]
- 151.Raad H, Eskalli Z, Corvilain B, Miot F, De DX. Thyroid hydrogen peroxide production is enhanced by the Th2 cytokines, IL-4 and IL-13, through increased expression of the dual oxidase 2and its maturation factorDUOXA2. Free Radic Biol Med. 2013;56:216–225. doi: 10.1016/j.freeradbiomed.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 152.Juhasz A, Markel S, Gaur S, Matsumoto L, Van balgooy J, Metz M, Doroshow JH. Inhibition of Nox1 gene expression with siRNA in human colon cancer cells decreases tumor growth and markers of angiogenesis in vivo. Proc Amer Assoc Cancer Res. 2005;46:Abstract #6108. [Google Scholar]