Abstract
Plants are chemical storehouses, a fact which has driven countless multidisciplinary quests for bioactive compounds. As the very first step of botanical research, the whole desire is to find “hit” plants with specific bioactivities. It is logical to use some strategies that can maximize the chances of finding these “hits” with limited time and resources. In addition to selecting the right plants for screening, how the plant extracts are prepared can also influence the bioactivity screening outcomes. An extract from the same plant material can be quite different in chemical composition having different preparations. Because of the complex mixture nature of plant extracts, it is possible artifact activities may be observed. Thus confirmatory activity tests are often necessary to warrant the next laborious isolation step. A bioassay directed isolation approach may be the most efficient in identifying the bioactive compounds because of the narrowed focus at each isolation step, but a phytochemistry isolation approach is appropriate to characterize a purified bioactive extract. In fact, these two approaches can be taken intermittently whenever efficiency can be improved. Finally, use of the identified active compounds is now broader. In addition to determining a lead compound to continue a drug development path, there is an increasing interest in support for the use of botanical extracts as botanical drugs. Instead of dropping the extract after extracting the lead compound, the natural analogues representing the purified extract now have a chance to become leading compounds in the pursuit of novel therapies for metabolic syndrome and other diseases.
INTRODUCTION
Many plants have medicinal properties and are in use by various cultures. Collectively these uses cover approximately 80% of the world population. The importance and contributions of medicinal plants to health maintenance and treatment of diseases are beyond any controversy. That heritage has seen expansion into modern medicines in recent years. There is plenty of scientific evidence that plants are good sources of important pharmaceutical compounds and drugs. It is no surprise that most of the drug discoveries from natural sources reported so far come from research institutions rather than pharmaceutical companies. In fact, the research institutions have always been the source of intensive investigations with botanical extracts. There may be some interesting reasons for this observation. First, preparations of botanical samples can be easy and inexpensive. This has allowed many laboratories to step into the investigation arena of botanical samples or to initiate collaborations. Second, unique plants and availability of plant resources give local investigators “first come first serve” opportunities, which also help spread the investigation base to a global scale. Drug discovery from plants has remained a constant interest in developed countries, and participation in exploring new molecules seems to be a worldwide effort. It is safe to say that is widespread interest and these investigations pave solid foundations for the discovery of pharmaceutical lead compounds and scientifically sound and proven modern medicines.
Most pharmaceutical research efforts have an ultimate goal of identifying bioactive compounds from botanical sources. Once the knowledge of active compounds is gained, then a traditional drug development approach is taken to convert lead compounds into pharmaceutical drugs [1]. The most prominent and successful examples in recent history are best exemplified by the taxol and camptothecin stories. In 1992 and 1996, taxol, originating from Pacific Yew bark, and semi-synthetic analogues of camptothecin derived from the Camptotheca acuminate tree material, were approved for cancer treatments, respectively, by the U.S. Food and Drug Administration. Although the use of botanical extracts for health care enjoyed wide acceptance in the world, including developed countries in Europe and Japan, the use of such commercial products in the United States really took off after 1994. It is the regulatory issues that have played catalytic roles in changing the use of botanical extracts. The 1994 Dietary Supplement Health and Education Act (DSHEA) prompted rapid marketing of numerous herbal dietary products in the U.S. These DSHEA products are basically exhibits of empirical knowledge being presented in modern product forms. In June 2004 when the FDA released the Guidance for Industry: Botanical Drug Products, regulatory hurdles were removed to allow the development of botanical extracts into pharmaceutical drugs. In early 2007, FDA approved the first botanical drug (EGCG) for transdermal treatment of genital warts. This symbolizes the sweeping change in attitudes towards botanical extracts as complex mixtures. Plants are no longer just sources of bioactive compounds and pharmaceutical drug lead compounds like taxol and camptothecin, they themselves now can be drug candidates without being broken apart.
The added enthusiasm of using botanical extracts as health care products and pharmaceutical drugs has prompted and will continue to invite investment from loyal investigators scattered in laboratories throughout the world. A strong call is heard for multiple disciplinary collaborations between phytochemists alike and those who are running in vitro and in vivo activity evaluations. Metabolic syndrome has become increasingly a health problem as shortage of foods in many countries is no longer a major issue [2]. Because this problem is largely caused by improper food intake, the foods themselves become target sources of problems and solutions at the same time. A search for traditional foods and herbal sources is obvious. In a comprehensive review article, Houston [3] pointed out the importance of lifestyle modifications in conjunction with vitamins, minerals, antioxidants, and nutraceutical supplements in maintaining a healthy blood pressure and reversing hypertension. Food ingredients that may be low in nutritive values such as rice bran are now receiving more investigations on their functional properties for improving metabolic syndrome in laboratory animal experiments [4]. Common herbs that offer many health benefits in traditional uses are now under investigation for potential benefits to metabolic syndrome. Ginseng root extract, a tonic in traditional use, was predicted and then proved in laboratory experiments to relieve metabolic syndrome; vinegar, traditional use for reducing hypertension, augmented ginseng’s effect [5]. Extracts of pomegranate fruits showed significant effect on alleviating metabolic syndrome in animal models [6]. Kang et al. [7] found that a traditional herb, Sorbus commixta Hedl., alleviated metabolic syndrome in experimental animals by reducing vascular inflammation. Cochlospermum vitifolium (Willd.) Sprengel, a Mexican medicinal plant that is used in the folk medicine for the treatment of hypertension, diabetes, hepatitis, and related diseases was tested in bioassays related to metabolic syndrome and is confirmed with its hypoglycemic property [8]. Recently, the effects of dietary patterns, specific food consumptions, and fruit and vegetable consumptions on metabolic syndrome have been investigated in human clinical trials [9-12].
Needless to say, there is no lack of discoveries of bioactive and/or potentially bioactive plant extracts, but there are significant delays in seeing reliable and viable botanical drug products. One of the major reasons may be attributed to the lack of effective multiple disciplinary collaborations that merge the expertise with the plant extracts and bioassays. Therefore, it is imperative to lay out the strategies for effective and efficient collaborations. When it comes to collaborations, there are many questions from all sides involved. This article attempts to address some of the underlying issues that collaborators would want to know. For examples, how can investigators prepare botanical samples for maximum initial screening output? How to select plants to feed the screening pipelines? How to interpret the results and make a wise decision? When to choose the bioactivity-directed isolation approach over the phytochemistry isolation approach? How to develop a bioactive plant extract towards a product itself rather than a source of a single active compound? How to control quality of a bioactive plant extract to support clinical investigations? With these questions in mind and focus on the in vitro screening needs, this article seeks answers from published work as well the author’s own experience, hoping to achieve the ultimate goal of maximizing positive screening outputs.
SELECTION STRATEGIES FOR THE TEST PIPELINE: WHY TO TEST THIS PLANT OVER THAT?
For many investigators who wish to screen plants for certain bioactivities, the exciting outcomes of course are the news of some “hits” or potent bioactivity. There are hundreds of thousands of plants to choose from, so the selection pool is rather large. However, in most cases, limited resources in the preparations of botanical samples and screening capabilities seem to discourage a random selection approach for initial screening among many anxious investigators. It is obvious that some guidelines may be helpful to, at least, narrow down the list of collections so chances of finding “hit” plants are increased, if not maximized. Although there is no guarantee to find hits, many have found the following criteria practical in selecting plants for screening tests. The very first strategy is to look at the traditional usage. In every culture, herbs are found useful in alleviating symptoms or treating many kinds of diseases. Although many herbal uses are mostly anecdotal, they are the best place to start. For example, if one wants to screen plants for anti-inflammatory activities, there are plenty of empirical folk medicines in the history of human use. There are many written records about herbal uses. For example, the Traditional Chinese Medicine, particularly the Compendium of Materia Medica (Bencao Gangmu, Li Shizhen, Ming Dynasty 1518-1593) detailing 1900 herbal species, the Ayurvedic medicines, United States Pharmacopeia and the National Formulary, Egyptian medicines are good sources of information. In Mexico, medicinal plants are actually selected based on the taste and smell [8]. On the other hand, if one wants to look for plants that are potentially useful for treating metabolic syndrome, there may not be as many. The simple reason might be that metabolic syndrome is more of a modern health problem. Investigators need to carefully sieve through the historical knowledge in order to select the right plants for screening tests.
The second selection strategy comes from modern literature search. With rapid developments in new bioassays there are clear needs to re-test the plants reported previously.
The third selection strategy is to look for the uniqueness of sources. The medicinal compounds synthesized in plants are often uniquely associated with their growth habitats. Plants grown in high elevations such as the Himalayas could be the sources of some unique chemical structures. The halophytes that grow in salty coasts of Louisiana and other states along the Gulf of Mexico may also have unique compounds.
Selecting plants based on chemical composition is another selection strategy. For examples, diterpenoids are known to be anti-cancer agents. Plants that are reported to contain diterpenoids would be candidates for a screening test of this activity. Anthocyanins are known to be anti-oxidant, so new sources of plants rich in anthocyanins are worthy collections. Additionally, one can select plants based on a specific biological activity, e.g., anti-cancer, anti-hypertension, anti-inflammation, cholesterol-lowering activity, NF kappa B inhibition. Moreover, one can select plants based on a biomedical mechanism. For example, over 100 diseases are angiogenesis driven. If looking for anti-angiogenic activities, one would focus on the traditional uses to treat these diseases for clues of potential hits. Of course, any combination of the above selection strategies would be desirable, e.g., based on traditional knowledge and available chemical expertise and facilities.
PREPARATION OF BOTANICAL SAMPLES FOR INITIAL SCREENING TESTS – MAKE IT SIMPLE
Once plants are selected, raw materials need to be extracted into test samples. At first, it may appear to be fairly easy processes as many herbs are either decocted or tinctured in traditional uses. Before starting extractions, the outcomes of the extraction should be well considered. If the target components are known, then the appropriate extraction method should be used, so that the extraction of those components can be maximized and other components minimized. For example, there is existing knowledge on anthocyanins from black raspberry, flavonoids from Ginkgo biloba leaves, camptothecin alkaloids from Camptotheca trees, gallotannins from persimmons, and many others. Natural product journals and publications are rich places for this knowledge. Williamson et al. [13] described specific methods for extracting specific components from plant materials. On the other hand, if the target components are not known, then the strategy would be to extract each and every component as much as possible. In this case, the plant materials may be subjected to repeated extractions until a majority of the components can be extracted, leaving residual plant materials as mostly structural components such as cellulose and hemi-cellulose. Why would one want to digest further those components that have little value in pharmaceutical activities?
It is found that most investigators use alcoholic extraction as their initial crude extraction method [14]. Li et al. [15] used methanol to obtain their first crude extract and then proceeded with further fractionation. Huang et al. [16] used 95% ethanol to obtain their crude extract. Traditional methods used in the extraction of botanical samples for phytochemistry investigations often use sequential solvent extraction methods starting with less polar solvents, such as hexane, petroleum ether, ethyl acetate, and chloroform, followed by solvents with increasing polarity such as ethanol and water. Obviously, each use of different solvents resulted in crude extracts of somewhat different chemical compositions. For bioactivity screening then, several samples of the same botanical raw material will have to be screened. One can combine these sequentially obtained extracts to make one sample that contains a majority, if not all, of the components of a particular botanical material for bioactivity screening. In herbal medicines, a decoction to form an herbal tea is the common use, and tincture is another. The decoction often contains polar components, but some less polar components may not be extracted readily. For example, oleanolic acid, ursolic acid, and tanshinone IIA are extracted with difficulty in boiling water but are extractable in alcohol. Is there a simple and universal way to extract plants for the purpose of extracting majority of the components? Since our goal is to extract as much and as many components as possible to make a truly representative extract sample for a plant material, combining the two solvents appears to be simple and practical. Using aqueous alcohol (e.g., 70% when solvent penetration ability is strong), therefore, may actually be a simple and effective way of extracting polar and non-polar components in one extraction, thus recovering major, if not all, components for bioactivity screening. This simple method does not favor the extraction of any particular components, but rather broadband components. The major advantage of testing such a comprehensive sample is the simplicity for screening tests. The major disadvantage is the possible dilution of the active components.
Of course there are other extraction methods by which crude botanical samples could be made faster. Pressurized liquid extraction (PLE) has gained popularity in recent years. PLE uses pressure to accelerate the dissolution and release of the compounds in the raw plant materials into the extraction solution. This extraction method has been used in the extractions of many medicinal plants, especially when there are chemical markers to measure. But even without the markers, one can still use this method to quickly prepare crude extracts for bioactivity screening. Anand et al. [17] compared three methods including PLE for extracting the bioactive molecules in Hypericum perforatum L. PLE was found to be more effective in extracting caffeine, phytol, palmitic, and stearic acid in mate tea leaves (Ilex paraguariensis) and in shortening the total extraction time and reducing solvent consumption [18], the same conclusions drawn by many other investigators [19-22]. PLE was also effective in extracting terpenes, fatty acids and Vitamin E [23] as well as giving a high total extract yield [24] but less effective in extracting sterols [25] and caffeine from green tea leaves [26]. PLE can also be combined with heat to further accelerate the extraction process, but concerns over the possible loss of temperature-sensitive compounds such as those phenolic compounds having a greater number of hydroxyl-type substitutes [27] may limit the use. PLE plus high temperature may be a risky method when unknown compounds are extracted for bioactivity screening. Microwave-assisted extraction (MAE) is another way of accelerating the extraction of compounds from plant materials. MAE showed high efficiency in extracting essential oils [28-30], camptothecin alkaloids [31], and limonoids in neem trees [32]. MAE was also effective in extracting the phytochemicals in peanut [33]. A good illustration of the MAE method may be seen in the development of an optimized extraction process for extracting phenolic compounds from grape seed [34]. Supercritical Fluid Extraction (SFE) has been widely used in recent years in extracting components in botanical samples. It has been shown that SFE was effective in extracting some botanical components, although ineffective in others. SFE was found to extract more apigenin and biochanin A [19] and flavonoidal aglycones [35] but less daidzein and genistein compared with other methods. Adding modifier solvents to the CO2 often results in dynamic changes of chemical composition in the extracts, similar to other solvent extraction methods. It could be that the raw materials are not fully extracted with the SFE method, even with the help of solvent modifiers, so the raw materials may have to be extracted again with other methods to recover additional components for the bioactivity screening. Other extraction methods, including ultrasound-assisted extraction, steam distillation, and superheated-water extraction are summarized and compared in other review articles [36-38].
Clearly, there are crude extraction methods with tremendous advantages, and it seems the best utilization of these methods resides in the knowledge of the target components desired to be extracted. The optimization of crude extraction methods may find these methods extremely useful when active compounds are identified and desired for extraction.
PLANT EXTRACTS FOR BIOACTIVITY TESTS – MAKE THEM COMPATIBLE
Before handing the initial crude extracts for bioactivity screening, some understanding of the characteristics of such extracts is helpful in predicting their future behaviors. The first characteristic is water solubility. Most of the in vitro bioassays use culture media that are water-based and at pH of 6.8 to 7.4. This test condition requires that the extracts are water-soluble and stable at the media pH. If an extract is not water soluble, additional measures are needed. DMSO or ethanol is often used to help break the thermodynamics of extract components first, followed by dilution with culture media to the final volume. However, there are limits in the amount of DMSO or ethanol in the final volume of culture media before they themselves become a potential effecter. Additional blank control containing the same concentrations of DMSO or ethanol needs to be included to account for the independent effect.
The second characteristic is the stability of extract components in the basic media. The pH of the culture media may affect the structural stability of some compounds, such as phenolic acid and gallotannins. For extracts prepared by acidic solvent there might be structural changes. Another stability issue has to do with the form of the extract. Components in the extracts are presumably much more stable structurally in powder forms than in solutions, particularly in the culture media solutions. As a general rule, extracts in culture media solutions would be at minimal risk within two weeks in a refrigerated environment. If there is suspicion that bioactivity fades with time, solution stability study may be warranted. Fig. (1) illustrates a hypothetical situation where the inhibitory activities faded on Day 17 after the extract solution was added to the culture media. The anti-angiogenic activity against new blood vessel formation (upper) and growth (lower) was immediately active and maintained until the end of the experiment (Day 27). The control group continued the formation and growth of new blood vessels. Had the bioactivity faded (dotted line) after the observed initial inhibition, extract stability in solution would be a subject of examination, because the extract was not prepared freshly in culture media solution at each use. The real situation was that the bioactivity was stable throughout the experiment and did not suggest a solution stability issue [39].
Fig. (1).
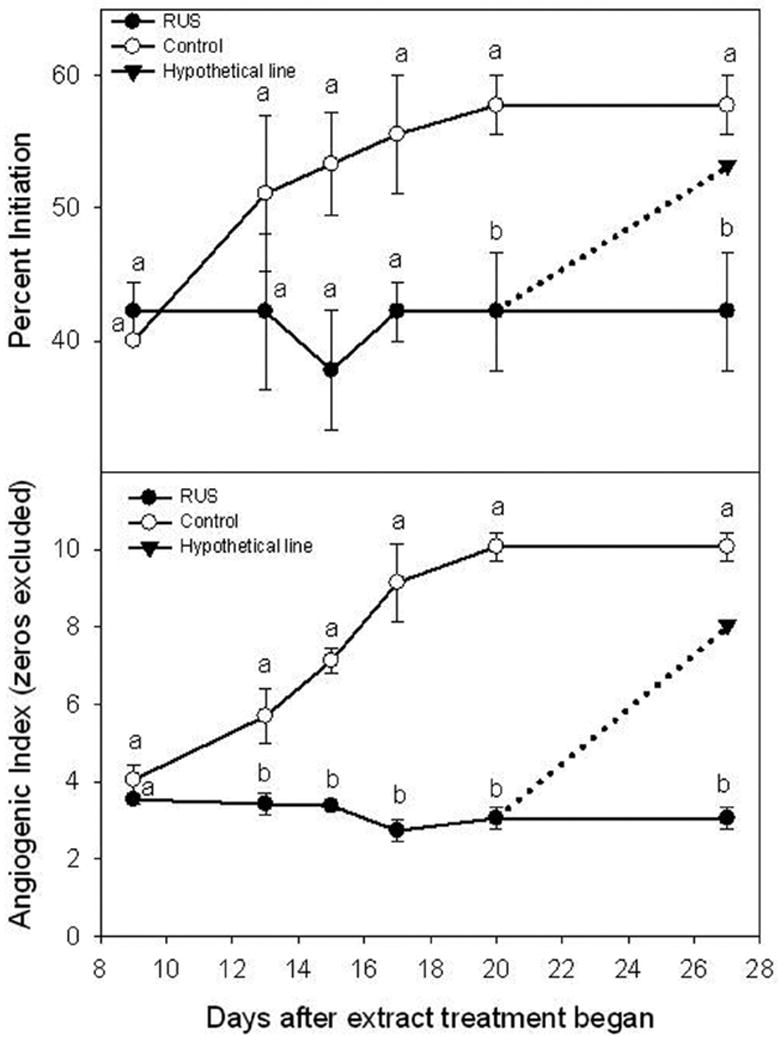
Hypothetical bioactivity results that could indicate sample stability issue. The effect of the crude leaf extract (RUS) from R. suavissimus on the pre-existing human angiogenesis developed in the pre-treatment culture. All samples were tested at 0.1% (w/v). human placental vein tissues in each well were allowed to develop new blood vessels for 9 days when RUS treatment started. Vertical bars at each data point represent one unit of standard error of the mean (n = 45). Different letter on each measurement day indicate a significant defference at p ≤ 0.05 (adapted from Liu et al. Phytotherapy Research 2006).
IDENTIFICATION OF ACTIVE MOLECULES IN BOTANICAL SAMPLES – IS IT WARRANTED?
If an exciting bioactive was observed in the screening assays and was real, the expensive bioassay directed isolation approach is warranted. In a recent review a typical approach to identifying the bioactive compounds in botanical extracts was through fractionation [1]. Fractionation is a key step for separating the bioactive compounds from a bioactive extract. Using liquid phase extraction, where two solvents do not mix and form separate layers, components of an extract can be easily fractioned based on their affinity to a solvent. This is often used after the initial crude extract is obtained [16, 40]. A typical example is the work by Jutiviboonsuk et al. [14], where the crude methanol extract was extracted again with petroleum ether to remove the fatty constituents, followed by partitioning with chloroform. This liquid phase extracted fraction with bioactivity moved forward to a series of solid phase extractions, as shown in column chromatography, that led to the isolation and purification of the bioactive molecules. Another good example is showcased in the work of isolating the isoflavonoids that promoted the proliferation of the osteoblast [21]. In this work, the stem bark was first extracted with 65% ethanol to obtain a crude extract. This extract was then subjected to liquid phase extraction (partitioning) with ethyl acetate and n-butanol sequentially, obtaining three fractions. Among these the water fraction showed no bioactivity, whereas the other two organic fractions were found active. At this point, almost 70% of the crude extract by weight had no interesting activity for further fractionation, leaving the investigations to focus on the other 30% extract, where the active molecules reside. The most active ethyl acetate fraction was further fractionated with solid phase extraction as in column chromatography, which led to the isolation of the active isoflavonoids. Solid phase extraction is also routine in many laboratories. For example, by using macroporous absorption resin, bioactive black raspberry extract was separated into four fractions, of which only one fraction retained promising bioactivity [41]. Not only did this occur, this fraction accounted for only 6.5% of the weight of its parent extract. This round of fractionation has substantially purified the extract and guided future fractionation towards this fraction instead of the other 93.5%. One could spend much time in characterizing the 93.5% inactive extract if justified. Of the active fraction, another round of fractionation was conducted with C18 sorbent. The bioassay result showed that none of the subfractions outperformed its parent extract, and the activity was rather evenly distributed among the four fractions. At this point, a decision has to be made on whether change of the fractionation method is needed and repeated, or the phytochemistry isolation approach should start now that this subfraction was no longer separable in bioactivity.
Column chromatography is widely used to isolate natural compounds. An allergy preventative plant extract was fractionated in column chromatography resulting in the purification and identification of eight flavonoidal compounds, all of which contributed to the observed allergy preventative activities [42]. Sánchez-Salgado et al. [8] investigated the effect of Cochlospermum vitifolium (Willd.) Sprengel on metabolic syndrome. This is a Mexican medicinal plant that is used in folk medicine for the treatment of hypertension, diabetes, hepatitis, and related diseases. Based on this traditional use, raw plant materials were extracted sequentially with hexane, dichloromethane, and methanol, and derived extracts were tested in bioassays to determine their vasorelaxant and hypoglycemic activity. They found varied activities among the three extracts, indicative of different chemical compositions and perhaps a success of separation.
Fig. (2a) summarizes a typical procedure in the preparation of botanical samples and their subsequent fractionation and isolation based on a single initial crude extract. Authenticated raw plant material is often extracted with 70% alcohol (aqueous methanol or ethanol). This common extraction method is aimed at extracting components as completely as possible yet maintaining simplicity. Such derived crude extracts can then be assessed by specific assays. Only those extracts deemed to have interesting activities are continued, whereas inactive extracts are often dropped out of the screening pipelines. The active crude extracts undergo liquid phase extraction using organic solvents (e.g., ethyl acetate, n-butanol, chloroform) in water, which form distinct layers to derive organic and water fractions. These fractions are evaluated again to confirm the activities. The inactive fractions are excluded from further fractionation, whereas those active become the focus of solid phase extraction such as column chromatography. This process of solid phase extraction may take several rounds to get to the point where pure compounds are obtainable. It can also be an expensive and laborious process. Therefore, the resultant fractions produced at the liquid phase extraction step also serve to confirm the activities found in the crude extracts. It is expected that bioactivity would improve at this step compared with the crude extract; otherwise, it should be a concern before initiating additional fractionation steps. Purified compounds are then identified using spectroscopy techniques, and their activities are evaluated conclusively. As illustrated previously, bioassay-directed isolation can always help eliminate the inactive compounds early on, thus avoiding unnecessary work in the search for active compounds. Therefore, readily available and reliable bioassays contribute to greater efficiency.
Fig. (2).
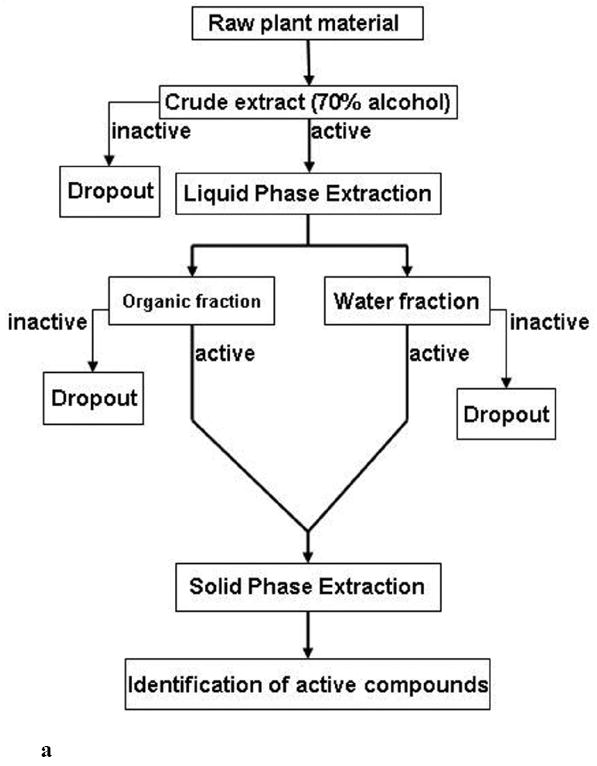
a. A typical procedure in the preparation and fractionation of botanical extract samples, leading to the identification of bioactive compounds.
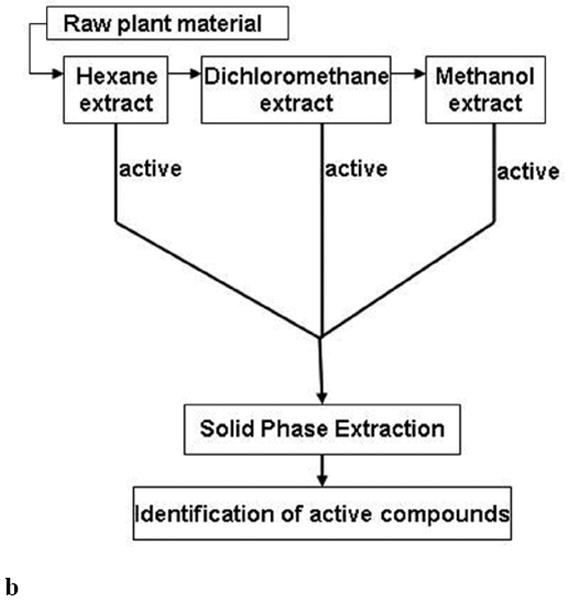
b. An alternative procedure in the preparation and fractionation of botanical extract samples, leading to the identification of bioactive compounds.
Fig. (2b) summarizes an alternative procedure in the preparation of botanical samples and their subsequent fractionation and isolation, based on multiple initial crude extracts. Authenticated raw plant material is often sequentially extracted with increasing polarity of the solvents from hexane, dichloromethane, and methanol. Therefore, the initial crude extracts are three in this case. Each sample is tested in bioassays. The active crude extracts proceed to further fractionation and isolation, similar to the typical procedure illustrated in Fig. 2a. In fact, the sequential extractions can be viewed as the liquid phase extraction described in Fig. 2a, except the plant material is raw instead of a crude extract. The primary advantage of this method is that the crude extract has already been partitioned, but the primary disadvantage is tripling of the number of extracts that have to be tested in the initial screening test.
There are many variations in the adopted initial extraction and subsequent fractionation procedures. Therefore, to have a uniform method is not necessary. Burdette et al. [43] prepared the black cohosh roots/rhizomes by sequentially extracting with methanol and aqueous methanol (60%), resulting in two crude extracts. After confirmed with bioactivities, these two extracts then branched out to liquid phase partitioning and column chromatography, leading to the isolation and identification of active compounds that have anti-oxidant properties against cellular DNA damage.
Sophisticated inline or hyphenated tools for the identification of molecules in complex extracts are available for high throughput drug discovery and obviously offer tremendous advantages, one of which is the reduction or elimination of laborious isolation work. However, that technology is still evolving and meeting challenges. To many natural product investigations, the expensive nature would further discourage many from routine drug discovery work.
INTERPRETATION OF THE SCREENING RESULTS – KEEP OR DROP?
If an extract showed an exciting activity in a bioassay, several possibilities may come into play. The unknown compound(s) contributing to the bioactivity may be: (1) very potent. This may also mean that they are at low concentrations; (2) at high concentrations. The fact that many plants are known for their signatory, characteristic, or major constituents can provide a quick answer. For example, ginger roots are known for their major constituents of gingerol and its many analogues. If ginger root extracts have specific activity, gingerol would be a primary target for quick assessment; (3) toxic, so that the cells in the bioassays may have been destroyed. For example, no vessel formation and growth is the desirable outcome of a bioassay in searching for anti-angiogenic agent. When measuring phenotypic responses of human placental vein tissues for new blood vessel formation, the non-sprouting vessels may be due to the toxicity (cell death) rather than the inhibition of the compounds in the extract; and/or (4) having interactions with the culture media that lead to artifact activity. This is particularly annoying when an extract is unknown and complex. The colorimetric measurement of glycerol is such an example. After co-culture of human adipose cells with a plant extract, the conditioned media where the release of glycerol from the adipose cells is desirable are harvested and reacted with reagents to form a color-forming product, which is then measured using a colorimetric method. In addition to background interference, there is a possibility that some constituents may react to form the colors at the save wavelength as glycerol. As a result, artifactual bioactivity could be generated (increased glycerol release and lipolysis) by use of this method for testing plant extracts. Lapidot et al. [44-45] examined in greater details the possibility of phenolics in apple extracts interacting with components in the cell culture media that resulted in the generation of hydrogen peroxide (H2O2). This newly formed product was found to cause the inhibition of the proliferation of tumor cells in vitro. It is therefore suggested that the phenolics/flavonoids in the apple extracts may not have directly caused the inhibition of tumor cell proliferation, and thus the results are artifacts. The results warn against many previously reported effects of flavonoids and phenolic compounds in cultured cells against cancer cell proliferation. These authors suggest that in order to prevent such artifacts, the use of catalase and/or metmyoglobin in the presence of reducing agents should be considered as a method to decompose H2O2 and prevent generation of other reactive oxygen species, which could affect cell proliferation. Many plant extracts contain phenolics and are complex in chemical composition. Exciting revelations of bioactivities exhibited in plant extracts must be dealt with cautiously to avoid potentially misleading results.
If a crude extract showed some interesting activity but not as potent as a known positive control, the question is often asked if this is a hit in the screening phase. It is not surprising to see the relatively weak bioactivity of a botanical extract, because it is a complex mixture. Assuming that the unknown, responsible compounds exist but are in a relatively low concentration, a fractionation can be easily performed to raise the relative levels of those responsible constituents, hoping that improved bioactivity will be observed. If there is no improvement, the extract may not be worthy of further investigations.
INITIAL FINGERPRINTING ANALYSIS OF THE BIOACTIVE BOTANICAL EXTRACTS – LAYING THE FOUNDATION FOR SEPARATION WORK
It is expensive to develop good chromatographic fingerprints for each and every botanical extract that have been screened. It is not necessary nor worth the resources at this point. However, for a confirmed “hit” plant extract, it goes a long way to have a chromatographic fingerprint in place. First, it helps establish a chemical authentication of this plant. It is common to see variations of chemical constituents within an authenticated plant due to different sources and batches. A fingerprint with good separation of peaks allows future collection of this plant material to be chemically verified and adulterants detected. This is especially useful when the knowledge of characteristic constituents is available for this plant. Second, one can easily track the fractionation results and see how constituents are separated into fractions and sub-fractions. And third, it serves as a roadmap for the isolation and purification processes. In a ginger (Zingiber officinale) screen test for potential metabolic syndrome therapy, for example, a chromatographic fingerprint was developed for the bioactive crude extract. Due to the various sources of ginger, it is necessary to establish a chromatographic fingerprint as a chemical template for future fractionation and new batches from the same source or new sources. Fig. (3) illustrates the ginger crude extract from a Chinese source, prepared by 70% aqueous ethanol, which contains 1.6% w/w 6-gingerol. Other gingerol analogues may be present as judged by their UV absorption spectra as well as many unidentified compounds. With the same extraction and HPLC fingerprinting analysis, another Chinese source (authenticated) showed similar fingerprinting patterns but drastic variation in the levels of 6-gingerol (Fig. 4). Obviously there are many pieces of puzzles ahead, but the chromatographic fingerprint will be very helpful in monitoring the chemical compositions of ginger root extracts.
Fig. (3).
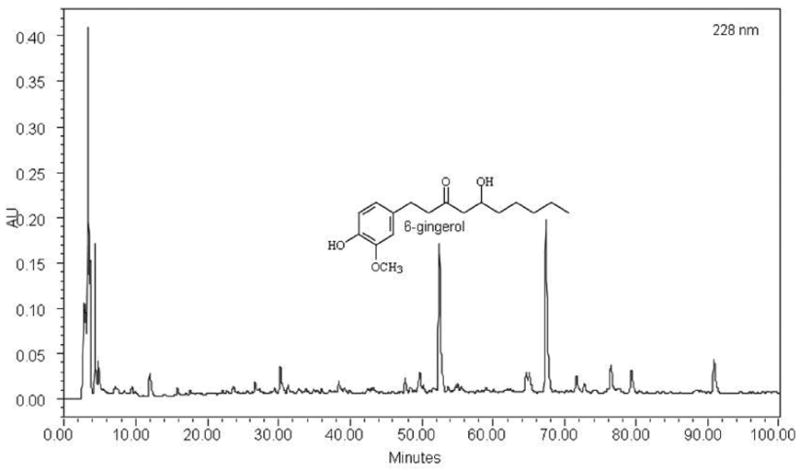
Chromatographic fingerprint of ginger root crude extract prepared with 70% ethanol. The fingerprint was developed at 228nm. 6-gingerol was eluted at 52.80 min and was 1.6% w/w in this extract sample.
Fig. (4).
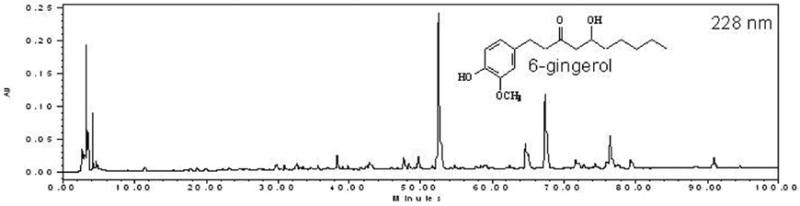
Chromatographic fingerprint of ginger root extract prepared with 70% ethanol. The authenticated ginger root came from a new source in China which is defferent from the source used in Fig. 3. The concentration of 6-gingerol was 3.04% w/w. HPLC analysis was performed on a Water 600E system with an auto sampler and a photodiode array detector. The analysis was conducted on a Symmetry C18 column (250mm×4.6mm, 5um). Mobile phase A consisted of HPLC grade acetonitrile and mobile phase B consisted of HPLS-grade water containing 0.3% phosphoric acid. The gradient eluting mobile phase ranged from 0 to 90 min linearly from A/B (10:90, v/v) to A/B (90:10, v/v), followed by another gradient elution period from 90 to 100 min linearly from A/B (90:10, v/v)to A/B (100:0, v/v). flow rate was set at 1.00 mL/min, clumn temperature was maintained at 25 °C, and injection volume was 10.0 mL. PDA detection range was set from 200 to 400 nm and the chromatogram was generated at 228 nm. The 6-gingerol standard (Fisher Scientific) was eluted at 52.8 min and calibration curve was established to quantify the 6-gingerol concentrations in the samples from different sources in China.
BIOACTIVITY DIRECTED ISOLATION APPROACH VS. PHYTOCHEMISTRY ISOLATION APPROACH: ARE WE EFFICIENT?
The phytochemistry isolation approach is widely used in natural product research. This approach focuses on the isolation of naturally occurring compounds, particularly novel structures. The isolated compounds are then screened for activities. The bioactivity directed isolation approach focuses on the bioactive compounds, regardless of structural novelty. The two approaches are not running against each other, but rather converge to identify active compounds, novel as the most desirable (Fig. 5). The phytochemistry isolation approach works best when a final, purified extract is obtained such that any further purification would reduce its therapeutic efficiency. Therapeutic efficiency can be defined as the collective advantage of a bioactive extract contributing to therapeutic effects. Factors such as synergistic and additive effects, structural stabilizing effect, and high bioavailability effect would contribute to high therapeutic efficiency. Phytochemistry isolation approach is then undertaken to characterize the bioactive extract with a defined bioactivity and strength.
Fig. (5).
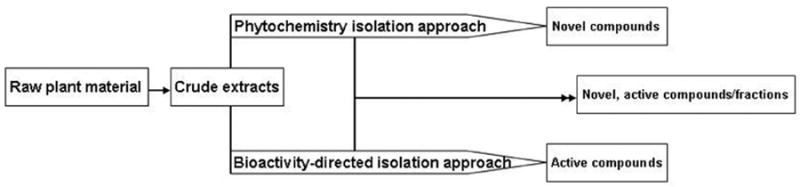
Schematic illustration of the two commonly used isolation approaches in identifying bioactive compounds and their convergence to discover novel and bioactive compounds and characterize a bioactive fraction.
ELUCIDATION OF ACTIVE COMPOUNDS – LEAD COMPOUNDS OR LEADING COMPOUNDS?
In conventional drug discovery, often a natural analogue of a pharmacophore that is most potent and/or of highest potential of therapeutic index is classified as a lead compound, whereas other natural analogues that are less potent or having undesirable properties are dropped out for further consideration. There are tens of natural analogues of the anti-cancer alkaloid camptothecin and tens of chemically modified analogues. Among them only the semi-synthetic Hycamtin® and Camptosar® have become clinically approved drugs for treating cancers. Increasingly, there are good possibilities that natural analogues in a plant extract with variable bioactivity and potency can be retained altogether to exert bioactivity. These compounds may be termed as leading compounds of a bioactive extract. Biologically, a class of analogues may be more bioavailable to reach target receptors. Different classes of compounds may be even more advantageous than a single class of bioactive compounds if they target different pathways in a disease process. Shutting down major or multiple pathways simultaneously makes it harder to develop alternative bypass pathways that cause drug resistance. Yet, there are many compounds in plant extracts or fractions that are neither active nor harmful and would have been termed as the impurities in a single entity drug development approach. These may be beneficial compounds if their presence enhances solubility of the insoluble active compounds. Several observations in the author’s lab tend to support this notion. Pure camptothecin, for example, has poor water solubility, but its water solubility is increased 145-fold when it remains in its naturally occurring leaf-extract form. Water-insoluble curcumin, an antiangiogenic coumarin [46-47], has increased water solubility in a naturally semi-pure extract [48]. Rutin, a glycoside flavonol, is water soluble in an active antiangiogenic Noni fraction but otherwise water insoluble in purer form. These observations strongly suggest that plant extracts or fractions may offer solutions to insoluble active compounds, perhaps achieving similar effects via the chemical-modification approach prevalent in the pharmaceutical arena.
QUALITY CONTROL OF BIOACTIVE EXTRACTS: BE SERIOUS ABOUT BATCH TO BATCH VARIATIONS
To use a bioactive extract for in-depth study in clinical settings, the biggest challenge may lie in the quality control over the batch-to-batch variations. The chromatographic fingerprinting analysis of a standardized extract is one of the convenient and reliable tools. Chromatographic fingerprints can be established to monitor the whole manufacturing process from the raw materials to semi-purified to the final purified extract. Obviously the quality of raw materials is of uttermost importance in this whole equation, thus the concept of good agricultural and medicinal practices is advanced.
CONCLUSIONS
In order to increase the chances of identifying bioactive plant extracts, selection strategies can be adopted. These strategies include the wise use of traditional knowledge as clues, literature search for knowledge of major constituents, focus on plants at unique growth habitats, selection of plants rich in specific classes of constituents (e.g., gallotannins) that have specific biological activity (e.g., anti-hypertension), and knowledge of a common mode of action (e.g., angiogenesis).
It is simple to prepare initial crude extracts for screening purposes but appropriate methods often result in efficiency in determining the activities. If the classes of target compounds are known, obviously the initial crude extraction method should be designed to maximize that extraction. In many cases, the classes of target compounds are not known. Therefore, the strategy would be to extract as many components as possible. For simplicity and for reducing the number of samples in bioassays, a simple 70% ethanol extraction method can be employed. The activity, if observed, can be then improved through liquid and/or solid phase partitioning methods.
Special attention should be paid to the highly potent bioactivity associated with a crude plant extract. This is because that this extract is a complex mixture. As such, possible interactions with the culture media could lead to artifact activity. Extreme caution must be taken to validate the bioactivity before initiating the isolation processes. This is especially important in the highly sensitive in vitro assays compared with the less sensitive animal models. The bioactivity-directed isolation approach can be very efficient but the phytochemistry isolation approach can be used intermittently to increase the efficiency in identifying the active compounds.
The use of active compounds may have been broadened by the recent botanical drug pathway in the U.S. The conventional drug discovery approach often abandons natural analogues after classifying a lead compound(s). In a bioactive botanical extract, the whole series of natural analogues of a core structure in the extract itself could be used as leading compounds to exert a defined bioactivity. This may be exactly where botanical extracts as novel therapies have an advantage over single entity drugs.
Acknowledgments
This work is partially funded through the Botanical Research Center funded by P50AT002776-01 from the National Center for Complementary and Alternative Medicine and the Office of Dietary Supplements. Thanks goes to Dr. Paul Y. Burns for gracious editorial review and to Gar Yee Koh for assistance in the collection of extraction papers.
References
- 1.Koehn FE, Carter GT. Nat Rev Drug Discov. 2005;4(3):206–220. doi: 10.1038/nrd1657. [DOI] [PubMed] [Google Scholar]
- 2.Panagiotakos DB, Pitsavos C, Skoumas Y, Stefanadis C. J Am Diet Assoc. 2007;107(6):979–987. doi: 10.1016/j.jada.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Houston MC. Expert Rev Cardiovasc Ther. 2007;5(4):681–691. doi: 10.1586/14779072.5.4.681. [DOI] [PubMed] [Google Scholar]
- 4.Ardiansyah, Shirakawa H, Koseki T, Hashizume K, Komai M. Br J Nutr. 2007;97(1):67–76. doi: 10.1017/S000711450721013X. [DOI] [PubMed] [Google Scholar]
- 5.Yun SN, Ko SK, Lee KH, Chung SH. Arch Pharm Res. 2007;30(5):587–595. doi: 10.1007/BF02977653. [DOI] [PubMed] [Google Scholar]
- 6.de Nigris F, Balestrieri ML, Williams-Ignarro S, D’Armiento FP, Fiorito C, Ignarro LJ, Napoli C. Nitric Oxide. 2007;17(1):50–54. doi: 10.1016/j.niox.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Kang DG, Sohn EJ, Lee AS, Kim JS, Lee DH, Lee HS. Am J Chin Med. 2007;35(2):265–277. doi: 10.1142/S0192415X07004801. [DOI] [PubMed] [Google Scholar]
- 8.Sánchez-Salgado JC, Ortiz-Andrade RR, Aguirre-Crespo F, Vergara-Galicia J, León-Rivera I, Montes S, Villalobos-Molina R, Estrada-Soto S. J Ethnopharmacol. 2007;109(3):400–405. doi: 10.1016/j.jep.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Mukuddem-Petersen J, Stonehouse Oosthuizen W, Jerling JC, Hanekom SM, White Z. Br J Nutr. 2007;97(6):1144–1153. doi: 10.1017/S0007114507682944. [DOI] [PubMed] [Google Scholar]
- 10.Davis L, Stonehouse W, Loots du T, Mukuddem-Petersen J, van der Westhuizen FH, Hanekom SM, Jerling JC. Eur J Nutr. 2007;46(3):155–164. doi: 10.1007/s00394-007-0647-x. [DOI] [PubMed] [Google Scholar]
- 11.Esmaillzadeh A, Kimiagar M, Mehrabi Y, Azadbakht L, Hu FB, Willett WC. Am J Clin Nutr. 2007;85(3):910–918. doi: 10.1093/ajcn/85.3.910. [DOI] [PubMed] [Google Scholar]
- 12.Esmaillzadeh A, Kimiagar M, Mehrabi Y, Azadbakht L, Hu FB, Willett WC. Am J Clin Nutr. 2006;84(6):1489–1497. doi: 10.1093/ajcn/84.6.1489. [DOI] [PubMed] [Google Scholar]
- 13.Williamson EM, Okpako DT, Evans FJ. Pharmacological Methods in Phytotherapy Research V1. John Wiley & Sons Ltd; West Sussex: 1996. pp. 15–23. [Google Scholar]
- 14.Jutiviboonsuk A, Zhang H, Tan GT, Ma C, Van Hung N, Manh Cuong N, Bunyapraphatsara N, Soejarto DD, Fong HH. Phytochemistry. 2005;66(23):2745–2751. doi: 10.1016/j.phytochem.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 15.Li JX, Hareyama T, Tezuka Y, Zhang Y, Miyahara T, Kadota S. Planta Med. 2005;71(7):673–9. doi: 10.1055/s-2005-871275. [DOI] [PubMed] [Google Scholar]
- 16.Huang HC, Tsai WJ, Liaw CC, Wu SH, Wu YC, Kuo YH. Chem Pharm Bull (Tokyo) 2007;55(9):1412–1415. doi: 10.1248/cpb.55.1412. [DOI] [PubMed] [Google Scholar]
- 17.Anand R, Verma N, Gupta DK, Puri SC, Handa G, Sharma VK, Qazi GN. J Chromatogr Sci. 2005;43(10):530–531. doi: 10.1093/chromsci/43.10.530. [DOI] [PubMed] [Google Scholar]
- 18.Assis Jacques R, dos Santos Freitas L, Flores Peres V, Dariva C, de Oliveira JV, Bastos Caramão E. J Sep Sci. 2006;29(18):2780–2784. doi: 10.1002/jssc.200600024. [DOI] [PubMed] [Google Scholar]
- 19.Benthin B, Danz H, Hamburger M. J Chromatogr, A. 1999;837(1-2):211–219. doi: 10.1016/s0021-9673(99)00071-0. [DOI] [PubMed] [Google Scholar]
- 20.Bajer T, Adam M, Galla L, Ventura K. J Sep Sci. 2007;30(1):122–127. doi: 10.1002/jssc.200600306. [DOI] [PubMed] [Google Scholar]
- 21.Li P, Li SP, Lao SC, Fu CM, Kan KK, Wang YT. J Pharm Biomed Anal. 2006;40(5):1073–1079. doi: 10.1016/j.jpba.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 22.Warburton E, Norris PL, Goenaga-Infante H. Phytochem Anal. 2007;18(2):98–102. doi: 10.1002/pca.955. [DOI] [PubMed] [Google Scholar]
- 23.Péres VF, Saffi J, Melecchi MI, Abad FC, de Assis Jacques R, Martinez MM, Oliveira EC, Caramão EB. J Chromatogr A. 2006;1105(1-2):115–118. doi: 10.1016/j.chroma.2005.07.113. [DOI] [PubMed] [Google Scholar]
- 24.Zhang S, Chen R, Wu H, Wang C. J Pharm Biomed Anal. 2006;41(1):57–63. doi: 10.1016/j.jpba.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 25.Shen J, Shao X. Anal Bioanal Chem. 2005;383(6):1003–1008. doi: 10.1007/s00216-005-0078-6. [DOI] [PubMed] [Google Scholar]
- 26.Dawidowicz AL, Wianowska D. J Pharm Biomed Anal. 2005;37(5):1155–1159. doi: 10.1016/j.jpba.2004.10.038. [DOI] [PubMed] [Google Scholar]
- 27.Liazid A, Palma M, Brigui J, Barroso CG. J Chromatogr A. 2007;1140(1-2):29–34. doi: 10.1016/j.chroma.2006.11.040. [DOI] [PubMed] [Google Scholar]
- 28.Baker GR, Lowe RF, Southwell IA. J Agric Food Chem. 2000;48(9):4041–4043. doi: 10.1021/jf0004356. [DOI] [PubMed] [Google Scholar]
- 29.Chiasson H, Bélanger A, Bostanian N, Vincent C, Poliquin A. J Econ Entomol. 2001;94(1):167–171. doi: 10.1603/0022-0493-94.1.167. [DOI] [PubMed] [Google Scholar]
- 30.Martino E, Ramaiola I, Urbano M, Bracco F, Collina S. J Chromatogr A. 2006;1125(2):147–151. doi: 10.1016/j.chroma.2006.05.032. [DOI] [PubMed] [Google Scholar]
- 31.Fulzele DP, Satdive RK. J Chromatogr A. 2005;63(1-2):9–13. doi: 10.1016/j.chroma.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 32.Dai J, Yaylayan VA, Raghavan GS, Paré JR, Liu Z, Bélanger JM. J Agric Food Chem. 2001;49(10):4584–4588. doi: 10.1021/jf010592k. [DOI] [PubMed] [Google Scholar]
- 33.Chukwumah YC, Walker LT, Verghese M, Bokanga M, Ogutu S, Alphonse K. J Agric Food Chem. 2007;55(2):285–290. doi: 10.1021/jf062148t. [DOI] [PubMed] [Google Scholar]
- 34.Hong N, Yaylayan VA, Raghavan GS, Paré JR, Bélanger JM. Nat Prod Lett. 2001;15(3):197–204. doi: 10.1080/10575630108041280. [DOI] [PubMed] [Google Scholar]
- 35.Bergeron C, Gafner S, Clausen E, Carrier DJ. J Agric Food Chem. 2005;53(8):3076–3080. doi: 10.1021/jf048408t. [DOI] [PubMed] [Google Scholar]
- 36.Deng C, Liu N, Gao M, Zhang X. J Chromatogr A. 2007;1153(1-2):90–96. doi: 10.1016/j.chroma.2007.01.081. [DOI] [PubMed] [Google Scholar]
- 37.Huie CW. Anal Bioanal Chem. 2002;373(1-2):23–30. doi: 10.1007/s00216-002-1265-3. [DOI] [PubMed] [Google Scholar]
- 38.Ong ES. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;812(1-2):23–33. doi: 10.1016/j.jchromb.2004.07.041. [DOI] [PubMed] [Google Scholar]
- 39.Liu Z, Schwimer J, Liu D, Lewis J, Greenway FL, York DA, Woltering EA. Phytotherapy Res. 2006;20(9):806–813. doi: 10.1002/ptr.1966. [DOI] [PubMed] [Google Scholar]
- 40.Pantev A, Ivancheva S, Staneva L, Serkedjieva J. Z Naturforsch [C] 2006;61(7-8):508–516. doi: 10.1515/znc-2006-7-807. [DOI] [PubMed] [Google Scholar]
- 41.Liu Z, Schwimer J, Liu D, Greenway FL, Anthony CT, Woltering EA. J Agri Food Chem. 2005;53(10):3909–3915. doi: 10.1021/jf048585u. [DOI] [PubMed] [Google Scholar]
- 42.Ogawa Y, Oku H, Iwaoka E, Iinuma M, Ishiguro K. Chem Pharm Bull (Tokyo) 2007;55(4):675–678. doi: 10.1248/cpb.55.675. [DOI] [PubMed] [Google Scholar]
- 43.Burdette JE, Chen SN, Lu ZZ, Xu H, White BE, Fabricant DS, Liu J, Fong HH, Farnsworth NR, Constantinou AI, Van Breemen RB, Pezzuto JM, Bolton JL. J Agric Food Chem. 2002;50(24):7022–7028. doi: 10.1021/jf020725h. [DOI] [PubMed] [Google Scholar]
- 44.Lapidot T, Walker MD, Kanner J. J Agric Food Chem. 2002;50(11):3156–3160. doi: 10.1021/jf011522g. [DOI] [PubMed] [Google Scholar]
- 45.Lapidot T, Walker MD, Kanner J. J Agric Food Chem. 2002;50(25):7220–7225. doi: 10.1021/jf020615a. [DOI] [PubMed] [Google Scholar]
- 46.Arbiser JL, Klauber N, Rohan R, van Leeuwen R, Huang MT, Fisher C, Flynn E, Byers HR. Mol Med. 1998;4(6):376–383. [PMC free article] [PubMed] [Google Scholar]
- 47.Gao C, Ding Z, Liang B, Chen N, Cheng D. Zhong Yao Cai. 2003;26(7):499–502. [PubMed] [Google Scholar]
- 48.Liu D, Schwimer J, Liu Z, Woltering E, Greenway FL. Pharmaceutical Biology. 2007;45(9 or 10):000–000. in press. [Google Scholar]


