Abstract
Human immunodeficiency virus (HIV) infection substantially elevates diffuse large B-cell lymphoma (DLBCL) risk, but its impact on the distinct DLBCL subtypes defined by cell of origin is unclear. We compared DLBCL molecular characteristics and prognosis in 51 HIV-infected and 116 HIV-uninfected cases diagnosed during 1977-2003. Using immunohistochemistry to classify cell of origin based on the Tally algorithm, activated B-cell (ABC)-DLBCL was substantially more common in HIV-infected (83%) than in HIV-uninfected (54%) cases (p< 0.001). Epstein-Barr virus (EBV) was detected in 63% of DLBCLs in HIV-infected cases, occurring almost exclusively in ABC-DLBCL (74% vs. 13% of germinal center B-cell [GCB]-DLBCL, p=0.002), but was rarely detected in DLBCLs among HIV-uninfected cases (3%). Among HIV-uninfected cases, MYC/IgH[t(8;14)(q24;q32)] and IgH/BCL2[t(14;18)(q32;q21)] translocations were significantly more common and BCL6/IgH[t(3;14)(q27;q32)] significantly less common in GCB-DLBCL than in ABC-DLBCL (p= 0.010, < 0.001 and = 0.039, respectively). Among HIV-infected cases, translocations other than MYC/IgH[t(8;14)(q24;q32)] (21%) were rare (≤6%) and unrelated to cell of origin. ABC-DLBCL was associated with adverse overall survival compared with GCB-DLBCL regardless of HIV status (pHIV-infected= 0.066;pHIV-uninfected= 0.038). Our data demonstrate key differences in the molecular characteristics, cell of origin and prognosis of DLBCL by HIV status in the pre-highly active antiretroviral therapy (HAART) and pre-rituximab era, supporting biologic differences in lymphomagenesis in the presence of HIV.
Keywords: Diffuse large B-cell lymphoma, Epstein-Barr virus, activated B-cell, germinal center B-cell
Introduction
Diffuse large B-cell lymphoma (DLBCL), the most common histologic type of non-Hodgkin lymphoma (NHL) in both immunocompetent and immunosuppressed individuals, is a clinically and molecularly heterogeneous entity. In the general population, 5-year relative survival is 59% [1], though the clinical course is highly variable. Gene expression profiling of DLBCLs has revealed distinct disease subtypes that derive from B-cells at varying stages of differentiation, specifically germinal center B-cell (GCB)-DLBCL, post-germinal center or activated B-cell (ABC)-DLBCL, and the rare primary mediastinal large B-cell lymphoma [2,3]. People with ABC-DLBCL have a substantially worse prognosis than people with GCB-DLBCL or primary mediastinal large B-cell lymphoma, and certain molecular characteristics appear distinctly related to the cell of origin, such as the association of BCL2and MYCtranslocations with GCB-DLBCL and constitutive activation of the nuclear factor- κB (NF-κB) pathway in ABC-DLBCL [4–7].
Human immunodeficiency virus (HIV) causes extensive immune dysfunction, including loss of T-cell function, altered cell signaling and chronic B-cell activation, with accompanying increases in B-cell proliferation, differentiation and apoptosis [8,9]. People with advanced HIV infection have 30-100-fold increased risk for DLBCL [10] and tend to present with aggressive disease that is more frequently of extranodal origin than in the general population [8]. Epstein- Barr virus (EBV), uncommon in DLBCLs in HIV-uninfected individuals, is detectable in over 80% of DLBCLs in HIV-infected individuals [11].
Previous studies of the cell of origin of DLBCLs in HIV-infected individuals have yielded varying results. Several studies have suggested the predominance of post-germinal center DLBCL based on gene expression profiling [12], immunohistochemistry [13] or demonstration of immunoglobulin gene somatic hypermutation [14], but other studies have not supported those findings [15–22]. Several gene expression [12,23] and immunohistochemistry-based [13,17,18,24] studies have reported an intermediate phenotype with increased co-expression of GCB and ABC markers, which complicates classification by cell of origin. Finally, studies of the prognostic significance of molecular characteristics have been conflicting [17–19], and few studies have related cell of origin to other clinicopathologic characteristics [13,17,20].
To advance understanding of the pathogenetic contribution of HIV to lymphomagenesis, we classified DLBCLs by cell of origin in archived specimens from HIV-infected and HIV-uninfected cases using the most current immunohistochemistry-based methods [25], evaluated the prognostic significance of this cell of origin classification, and compared the frequency of key molecular and viral characteristics.
Methods
Study population
Cases were identified through the Los Angeles Residual Tissue Repository (LA RTR), directed and maintained at the University of Southern California Keck School of Medicine. The LA RTR collects formalin-fixed, paraffin-embedded tissue specimens for Los Angeles County patients that otherwise would have been discarded by local area pathology departments (e.g. for logistical or storage constraints), and links the specimens to the University of Southern California Cancer Surveillance Program (USC-CSP, the population-based cancer registry for Los Angeles, one of the National Cancer Institute Surveillance, Epidemiology and End-Results [SEER] cancer registries), to obtain patient information [26]. The linkage is possible because the USC-CSP collects pathology reports on patients diagnosed in Los Angeles County since 1972 as part of the cancer reporting process.
For all patients with a primary diagnosis of NHL who had an available specimen with sufficient tumor tissue in the LA RTR as of 2010 (n= 351), multi-tumor blocks were created by assembling a tumor tissue specimen (up to 8 mm × 3 mm) from each patient in array fashion, with 8–10 samples per block. Samples were organized by NHL subtype, based on initial review of pathology reports and hematoxylin and eosin stained slides by an expert hematopathologist (L. Weiss), and by HIV status, as confirmed by pathology report and USC-CSP abstract review (D. Hawes).
Eligible patients for this study included all patients diagnosed with primary DLBCL based on pathology report and diagnostic slide review (n= 180). DLBCL diagnoses were confirmed with additional immunophenotyping (see laboratory methods below), resulting in the exclusion of three patients, who were determined to have another NHL subtype, yielding a population of 177 patients for molecular studies (described below). Linkage with the SEER registry database provided information on sex; race/ethnicity; socioeconomic status; age, date and primary site of DLBCL diagnosis; occurrence of other primary malignancies before or after DLBCL diagnosis; first course of DLBCL treatment (chemotherapy, immunotherapy, radiotherapy); and follow-up for vital status. Socioeconomic status was derived from census tract-level information on educational attainment and household income. All census tracts were ranked twice, first by the weighted average highest level of education attained by adult residents, and second by median household income. These two ranks were summed, and the socioeconomic status was assigned by dividing the summed ranking into quintiles. The study was conducted according to a protocol reviewed and approved by the University of Southern California Institutional Review Board and National Cancer Institute Office of Human Subjects Research.
Laboratory analysis
Immunohistochemical staining was conducted for CD20 and nine key markers of B-cell differentiation, including four germinal center B-cell markers (BCL6, CD10, GCET1, LMO2) and five activated B-cell markers (BCL2, FOXP1, CD138, MUM1, TP53), according to standard methods. Briefly, 5 μm sections were cut from the multi-tumor blocks, deparaffinized and pretreated for antigen retrieval. Immunohistochemical staining was conducted using a Dako autostainer, with incubation of primary antibodies for 20–30 min at room temperature, followed by a secondary antibody. Details of the antibodies and antigen retrieval are provided in Table II. All reactivities were visualized using chromogen diaminobenzidine tetrahydrochloride (DAB), with hematoxylin as a counterstain.
Table II.
Expression frequency of individual markers of B-cell differentiation in 51 HIV-infected and 116 HIV-uninfected individuals with DLBCL.
| Protein* | HIV-infected | HIV-uninfected | p-Value† | ||
|---|---|---|---|---|---|
|
|
|
||||
| n/total | (%) | n/total | (%) | ||
| Germinal center B-cell markers | |||||
| BCL6 | 14/50 | (28) | 53/116 | (46) | 0.039 |
| CD10 (MME) | 13/49 | (27) | 42/113 | (37) | 0.210 |
| GCET1 (SERPINA9) | 6/51 | (12) | 31/116 | (27) | 0.042 |
| LM02 | 22/44 | (50) | 69/114 | (61) | 0.282 |
| Activated B-cell markers | |||||
| BCL2 | 24/50 | (48) | 67/116 | (58) | 0.308 |
| FOXP1 | 31/50 | (62) | 53/114 | (46) | 0.090 |
| CD138(SDC1) | 5/49 | (10) | 5/114 | (4) | 0.169 |
| MUM1 (IRF4) | 35/51 | (69) | 55/115 | (48) | 0.018 |
| TP53 | 6/51 | (12) | 14/116 | (12) | 1.000 |
HIV, human immunodeficiency virus; DLBCL, diffuse large B-cell lymphoma.
Primary antibodies included BCL6 clone PG-B6p Dako7211, CD10 clone 56C6 Dako M7308, GCET1 courtesy of Dr. Miguel Piris (Santander, Spain), LMO2 clone ab82090 Abcam, BCL2 clone 124 Dako M0887, FOXP1 clone #3210-1 EPITOMICS, CD138 clone MI15 Dako M7228, MUM1 clone Dako M7259 and p53 clone D07 M7001. Secondary antibodies were derived from the Flex Kit from Dako for all except GCET1, LMO2 and FOXP1, for which we used EnVision + polymer from Dako. Antigen retrieval was done using High Ph (Dako) for all antibodies except LMO2 and FOXP1, for which we used DIVA buffer (Biocare). Appropriate positive and negative controls were included in each run.
p-Value derived from Fisher's exact test.
Slides were evaluated by an expert hematopathologist (L. Weiss). Ten people were excluded due to insufficient or poor quality tissue, resulting in a final analytic population of 167 individuals (51 HIV-infected, 116 HIV-uninfected). Within this analytic population, successful staining was achieved for ≥ 95% of samples for all markers. Based on consensus in the literature at the time of slide review, samples with ≥ 30% tumor cells stained were considered positive for BCL6, CD10, LMO2 and CD138; ≥ 60% for GCET1 and BCL2; or ≥ 75% for FOXP1, MUM1 and TP53 [25]. Slides from 16 randomly selected individuals were re-evaluated separately without knowledge of the original interpretation. Agreement between the independent evaluations was ≥ 94% for all markers except LMO2 (88%) and CD138 (81%).
The presence of EBV was evaluated using EBV-encoded RNA-1 (EBER) in situhybridization according to standard methods [27], with successful completion for all 167 samples. Cases were considered EBV+ if all or nearly all tumor cells were stained. Fluorescence in situhybridization (FISH) was used to detect common NHL-associated translocations including MYC/IgH[t(8;14)(q24;q32)], IgH/BCL2[t(14;18)(q32;q21)] and BCL6/IgH[t(3;14)(q27;q32)], according to standard methods [28]. Probes included the LSI BCL6 (ABR) dual color breakapart probe; the LSI IGH/BCL2 dual color, dual fusion translocation probe; and the LSI MYC dual color, breakapart rearrangement probe, all obtained from Abbott Molecular (Des Plaines, IL). FISH was conducted successfully for 89% of patients for MYC/IgH[t(8;14)(q24;q32)], 93% for IgH/BCL2[t(14;18)(q32;q21)] and 86% for BCL6/IgH[t(3;14)(q27;q32)].
Statistical analysis
DLBCLs were distinguished by cell of origin into GCB- and ABC-DLBCL according to the Tally algorithm [25]. Briefly, cases were classified by counting the number of positive GCB (GCET1 and CD10) and ABC (FOXP1 and MUM1) antibodies and assigning the category with the higher score [25]. In the case of an equal number of GCB and ABC antibodies, expression of another GCB marker (LMO2) determined the cell of origin (15 HIV-infected, 26 HIV-uninfected).
Fisher's exact test was used to compare: (1) expression frequency of individual markers by HIV status, (2) cell of origin category by HIV status and (3) correlates of cell of origin (i.e. presence of translocations, EBV status and primary site) for HIV-infected and HIV-uninfected people separately. Overall survival (OS), defined as the time from DLBCL diagnosis to death or last follow-up, by cell of origin and HIV status was estimated using the Kaplan-Meier method.
Differences among groups were assessed using the (univariate) log-rank test and multivariate Cox proportional hazards regression, adjusting for race/ethnicity (White, non-Hispanic; Hispanic; other/unknown), socioeconomic status, age at DLBCL diagnosis (< 40, ≥ 40 years), year of DLBCL diagnosis (≤ 1990, 1991–1995, ≥ 1996) and receipt of initial therapy. We conducted two sensitivity analyses. First, because of demographic differences among HIV-infected and HIV-uninfected individuals, all comparisons by HIV status were repeated restricting HIV-uninfected individuals to males aged < 60 years. Second, we restricted analyses of OS to the 72% of cases (40 HIV-infected, 80 HIV-uninfected) who were reported to have received initial immunotherapy and/or chemotherapy. Results from both of these sensitivity analyses were consistent with those obtained in our main analyses with the full study population and thus are not presented. Two-sided p-values < 0.05 were considered statistically significant. Analyses were conducted using SAS software version 9.2 (SAS Institute, Cary, NC).
Role of the funding source
The funding source had no role in the study design; collection, analysis and interpretation of data; writing of the report; or decision to submit the paper for publication.
Results
All HIV-infected cases were male, with a median age at DLBCL diagnosis of 37 years (range 25–58 years) and median year of diagnosis of 1992 (range 1987–1998; largely prior to the introduction of highly active antiretroviral therapy [HAART] to treat HIV and rituximab to treat DLBCL) (Table I). In contrast, HIV-uninfected cases were evenly divided by sex, tended to be older (median age: 65 years, range 16–90) and were diagnosed over a wider range of time (median year: 1992, range 1977–2003; also largely in the pre-rituximab era). HIV-infected individuals were predominantly diagnosed with DLBCL of primary extranodal origin (61%), with 20% occurring in the brain or central nervous system, 14% in the lower gastrointestinal tract and 8% in the stomach, whereas only 39% of HIV-uninfected individuals were diagnosed with DLBCL of primary extranodal origin, with no particularly common sites. All HIV-infected and 99 (85%) HIV-uninfected cases were deceased as of 2011.
Table I.
Selected characteristics of 51 HIV-infected and 116 HIV-uninfected individuals with DLBCL.
| Characteristic | HIV-infected | HIV-uninfected | ||
|---|---|---|---|---|
|
|
|
|||
| n | (%) | n | (%) | |
| Sex | ||||
| Male | 51 | (100) | 56 | (48) |
| Female | 0 | (0) | 60 | (52) |
| Race/ethnicity | ||||
| White, non-Hispanic | 25 | (49) | 59 | (51) |
| Hispanic | 17 | (33) | 25 | (22) |
| Asian | 2 | (4) | 14 | (12) |
| Black | 4 | (8) | 9 | (8) |
| Other/unknown | 3 | (6) | 9 | (8) |
| Socioeconomic status* | ||||
| Highest | 5 | (10) | 20 | (17) |
| Higher middle | 9 | (18) | 16 | (14) |
| Middle | 10 | (20) | 21 | (18) |
| Lower middle | 11 | (22) | 27 | (23) |
| Lowest | 12 | (24) | 23 | (20) |
| Unknown/missing | 4 | (8) | 9 | (8) |
| Age at DLBCL diagnosis (years) | ||||
| <40 | 31 | (61) | 11 | (9) |
| 40&–59 | 20 | (39) | 31 | (27) |
| 60–69 | 0 | (0) | 26 | (22) |
| 70–79 | 0 | (0) | 30 | (26) |
| 80+ | 0 | (0) | 18 | (16) |
| Year of DLBCL diagnosis | ||||
| <1990 | 11 | (22) | 47 | (41) |
| 1991–1995 | 36 | (71) | 44 | (38) |
| >1996 | 4 | (8) | 25 | (22) |
| Primary site of DLBCL | ||||
| Nodal | 20 | (39) | 71 | (61) |
| Extranodal | ||||
| Brain/central nervous system | 10 | (20) | 3 | (3) |
| Lower gastrointestinal tract† | 7 | (14) | 7 | (6) |
| Stomach | 4 | (8) | 10 | (9) |
| Other‡ | 10 | (20) | 25 | (22) |
| Vital status (as of 2011) | ||||
| Deceased | 51 | (100) | 99 | (85) |
| Alive | 0 | (0) | 17 | (15) |
HIV, human immunodeficiency virus; DLBCL, diffuse large B-cell lymphoma.
Derived from education and income levels in each patient's census tract.
Lower gastrointestinal tract includes small or large intestine, anus and rectum.
Other extranodal sites include lip, mouth, tonsil, nasal cavity, accessory sinuses, pleura, bone (n= 3), spleen, peritoneum (n= 2), connective tissue (n= 3), vagina, testis (n = 3), orbit and thyroid (n= 5) among HIV-uninfected cases, and tongue, parotid gland, tonsil, nasopharynx, liver (n= 2), accessory sinuses, lung (n= 2) and skin among HIV-infected cases.
We compared the expression frequency of individual markers of B-cell differentiation by HIV status (Table II). Compared with HIV-uninfected cases, DLBCLs in HIV-infected cases generally were less likely to express germinal center markers, particularly BCL6 (28% vs. 46%, p= 0.039) and GCET1 (12% vs. 27%, p= 0.042) and more likely to express activated B-cell markers, particularly MUM1 (69% vs. 48%, p= 0.018).
Using the Tally algorithm to classify DLBCLs according to cell of origin [25], HIV-infected cases were more likely to have ABC-DLBCL than HIV-uninfected cases (HIV-infected: 83%, HIV-uninfected: 54%, p< 0.001). Among HIV-infected individuals, only 31% of the DLBCLs had one of the three translocations we evaluated, most commonly MYC/IgH, and the presence of translocations was not related to cell of origin (p= 0.648; Table III). In contrast, 47% of the DLBCLs among HIV-uninfected individuals had at least one of the three translocations we evaluated, and the presence of translocations was strongly related to cell of origin (p= 0.009). Specifically, among HIV-uninfected cases, the MYC/IgH[t(8;14)(q24;q32)] and IgH/BCL2[t(14;18)(q32;q21)] translocations were significantly more common in GCB- than ABC-DLBCL (p= 0.010 and < 0.001, respectively), and the BCL6/IgH[t(3;14)(q27;q32)] translocation was significantly more common in ABC-DLBCL (p= 0.039). EBV was detected in 63% of DLBCLs in HIV-infected cases and occurred almost exclusively in the ABC type (p= 0.002), whereas EBV was rarely detected in DLBCLs of HIV-uninfected cases (3%). In all cases, regardless of HIV status, ABC-DLBCL was somewhat more likely to be of primary extranodal origin than GCB-DLBCL, but these differences were not statistically significant. Patient characteristics such as sex and age were not significantly related to cell of origin among HIV-infected and HIV-uninfected cases.
Table III.
Molecular characteristics of DLBCLs by HIV status and cell of origin*.
| Characteristic | HIV-infected cases | HIV-un infected cases | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| GCB | ABC | GCB | ABC | |||||||
|
|
|
|
|
|||||||
| n | (%) | n | (%) | p-Value† | n | (%) | n | (%) | p-Value† | |
| Total | 8 | (17)‡ | 39 | (83)‡ | 51 | (46)‡ | 60 | (54)‡ | ||
| Translocations | ||||||||||
| MYC/IgH[t(8;14)(q24;q32)] | ||||||||||
| − | 4 | (57) | 26 | (84) | 0.146 | 36 | (77) | 53 | (95) | 0.010 |
| + | 3 | (43) | 5 | (16) | 11 | (23) | 3 | (5) | ||
| IgH/BCL2[t(14;18)(q32;q2l)] | ||||||||||
| − | 8 | (100) | 33 | (100) | –** | 26 | (55) | 54 | (92) | <0.001 |
| + | 0 | (0) | 0 | (o) | 21 | (45) | 5 | (8) | ||
| BCL6/IgH[t(3;14)(q27;q32)] | ||||||||||
| − | 8 | (100) | 26 | (93) | 1.000 | 41 | (91) | 41 | (73) | 0.039 |
| + | 0 | (0) | 2 | (7) | 4 | (9) | 15 | (27) | ||
| No. of translocations§ | ||||||||||
| 0 | 4 | (57) | 18 | (72) | 0.648 | 20 | (44) | 33 | (60) | 0.009 |
| 1 | 3 | (43) | 7 | (28) | 16 | (36) | 21 | (38) | ||
| 2 or 3 | 0 | (0) | 0 | (0) | 9 | (20) | 1 | (2) | ||
| EBV | ||||||||||
| − | 7 | (88) | 10 | (26) | 0.002 | 50 | (98) | 59 | (98) | 1.000 |
| + | 1 | (13) | 29 | (74) | 1 | (2) | 1 | (2) | ||
| Primary DLBCL site | ||||||||||
| Nodal | 5 | (63) | 14 | (36) | 0.240¶ | 35 | (69) | 34 | (57) | 0.240¶ |
| Extranodal | 3 | (38) | 25 | (64) | 16 | (31) | 26 | (43) | ||
| Stomach | 0 | (0) | 4 | (10) | 2 | (4) | 7 | (12) | ||
| Lower gastrointestinal tract | 1 | (13) | 6 | (15) | 1 | (2) | 6 | (10) | ||
| Brain/central nervous system | 1 | (13) | 8 | (21) | 2 | (4) | 1 | (2) | ||
| Other | 1 | (13) | 7 | (18) | 11 | (22) | 12 | (20) | ||
DLBCL, diffuse large B-cell lymphoma; HIV, human immunodeficiency virus; ABC, activated B-cell; GCB, germinal center B-cell.
Cell of origin was categorized using the Tally algorithm [25] and could not be determined for four HIV+ and five HIV- patients due to missing data on CD10, GCET1, LMO2, MUM1 or FOXP1.
p-Value derived from Fisher's exact test.
Row percentages. All other percentages reflect column percentages.
15 HIV+ and 11 HIV- patients with missing data for any translocation were excluded from this analysis.
Compares nodal versus extranodal.
Statistic not calculated due to zero observed HIV+ patients with the IgH/BCL2[t(14;18)(q32;q21)] translocation.
HIV-infected cases had worse OS compared with HIV-uninfected cases (hazard ratio [HR] = 3.82, 95% confidence interval [CI] 2.57-5.67). Among both HIV-infected and HIV-uninfected cases, ABC-DLBCL was associated with adverse OS compared with GCB-DLBCL (HIV-uninfected: HR = 1.64, 95% CI 1.03-2.62, p= 0.038; HIV-infected: HR = 2.38, 95% CI 0.95–5.99,p= 0.066) (Figure 1). Patterns were similar in analyses stratified by calendar year of DLBCL diagnosis (≤ 1990, 1991-1995, ≥ 1996), and for analyses of HIV-uninfected individuals restricted to males aged < 60 years.
Figure 1.
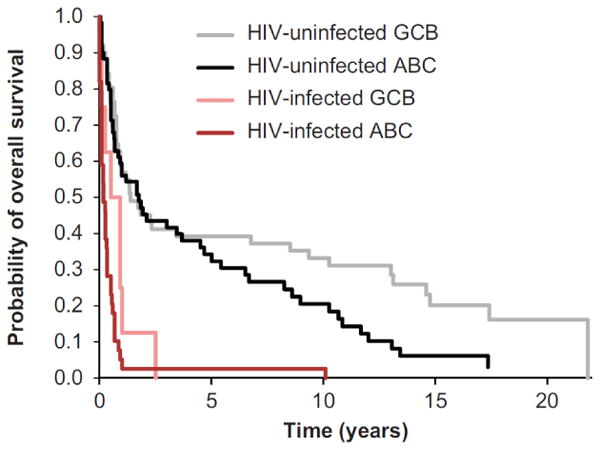
Overall survival following DLBCL diagnosis, by HIV status and cell of origin*. ABC, activated B-cell; DLBCL, diffuse large B-cell; GCB, germinal center B-cell. *Cell of origin was categorized using the Tally algorithm [25].
To address the potential for increased frequency of an intermediate phenotype (co-expression of GCB and ABC markers) in DLBCLs in HIV-infected individuals, we compared our classification of cell of origin to the immunostain algorithms reported in previous studies. Most patients with the phenotype BCL6–/MUM1+/CD138 – described by Carbone et al.[13] were classified as ABC-DLBCL using the Tally algorithm (among HIV-infected: 22 [43%] cases were BCL6-/MUM1+/CD138–, of which 21 were classified as ABC-DLBCL; among HIV-uninfected: 32 [28%] were BCL6-/MUM1+/CD138–, of which 20 were classified as ABC-DLBCL). The pattern was similar for the phenotype described by Hoffmann et al.[18](CD10 + and/or BCL6 + and MUM1 + and/or CD138+; nine [18%] HIV-infected, all classified as ABC-DLBCL; 24 [21%] HIV-uninfected, of which 18 were classified as ABC-DLBCL). In contrast, few cases were identified as CD10+/BCL6+/MUM1 + based on Chadburn et al.[17](one [2%] HIV-infected, five [4%] HIV-uninfected) in our study, and we could not directly compare our results to Madan et al.[24] because we did not include all necessary immunostains.
Discussion
In a direct comparison of DLBCLs in HIV-infected and HIV-uninfected individuals, we observed striking differences in the distributions of certain molecular characteristics, supporting alternative pathways of lymphomagenesis in disease subgroups defined by both cell of origin and HIV status. Specifically, DLBCLs in HIV-infected cases were predominantly of ABC origin, with EBV-positivity almost completely concordant with the ABC phenotype. DLBCLs in HIV-infected cases tended to lack IgH/BCL2and BCL6/IgHtranslocations, regardless of the cell of origin. In contrast, DLBCLs in HIV-uninfected cases were approximately evenly divided by cell of origin, with the BCL6/IgHtranslocation more common in ABC-DLBCL and the IgH/BCL2translocation slightly more common in GCB-DLBCL. The MYC/IgHwas more common in GCB-DLBCL, regardless of HIV status. These results provide further insight into the pathogenetic mechanisms of lymphomagenesis in HIV-infected DLBCLs occurring during the pre-HAART and pre-rituximab era. The first distinct pattern of lymphomagenesis in HIV-infected cases, constituting the majority of DLBCLs, is driven by EBV, lacks typical lymphomagenic lesions and is of the ABC-type, with the central nervous system being the most frequent extranodal site. In contrast, the second pattern, similar to HIV-uninfected cases, is independent of EBV, includes typical genetic alterations, and is evenly distributed between ABC- and GCB-types.
The predominance of the ABC phenotype that we observed in HIV-infected cases is consistent with some [12–14], but not all [15-22], previous studies of DLBCL by HIV status, though direct comparison of our results with some studies is hindered by inclusion of histologies other than DLBCL, evaluation of relatively few cases or, for immunohistochemistry-based studies, use of various antibodies (alone or in combination, with a range of cut-offs to determine positivity) to identify the cell of origin. Although we identified some DLBCLs with the “intermediate” phenotypes described previously, most of these patients were classified as ABC-DLBCL by the Tally algorithm in our study. Thus, our data do not clearly support previous studies that have suggested a particular propensity of DLBCLs in HIV-infected individuals to express an intermediate phenotype with increased co-expression of GCB and ABC markers [12,13,17,18,23,24]. However, DLBCLs that cannot be classified by cell of origin also have been reported in the general population [7], suggesting that further gene expression profiling studies with the ability to evaluate an intermediate phenotype with sufficient numbers of HIV-infected and HIV-uninfected cases are warranted.
Constitutive activation of the NF-κB pathway is one of the most important features of ABC-DLBCL, based on increased expression of a range of NF-κB target genes in microarray analyses and dependence of ABC-DLBCL cell lines on NF-κB activity [7]. EBV is known to be a key driver of lymphomagenesis in immunosuppressed individuals [11], and we demonstrated a nearly exclusive association between EBV and ABC-DLBCL in the HIV-infected cases in our study. Our results are consistent with several previous studies that also reported higher EBV prevalence in ABC- than GCB-DLBCL [13,17,20,22], and may support the importance of the transforming EBV protein LMP1 in activating the NF-κB pathway [11], preferentially leading to ABC-DLBCL, although data on NF-κB were not available. EBV also may preferentially lead to ABC-DLBCL by down-regulating BCL6 and up-regulating BCL2 [29-32]. In HIV-uninfected individuals, we observed a higher frequency of the BCL6/IgHin ABC-DLBCL than in GCB-DLBCL, which also is consistent with previous research [33]. Interestingly, although the BCL6/IgHoccurred in DLBCLs of only two HIV-infected cases, both were EBV- and had the ABC phenotype.
GCB-DLBCL is also associated with a range of genetic alterations, including translocations involving the BCL2and MYConcogenes [6,7]. In our study, the MYC/IgHwas indeed more common in GCB-DLBCL, regardless of HIV status. In contrast, we observed a higher frequency of the IgH/BCL2translocation in GCB-DLBCL only in HIV-uninfected cases.
The ABC phenotype was associated with adverse prognosis compared with GCB-DLBCL in our study, regardless of HIV status, although the associations were of borderline statistical significance. Although the prognostic significance of the cell of origin classification independent of the International Prognostic Index is well-established in HIV-uninfected cases [25], some studies [18,19,22], but not all [17], have reported a similar association in HIV-infected cases. These inconsistent results may be related to the use of different classification approaches to identify the cell of origin. Additionally, the role of underlying differences in patients' immune status may explain some inconsistencies, because the risk of DLBCL in HIV-infected individuals is related to immune suppression [34], and DLBCL risks have declined since the introduction of HAART [10]. Almost all cases in our study were diagnosed before 1996, so further studies in the HAART era that can directly evaluate the relationship of molecular characteristics to the degree of immunosuppression are warranted, and would be hypothesized to observe a higher proportion of GCB-DLBCL in HIV-related cases due to improved immune function.
The cell of origin may have been misclassified for some cases in our study because we used immunohistochemistry rather than gene expression profiling [35]. However, the Tally algorithm has the best concordance with gene expression profiling studies to date [25], and does not rely on BCL6, the germinal center marker associated with the most rapid antigen degradation [36]. In addition, we included blinded duplicates to ensure the reproducibility of our immunostain interpretation. Some misclassification also could have occurred because we used different cut-offs to determine positivity for FOXP1 (80%), MUM1 (80%) and GCET1 (60%) since the Tally algorithm (30% cut-off for all immunostains) was published after we completed immunostain interpretation. The difference in the cut-offs would bias away from classification of ABC-DLBCL because we used more stringent criteria for the two ABC markers, and thus our identification of a high proportion of ABC-DLBCL in HIV-infected cases is likely conservative. Our analyses of OS should be interpreted cautiously because we lacked detailed clinical information (e.g. International Prognostic Index parameters, DLBCL treatment, CD4 count, HIV treatment) and because nearly all HIV-infected cases were diagnosed before the introduction of HAART, and nearly all patients were diagnosed prior to the introduction of rituximab as standard therapy for DLBCL. Finally, because cases identified for this study had tissue specimens that otherwise would have been discarded by local area pathology departments (e.g. for logistical or storage constraints), our population was not necessarily representative of all DLBCL cases diagnosed in the LA SEER registry.
In summary, our results provide strong evidence for both similarities and differences in the molecular characteristics of DLBCLs in HIV-infected and HIV-uninfected individuals. Specifically, among HIV-infected cases, DLBCLs were predominantly EBV+, with nearly all EBV+ DLBCLs of the ABC-type and lacking typical lymphomagenic translocations. In contrast, a small proportion of DLBCLs in HIV- infected cases and nearly all DLBCLs in HIV-uninfected cases were EBV–, with near even distribution of ABC and GCB phenotypes, and increased frequency of the MYC/IgHand IgH/BCL2translocations in GCB-DLBCL. Future research evaluating the distribution and prognostic significance of molecular characteristics of DLBCLs in HIV-infected individuals should include patients treated with HAART and be able to measure the relationship of molecular characteristics to the degree of immunosuppression. In addition, analyses that directly compare a wider range of genetic alterations in DLBCLs by HIV status would provide further insight into the pathogenetic mechanisms of lymphomagenesis.
References
- 1.Howlader N, Noone A, Krapcho M, et al. Bethesda, MD: National Cancer Institute; SEER Cancer Statistics Review, 1975-2008. Available from: http://seer.cancer.gov/csr/1975_2008/.2011. [Google Scholar]
- 2.Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 3.Rosenwald A, Wright G, Leroy K, et al. Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. J Exp Med. 2003;198:851–862. doi: 10.1084/jem.20031074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:1937–1947. doi: 10.1056/NEJMoa012914. [DOI] [PubMed] [Google Scholar]
- 5.Lenz G, Wright GW, Emre NC, et al. Molecular subtypes of diffuse large B-cell lymphoma arise by distinct genetic pathways. Proc Natl Acad Sci USA. 2008;105:13520–13525. doi: 10.1073/pnas.0804295105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pasqualucci L, Trifonov V, Fabbri G, et al. Analysis of the coding genome of diffuse large B-cell lymphoma. Nat Genet. 2011;43:830–837. doi: 10.1038/ng.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schneider C, Pasqualucci L, Dalla-Favera R. Molecular pathogenesis of diffuse large B-cell lymphoma. Semin Diagn Pathol. 2011;28:167–177. doi: 10.1053/j.semdp.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knowles DM. Etiology and pathogenesis of AIDS-related non-Hodgkin's lymphoma. Hematol Oncol Clin North Am. 2003;17:785–820. doi: 10.1016/s0889-8588(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 9.Moir S, Fauci AS. B cells in HIV infection and disease. Nat Rev Immunol. 2009;9:235–245. doi: 10.1038/nri2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engels EA, Pfeiffer RM, Goedert JJ, et al. Trends in cancer risk among people with AIDS in the United States 1980-2002. AIDS. 2006;20:1645–1654. doi: 10.1097/01.aids.0000238411.75324.59. [DOI] [PubMed] [Google Scholar]
- 11.Carbone A, Cesarman E, Spina M, et al. HIV-associated lymphomas and gamma-herpesviruses. Blood. 2009;113:1213–1224. doi: 10.1182/blood-2008-09-180315. [DOI] [PubMed] [Google Scholar]
- 12.Deffenbacher KE, Iqbal J, Liu Z, et al. Recurrent chromosomal alterations in molecularly classified AIDS-related lymphomas: an integrated analysis of DNA copy number and gene expression. J Acquir Immune Defic Syndr. 2010;54:18–26. doi: 10.1097/QAI.0b013e3181d3d9eb. [DOI] [PubMed] [Google Scholar]
- 13.Carbone A, Gloghini A, Larocca LM, et al. Expression profile of MUM1/IRF4, BCL-6, and CD138/syndecan-l defines novel histogenetic subsets of human immunodeficiency virus-related lymphomas. Blood. 2001;97:744–751. doi: 10.1182/blood.v97.3.744. [DOI] [PubMed] [Google Scholar]
- 14.Capello D, Martini M, Gloghini A, et al. Molecular analysis of immunoglobulin variable genes in human immunodeficiency virus-related non-Hodgkin's lymphoma reveals implications for disease pathogenesis and histogenesis. Haematologica. 2008;93:1178–1185. doi: 10.3324/haematol.12705. [DOI] [PubMed] [Google Scholar]
- 15.Delecluse HJ, Hummel M, Marafioti T, et al. Common and HIV-related diffuse large B-cell lymphomas differ in their immunoglobulin gene mutation pattern. J Pathol. 1999;188:133–138. doi: 10.1002/(SICI)1096-9896(199906)188:2<133::AID-PATH349>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 16.Kurosu K, Weiden MD, Takiguchi Y, et al. BCL-6 mutations in pulmonary lymphoproliferative disorders: demonstration of an aberrant immunological reaction in HIV-related lymphoid interstitial pneumonia. J Immunol. 2004;172:7116–7122. doi: 10.4049/jimmunol.172.11.7116. [DOI] [PubMed] [Google Scholar]
- 17.Chadburn A, Chiu A, Lee JY, et al. Immunophenotypic analysis of AIDS-related diffuse large B-cell lymphoma and clinical implications in patients from AIDS Malignancies Consortium clinical trials 010 and 034. J Clin Oncol. 2009;27:5039–5048. doi: 10.1200/JCO.2008.20.5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffmann C, Tiemann M, Schrader C, et al. AIDS-related B-cell lymphoma (ARL): correlation of prognosis with differentiation profiles assessed by immunophenotyping. Blood. 2005;106:1762–1769. doi: 10.1182/blood-2004-12-4631. [DOI] [PubMed] [Google Scholar]
- 19.Xicoy B, Ribera JM, Mate JL, et al. Immunohistochemical expression profile and prognosis in patients with diffuse large B-cell lymphoma with or without human immunodeficiency virus infection. Leuk Lymphoma. 2010;51:2063–2069. doi: 10.3109/10428194.2010.520772. [DOI] [PubMed] [Google Scholar]
- 20.Vilchez RA, Lopez-Terrada D, Middleton JR, et al. Simian virus 40 tumor antigen expression and immunophenotypic profile of AIDS-related non-Hodgkin's lymphoma. Virology. 2005;342:38–46. doi: 10.1016/j.virol.2005.06.053. [DOI] [PubMed] [Google Scholar]
- 21.Little RF, Pittaluga S, Grant N, et al. Highly effective treatment of acquired immunodeficiency syndrome-related lymphoma with dose-adjusted EPOCH: impact of antiretroviral therapy suspension and tumor biology. Blood. 2003;101:4653–4659. doi: 10.1182/blood-2002-11-3589. [DOI] [PubMed] [Google Scholar]
- 22.Dunleavy K, Little RF, Pittaluga S, et al. The role of tumor histogenesis, FDG-PET, and short-course EPOCH with dose-dense rituximab (SC-EPOCH-RR) in HIV-associated diffuse large B-cell lymphoma. Blood. 2010;115:3017–3024. doi: 10.1182/blood-2009-11-253039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klein U, Gloghini A, Gaidano G, et al. Gene expression profile analysis of AIDS-related primary effusion lymphoma (PEL) suggests a plasmablastic derivation and identifies PEL-specific transcripts. Blood. 2003;101:4115–4121. doi: 10.1182/blood-2002-10-3090. [DOI] [PubMed] [Google Scholar]
- 24.Madan R, Gormley R, Dulau A, et al. AIDS and non-AIDS diffuse large B-cell lymphomas express different antigen profiles. Mod Pathol. 2006;19:438–446. doi: 10.1038/modpathol.3800493. [DOI] [PubMed] [Google Scholar]
- 25.Meyer PN, Fu K, Greiner TC, et al. Immunohistochemical methods for predicting cell of origin and survival in patients with diffuse large B-cell lymphoma treated with rituximab. J Clin Oncol. 2011;29:200–207. doi: 10.1200/JCO.2010.30.0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goodman MT, Hernandez BY, Hewitt S, et al. Tissues from population-based cancer registries: a novel approach to increasing research potential. Hum Pathol. 2005;36:812–820. doi: 10.1016/j.humpath.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 27.Chang K, Chen YY, Shibata D, et al. In situ hybridization methodology for the detection of EBV EBER-1 RNA in paraffin-embedded tissues, as applied to normal and neoplastic tissues. Diagn Mol Pathol. 1992;1:246–255. [PubMed] [Google Scholar]
- 28.Bedell V, Forman S, Gaal K, et al. Successful application of a direct detection slide-based sequential phenotype/genotype assay using archived bone marrow smears and paraffin embedded tissue sections. J Mol Diagn. 2007;9:589–597. doi: 10.2353/jmoldx.2007.070050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carbone A, Gaidano G, Gloghini A, et al. BCL-6 protein expression in AIDS-related non-Hodgkin's lymphomas: inverse relationship with Epstein-Barr virus-encoded latent membrane protein-1 expression. Am J Pathol. 1997;150:155–165. [PMC free article] [PubMed] [Google Scholar]
- 30.Martin-Perez D, Vargiu P, Montes-Moreno S, et al. Epstein-Barr virus microRNAs repress BCL6 expression in diffuse large B-cell lymphoma. Leukemia. 2012;26:180–183. doi: 10.1038/leu.2011.189. [DOI] [PubMed] [Google Scholar]
- 31.Finke J, Fritzen R, Ternes P, et al. Expression of bcl-2 in Burkitt's lymphoma cell lines: induction by latent Epstein-Barr virus genes. Blood. 1992;80:459–469. [PubMed] [Google Scholar]
- 32.Henderson S, Rowe M, Gregory C, et al. Induction of bcl-2 expression by Epstein-Barr virus latent membrane protein 1 protects infected B cells from programmed cell death. Cell. 1991;65:1107–1115. doi: 10.1016/0092-8674(91)90007-l. [DOI] [PubMed] [Google Scholar]
- 33.Iqbal J, Greiner TC, Patel K, et al. Distinctive patterns of BCL6 molecular alterations and their functional consequences in different subgroups of diffuse large B-cell lymphoma. Leukemia. 2007;21:2332–2343. doi: 10.1038/sj.leu.2404856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Biggar RJ, Chaturvedi AK, Goedert JJ, Engels EA. AIDS-related cancer and severity of immunosuppression in persons with AIDS. J Natl Cancer Inst. 2007;99:962–972. doi: 10.1093/jnci/djm010. [DOI] [PubMed] [Google Scholar]
- 35.Gutierrez-Garcia G, Cardesa-Salzmann T, Climent F, et al. Gene-expression profiling and not immunophenotypic algorithms predicts prognosis in patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Blood. 2011;117:4836–4843. doi: 10.1182/blood-2010-12-322362. [DOI] [PubMed] [Google Scholar]
- 36.de Jong D, Xie W, Rosenwald A, et al. Immunohistochemical prognostic markers in diffuse large B-cell lymphoma: validation of tissue microarray as a prerequisite for broad clinical applications (a study from the Lunenburg Lymphoma Biomarker Consortium) J Clin Pathol. 2009;62:128–138. doi: 10.1136/jcp.2008.057257. [DOI] [PubMed] [Google Scholar]


