Abstract
Understanding the cognitive impact of endogenously derived, and exogenously administered, hormone alterations is necessary for developing hormone treatments to support healthy brain function in women, especially during aging. The increasing number of studies in the burgeoning area of translational cognitive neuroendocrinology has revealed numerous factors that influence the extent and direction of female steroid effects on cognition. Here, we discuss the decision processes underlying the design of rodent hormone manipulation experiments evaluating learning and memory. It is noted that even when beginning with a clear hypothesis-driven question, there are numerous factors to consider in order to solidify a sound experimental design that will yield clean, interpretable results. Decisions and considerations include: age of animals at hormone administration and test, ovariectomy implementation, when to administer hormones relative to ovarian hormone loss, how and whether to monitor the estrous cycle if animals are ovary-intact, dose of hormone, administration route of hormone, hormone treatment confirmation protocols, handling procedures required for hormone administration and treatment confirmation, cognitive domains to be tested and which mazes should be utilized to test these cognitive domains, and control measures to be used. A balanced view of optimal design and realistic experimental practice and protocol is presented. The emerging results from translational cognitive neuroendocrinology studies have been diverse, but also enlightening and exciting as we realize the broad scope and powerful nature of ovarian hormone effects on the brain and its function. We must design, implement, and interpret hormone and cognition experiments with sensitivity to these tenets, acknowledging and respecting the breadth and depth of the impact gonadal hormones have on brain functioning and its rich plasticity.
Keywords: Memory, Cognition, Hormones, Aging, Behavior
1. Introduction
Elucidating the cognitive effects of endogenously released, and exogenously delivered, female steroids is a crucial step towards the goal of facilitating healthy brain aging in women. Both endogenous and exogenous hormone exposures occur throughout the life span in women; hormone levels naturally change with the reproductive cycle, age, and menopause, and they are purposefully altered with clinical regimens such as hormonal birth control and hormone therapy (HT). Preclinical and clinical hormone researchers have made substantial progress on this front, illuminating the impact that various factors related to aging, menopause, and hormone-containing treatments have on cognitive outcome.
A collection of methodical, well-designed research studies has led to an understanding that hormones and cognition are impacted by a multitude of factors, including many previously unexplored until the past decade. Because of the countless factors that can influence the outcome of a rodent hormone and cognition study, designing such a study can initially seem like a daunting task. When designing experiments to test effects of steroid hormones on memory, optimally, one begins with a hypothesis– or prediction – driven question regarding the effects of a hormone on a specific set of cognitive functions. However, even when beginning with what seems like a clear question, there are inevitably numerous factors to consider in order to solidify a sound experimental design that will yield clear and interpretable results. Decisions one must make include: age of animals at hormone administration and at test, whether animals should undergo ovariectomy (Ovx), when to administer hormones relative to Ovx, whether to monitor the estrous cycle if animals are ovary-intact, what dose of hormone should be given, how the hormone should be administered, which measures should be taken to confirm hormone administration, whether handling procedures required for administration and confirmation of hormone treatment impact the outcome measurement, which cognitive domains should be tested, which mazes should be utilized to test these cognitive domains, and what control measures should be used for the cleanest and most accurate interpretation possible. The goal of this paper is to address these important facets. We outline and discuss the decision processes that go into designing hormone experiments to evaluate learning and memory in the rodent. A balanced view of optimal design and realistic experimental practice and protocol is presented.
2. Estrogens are the examples primarily used in this discussion, but other hormones play more than just supporting roles
Most cognitive research evaluating ovarian hormone effects in the rodent focuses on estrogens, a class of hormones including 17β-estradiol (E2), estrone, and estriol. Thus, various estrogens are the majority of examples used herein, primarily the most potent endogenous form of estrogen, E2 (Kuhl, 2005), and the most commonly prescribed HT estrogen formulation, Conjugated Equine Estrogens (CEE, tradename Premarin) (Hersh et al., 2004). It is important to note that other ovarian hormones, such as progesterone, androgens, and metabolites of each, are becoming an increasingly popular area of study as their cognitive impact, both independently and interactively with estrogens, is becoming evident. Such newly noted estrogenic interactions are also true for synthetic hormones that are administered as part of contraceptives and/or HTs. These effects are undoubtedly complex, and may be task dependent. For example, there is evidence that progestogens and estrogens interact for cognition. Progesterone reverses the beneficial effects of E2 when they are co-administered (Bimonte-Nelson et al., 2006; Harburger et al., 2007). Recent work from the laboratory of Dr. Janice Juraska has shown that long-term treatment (approximately 6 months) with the combination of E2 and the synthetic progestin medroxyprogesterone acetate (MPA) can alter maze performance, with the direction of the effect possibly depending on the task requirements and solving strategy possibilities. Specifically, enhancements with E2 and MPA combination treatment have been seen on a T-maze alternation task (Chisholm and Juraska, 2012), which can tap spatial or non-spatial memory depending on the animal’s strategy. In contrast, compared to E2 treatment alone, E2 and MPA combination treatment impaired performance on other tasks, such as the spatial reference memory Morris water maze (MM) (Lowry et al., 2010). It is notable that in both Chisholm and Juraska (2012) and Lowry et al. (2010), effects seen with the combination treatment were not seen with any estrogen regimen when given alone. However, neither MPA nor progesterone were tested alone, so it cannot be determined whether the E2/progestogen interaction effects were accounted for by the progestogen component solely. Studies evaluating the effects of progestogens given alone indicate this could account for the noted impairments in these experiments. For example, treatment with MPA alone impaired performance on several measures of spatial memory in older Ovx rats (Braden et al., 2010), and these detrimental effects may be non-reversible (Braden et al., 2011). Collectively, these studies illustrate that it is important to test individual components of current and potential HTs, as well as to test them together as combination therapies. Teasing apart which individual components are beneficial or detrimental can aid in formulating a potentially “optimal” HT; however, interactions between several components must also be studied since this is the manner in which most women will be taking HT. Indeed, combination estrogen/progestin therapy is most commonly used since all women with an intact uterus must include a progestin in their regimen to offset the increased risk of endometrial hyperplasia with unopposed estrogen treatment, and the majority of women have not had a hysterectomy (NAMS, 2012).
3. Incorporating aging into hormone studies
The benefits of testing hormone effects in young animals are manifold, ranging from practical issues such as cost efficiency, to increased ease of scientific interpretation. Testing in young animals also means hormone effects can be interpreted independent from effects of old age. However, over the past decade it has become increasingly clear that ovarian hormone effects on cognition vary with age. As such, age is an important factor to consider when designing a study to test hormone effects on learning and memory.
3.1. Cognitive decline with aging
In the United States, the proportion of the population that is over 65 is increasing. Currently, about 13% of the population is over 65, and by the year 2020 this percentage is expected to increase to 20% due to aging of the “baby boomer” generation (U.S. Census Bureau, 2007, 2010). This underscores the importance of: (1) performing aging research, and (2) acknowledging that gonadal hormones change with age in both males and females, and that these changes might impact age-related cognitive decline. In fact, it is well documented that some memory loss is observed as aging ensues, although broad, severe learning and memory deficiencies are surely not an inevitable part of aging (Erickson & Barnes, 2003; Tulving & Craik, 2000). Of further note, rather than a uniform deterioration of cognitive function with age, aging tends to influence some types of learning and memory more than others.
Perhaps most notably altered with age is spatial learning and memory. Describing spatial memory to navigate through an environment has been an area of study for many decades. In 1948, the historic work of Tolman laid the foundation for discussing such processes in terms of a “cognitive map” (Tolman, 1948). Since then, we have learned that medial temporal lobe structures, such as the hippocampus, are involved in an animal’s ability to navigate effectively through an environment by acquiring, integrating, retaining and retrieving environmental features such as landmarks and other prominent cues (Barnes, 1998). Spatial working memory declines with normal aging, resulting in a memory impairment that becomes more severe as task difficulty increases (Balota et al., 2000; Bimonte et al., 2003). Working memory requires updating of information, and can be noted as manipulation of information kept “on-line”.
3.2. Age-related changes in cognitive responsiveness to hormone loss and replacement
Effects of female steroid hormones have been tested primarily in young animals, although there has been a recent increase in evaluations during aging. Generally, E2 has been shown to have a positive effect on spatial working memory tasks; however, some studies suggest that whether E2 treatment improves performance depends on memory demand, although this effect has not been tested at all ages. For example, in young Ovx rats, tonic E2-induced spatial working memory improvements are most pronounced when working memory demand is high (Bimonte and Denenberg, 1999). Performance enhancements have also been reported on working memory tasks with tonic E2 treatment in middle-aged Ovx rats (Rodgers at el., 2010; Witty et al., 2013), as well as aged Ovx rats, a finding that may be specific to task learning (Gibbs, 2000). Whether benefits of E2 treatment are more prominent at a higher working memory load in aging animals has not yet been determined.
Age-related changes in responsiveness to estrogen treatment have been shown for spatial reference memory. Reference memory is a form of long-term memory whereby information remains constant. In a study methodically testing E2 treatment at different ages ranging from young adulthood to reproductive senescence, aged Ovx rats were not responsive to the E2 treatment regimen that effectively enhanced spatial reference memory in young and middle-aged Ovx rats (Talboom et al., 2008). These findings concur with an elegantly performed systematic comparison of studies evaluating cognitive effects of estrogen-only or combination estrogen-progestin containing hormone therapies in women under the age of 65, versus in older women. Results suggest that there is little cognitive benefit from HT in women over the age of 65, while in younger women there is potential for HT to benefit specific cognitive domains (Maki, 2005). Thus, there seems to be a decline in responsiveness to estrogen treatment with advancing age, and time since menopause. Why, then, have some animal studies found that aged female rodents can exhibit cognitive enhancements in response to E2 treatment? For example, E2 injections enhanced spatial reference memory in 27–28 month old ovary-intact mice (Frick et al., 2002). The discrepancy may relate to whether E2 administration was cyclic versus tonic, as priming with cyclic E2 was shown to enhance responsiveness to tonic E2 in older Ovx rats (Markowska and Savonenko, 2002).
It also appears that sensitivity and responsiveness to ovarian hormone loss changes with age, and does not predict sensitivity and responsiveness to exogenous E2 treatment. For example, Talboom et al. (2008) found that young animals were responsive to both ovarian hormone removal and E2 replacement, middle-aged animals were not responsive to ovarian hormone removal but were responsive to E2 replacement, and aged animals were not responsive to ovarian hormone removal or E2 replacement for the test trials of a spatial reference memory task. In this study the middle-aged group, in particular, demonstrates that responsiveness to Ovx does not predict responsiveness to E2 treatment, since these animals benefitted from E2 administration but Ovx did not impact performance.
3.3. Changes in cognitive brain regions with aging
Specific brain areas known to mediate learning and memory function have been shown to be susceptible to aging, and these regions are also especially responsive to gonadal hormones. In particular, the basal forebrain and hippocampus are areas that appear to be particularly vulnerable to age-related changes (Luine et al., 1986; Burke & Barnes, 2006).
E2 has been shown to impact learning and memory through the basal forebrain cholinergic system, and E2-induced enhancements require this system to be intact for cognitive benefits to be realized (Luine et al., 1986; Gibbs, 2002, 2007; Shughrue and Merchenthaler, 2000). Estrogens and progesterone impact hippocampal morphology, including increasing hippocampal CA1 spine density in rats (Gould et al., 1990; Silva et al., 2000; Woolley and McEwen, 1992, 1993). Dendritic spines have been theorized to be a structural representation of learning and memory (Geinisman, 2000; Kasai et al., 2003; Moser, 1999; Nimchinsky et al., 2002; Sorra and Harris, 2000), leading to wide-reaching implications regarding ovarian hormones’ influence on cognitive function. This tenet is supported by recent rodent work showing that spatial working and reference memory performance is enhanced after E2 injections (McLaughlin et al., 2008; Sandstrom and Williams, 2001, 2004) within the timeframes corresponding to E2-induced increases in hippocampal dendritic spines (Gould et al., 1990; Silva et al., 2000; Woolley and McEwen, 1992, 1993) and other markers of synaptic plasticity (Foy, 2001). Consequently, age-related changes in dendritic structure and synapses may relate to the functional outcomes of hormone-containing treatments on hippocampal-dependent cognition.
The past two decades have built upon a prior framework of evidence, which is now beginning to provide clear, plausible, and mechanistic insights into interactions between aging, memory, and ovarian hormones. Evidence suggests that there are complex interactions between hormone-induced area-specific brain changes and aging. This complexity underscores the tenet that age is a critical factor and pivotal decision point of the experimental design process for rodent studies evaluating ovarian hormone effects on learning and memory. In order to be translational, the question of age must be considered and results must be interpreted in the context of age-related changes. For example, preclinical studies testing cognitive effects of contraceptives would then primarily focus on young adulthood and the most fertile age ranges. Likewise, studies testing cognitive effects of menopause and hormone therapies would primarily focus on older animals, since menopause is an age-related event in women and most HT exposure occurs during and after midlife. The latter point is true, of course, only if the researcher is interested in the effects of hormone therapies in the context of an aging brain. To evaluate effects of the hormone/s in question independent of aging, young animals should be utilized.
4. Baseline hormone level and reproductive senescence status
Baseline hormone level must be taken into account when designing studies to test the behavioral effects of endogenously circulating ovarian hormones, and exogenously administered hormones, in the rodent. When assessing the cognitive impact of steroid hormones in humans, researchers have been very creative in their study designs. Effects have been reported before versus after HT treatment in surgically menopausal women (Sherwin, 2006), across menopause transition stages (Luetters et al., 2007), as well as with sex-change operations and associated steroid hormone treatment (Gómez-Gil et al., 2009). To test the cognitive effects of steroid hormones in rodent models, the traditional procedure is to remove the ovaries (Ovx), which is the source of major endogenous synthesis and release, followed by exogenous delivery of the steroid of question as a treatment regimen.
It is notable that the ultimate hormone profile of the older, reproductively senescent ovary-intact woman differs from that of the aged, reproductively senescent ovary-intact female rat. In women, menopause, typically occurring in the fifth decade of life, is characterized by loss of ovarian-derived circulating hormones, including estrogen and pro-gesterone, due to decreased ovarian follicular reserves (Timaras et al., 1995). In contrast, the aging rat undergoes estropause, a persistent estrus state due to chronic anovulation, rendering intermediate estrogen levels, or a pseudo-pregnant/persistent diestrus state characterized by high progesterone levels, due to increased ovulation and number of corpora lutea (Meites and Lu, 1994). These ovarian changes in the rat are mainly due to hypothalamic/pituitary axis alterations (Meites and Lu, 1994). Thus, the hypothalamic-pituitary axis is the primary mechanism underlying reproductive senescence and circulating hormone changes in the rat, while follicular depletion in the ovary is what ultimately causes the major hormonal changes seen during menopause in women. This limits the use of the ovary-intact female aged rat as a model for aging in women, as it is not an optimal model of human menopause for evaluating cognition. The Ovx model of menopause can be interpreted as problematic as well, as the majority of women do not undergo surgical menopause via oophorectomy (i.e., removal of the ovaries). Rather, most women experience menopause as a transitional hormone loss following age-related alterations of the hypothalamus, pituitary, and ovary, which ultimately result in follicular depletion and a drastic decline in ovarian release of estrogen and progesterone (Timaras et al., 1995). The Ovx rodent model is an appropriate model for surgical menopause in women, and it is, in fact, optimal to create a “blank slate” when attempting to isolate the effects of individual female steroid hormones and their specific interactions in preclinical studies.
A model of transitional menopause in rodents has recently become available. The industrial chemical 4-vinylcyclohexene diepoxide (VCD) can be used to reliably produce follicular depletion in rodents (Borman, et al., 1999; Flaws et al., 1994; Hu et al., 2001a, 2001b; Hu et al., 2002; Kao et al., 1999; Mayer et al., 2002, 2004, 2005; Springer et al., 1996a, 1996b, 1996c). VCD selectively destroys primary and primordial follicles by accelerating the natural process of atresia (Mayer et al., 2004; Springer et al., 1996b). Since the pool of primordial follicles is finite and cannot proliferate, destruction of these follicles results in ovarian failure (Hirshfield, 1991). Following treatment with VCD, similar to natural menopause in women, progesterone and androstenedione levels decrease and estrogen is depleted (Mayer et al., 2004, 2005). Androstenedione becomes the primary hormone released by the ovaries, resulting in a relatively androgen-rich hormone profile. The VCD model is a more accurate model of natural menopause in women, and has the potential to be very useful for methodical evaluation of the menopausal transition and subsequent HT administration effects on cognition (e.g., Acosta et al., 2009, 2010).
5. Timing of hormone administration
New data indicate that the cognitive benefit of estrogen administration is impacted by the delay between ovarian hormone loss and estrogen treatment. Thus, timing of hormone administration relative to ovarian hormone loss is a critical decision in designing a study to test ovarian hormone effects on learning and memory. In general, research indicates that the benefits become more limited when there is an extended window of time between hormone loss and hormone treatment. This has been seen in rat work evaluating spatial memory; for example, the Daniel laboratory showed that E2 treatment initiated immediately after Ovx enhanced spatial memory performance in middle-aged rats, but imparted no benefit when given after an extended window of 5 months after Ovx (Daniel et al., 2006). A similar “window of opportunity” for HT initiation is seen in women (Maki et al., 2011). It is noteworthy that these cognitive findings correspond with neurochemical data from young and middle-aged rats, showing E2 treatment given immediately after Ovx increased choline acetyltransferase (ChAT) levels in the hippocampus; yet, this increase was not seen when initiated 5 months after Ovx (Bohacek et al., 2008). We have also shown evidence of a critical window for the well-established regulation of hippocampal dendritic spines by E2 (Woolley, 2000), with a 10-week delay after Ovx attenuating the ability of E2 to increase CA1 apical spine density, compared to treatment given immediately, in young rats (McLaughlin et al., 2008). Thus, timing of hormone administration appears to be crucial when evaluating the cognitive impact of ovarian hormones, as well as the potential mechanisms underlying such effects. Researchers have noted the importance of this variable, as underscored by the recent increase in studies evaluating specific parameters and brain mechanisms underlying this “window of opportunity” for HT initiation (for review, see Daniel, 2012). In fact, some of the leading HT researchers recently organized a satellite conference to the annual American Aging Association meeting for research dedicated to optimizing HT and, specifically, elucidating the window of opportunity for HT initiation (Window of Opportunity Conference 2012, Fort Worth, TX). Utilizing basic science approaches to discover the parameters and brain mechanisms driving the window of opportunity of hormone effects on cognition will be critical to successful outcomes that could translate to the clinic. The hope is that these findings will ultimately enhance brain health and function in women.
6. Dosing and route of hormone administration
Dose is an important, and perhaps obvious, factor to consider when administering a female steroid hormone treatment. For example, low doses of E2 have been shown to improve, while high doses impair, spatial and non-spatial working memory in rodents (Holmes et al., 2002, Wide et al., 2004). When discussing the idea of dose-specific effects, it is important to consider to which “low” and “high” is relative. For example, in the Holmes et al. (2002) paper, the low dose was a daily injection of 0.32 μg of E2 benzoate, which results in low physiological circulating E2 levels for a young female rat, and the high dose was a daily injection of 1.00 or 5.00 μg of E2 benzoate, which results in high physiological circulating E2 levels for a young rat. Thus, in this case “low” and “high” are representative of physiological levels as seen across a rat estrous cycle; however, in some cases “high” can mean supraphysiological levels that are not seen within the range of naturally occurring endogenous levels. It is important to interpret studies within the appropriate relative dose framework. For example, if a higher supraphysiological E2 dose were included in the Holmes et al. study, the formerly two highest doses would now be “middle” doses, and the “highest” dose might have a different effect.
This kind of relative framework and structure is important because many hormones exhibit an inverted-U shaped relationship with cognition. For example, in some cases low and high levels fail to positively impact, or might even impair, cognition, while mid-range levels improve cognition. Several human studies testing cognition illustrate this point well; indeed, in older adult men, higher and lower levels of circulating testosterone are associated with impaired performance, while levels in between are associated with optimal performance (Barrett-Connor et al., 1999; Yaffe et al., 2002; Cherrier et al., 2007; Martin et al., 2008; Gouchie and Kimura, 1991). Interestingly, there is also evidence for a U-shaped relationship between serum luteinizing hormone (LH) and spatial memory performance, with moderate LH levels associated with poorest performance (Acosta et al., 2009); however, this quadratic relationship between hormones and memory is not always seen (e.g., with serum follicle stimulating hormone, FSH, Acosta et al., 2009).
Route of administration is another key factor to consider when designing a hormone and cognition study in the rodent. Behavioral endocrinology researchers have taken advantage of the many methods available to administer hormones. The majority of rodent studies testing female steroid hormones and memory use cyclic subcutaneous injections (e.g. Fader et al., 1998; Daniel and Dohanich, 2001; Frick et al., 2002; Gresack and Frick, 2006; Bimonte-Nelson et al., 2006; Acosta et al., 2009; Braden et al., 2011; McClure et al., 2012) or tonic delivery methods such as subcutaneous silastic capsules or osmotic pumps (e.g. Daniel et al., 1997; Fader and Johnson 1999; Gibbs, 2002; Talboom et al., 2008; Braden et al., 2010; Gibbs et al., 2011a, 2011b; Engler-Chiurazzi et al., 2011). Of note, the subcutaneous route is not the most utilized in humans; the most commonly prescribed contraceptive and HT formulations are taken orally (Curtis et al., 2005). However, while oral administration is not common in rodent studies, there has been some work done using this method. A study in middle-aged Ovx mice that delivered E2 in drinking water found the medium dose to impair spatial reference memory, while all doses improved non-spatial object recognition performance relative to vehicle treatment (Fernandez and Frick, 2004). Other studies in middle-aged Ovx rats that delivered hormones in the drinking water found enhanced acquisition of a non-spatial alternation task following administration of E2 in combination with MPA, but not with E2 alone (Chisholm and Juraska, 2012), and enhanced performance on the spatial MM with chronic E2 treatment, but not with cyclic E2 or E2 plus MPA treatment (Lowry et al., 2010). These findings suggest that orally administered E2 can impact cognitive performance in the rat, but that effects are likely quite specific depending on both dose and task.
While we do note here that most human studies focus on orally administered hormones, we would be remiss if we did not mention that there is accumulating evidence that transdermal regimens can be beneficial. In fact, the human literature testing transdermal methods of administration is among the most consistent in the field. For example, in randomized, placebo controlled studies assessing women with mild-moderate probable Alzheimer’s disease (AD), treatment with E2-containing transdermal patches had a positive effect on attention, verbal memory, visual memory and semantic memory (Asthana et al., 1999; Wharton et al., 2011). Additionally, the pharmacokinetics of transdermally delivered E2 differ from oral administration, as oral administration results in an estrone to E2 ratio between 5:1 and 7:1, while transdermal delivery circumvents hepatic metabolism in the liver, resulting in a ratio of estrone to E2 of about 1:1, closer to that of a menopausal woman (Coelingh Bennink, 2004; for full review of E2 dosing and route of administration literature see Gleason et al., 2005).
In animal research, subcutaneous capsules and osmotic pumps are methods of administration designed to deliver treatment at a steady rate for a relatively long period of time. In humans, transdermal patches and vaginal inserts are available methods of achieving tonic hormone delivery. These are in contrast to the cyclic pattern achieved with administration via injection or with oral administration, which can be used for either acute or long-term treatment durations. Tonic versus cyclic administration may influence the cognitive impact of hormone treatments, possibly through cycling of estrogen receptors (ERs). Several rodent studies have shown that cyclic treatment with E2 produces a decrease in number of cytoplasmic ERs 1 h after administration, accompanied by an increase in nuclear ER levels, presumably as the bound estrogen receptor complex travels to the nucleus. From 2 to 4 h after E2 delivery, nuclear ER levels decrease by 60–70% and cytoplasmic ER levels remain low. Finally, there is an overall increase in ER number 12 h after administration and ER levels return to control levels by 16 h after E2 administration (Sarff and Gorski, 1971; Kassis and Gorski, 1981; Li et al., 1993, Rosser et al., 1993). It is unknown how ERs react to a tonic release of hormone, however.
7. Measures to confirm hormone phase and treatment, and “controlling for” estrous cycle phase
To determine estrous cycle phase, or verify Ovx and subsequent hormone treatment, vaginal smears can be taken before and/or after behavioral testing. There is evidence that performance on spatial memory tasks, such as the MM, varies across the estrous cycle in young animals, with rats in the proestrus phase outperforming rats in the estrus phase (Warren and Juraska, 1997). If ovary-intact young animals are being used, the estrous cycle could be accounted for or randomized across treatment groups. However, in reality this is a challenging endeavor, and experimental protocols end up fraught with timing conflicts relating to cycle phase. Indeed, even though animals that share an air supply tend to have similar estrous cycles, it is rare for all animals to completely synchronize cycling (McClintock, 1978). In addition, many studies, even with rigorous and methodical experimental comparisons across animals in varied estrous cycle phases, still find null estrous cycle effects on cognition, as tested on a delayed non-match to sample working memory task for example (Stackman et al., 1997), possibly due to high variability within group of estrous cycle phase. For these reasons, in many laboratories testing ovary-intact females, including ours, a “natural” counterbalancing of estrous cycle phase across ovary-intact treatment groups is assumed with the resulting variability acknowledged as error variance in the data. However, in ovary-intact aged female rodents, estropause phase should be taken into account when possible, as there is clearer evidence that it impacts cognitive aging (Markowska, 1999; Warren and Juraska, 2000).
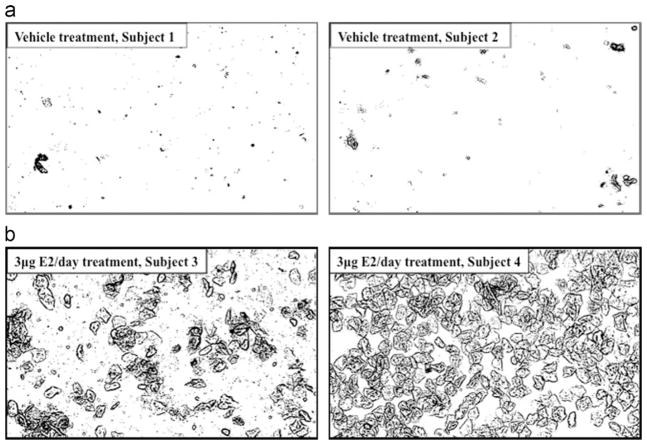
In ovary-intact animals, vaginal smears can be classified as proestrus, estrus, metestrus, or diestrus, according to vaginal cytology (Goldman et al., 2007). If Ovx animals are being utilized, vaginal smears can be done to determine whether Ovx and subsequent hormone treatment were “successful”. Fig. 1 provides representative pictures from our laboratory of four Ovx animals, two of which received vehicle treatment (Fig. 1a), and two of which received 3 μg of E2 per day for 19 days via a subcutaneous Alzet osmotic pump (Fig. 1b). In the vehicle-treated animals, very few cells are present in general, indicating an estrogen “blank” animal, while the smears from animals receiving E2 treatment contain many cells, most of which are cornified cells, indicating estrogenic stimulation and “successful” E2 treatment.
Fig. 1.
Vaginal smears at 10 ×magnification from representative animals receiving: (a) vehicle treatment (polyethylene glycol) and (b) 3 μg/day 17β-estradiol treatment in vehicle (polyethylene glycol).
Ovx and hormone treatments are also known to alter body weights. Specifically, Ovx or treatment with progesterone produces an increase in body weights, while treatment with estrogens or androgens promote weight loss and decrease body weights; of note, early pre-pubertal exposure to androgens or estrogens can alter these relationships in adulthood (Bell and Zucker, 1971; Wade, 1972). These effects can be used as a confirmation method of Ovx status or hormone treatment.
There are other procedures that can help to confirm treatments. At sacrifice, uterine wet weights can be recorded as another marker of peripheral stimulation, once uteri have been removed and trimmed of all visible fat (Westerlind et al., 1998). Osmotic pumps can be removed and visually inspected upon sacrifice to verify pump integrity. Obtaining serum hormone levels in preclinical studies can help with clinical translation, and can lead to new hypotheses and predictions about relations between hormones and memory. For example, we performed a study testing MPA in female rats (Braden et al., 2010). In this study, we confirmed that serum levels of MPA were within the range of serum levels measured in women taking the hormone. This was particularly important in this study since we were testing MPA, which is a synthetic hormone that is not endogenously released in women or rats. As an illustration of how obtaining serum hormone levels can help form new hypotheses, in Acosta et al. (2010), we found a positive correlation between serum androstenedione levels and working memory errors using the transitional VCD model of menopause. This led to the hypothesis that androstenedione released from the follicle-deplete ovary was impacting spatial learning and memory, and resulted in a methodical manipulation of androstenedione levels in a subsequent study which found profound androstenedione effects on cognition (Camp et al., 2012). Serum hormone levels can also be used to provide a comparison to physiological levels that would be seen across the estrous cycle in the rodent, or to rule out systematic group differences in estrous cycle stage (Gibbs et al., 1998; Hammond et al., 2012). Thus, if possible, serum should be collected during the study, or at sacrifice, to be analyzed for the various hormones of interest in order to confirm treatment measures, to provide insight into the translational validity of the experimental design, and to potentially yield new directions for research.
8. Acknowledge complexity in your dependent variables, and entertain alternate interpretations of results, because hormones impact… well… close to everything
In order to test cognition, researchers must operationally define performance, and use these definitions to interpret results. As prudent experimentalists we must, of course, acknowledge that there is always the potential for modifications to be made to optimize our working definitions or task designs. A superb example is the beautifully creative study by van Haaren and van de Poll (1984). In this study, it was shown that the addition of an alternative choice (a third chamber) in a passive avoidance shock task, traditionally offering only two chambers, abolished the well-established sex difference in task performance. This study indicates that the previously observed sex difference of female “impairment” on this task was not due to a memory deficit in females. Given the established finding that females are more exploratory than males (Anderson, 1941; Bengelloun et al., 1976), in the two-chambered task it was possible that females moved to the shock-paired chamber due to this increased motor activity, and not due to a memory deficit. In fact, once given an alternate option, female rats no longer returned to the shock-paired chamber. Instead, the females preferred the third chamber that was not previously associated with a foot shock. Thus, the two-chamber version of this task produced a clear sex difference, which had been attributed to a lack of memory of the shock location in the female rats. However, once given another option, it was clear that females preferred to avoid the chamber where the shock had previously been administered (since they moved to the now present optional third chamber), thereby indicating memory of the shock location. Simply put, the operational definition of memory performance in the traditional task, leading to interpretation of a female memory impairment, was in actuality an increase in female ambulatory and exploratory behavior.
There are several tasks available to control for possible confounds in interpretation of behavioral data. For laboratories that use water escape tasks, to avoid potential confounds with swim ability, the number of incorrect arm entries or swim distance to the platform should be used as outcome measures rather than latency to the platform, which can be difficult to interpret due to potential confounds with swim ability. Generally, the number of errors and swim distance are minimally impacted by motor ability, so long as subjects are able to locate the platform within the allotted trial time. However, if subjects are experiencing motor impairments to such a degree that they are unable to locate the platform during a given trial, the low number of incorrect arm entries or low swim distance could potentially be incorrectly interpreted as superior cognitive performance. The addition of a visible platform task can be used to rule out performance deficits on cognitive swim tasks, including motor and vision deficiencies (Morris et al., 1982; Hunter et al., 2003).
For Morris maze testing, a probe trial should be administered at the end of testing, whereby the platform is removed from the maze and animals are allowed to swim for the maximum trial time. Percent swim distance in the previously platformed quadrant should be greater than in the diagonally opposite quadrant, indicating that a spatial strategy was used to locate the platform, rather than a motoric strategy. A finer measure of place learning that can be recorded during the probe trial is the number of platform crossings. Crossings through the platform area itself, as well as through enlarged annuli around the platform, can be used to determine the extent of spatial localization of the platform that animals were able to learn. These analyses allow one to ask whether animals were able to navigate to the exact location of the platform, or to only the more general, less localized, area that contained the platform (the quadrant or a larger annulus around the platform).
If further analysis of the probe trial reveals an interaction with percent distance and treatment, this may indicate a bias toward a non-spatial strategy in some groups. In fact, several studies have assessed the impact of circulating estrogen on strategy choice in rodents, reporting findings that E2-treated Ovx rats outperformed vehicle-treated rats on a spatial task while vehicle treated rats excelled at a response task (Korol and Kolo, 2002), that rats are more likely to use place strategies during proestrus when endogenous estrogen levels are high (Korol, 2004), and that treatment with E2 influences strategy choice (Daniel and Lee, 2004). Of note, inhibiting hippocampal activity through a GABA receptor agonist in the hippocampus shifts preference to a response strategy thereby indicating that hippocampal inhibition is involved in strategy selection. (McElroy and Korol, 2005). Together, these studies suggest that circulating hormones levels and hippocampal inhibition can influence strategy preference, manifesting as better or worse performance on spatial tasks. It is additionally noted that recent work has indicated that these strategy preferences may be limited to the learning phase of the task (Schmidt et al., 2009).
It is also possible for the experimental procedures, including those necessary to implement the experiment, to impact the cognitive scores of animals given various hormone manipulations. An excellent example of such an effect is the finding that handling of animals necessary for experimental procedures can impact the cognitive effects of hormone treatments; specifically, increased handling has been shown to enhance performance on a working memory task and to obviate the benefits of E2 treatment following a delay between trials (Bohacek and Daniel, 2007). The potential of handling effects is especially relevant when comparing different routes of administration as well as when choosing a behavioral task to measure cognition. Additionally, the dependent measures of cognitive function may interact with the cognitive impact of hormone treatment. In fact, it has been shown that a single day of Morris maze testing can abolish E2 benzoate’s ability to increase dendritic spine density in the rat hippocampus (Frick et al., 2004), an effect that has been replicated many times in untested animals (Woolley and McEwen, 1993).
Fig. 2 shows the various factors discussed herein that are likely to influence the cognitive outcome of any hormone treatment; many of these factors should be taken into account when designing any behavior study, but these issues become especially salient when studying hormones and cognition. Indeed, hormones interact with numerous brain systems related to cognition and other functions, and as a result, there are ample opportunities for unanticipated interactions to arise. It is likely that at least some of these factors, which could be considered “nuances” of hormone effects, will one day be key for understanding the cognitive effects of hormones.
Fig. 2.
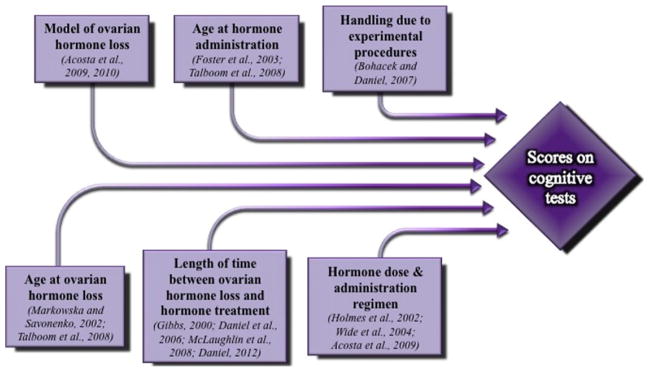
Putative factors that may influence the cognitive outcome of hormone treatment, as determined via methodical manipulation in rodent studies. Examples from the rat literature are in parentheses.
9. Carefully designed hormone and behavior studies can ultimately inform clinical decisions
At the beginning of the 20th century, steroid hormones were referred to as “internal secretions”, and were argued to be chemical mediators of the phenotype (Parkes, 1927). Since this time, exciting revelations have come to light showing that gonadal hormones are key not just for classic reproductive actions (Beach, 1947), but also for affording neural plasticity and influencing brain functions. To the excitement of behavioral neuroendocrinologists and clinicians alike, research studying female steroid hormones has been increasing in intensity, as well as in breadth and depth. It is likely that much of this is because of the recent research and debate regarding whether HTs impact women undergoing normal aging, or those with Alzheimer’s disease. For example, a meta-analysis found that estrogen-containing HTs decreased the risk of AD by 29% (Yaffe et al., 1998) and placebo-controlled studies have shown that estrogens enhanced memory, or improved dementia scores in female AD patients (Asthana et al., 1999, 2001; Ohkura et al., 1994, 1995). Other studies have found that menopause could exacerbate age-related cognitive changes in visuospatial abilities and possibly other domains as well (Halbreich et al., 1995). More recently, the outcome of the Women’s Health Initiative studies showed null or detrimental effects on cognition or dementia from the most commonly used HTs (for discussion, see Sherwin and Henry, 2008). The clinical implications of understanding the effects of ovarian hormone administration during or after menopause, as well as changes associated with ovarian hormone loss, have been underscored by realization that life expectancy of women has increased from an average of 54 years in 1900, to a recent estimate of 81 years, in the United States (Singh et al., 1996; World Factbook 2012 estimate). Indeed, even though life expectancy is increasing, the age of spontaneous menopause has remained relatively stable. Thus, women are now living approximately one-third of their lives in a hypo-estrogenic menopausal state (Amundsen and Diers, 1970, 1973; Sherwin, 2003). This is quite poignant when we recognize how much we have to learn about ovarian hormone loss with menopause, the effects of HTs, and optimizing HTs including a perspective of options tailored to each individual woman. The overarching goal of this research is to explore the impact of available hormones using a systems approach in order to create the most effective and safe HT formulations, taking into account the many important aspects of women’s health. These aspects include brain health during aging. Further evaluations of the mechanisms underlying these hormone effects can also open the door to novel non-hormonal therapies that have yet to be utilized, a tactic that is beginning to be cleverly implemented by some research groups (for review see Frick, 2012).
The emerging results from the field have been diverse, but also enlightening and exciting as we realize the broad scope and powerful nature of ovarian hormone effects on the brain and its function. In fact, data have shown that several experimental parameters can influence the extent, and even the direction, of ovarian hormone effects on learning and memory. We must design, implement, and interpret hormone and cognition experiments with sensitivity to these tenets, acknowledging and respecting the breadth and depth of the impact gonadal hormones have on brain functioning and its rich plasticity.
Acknowledgments
This work was funded by NIH Grant no. AG028084; state of Arizona, ADHS and the Arizona Alzheimer’s Disease Core Center. This work was also supported in part by funds from the IMSD Program at Arizona State University.
References
- Acosta J, Mayer L, Braden B, Nonnenmacher S, Mennenga S, Bimonte-Nelson HA. The cognitive effects of conjugated equine estrogens depend on whether menopause etiology is transitional or surgical. Endocrinology. 2010;151 (8):3795–3804. doi: 10.1210/en.2010-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta J, Mayer L, Talboom J, Tsang C, Smith C, Enders C, Bimonte-Nelson HA. Transitional versus surgical menopause in a rodent model: etiology of ovarian hormone loss impacts memory and the acetylcholine system. Endocrinology. 2009;150(9):4248–4259. doi: 10.1210/en.2008-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amundsen D, Diers C. The age of menopause in classical Greece and Rome. Human Biol. 1970;42 (1):79–86. [PubMed] [Google Scholar]
- Amundsen D, Diers C. The age of menopause in medieval Europe. Human Biol. 1973;45 (4):605–612. [PubMed] [Google Scholar]
- Anderson EE. Sex Differences in Timidity in Normal and Gonadectomized Rats. Pedagogical Seminary and J Genet Psychol. 1941:59. [Google Scholar]
- Asthana S, Baker L, Craft S, Stanczyk F, Veith R, Raskind M, Plymate S. High-dose estradiol improves cognition for women with AD: results of a randomized study. Neurology. 2001;57 (4):605–612. doi: 10.1212/wnl.57.4.605. [DOI] [PubMed] [Google Scholar]
- Asthana S, Craft S, Baker L, Raskind M, Birnbaum R, Lofgreen C, Veith R, et al. Cognitive and neuroendocrine response to transdermal estrogen in postmenopausal women with Alzheimer’s disease: results of a placebo-controlled, double-blind, pilot study. Psychoneuroendocrinology. 1999;24 (6):657–677. doi: 10.1016/s0306-4530(99)00020-7. [DOI] [PubMed] [Google Scholar]
- Balota DA, Dolan PO, Duchek JM. Memory changes in healthy young and older adults. In: Tulving E, Craik FIM, editors. Handbook of Memory. Oxford University Press; 2000. pp. 395–410. [Google Scholar]
- Barnes CA. Memory changes during normal aging: Neurobiological correlates. In: Martinez JA Jr, Kesner RP, editors. Neurobiology of Learning and Memory. Academic Press; San Diego, CA: 1998. pp. 247–273. [Google Scholar]
- Barrett-Connor E, Goodman-Gruen D, Patay B. Endogenous sex hormones and cognitive function in older men. J Clin Endocrinol Metab. 1999;84 (10):3681–3685. doi: 10.1210/jcem.84.10.6086. [DOI] [PubMed] [Google Scholar]
- Beach FA. Hormones and mating behaviora in vertebrates, Recent Progress in Hormone Research. Academic Press; New York, NY: 1947. [DOI] [PubMed] [Google Scholar]
- Bell D, Zucker I. Sex differences in body weight and eating: organization and activation by gonadal hormones in the rat. Physiol Behav. 1971;7 (1):27–34. doi: 10.1016/0031-9384(71)90231-9. [DOI] [PubMed] [Google Scholar]
- Bengelloun W, Nelson D, Zent H, Beatty W. Behavior of male and female rats with septal lesions: influence of prior gonadectomy. Physiol Behav. 1976;16 (3):317–330. doi: 10.1016/0031-9384(76)90139-6. [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Nelson ME, Granholm AC. Age-related deficits as working memory load increases: relationships with growth factors. Neurobiol Aging. 2003;24:37–48. doi: 10.1016/s0197-4580(02)00015-5. [DOI] [PubMed] [Google Scholar]
- Bimonte H, Denenberg V. Estradiol facilitates performance as working memory load increases. Psychoneuroendocrinology. 1999;24 (2):161–173. doi: 10.1016/s0306-4530(98)00068-7. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Francis K, Umphlet C, Granholm AC. Progesterone reverses the spatial memory enhancements initiated by tonic and cyclic oestrogen therapy in middle-aged ovariectomized female rats. Eur J Neurosci. 2006;24 (1):229–242. doi: 10.1111/j.1460-9568.2006.04867.x. [DOI] [PubMed] [Google Scholar]
- Bohacek J, Bearl A, Daniel J. Long-term ovarian hormone deprivation alters the ability of subsequent oestradiol replacement to regulate choline acetyltransferase protein levels in the hippocampus and prefrontal cortex of middle-aged rats. J Neuroendocrinol. 2008;20 (8):1023–1027. doi: 10.1111/j.1365-2826.2008.01752.x. [DOI] [PubMed] [Google Scholar]
- Bohacek Johannes, Daniel J. Increased daily handling of ovariectomized rats enhances performance on a radial-maze task and obscures effects of estradiol replacement. Horm Behav. 2007;52 (2):237–243. doi: 10.1016/j.yhbeh.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Borman S, BV, Kao S, Thompson K, Sipes I, Hoyer P. A single dose of the ovotoxicant 4-vinylcyclohexene diepoxide is protective in rat primary ovarian follicles. Toxicol Appl Pharmacol. 1999;158 (3):244–252. doi: 10.1006/taap.1999.8702. [DOI] [PubMed] [Google Scholar]
- Braden B, Garcia A, Mennenga S, Prokai L, Villa S, Acosta J, Lefort N, et al. Cognitive-impairing effects of medroxyprogesterone acetate in the rat: independent and interactive effects across time. Psychopharmacology. 2011;218 (2):405–418. doi: 10.1007/s00213-011-2322-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braden B, Talboom J, Crain I, Simard A, Lukas R, Prokai L, Scheldrup M, et al. Medroxyprogesterone acetate impairs memory and alters the GABAergic system in aged surgically menopausal rats. Neurobiol Learn Mem. 2010;93 (3):444–453. doi: 10.1016/j.nlm.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke S, Barnes C. Neural plasticity in the ageing brain. Nat Rev Neurosci. 2006;7 (1):30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- Camp BW, Gerson JE, Tsang CW, Villa SR, Acosta JI, Braden BB, Hoffman AN, Conrad CD, Bimonte-Nelson HA. High serum androstenedione levels correlate with impaired memory in the surgically menopausal rat: a replication and new findings, Eur. J Neurosci. 2012;36(8):3086–3095. doi: 10.1111/j.1460-9568.2012.08194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrier M, Matsumoto A, Amory J, Johnson M, Craft S, Peskind E, Raskind M. Characterization of verbal and spatial memory changes from moderate to supraphysiological increases in serum testosterone in healthy older men. Psychoneuroendocrinology. 2007;32 (1):72–79. doi: 10.1016/j.psyneuen.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm N, Juraska J. Long-term replacement of estrogen in combination with medroxyprogesterone acetate improves acquisition of an alternation task in middle-aged female rats. Behav Neurosci. 2012;126 (1):128–136. doi: 10.1037/a0026461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelingh Bennink H. Are all estrogens the same? Maturitas. 2004;47 (4):269–275. doi: 10.1016/j.maturitas.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Curtis MG, Overholt S, Hopkins M, DelConte A. Contraception. Glass’ Office Gynecology. Lippincott Williams & Wilkins; 2005. pp. 347–383. [Google Scholar]
- Daniel J. Estrogens, estrogen receptors, and female cognitive aging: The impact of timing. Horm Behav. 2012 doi: 10.1016/j.yhbeh.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Daniel J, Dohanich G. Acetylcholine mediates the estrogen-induced increase in NMDA receptor binding in CA1 of the hippocampus and the associated improvement in working memory. J Neurosci. 2001;21 (17):6949–6956. doi: 10.1523/JNEUROSCI.21-17-06949.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel J, Fader A, Spencer A, Dohanich G. Estrogen enhances performance of female rats during acquisition of a radial arm maze. Horm Behav. 1997;32 (3):217–225. doi: 10.1006/hbeh.1997.1433. [DOI] [PubMed] [Google Scholar]
- Daniel J, Hulst J, Berbling J. Estradiol replacement enhances working memory in middle-aged rats when initiated immediately after ovariectomy but not after a long-term period of ovarian hormone deprivation. Endocrinology. 2006;147 (1):607–614. doi: 10.1210/en.2005-0998. [DOI] [PubMed] [Google Scholar]
- Daniel J, Lee C. Estrogen replacement in ovariectomized rats affects strategy selection in the Morris water maze. Neurobiol Learn Mem. 2004;82 (2):142–149. doi: 10.1016/j.nlm.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Engler-Chiurazzi E, Tsang C, Nonnenmacher S, Liang W, Corneveaux J, Prokai L, et al. Tonic Premarin dose-dependently enhances memory, affects neurotrophin protein levels and alters gene expression in middle-aged rats. Neurobiol Aging. 2011;32 (4):680–697. doi: 10.1016/j.neurobiolaging.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson C, Barnes C. The neurobiology of memory changes in normal aging. Exp Gerontol. 2003;38 (1–2):61–69. doi: 10.1016/s0531-5565(02)00160-2. [DOI] [PubMed] [Google Scholar]
- Fader A, Hendricson A, Dohanich G. Estrogen improves performance of reinforced T-maze alternation and prevents the amnestic effects of scopolamine administered systemically or intrahippocampally. Neurobiol Learn Mem. 1998;69 (3):225–240. doi: 10.1006/nlme.1998.3820. [DOI] [PubMed] [Google Scholar]
- Fader A, Johnson PEM. Estrogen improves working but not reference memory and prevents amnestic effects of scopolamine on a radial-arm maze. Pharmacol Biochem Behav. 1999;62(4):711–717. doi: 10.1016/s0091-3057(98)00219-6. [DOI] [PubMed] [Google Scholar]
- Fernandez S, Frick K. Chronic oral estrogen affects memory and neurochemistry in middle-aged female mice. Behav Neurosci. 2004;118 (6):1340–1351. doi: 10.1037/0735-7044.118.6.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaws J, Doerr J, Sipes I, Hoyer P. Destruction of preantral follicles in adult rats by 4-vinyl-1-cyclohexene diepoxide. Reprod Toxicol. 1994;8 (6):509–514. doi: 10.1016/0890-6238(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Foy MR. 17β-estradiol: effect on CA1 hippocampal synaptic plasticity. Neurobiol Learn Mem. 2001;76 (3):239–252. doi: 10.1006/nlme.2001.4018. [DOI] [PubMed] [Google Scholar]
- Frick KM. Building a better hormone therapy? How understanding the rapid effects of sex steroid hormones could lead to new therapeutics for age-related memory decline. Behav Neurosci. 2012;126 (1):29–53. doi: 10.1037/a0026660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Burlingame L, Delaney S. Sex differences in neurochemical markers that correlate with behavior in aging mice. Neurobiol Aging. 2002;23(1):145–158. doi: 10.1016/s0197-4580(01)00237-8. [DOI] [PubMed] [Google Scholar]
- Frick KM, Fernandez S, Bennett, Prange-Kiel J, MacLusky MJ, Leranth C. Behavioral training interfereswith the abilityof gonadal hormones to increase CA1 spine synapse density in ovariectomized female rats. Eur J Neurosci. 2004;19 (11):3026–3032. doi: 10.1111/j.1460-9568.2004.03427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geinisman Y. Structural synaptic modifications associated with hippocampal LTP and behavioral learning. Cereb Cortex. 2000;10 (10):952–962. doi: 10.1093/cercor/10.10.952. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiol Aging. 2000;21:107–116. doi: 10.1016/s0197-4580(00)00103-2. [DOI] [PubMed] [Google Scholar]
- Gibbs R. Basal forebrain cholinergic neurons are necessary for estrogen to enhance acquisition of a delayed matching-to-position T-maze task. Horm Behav. 2002;42 (3):245–257. doi: 10.1006/hbeh.2002.1825. [DOI] [PubMed] [Google Scholar]
- Gibbs R. Estradiol enhances DMP acquisition via a mechanism not mediated by turning strategy but which requires intact basal forebrain cholinergic projections. Horm Behav. 2007;52 (3):352–359. doi: 10.1016/j.yhbeh.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Gibbs R, Burke A, Johnson D. Estrogen replacement attenuates effects of scopolamine and lorazepam on memory acquisition and retention. Horm Behav. 1998;34 (2):112–125. doi: 10.1006/hbeh.1998.1452. [DOI] [PubMed] [Google Scholar]
- Gibbs R, Chipman A, Hammond R, Nelson D. Galanthamine plus estradiol treatment enhances cognitive performance in aged ovariectomized rats. Horm Behav. 2011a;60 (5):607–616. doi: 10.1016/j.yhbeh.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs R, Chipman A, Nelson D. Donepezil plus estradiol treatment enhances learning and delay-dependent memory performance by young ovariectomized rats with partial loss of septal cholinergic neurons. Horm Behav. 2011b;59 (4):503–511. doi: 10.1016/j.yhbeh.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason C, Carlsson C, Johnson S, Atwood C, Asthana S. Clinical pharmacology and differential cognitive efficacy of estrogen preparations. Ann NY Acad Sci. 2005;1052:93–9115. doi: 10.1196/annals.1347.007. [DOI] [PubMed] [Google Scholar]
- Goldman J, Murr A, Cooper R. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res Part B, Develop Reprod Toxicol. 2007;80 (2):84–97. doi: 10.1002/bdrb.20106. [DOI] [PubMed] [Google Scholar]
- Gómez-Gil E, Cañizares S, Torres A, de la Torre F, Halperin I, Salamero M. Androgen treatment effects on memory in female-to-male transsexuals. Psychoneuroendocrinology. 2009;34 (1):110–117. doi: 10.1016/j.psyneuen.2008.08.017. [DOI] [PubMed] [Google Scholar]
- Gouchie C, Kimura D. The relationship between testosterone levels and cognitive ability patterns. Psychoneuroendocrinology. 1991;16 (4):323–334. doi: 10.1016/0306-4530(91)90018-o. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley C, Frankfurt M, McEwen B. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10 (4):1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresack J, Frick K. Effects of continuous and intermittent estrogen treatments on memory in aging female mice. Brain Res. 2006;1115 (1):135–147. doi: 10.1016/j.brainres.2006.07.067. [DOI] [PubMed] [Google Scholar]
- Hammond R, Nelson D, Kline E, Gibbs RB. Chronic treatment with a GPR30 antagonist impairs acquisition of a spatial learning task in young female rats. Horm Behav. 2012;62:367–374. doi: 10.1016/j.yhbeh.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbreich U, Lumley L, Palter S, Manning C, Gengo F, Joe S. Possible acceleration of age effects on cognition following menopause. J Psych Res. 1995;29 (3):153–163. doi: 10.1016/0022-3956(95)00005-p. [DOI] [PubMed] [Google Scholar]
- Harburger L, Bennett J, Frick K. Effects of estrogen and progesterone on spatial memory consolidation in aged females. Neurobiol Aging. 2007;28 (4):602–610. doi: 10.1016/j.neurobiolaging.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Hersh A, Stefanick M, Stafford R. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. Journal Am Med Assoc. 2004;291 (1):47–53. doi: 10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
- Hirshfield A. Development of follicles in the mammalian ovary. Int Rev Cytol. 1991;124:43–4101. doi: 10.1016/s0074-7696(08)61524-7. [DOI] [PubMed] [Google Scholar]
- Holmes M, Wide J, Galea L. Low levels of estradiol facilitate, whereas high levels of estradiol impair, working memory performance on the radial arm maze. Behav Neurosci. 2002;116 (5):928–934. doi: 10.1037//0735-7044.116.5.928. [DOI] [PubMed] [Google Scholar]
- Hu X, Christian P, Sipes I, Hoyer P. Expression and redistribution of cellular Bad, Bax, and Bcl-X(L) protein is associated with VCD-induced ovotoxicity in rats. Biol Reprod. 2001a;65 (5):1489–1495. doi: 10.1095/biolreprod65.5.1489. [DOI] [PubMed] [Google Scholar]
- Hu X, Christian P, Thompson K, Sipes I, Hoyer P. Apoptosis induced in rats by 4-vinylcyclohexene diepoxide is associated with activation of the caspase cascades. Biol Reprod. 2001b;65 (1):87–93. doi: 10.1095/biolreprod65.1.87. [DOI] [PubMed] [Google Scholar]
- Hu X, Flaws J, Sipes I, Hoyer P. Activation of mitogen-activated protein kinases and AP-1 transcription factor in ovotoxicity induced by 4-vinylcyclohexene diepoxide in rats. Biol Reprod. 2002;67 (3):718–724. doi: 10.1095/biolreprod.102.004259. [DOI] [PubMed] [Google Scholar]
- Hunter C, Bimonte H, Granholm A. Behavioral comparison of 4 and 6 month-old Ts65Dn mice: age-related impairments in working and reference memory. Behav Brain Res. 2003;138 (2):121–131. doi: 10.1016/s0166-4328(02)00275-9. [DOI] [PubMed] [Google Scholar]
- Kao S, Sipes I, Hoyer P. Early effects of ovotoxicity induced by 4-vinylcyclohexene diepoxide in rats and mice. Reprod Toxicol. 1999;13 (1):67–75. doi: 10.1016/s0890-6238(98)00061-6. [DOI] [PubMed] [Google Scholar]
- Kasai H, Matsuzaki M, Noguchi J, Yasumatsu N, Nakahara H. Structure-stability-function relationships of dendritic spines. Trends Neurosci. 2003;26 (7):360–368. doi: 10.1016/S0166-2236(03)00162-0. [DOI] [PubMed] [Google Scholar]
- Kassis J, Gorski J. Estrogen receptor replenishment. Evidence for receptor recycling. J Biol Chem. 1981;256 (14):7378–7382. [PubMed] [Google Scholar]
- Korol DL. Role of estrogen in balancing contributions from multiple memory systems. Neurobiol Learn Mem. 2004;82 (3):309–323. doi: 10.1016/j.nlm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Korol DL, Kolo LL. Estrogen-induced changes in place and response learning in young adult female rats. Behav Neurosci. 2002;116 doi: 10.1037//0735-7044.116.3.411. [DOI] [PubMed] [Google Scholar]
- Kuhl H. Breast cancer risk in the WHI study: the problem of obesity. Maturitas. 2005;51 (1):83–97. doi: 10.1016/j.maturitas.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Li H, Blaustein J, De Vries G, Wade G. Estrogen-receptor immunoreactivity in hamster brain: preoptic area, hypothalamus and amygdala. Brain Res. 1993;631 (2):304–312. doi: 10.1016/0006-8993(93)91549-8. [DOI] [PubMed] [Google Scholar]
- Lowry N, Pardon L, Yates M, Juraska J. Effects of long-term treatment with 17 beta-estradiol and medroxyprogesterone acetate on water maze performance in middle aged female rats. Horm Behav. 2010;58 (2):200–207. doi: 10.1016/j.yhbeh.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luetters C, Huang MH, Seeman T, Buckwalter G, Meyer P, Avis N, Sternfeld B, et al. Menopause transition stage and endogenous estradiol and follicle-stimulating hormone levels are not related to cognitive performance: cross-sectional results from the study of women’s health across the nation SWAN. J Women’s Heal (2002) 2007;16 (3):331–344. doi: 10.1089/jwh.2006.0057. [DOI] [PubMed] [Google Scholar]
- Luine V, Renner K, Heady S, Jones K. Age and sex-dependent decreases in ChAT in basal forebrain nuclei. Neurobiol Aging. 1986;7 (3):193–198. doi: 10.1016/0197-4580(86)90042-4. [DOI] [PubMed] [Google Scholar]
- Maki P. A systematic review of clinical trials of hormone therapy on cognitive function: effects of age at initiation and progestin use. Ann NY Acad Sci. 2005;1052:182–197. doi: 10.1196/annals.1347.012. [DOI] [PubMed] [Google Scholar]
- Maki P, Dennerstein L, Clark M, Guthrie J, Pamela L, Fornelli D, Little D, et al. Perimenopausal use of hormone therapy is associated with enhanced memory and hippocampal function later in life. Brain Res. 2011;1379:232–243. doi: 10.1016/j.brainres.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowska AL. Sex dimorphisms in the rate of age-related decline in spatial memory: relevance to alterations in the estrous cycle. J Neurosci. 1999;19 (18):8122–8133. doi: 10.1523/JNEUROSCI.19-18-08122.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowska A, Savonenko A. Protective effect of practice on cognition during aging: implications for predictive characteristics of performance and efficacy of practice. Neurobiol Learn Mem. 2002;78 (2):294–320. doi: 10.1006/nlme.2002.4064. [DOI] [PubMed] [Google Scholar]
- Martin D, Wittert G, Burns N, Jason M. Endogenous testosterone levels, mental rotation performance, and constituent abilities in middle-to-older aged men. Horm Behav. 2008;53 (3):431–441. doi: 10.1016/j.yhbeh.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Mayer L, Devine P, Dyer C, Hoyer P. The follicle-deplete mouse ovary produces androgen. Biol Reprod. 2004;71 (1):130–138. doi: 10.1095/biolreprod.103.016113. [DOI] [PubMed] [Google Scholar]
- Mayer L, Dyer C, Eastgard R, Hoyer P, Banka C. Atherosclerotic lesion development in a novel ovary-intact mouse model of perimenopause. Arterioscl Throm Vas Biol. 2005;25 (9):1910–1916. doi: 10.1161/01.ATV.0000175767.46520.6a. [DOI] [PubMed] [Google Scholar]
- Mayer L, Pearsall N, Christian P, Devine P, Payne C, Margaret M, Marion S, et al. Long-term effects of ovarian follicular depletion in rats by 4-vinylcyclohexene diepoxide. Reprod Toxicol Elmsford, NY. 2002;16 (6):775–781. doi: 10.1016/s0890-6238(02)00048-5. [DOI] [PubMed] [Google Scholar]
- McClintock M. Estrous synchrony and its mediation by airborne chemical communication. Horm Behav. 1978;10 (3):264–275. doi: 10.1016/0018-506x(78)90071-5. [DOI] [PubMed] [Google Scholar]
- McClure R, Barha C, Galea L. 17β-Estradiol, but not estrone, increases the survival and activation of new neurons in the hippocampus in response to spatial memory in adult female rats. Horm Behav. 2012;63(1):144–157. doi: 10.1016/j.yhbeh.2012.09.011. [DOI] [PubMed] [Google Scholar]
- McElroy M, Korol D. Intrahippocampal muscimol shifts learning strategy in gonadally intact young adult female rats. Learn Mem (Cold Spring Harb NY) 2005;12(2):150–158. doi: 10.1101/lm.86205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K, Bimonte-Nelson H, Neisewander J, Conrad C. Assessment of estradiol influence on spatial tasks and hippocampal CA1 spines: evidence that the duration of hormone deprivation after ovariectomy compromises 17beta-estradiol effectiveness in altering CA1 spines. Horm Behav. 2008;54 (3):386–395. doi: 10.1016/j.yhbeh.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meites J, Lu JKH. Reproductive aging and neuroendocrine function. In: Charlton HM, editor. Oxford review of reproductive biology. Vol. 16. Oxford Press; New York: 1994. [Google Scholar]
- Morris R, Garrud P, Rawlins J, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297 (5868):681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Moser M. Making more synapses: a way to store information? Cell Mol Life Sci. 1999;55 (4):593–600. doi: 10.1007/s000180050317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAMS The North American Menopause Society. The 2012 hormone therapy position statement of: The North American Menopause Society. Menopause. 2012;19(3):257–271. doi: 10.1097/gme.0b013e31824b970a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimchinsky E, Sabatini B, Svoboda K. Structure and function of dendritic spines. Ann Rev Physiol. 2002;64:313–353. doi: 10.1146/annurev.physiol.64.081501.160008. [DOI] [PubMed] [Google Scholar]
- Ohkura T, Isse K, Akazawa K, Hamamoto M, Yaoi Y, Hagino N. Evaluation of estrogen treatment in female patients with dementia of the Alzheimer type. Endocr J. 1994;41 (4):361–371. doi: 10.1507/endocrj.41.361. [DOI] [PubMed] [Google Scholar]
- Ohkura T, Isse K, Akazawa K, Hamamoto M, Yaoi Y, Hagino N. Long-term estrogen replacement therapy in female patients with dementia of the Alzheimer type: 7 case reports. Dementia. 1995;6 (2):99–9107. doi: 10.1159/000106929. [DOI] [PubMed] [Google Scholar]
- Parkes A. The Internal Secretions of the Ovary. Proc Royal Soc Med. 1927;20 (10):1663–1667. [PMC free article] [PubMed] [Google Scholar]
- Rodgers SP, Bohacek J, Daniel JM. Transient estradiol exposure during middle age in ovariectomized rats exerts lasting effects on cognitive function and the hippocampus. Endocrinology. 2010;151:1194–1203. doi: 10.1210/en.2009-1245. [DOI] [PubMed] [Google Scholar]
- Rosser M, Chorich L, Howard E, Zamorano P, Mahesh V. Changes in rat uterine estrogen receptor messenger ribonucleic acid levels during estrogen- and progesterone-induced estrogen receptor depletion and subsequent replenishment. Biol Reprod. 1993;48 (1):89–98. doi: 10.1095/biolreprod48.1.89. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Williams CL. Memory retention is modulated by acute estradiol and progesterone replacement. Behav Neurosci. 2001;115 (2):384–393. [PubMed] [Google Scholar]
- Sandstrom N, Williams C. Spatial memory retention is enhanced by acute and continuous estradiol replacement. Horm Behav. 2004;45 (2):128–135. doi: 10.1016/j.yhbeh.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Sarff M, Gorski J. Control of estrogen binding protein concentration under basal conditions and after estrogen administration. Biochemistry. 1971;10 (13):2557–2563. doi: 10.1021/bi00789a022. [DOI] [PubMed] [Google Scholar]
- Schmidt B, Jacobson T, Markus E. Hippocampal and striatal dependent navigation: sex differences are limited to acquisition. Horm Behav. 2009;56 (2):199–205. doi: 10.1016/j.yhbeh.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin B. Steroid hormones and cognitive functioning aging men: a mini-review. J Mol Neurosci. 2003;20 (3):385–393. doi: 10.1385/JMN:20:3:385. [DOI] [PubMed] [Google Scholar]
- Sherwin B. Estrogen and cognitive aging in women. Neuroscience. 2006;138 (3):1021–1026. doi: 10.1016/j.neuroscience.2005.07.051. [DOI] [PubMed] [Google Scholar]
- Sherwin B, Henry J. Brain aging modulates the neuroprotective effects of estrogen on selective aspects of cognition in women: a critical review. Front Neuroendocrin. 2008;29 (1):88–8113. doi: 10.1016/j.yfrne.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Shughrue P, Merchenthaler I. Evidence for novel estrogen binding sites in the rat hippocampus. Neuroscience. 2000;99 (4):605–612. doi: 10.1016/s0306-4522(00)00242-6. [DOI] [PubMed] [Google Scholar]
- Silva I, Mello L, Freymüller E, Haidar M, Baracat E. Estrogen, progestogen and tamoxifen increase synaptic density of the hippocampus of ovariectomized rats. Neurosci Lett. 2000;291 (3):183–186. doi: 10.1016/s0304-3940(00)01410-5. [DOI] [PubMed] [Google Scholar]
- Singh GK, Kochanek KD, MacDorman MF. Advance report of final mortality statistics, 1994. Month Vital Stat Rep. 1996;45 (Suppl):1–80. [Google Scholar]
- Sorra K, Harris K. Overview on the structure, composition, function, development, and plasticity of hippocampal dendritic spines. Hippocampus. 2000;10 (5):501–511. doi: 10.1002/1098-1063(2000)10:5<501::AID-HIPO1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Springer L, Flaws J, Sipes I, Hoyer P. Follicular mechanisms associated with 4-vinylcyclohexene diepoxide-induced ovotoxicity in rats. Reprod Toxicol. 1996a;10 (2):137–143. doi: 10.1016/0890-6238(95)02056-x. [DOI] [PubMed] [Google Scholar]
- Springer L, McAsey M, Flaws J, Tilly J, Sipes I, Hoyer P. Involvement of apoptosis in 4-vinylcyclohexene diepoxide-induced ovotoxicity in rats. Toxicol Appl Pharmacol. 1996b;139 (2):394–401. doi: 10.1006/taap.1996.0180. [DOI] [PubMed] [Google Scholar]
- Springer L, Tilly J, Sipes I, Hoyer P. Enhanced expression of bax in small preantral follicles during 4-vinylcyclohexene diepoxide-induced ovotoxicity in the rat. Toxicol Appl Pharmacol. 1996c;139 (2):402–410. doi: 10.1006/taap.1996.0181. [DOI] [PubMed] [Google Scholar]
- Stackman RW, Blasberg ME, Langan CJ, Clark AS. Stability of spatial working memory across the estrous cycle of Long-Evans rats. Neurobiol Learn Mem. 1997;67 (2):167–171. doi: 10.1006/nlme.1996.3753. [DOI] [PubMed] [Google Scholar]
- Talboom J, Williams B, Baxley E, West S, Bimonte-Nelson HA. Higher levels of estradiol replacement correlate with better spatial memory in surgically menopausal young and middle-aged rats. Neurobiol Learn Mem. 2008;90 (1):155–163. doi: 10.1016/j.nlm.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timaras P, Quay W, Vernadakis A. Horm Aging. CRC Press; Boca Raton, NY: 1995. [Google Scholar]
- Tolman E. Cognitive maps in rats and men. Psychol Rev. 1948;55 (4):189–208. doi: 10.1037/h0061626. [DOI] [PubMed] [Google Scholar]
- Tulving E, Craik FI. The Oxford Handbook of Memory. Oxford University Press; New York, NY: 2000. [Google Scholar]
- U.S. Census Bureau. Age and Census Composition: 2010 Census Brief. 2011 Issued May 2011, http://www.census.gov/
- U.S. Census Bureau. World Population Information. 2007 Retrieved on April 21, 2007, http://www.census.gov/ipc/www/world.html.
- van Haaren F, van de Poll N. The effect of a choice alternative on sex differences in passive avoidance behavior. Physiol Behav. 1984;32 (2):211–215. doi: 10.1016/0031-9384(84)90131-8. [DOI] [PubMed] [Google Scholar]
- Wade G. Gonadal hormones and behavioral regulation of body weight. Physiol Behav. 1972;8 (3):523–534. doi: 10.1016/0031-9384(72)90340-x. [DOI] [PubMed] [Google Scholar]
- Warren S, Juraska J. Spatial and nonspatial learning across the rat estrous cycle. Behav Neurosci. 1997;111 (2):259–266. doi: 10.1037//0735-7044.111.2.259. [DOI] [PubMed] [Google Scholar]
- Warren SG, Juraska JM. Sex differences and estropausal phase effects on water maze performance in aged rats. Neurobiol Learn Mem. 2000;74 (3):229–240. doi: 10.1006/nlme.1999.3948. [DOI] [PubMed] [Google Scholar]
- Westerlind K, Gibson K, Malone P, Evans G, Turner R. Differential effects of estrogen metabolites on bone and reproductive tissues of ovariectomized rats. J Bone Min Res. 1998;13 (6):1023–1031. doi: 10.1359/jbmr.1998.13.6.1023. [DOI] [PubMed] [Google Scholar]
- Wharton W, Baker L, Gleason C, Dowling M, Barnet J, Johnson S, Carlsson C, et al. Short-term hormone therapy with transdermal estradiol improves cognition for postmenopausal women with Alzheimer’s disease: results of a randomized controlled trial. J Alzheimer’s Dis. 2011;26 (3):495–505. doi: 10.3233/JAD-2011-110341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witty CF, Gardella LP, Perez MC, Daniel JM. Short-Term Estradiol Administration in Aging Ovariectomized Rats Provides Lasting Benefits for Memory and the Hippocampus: A Role for Insulin-Like Growth Factor-I. Endocrinology. 2013;154:842–852. doi: 10.1210/en.2012-1698. [DOI] [PubMed] [Google Scholar]
- Wide J, Hanratty K, Ting J, Galea L. High level estradiol impairs and low level estradiol facilitates non-spatial working memory. Behav Brain Res. 2004;155 (1):45–53. doi: 10.1016/j.bbr.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Woolley CS. Effects of oestradiol on hippocampal circuitry. Novartis Found Symp. 2000;230:173–180. doi: 10.1002/0470870818.ch13. discussion 181–177. [DOI] [PubMed] [Google Scholar]
- Woolley C, McEwen B. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12 (7):2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 1993;336 (2):293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Lui LY, Zmuda J, Cauley J. Sex hormones and cognitive function in older men. J Am Geriat Soc. 2002;50 (4):707–712. doi: 10.1046/j.1532-5415.2002.50166.x. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Sawaya G, Lieberburg I, Grady D. Estrogen therapy in postmenopausal women: effects on cognitive function and dementia. J Am Med Assoc. 1998;279 (9):688–695. doi: 10.1001/jama.279.9.688. [DOI] [PubMed] [Google Scholar]