Abstract
Study Design: A randomized, double-blind, active-controlled trial.
Objective: To assess the effectiveness of cervical interlaminar epidural injections of local anesthetic with or without steroids for the management of axial or discogenic pain in patients without disc herniation, radiculitis, or facet joint pain.
Summary of Background Data: Cervical discogenic pain without disc herniation is a common cause of suffering and disability in the adult population. Once conservative management has failed and facet joint pain has been excluded, cervical epidural injections may be considered as a management tool. Despite a paucity of evidence, cervical epidural injections are one of the most commonly performed nonsurgical interventions in the management of chronic axial or disc-related neck pain.
Methods: One hundred and twenty patients without disc herniation or radiculitis and negative for facet joint pain as determined by means of controlled diagnostic medial branch blocks were randomly assigned to one of the 2 treatment groups. Group I patients received cervical interlaminar epidural injections of local anesthetic (lidocaine 0.5%, 5 mL), whereas Group II patients received 0.5% lidocaine, 4 mL, mixed with 1 mL or 6 mg of nonparticulate betamethasone.
The primary outcome measure was ≥ 50% improvement in pain and function. Outcome assessments included numeric rating scale (NRS), Neck Disability Index (NDI), opioid intake, employment, and changes in weight.
Results: Significant pain relief and functional improvement (≥ 50%) was present at the end of 2 years in 73% of patients receiving local anesthetic only and 70% receiving local anesthetic with steroids. In the successful group of patients, however, defined as consistent relief with 2 initial injections of at least 3 weeks, significant improvement was illustrated in 78% in the local anesthetic group and 75% in the local anesthetic with steroid group at the end of 2 years. The results reported at the one-year follow-up were sustained at the 2-year follow-up.
Conclusions: Cervical interlaminar epidural injections with or without steroids may provide significant improvement in pain and functioning in patients with chronic discogenic or axial pain that is function-limiting and not related to facet joint pain.
Keywords: Chronic neck pain, cervical discogenic pain, cervical axial pain, cervical disc herniation, cervical epidural injections, epidural steroids, local anesthetics.
Introduction
The State of U.S. Health: Burden of Diseases, Injuries, and Risk Factors 1 as published by the members of the U.S. Burden of Disease Collaboration showed that from 1990 to 2010, the United States made substantial progress in improving health, even though age-specific rates of years lived with disability remain stable. This report also showed that morbidity and chronic disability now account for nearly half of the U.S. health burden. Among the 30 leading diseases and injuries contributing to years lived with disability (YLD), low back pain was number one with neck pain occupying fourth place. Martin et al 2,3 evaluated health care expenditures for treatment of back and neck problems in the United States in 2005 and reported that these expenditures totaled approximately $86 billion, with an increase of 65% between 1997 and 2005 and a 49% increase in the number of patients seeking spine-related care. Studies of the prevalence of chronic neck pain and the impact it has on general health have shown that 14% of patients report Grade II to IV neck pain, with a high pain intensity leading to disability, with Grade 0 referring to no neck pain; Grade I representing pain of low intensity and few activity limitations; Grade II with neck pain of high intensity, but few activity limitations; Grade III with pain of high intensity and high levels of disability associated with moderate limitations in activities; and Grade IV referring to pain with high levels of disability and several activity limitations 4,5. It is important to note that Grade III and IV pain with disability is seen in 5% of patients 4. In addition, chronic recurrent neck pain is a common problem in the adult population, with a typical 12-month prevalence of 30% to 50% 6-9.
Among the multiple presentations of neck pain and upper extremity pain, cervical radicular pain is a common condition leading to interventional techniques and surgery 6,10-34. For patients without disc herniation, however, either related to discogenic pain from a degenerative disc or chemical irritation without facet joint pain, spondylosis, or spinal stenosis, the options are limited even though surgery and epidural injections are utilized in some cases 6,10,15-34. Consequently, all modalities of treatments, including cervical spine surgery and cervical epidural injections, have risen dramatically over the past 2 decades 6,10,16-20,22,23,25-34. Cervical interlaminar epidural injections have been applied only in recent years to manage chronic axial or discogenic pain without facet joint pain or radiculitis 6,10,15. The increase of cervical epidural injections in the fee-for-service (FFS) Medicare population from 2000 to 2011 of 123% per 100,000 population lags behind cervical transforaminal epidural injections 18 with increases of 182%, 662% for lumbar facet joint neurolysis 20, 836% for cervical facet joint neurolysis 20, and 665% for lumbar transforaminal epidural injections 18.
Axial neck pain may be related to either a disc or facet joint, spondylosis, or be musculoligamentous 6,9,21,24,25. In general, cervical epidural injections are not recommended for axial neck pain, but they are considered to be reasonable for disc herniation with radiculitis and spinal stenosis 6,10-14,16-18,35-37. A single report of a one year follow-up of discogenic neck pain after excluding cervical facet joint pain in 120 patients without disc herniation or radiculitis showed a primary outcome of significant pain relief and improvement in functional status (≥ 50%) in 72% of patients in Group I receiving local anesthetic and 68% in Group II receiving local anesthetic and steroids. In patients considered to be successful, with at least 3 weeks of significant improvement with the first 2 procedures, the results were superior with 78% in Group I and 73% in Group II. Overall, this study showed better results in patients not receiving steroids in contrast to the results of disc herniation in the lumbar spine 38,39. However, the results are similar to the treatment of disc herniation in the cervical spine 11,14 and lumbar discogenic pain 40-42 and superior to treatment of central spinal stenosis 12,43-45 and post-surgery syndrome 13,46.
In addition to epidural injections, multiple modalities of treatment are applied for axial neck pain including surgical interventions which are increasing rapidly along with other interventional techniques 16-34. The primary goals of surgical intervention, however, are to relieve radiating arm pain in the case of radiculopathy and to prevent the progression of a neurological deficit in case of myelopathy 26,30. Consequently, surgical interventions are focused on cases of radiculopathy, myelopathy, or a combination. Thus, although surgical interventions for discogenic pain are increasing rapidly, they fail to meet the fundamental premise, lack effectiveness, and are coupled with associated complications and off-label use of bone morphogenic protein 26-34.
This study has been designed to assess the effectiveness of cervical interlaminar epidural injections with local anesthetic with or without steroids in patients with axial neck pain after eliminating disc herniation, radiculitis, facet joint pain, and those suffering from chronic, function-limiting neck pain with or without upper extremity pain despite conservative management. This report consists of the results of 120 patients with a 2-year follow-up, and is a continuation of a previously published one-year follow-up report 15.
Materials and Methods
The study was conducted in an interventional pain management referral center in the United States. The randomized, double-blind, active-control design based on Consolidated Standards of Reporting Trials (CONSORT) guidelines 47,48 was approved by the Institutional Review Board (IRB) and was also registered with the U.S. Clinical Trial Registry with an assignment number of NCT01071369. There was no external funding from any sources in conduct of this study. Only internal resources of the practice were utilized.
Participants
A total of 120 patients were recruited to participate in this randomized, active-controlled, double-blind trial. They were recruited from new patients presenting for interventional pain management. All patients were informed of the IRB approved protocol and all patients signed the informed consent. The informed consent and IRB approved protocol also described the withdrawal process.
Interventions
Of the 120 patients participating in the study, 60 patients were allocated to each group with Group I patients receiving cervical interlaminar epidural injections of local anesthetic with 5 mL of 0.5% preservative-free lidocaine and Group II receiving cervical interlaminar epidural injections with lidocaine 0.5%, 4 mL, mixed with 1 mL or 6 mg of nonparticulate betamethasone with a total of 5 mL of injectate.
Pre-enrollment Evaluation
All patients with axial pain or those without a definite diagnosis of disc herniation, spinal stenosis, spondylosis, or radiculitis underwent controlled comparative local anesthetic blocks to exclude facet joint pain 6,24,49,50. Prior to subjecting them to controlled comparative local anesthetic blocks, data on all patients were assessed using demographic data, medical and surgical history with coexisting disease(s), radiologic investigations, physical examination, pain rating scores using the numeric rating scale (NRS) scale 51,52, work status, opioid intake, and functional status assessment by the Neck Disability Index (NDI) 53,54. Information was also obtained in reference to drug therapy and conservative management as well as all other failed treatment modalities 55.
Inclusion Criteria
Only patients without disc herniation, radiculitis, spinal stenosis, spondylosis, and those who were judged to have negative cervical facet joint pain by means of controlled, comparative local anesthetic blocks were included. Patients must have been over 18 years of age, with chronic function-limiting neck pain with or without upper extremity pain of at least 6 months duration, have failed conservative management including drug therapy, physical therapy and structured exercise programs, and have the ability to understand the study protocol and provide voluntary, written, informed consent.
Exclusion Criteria
Any patient with cervical disc herniation, radiculitis, spinal stenosis, significant spondylosis, uncontrollable or unstable opioid use, uncontrolled psychiatric disorders, and uncontrolled medical illness (acute or chronic) were excluded from the study. Furthermore, any patients with medical conditions or abnormalities which could interfere with the interpretation of outcome assessments, pregnant or lactating women, and those with a history of or potential for adverse reaction(s) to either local anesthetic or steroids were also excluded.
Description of Interventions
All patients underwent diagnostic facet joint nerve blocks. They were performed on 2 different occasions utilizing short-acting and long-acting local anesthetics, specifically 0.5 mL of 1% preservative-free lidocaine on the first occasion, and 0.5% preservative-free bupivacaine on the second occasion. The patient's response was considered positive if pain relief lasted for more than 2 hours following the lidocaine injection and lasted at least 3 hours or more or longer than the duration of relief with lidocaine when bupivacaine was used, plus the ability to perform previously painful movements 6,24,49,50.
After the initial process and enrollment, cervical interlaminar epidural injections were performed under fluoroscopy in a sterile operating room with patients in the prone position, with intravenous access and sedation as medically necessary with appropriate monitoring by one physician (LM). The epidural space was identified in all cases using the loss of resistance technique with intermittent fluoroscopic visualization. The entry into the epidural space and appropriate positioning of the needle was confirmed with an injection of nonionic contrast medium, generally entering the epidural space from C7-T1 to C5-C6.
After the confirmation of the appropriate location of the epidural space, each patient was injected with 5 mL of solution that was identical in both groups and consisted of 5 mL of preservative-free lidocaine hydrochloride in Group I and 4 mL of preservative-free lidocaine mixed with 6 mg of nonparticulate betamethasone for a total of 5 mL of injectate in Group II.
Additional Interventions
Even though the protocol did not specify physical therapy, occupational therapy or drug therapy, all patients were provided with a structured exercise program, along with the continuation of conservative management with drug therapy, as well as continuation of work if they were already working.
Repeat cervical epidural injections were provided when increased levels of pain were reported along with the deterioration of pain relief, along with the deterioration of functional status to below 50%. A patient was unblinded if an emergency situation arose or if they requested to be unblinded.
Those patients who were nonresponsive to epidural injections continued with conservative management and were followed without further epidural injections, with only medical management, unless they requested unblinding.
Objectives
This assessment was designed to evaluate the effectiveness of cervical epidural injections with or without steroids for managing chronic recalcitrant neck pain with or without upper extremity pain diagnosed as discogenic pain without disc herniation, radiculitis, spinal stenosis, or facet joint pain.
Outcomes
Outcome measures included NRS, NDI, work status, and opioid intake in terms of morphine equivalence, assessed at baseline and at 3, 6, 12, 18, and 24 months following the treatment.
The primary outcome was defined as at least 50% pain relief associated with 50% improvement in functional status measured by NDI. The NRS and NDI have been shown to be valid and reliable in patients with mechanical neck pain 51-54. Furthermore, the significant improvement utilized in this study is a robust measure in contrast to generally utilized measures of 20% to 30% measurable difference in outcomes 56-58.
Opioid intake was assessed in terms of morphine equivalence after converting dosages from various types of opioids 59.
All patients enrolled were categorized into different employment categories. Patients unemployed or employed on a part-time basis with limited or no employment due to pain were classified as employable. Consequently, patients who chose not to work, were retired, or were homemakers (not working but not due to pain) were not considered to be in the employment pool.
Sample Size
The sample size was calculated based on previous studies as well as significant pain relief. In calculating the sample size, a 0.05 two-sided significance level, a power of 80%, and an allocation of 1:1 was considered 60. An allowance was also considered for a 10% attrition-noncompliance rate. Thus, while 55 patients in each group were estimated to be necessary, 60 patients were required.
Randomization
Of the 120 patients willing to participate, 60 patients were randomly assigned into each group.
Sequence Generation
Random allocations sequence by simple randomization by computer-generated sequences was utilized.
Allocation Concealment
Multiple precautions were observed in order to maintain allocation concealment. Randomization was not revealed to the patient, to the physician performing the procedure or to any others involved in the care of the patients. Identical drugs were prepared by one of 3 coordinators and information was not provided to any others participating in the care of the patients. One of the 3 coordinators also randomized the patients into 2 groups.
Blinding and Masking
Appropriate blinding or masking was achieved in both groups throughout the study period. Group assignments were random and were not revealed to any of the caretakers. Both solutions were clear so as to not identify the group assignment. In addition, all patients in the study were mixed with other patients receiving routine treatment without informing the physician performing the procedure of the inclusion of the patients either in the study or their randomization status.
Statistical Methods
The statistical methods included chi-squared statistic, Fisher's exact test, t-test, and paired t-test. In this analysis, the chi-squared statistic was used specifically to test the differences in proportions, whereas results were considered statistically significant if the P value was less than 0.05. Further, Fisher's exact test was used whenever the expected value was less than 5 and a paired t-test was used to compare the pre-treatment and post-treatment results of average pain scores and NDI measurements at baseline versus 3, 6, 12, 18, and 24 months. The t-test was performed for comparison of mean scores between groups.
An intent-to-treat analysis was performed utilizing either the last follow-up data or initial data for patients who dropped out of the study and for whom no other data were available. A sensitivity analysis was performed utilizing best care, worst care, and lost follow case scenario.
RESULTS
Participant Flow
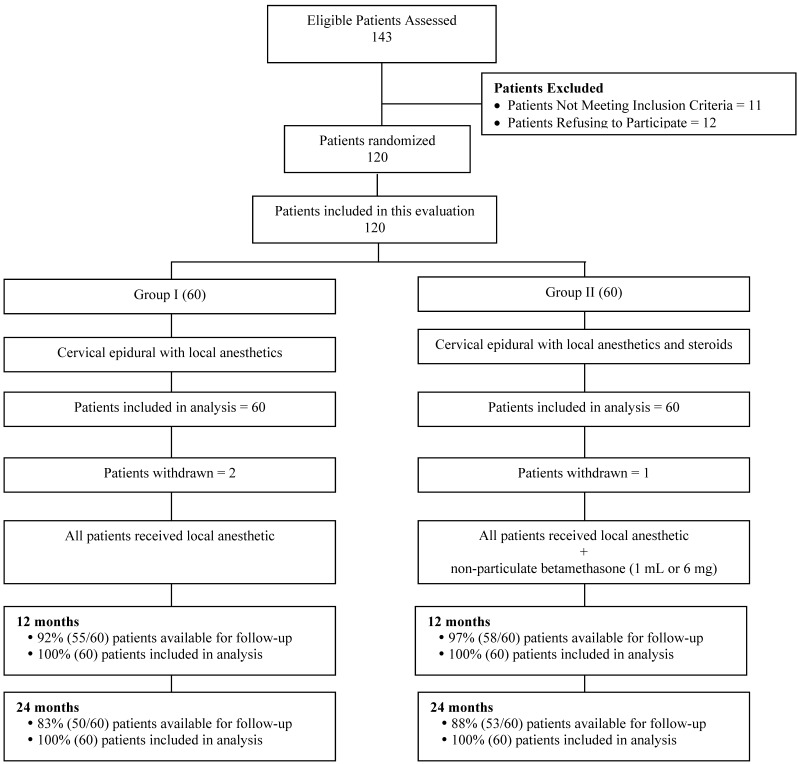
Figure 1 illustrates the patient flow. The recruitment period lasted from August 2007 to June 2010.
Figure 1.
Schematic presentation of patient flow at 2-year follow-up of 120 patients. Patients not available for follow-up: In Group I - 2 patients died, 2 withdrew, 5 lost to follow-up, and 1 developed MRSA due to unrelated surgery. Group II - 3 Lost to follow-up or moved away, 1 was discharged due to drug abuse; 1 had cardiac problems no treatment; 1 died; 1 was withdrawn.
Demographic Data
Baseline demographic data are summarized in Table 1. There were no significant differences among the groups, with the exception of weight with Group I patients weighing more than Group II patients.
Table 1.
Baseline demographic characteristics.
| Group 1 (60) | Group II (60) | P value | ||
|---|---|---|---|---|
| Gender | Male | 25% (15) | 32% (19) | 0.544 |
| Female | 75% (45) | 68% (41) | ||
| Age | Mean ± SD | 44.5 ± 12.6 | 41.8 ± 11.6 | 0.235 |
| Weight | Mean ± SD | 183.6 ± 57.5 | 164.7 ± 39.3 | 0.038 |
| Height | Mean ± SD | 65.6 ± 3.0 | 66.4 ± 3.5 | 0.184 |
| Duration of Pain (months) | Mean ± SD | 100.3 ± 94.3 | 95.8 ± 95.7 | 0.794 |
| Onset of the Pain | Gradual | 58% (35) | 47% (28) | 0.273 |
| Injury | 42% (25) | 53% (32) | ||
| Neck Pain Distribution | Neck pain only | 33% (20) | 43% (26) | 0.653 |
| Neck pain worse than upper extremity | 45% (27) | 37% (22) | ||
| Upper extremity pain worse than neck pain | 3% (2) | 2% (1) | ||
| Both equal | 18% (11) | 18% (11) | ||
| Numeric Rating Score | Mean ± SD | 7.9 ± 0.9 | 7.6 ± 0.8 | 0.074 |
| Neck Disability Index | Mean ± SD | 30.2 ± 4.7 | 28.6 ± 7.2 | 0.164 |
Therapeutic Procedural Characteristics
Epidural entry into the intervertebral spaces was 33% between C7 and T1, 58% between C6 and C7, and 9% between C5 and C6. Therapeutic procedural characteristics are shown in Table 2. There were no significant differences noted in average pain relief per year, with an average relief of 66.5 ± 35.0 weeks in Group I and 68.3 ± 33.6 weeks in Group II at the end of 2 years. The total number of injections per 2 years was 5.7 ± 2.4 in Group I and 5.8 ± 2.3 in Group II. At the end of one year, the total number was 3.6 ± 1.1 in Group I and 3.6 ± 1.0 in Group II. After the separation of patients into successful and failed groups, the number was slightly higher at the end of one year as well as at the end of 2 years; however, total relief also increased in the successful group, 73.3 ± 29.6 weeks in Group I and 71.2 ± 31.2 weeks in Group II at the end of 2 years. This was also different at end of one year with 39.2 ± 13.2 weeks versus 36.4 ± 15.9 weeks in Group I and 37.3 ± 37.7 weeks versus 34.8 ± 16.1 weeks in Group II. Further, the total relief was only 5.2 ± 8.4 and 0.8 ± 1 week in the failed groups at the end of one year; at the end of 2 years this was 13.2 ± 26.2 in Group I and 0.8 ± 1.0 in Group II.
Table 2.
Therapeutic procedural characteristics with procedural frequency, average relief per procedure, and average total relief in weeks over a period of 1-year.
| Successful Subjects | Failed Subjects | Combined | ||||
|---|---|---|---|---|---|---|
| Group I (55) |
Group II (56) |
Group I (5) |
Group II (4) |
Group I (60) |
Group II (60) |
|
| Average Number of Procedures for One Year | 3.7 ± 0.9 (55) |
3.7 ± 0.9 (56) |
2.4 ± 1.7 (5) |
2.2 ± 1.0 (4) |
3.6 ± 1.1 (60) |
3.6 ± 1.0 (60) |
| Average Number of Procedures for Two Years | 5.9 ± 2.3 | 6.1 ± 2.2 | 3.0 ± 2.9 | 2.3 ± 1.0 | 5.7 ± 2.4 | 5.8 ± 2.3 |
| Average Relief per Procedure for Initial Two Procedures in Weeks | 9.1 ± 5.5 (110) |
8.7 ± 7.0 (110) |
1.1 ± 1.0 (8) |
0.2 ± 0.4 (7) |
8.6 ± 5.7 (118) |
8.2 ± 7.0 (117) |
| Average Relief per Procedure after Initial Two Procedures | 13.4# ± 6.9 (95) |
11.8 ± 4.2 (98) |
4.6 ± 6.1 (4) |
1.0 ± 0 (2) |
13.1 ± 7.0 (99) |
11.5 ± 4.5 (100) |
| Average Relief per Procedure | 12.6 ± 8.8 | 12.0 ± 9.0 | 4.4 ± 5.5 | 0.4 ± 0.5 | 12.2 ± 8.8 | 11.7 ± 9.1 |
| Average Total Relief for One Year (Weeks) | 39.2 ± 13.2 (55) |
37.3 ± 13.7 (56) |
5.2 ± 8.4 (5) |
0.8 ± 1.0 (4) |
36.4 ± 15.9 (60) |
34.8 ± 16.1 (60) |
| Average Total Relief For Two Years (Weeks) | 73.3 ± 29.6 | 71.2 ± 31.2 | 13.2 ± 26.2 | 0.8 ± 1.0 | 66.5 ± 35.0 | 68.3 ± 33.6 |
Assessment of Pain Relief and Function
Table 3 shows the results of the NRS and NDI scores and the proportion of patients with significant (> 50%) pain relief in each category at baseline as well as at 3, 6, 12, 18, and 24 months. No significant difference was seen between the groups, although there was a significant difference between the baseline and various follow-up periods in each group.
Table 3.
Comparison of Numeric Rating Scale (NRS) for pain and Neck Disability Index (NDI) score summaries at 6 time points.
| Time Points | Numeric Pain Rating Score Mean ± SD |
Neck Disability Index Mean ± SD |
||
|---|---|---|---|---|
| Group I (N=60) |
Group II (N=60) |
Group I (N=60) |
Group II (N=60) |
|
| Baseline | 7.9 ± 0.9 | 7.6 ± 0.8 | 30.2 ± 4.7 | 28.6 ± 7.2 |
| 3 months | 3.7* ± 1.4 (73%) |
3.3* ± 1.0 (85%) |
15.5* ± 6.0 (70%) |
13.7* ± 5.4 (78%) |
| 6 months | 3.6* ± 1.4 (78%) |
3.5* ± 1.3 (77%) |
15.0* ± 5.6 (68%) |
14.2* ± 6.1 (73%) |
| 12 months | 3.7* ± 1.3 (80%) |
3.6* ± 1.4 (73%) |
14.6* ± 5.8 (73%) |
14.4* ± 6.5 (68%) |
| 18 months | 3.7* ± 1.4 (75%) |
3.6* ± 1.4 (77%) |
14.2* ± 5.5 (80%) |
13.9* ± 5.9 (73%) |
| 24 months | 3.7* ± 1.6 (75%) |
3.5* ± 1.4 (75%) |
14.1* ± 5.7 (75%) |
13.8* ± 6.5 (70%) |
| Group Difference | 0.346 | 0.129 | ||
| Baseline vs Follow-up Points | 0.001 | 0.001 | ||
| Group by Time Interaction# | 0.348 | 0.303 | ||
Percentages in parenthesis illustrate proportion with significant pain relief (≥ 50%) from baseline.
* indicates significant difference with baseline values (P < 0.01) within the group.
# Group by Time Interaction - There was no significant difference between groups at 3 months, 6 months, 12 months, 18 months and 24 months.
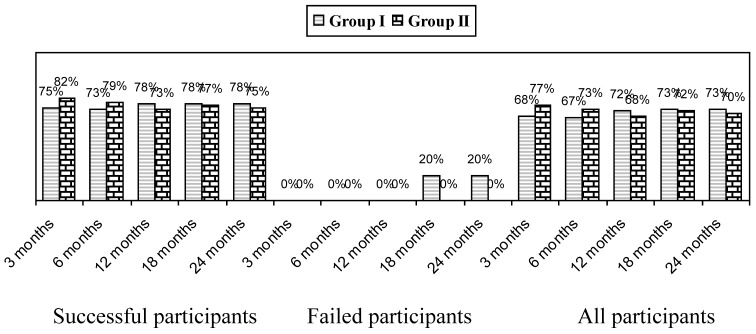
Significant improvement as defined by pain relief and functional status improvement of 50% is shown in Figure 2. This figure also shows improvement as assessed in all patients, the successful group, and the failed group. Patients reporting at least significant improvement for 3 weeks with the first 2 procedures were considered to be in the successful group, whereas, all others were included in the failed group.
Figure 2.
Proportion of patients with significant reduction in Numeric Rating Score and Neck Disability Index (>= 50% reduction from baseline).
Overall, improvement was seen in 73% in Group I and 70% in Group II at 2 years; whereas in the successful group the improvement was 78% in Group I and 75% in Group II.
Employment Characteristics
Table 4 shows employment characteristics in both groups. Overall there were a total of 17 patients in Group I and 25 patients in Group II eligible for employment. The employment total increased from 11 to 14 in Group I, and from 14 to 18 in Group II at the end of 2 years.
Table 4.
Employment characteristics.
| Employment Status | Group I | Group II | ||||
|---|---|---|---|---|---|---|
| Baseline | 12 months | 24 months | Baseline | 12 months | 24 months | |
| Employed part-time | 8 | 5 | 4 | 5 | 4 | 3 |
| Employed full-time | 3 | 12 | 10 | 14 | 18 | 18 |
| Unemployed | 6 | 2 | 3 | 6 | 4 | 4 |
| Eligible for employment at baseline | 17 | 17 | 17 | 25 | 25 | 25 |
| Total Employed | 11 | 17 | 14 | 19 | 22 | 21 |
| Housewife | 4 | 3 | 3 | 4 | 3 | 3 |
| Disabled | 37 | 36 | 38 | 27 | 27 | 28 |
| Retired or Over 65 | 2 | 2 | 2 | 4 | 4 | 4 |
| Total Number of Patients | 60 | 60 | 60 | 60 | 60 | 60 |
Opioid Intake
Table 5 illustrates opioid intake. There was no significant difference among the groups, although there was a significant decrease from baseline to all follow-up points in Group I only.
Table 5.
Opioid intake (morphine equivalence mg).
| Opioid Intake (Morphine Equivalence mg) |
Group I (60) | Group II (60) |
|---|---|---|
| Mean ± SD | Mean ± SD | |
| Baseline | 47.0 ± 35.0 | 39.1 ± 27.1 |
| 3 months | 37.1* ± 21.2 | 33.7 ± 22.0 |
| 6 months | 36.8* ± 21.0 | 33.8 ± 22.0 |
| 12 months | 36.9* ± 20.9 | 34.7 ± 23.5 |
| 18 months | 36.9* ± 20.9 | 34.5 ± 23.5 |
| 24 months | 36.9* ± 20.9 | 34.5 ± 23.5 |
| Group Difference | 0.281 | |
| Baseline vs follow-up points | 0.003 | |
| Group by Time Interaction# | 0.372 | |
* indicates significant difference with baseline values in group I (P < 0.01).
# Group by Time Interaction - There was no significant difference between groups at 3 months, 6 months, 12 months, 18 months and 24 months.
Changes in Weight
As shown in Table 6, there were no significant patterns of change in weight noted among the groups or from baseline to follow-up periods. Overall, at the end of 2 years, 45% of the patients in each group lost weight, 17% in Group I and 10% in Group II had no change in weight, and 38% in Group I and 45% in Group II gained weight.
Table 6.
Characteristics of changes in weight.
| Weight (lbs.) | Group I (60) | Group II (60) | P value |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Weight at beginning | 183.6 ± 57.5 | 164.7 ± 39.3 | 0.038 |
| Weight at one year | 182.6 ± 59.7 | 165.4 ± 41.8 | 0.070 |
| Change | -1.0 ± 9.7 | 0.7 ± 8.8 | 0.313 |
| Lost weight | 43% (26) | 38% (23) | 0.645 |
| No change | 20% (12) | 17% (10) | |
| Gained weight | 37% (22) | 45% (27) | |
| Weight at two years | 182.0 ± 63.1 | 165.4 ± 40.9 | 0.089 |
| Change | -1.5 ± 18.7 | 0.75 ± 12.0 | 0.428 |
| Lost weight | 45% (27) | 45% (27) | 0.517 |
| No change | 17% (10) | 10% (6) | |
| Gained weight | 38% (23) | 45% (27) |
Adverse Events
Of the 688 cervical epidural procedures performed, 6 subarachnoid punctures, 10 intravascular penetrations, and 3 nerve root irritations were observed without long-term sequelae. All patients experiencing transient nerve root irritation were given dexamethasone 8 mg intravenously. No postoperative headache was reported after subarachnoid punctures.
Discussion
The 2 year results of this randomized, double-blind, active control trial, assessing the effectiveness of cervical interlaminar epidural injections with local anesthetic with or without steroids in recalcitrant axial or discogenic pain without disc herniation, radiculitis, spinal stenosis, or facet joint pain, showed significant, clinically applicable results in interventional pain management settings. This assessment showed significant improvement, with improvement in pain relief and functional status of 50% or more observed in 73% of patients receiving local anesthetic only and in 70% of patients receiving local anesthetic with steroid at the end of 2 years. Furthermore, in the successful group with at least 3 weeks or relief with the first 2 procedures, significant improvement was seen in 78% in the local anesthetic group and in 75% in those patients who received local anesthetic and steroids. Overall, the average number of procedures per year was 3.6 at the end of the first year and 5.8 at the end of the second year with an average duration of total relief per year of 66.5 ± 35.0 weeks in Group I and 68.3 ± 33.6 weeks in Group II over a period of 104 weeks. In the successful group, the average total relief for 2 years was 73.3 ± 29.6 weeks in Group I with local anesthetic only and 71.2 ± 31.2 weeks in Group II with steroids. More importantly, there was no significant difference among the patients receiving local anesthetic only compared to those receiving steroids also. Both groups showed similar results in reference to significant improvement (50% pain relief and improvement in functional status). The results were similar to the one year follow-up of this study and the results of a similar study of the lumbar spine 40,41. The results were also superior to lumbar and cervical spine disc herniation patients in even though lumbar spine improvement was better in the steroid group 14,38,39. The results were superior to those of patients receiving either local anesthetic alone or with steroids in spinal stenosis and post-surgery syndrome in the cervical and lumbar spine 43-46.
The paucity of literature with respect to epidural injections in managing axial or discogenic neck pain continues, as this is the only trial 15. Studies performed in contemporary interventional pain management settings with appropriate assessment, proper design including the proper definition of placebo, performed under fluoroscopy with the repeat of injections as necessary at appropriate intervals are very few. There have been numerous published studies evaluating the role of cervical epidural injections performed without fluoroscopy. The importance of fluoroscopy and the nature of the setting where they are performed including contemporary interventional pain management settings, as well as selection criteria, have demonstrated, yielding superior results. Without fluoroscopy, the delivery of the injectate is not ceratin 61-66. In addition, this study also provides an insight into the selection of patients with identifying successful or failed groups based on positive results.
Even though multiple complications have been reported with cervical epidural injections, these complications are infrequent. Major complications are rare with fluoroscopically guided interlaminar cervical epidural injections 67-75. Reports of complications with transforaminal epidural injections have been significantly higher and devastating and the conclusions lack proven clinical superiority 76-85. Factors related to vasculature and discontinuity in the ligamentum flavum may contribute to an increased level of complications in the cervical spine compared to the lumbar spine 86,87.
While the underlying mechanism of action of epidurally administered local anesthetics and steroids is not clear, it is believed to be due to the antiinflammatory properties of corticosteroids 88-98. The evidence also indicates, however, that local anesthetics may have a similar effect to that of steroids 37,97-104. Furthermore, there is abundant literature based on clinical and experimental evidence that local anesthetics and steroids may provide long-term relief, often equally individually 6,10-15,25,38-46,97-104.
As with all trials, this trial has multiple strengths and limitations. The strengths include the study being a practical clinical trial conducted in a private practice setting, with inclusion of fairly large number of patients and an active-control design. Furthermore, this study was performed under fluoroscopy with repeat interventions provided only upon the return of pain and deterioration of functional status. In an era of comparative effectiveness, evidence-based medicine, and a cost-conscious environment 105,106, the current trial, even though limited to a single center, provides evidence generalizable to contemporary interventional pain management settings. Various measures have been undertaken to ensure proper patient selection, including only selecting patients with axial neck pain without facet joint pain, disc herniation, radiculitis, and spinal stenosis. Consequently, this study is typical of a practical clinical trial with an active-control group instead of a placebo-controlled trial. These types of trials in contemporary medicine are more effective in providing value-based interventional pain management 105.
Limitations include the lack of a placebo group. Having a placebo group, by design, with the appropriate inclusion of an injection of a placebo solution into a nonactive structure, has been debated 6,10,107-115. Placebo interventions have been misinterpreted based on the solution injected and the location of the injection, with some even interpreting local anesthetic injection as placebo, not realizing the inactive substances injected into active structures invariably result in a multitude of effects, with the majority of them being therapeutic 116-119. The effects of placebo, nocebo, Hawthorne effect, natural course of the disease which is not applicable in these chronic patients, and regression to mean have been extensively discussed in reference to placebo, nocebo, and pure, impure, and fake placeboes 120-123. The only appropriate placebo designs reported in interventional pain management were those of Ghahreman et al 111 and Gerdesmeyer 124. These trials showed that when proper placebo design is achieved with injection of an inactive solution into an inactive structure, it is not only considered as true placebo, but that the results are striking in the treatment groups. In addition, the other limitation in this study includes the higher weight of patients in Group I compared to that of Group II; however, this appears to have not resulted in any significant difference among the groups.
The implications of this trial for health care are enormous in an era of health care costs that have spiraled out of control, resulting in numerous public policies and restrictions on the provision of care 105. Thus, it is crucial that accountable interventional pain management include performing trials with appropriate methodology in practical settings. This promotes accountable interventional pain management with an improvement in patient care without curtailing patient access and assists in curbing health care costs. In fact, this is the opposite of the inappropriate utilization of health care resources and provides the necessary appropriate information for shared decision making to patients presenting into similar settings.
Conclusion
The 2-year follow-up of this randomized, double-blind, active control trial of 120 patients with chronic function-limiting axial or discogenic pain managed with fluoroscopically guided cervical epidural injections with local anesthetic with or without steroids showed effectiveness in 71% of patients. This trial also showed improvement in pain and functional status requiring an average of 6 procedures over a period of 2 years with relief for 72 weeks over a period of 2 years in the successful group.
Acknowledgments
The authors wish to thank Alvaro F. Gómez, MA, and Laurie Swick, BS, for manuscript review, and Tonie M. Hatton and Diane E. Neihoff, transcriptionists, for their assistance in preparation of this manuscript.
References
- 1.U.S. Burden of Disease Collaborators. The state of US health, 1990-2010: Burden of disease, injuries, and risk factors. JAMA. 2013;310:591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin BI, Deyo RA, Mirza SK. et al. Expenditures and health status among adults with back and neck problems. JAMA. 2008; 299:656-64. Erratum in: JAMA. 2008;299:2630. doi: 10.1001/jama.299.6.656. [DOI] [PubMed] [Google Scholar]
- 3.Martin BI, Turner JA, Mirza SK, Lee MJ, Comstock BA, Deyo RA. Trends in health care expenditures, utilization, and health status among US adults with spine problems, 1997-2006. Spine (Phila Pa 1976) 2009;34:2077–84. doi: 10.1097/BRS.0b013e3181b1fad1. [DOI] [PubMed] [Google Scholar]
- 4.Côté P, Cassidy JD, Carroll L. The Saskatchewan Health and Back Pain Survey. The prevalence of neck pain and related disability in Saskatchewan adults. Spine (Phila Pa 1976) 1998;23:1689–98. doi: 10.1097/00007632-199808010-00015. [DOI] [PubMed] [Google Scholar]
- 5.Côté P, Cassidy JD, Carroll LJ, Kristman V. The annual incidence and course of neck pain in the general population: A population-based cohort study. Pain. 2004;112:267–73. doi: 10.1016/j.pain.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Manchikanti L, Abdi S, Atluri S. et al. An update of comprehensive evidence-based guidelines for interventional techniques of chronic spinal pain: Part II: Guidance and recommendations. Pain Physician. 2013;16:S49–S283. [PubMed] [Google Scholar]
- 7.Hogg-Johnson S, van der Velde G, Carroll LJ. et al. The burden and determinants of neck pain in the general population: results of the Bone and Joint Decade 2000-2010 Task Force on Neck Pain and Its Associated Disorders. Spine (Phila Pa 1976) 2008;33:S39–S51. doi: 10.1097/BRS.0b013e31816454c8. [DOI] [PubMed] [Google Scholar]
- 8.Vos CJ, Verhagen AP, Passchier J, Koes BW. Clinical course and prognostic factors in acute neck pain: An inception cohort study in general practice. Pain Med. 2008;9:572–80. doi: 10.1111/j.1526-4637.2008.00456.x. [DOI] [PubMed] [Google Scholar]
- 9.Manchikanti L, Falco FJE, Benyamin RM. Manchikanti L, Christo PJ, Trescot AM, Falco FJE (eds) Clinical Aspects of Pain Medicine and Interventional Pain Management: A Comprehensive Review. Paducah, KY: ASIPP Publishing; 2011. Neck and cervical radicular pain; pp. 35–60. [Google Scholar]
- 10.Diwan SA, Manchikanti L, Benyamin RM. et al. Effectiveness of cervical epidural injections in the management of chronic neck and upper extremity pain. Pain Physician. 2012;15:E405–34. [PubMed] [Google Scholar]
- 11.Manchikanti L, Cash KA, Pampati V, Wargo BW, Malla Y. Management of chronic pain of cervical disc herniation and radiculitis with fluoroscopic cervical interlaminar epidural injections. Int J Med Sci. 2012;9:424–34. doi: 10.7150/ijms.4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manchikanti L, Malla Y, Cash KA, McManus CD, Pampati V. Fluoroscopic epidural injections in cervical spinal stenosis: Preliminary results of a randomized, double-blind, active control trial. Pain Physician. 2012;15:E59–70. [PubMed] [Google Scholar]
- 13.Manchikanti L, Malla Y, Cash KA, McManus CD, Pampati V. Fluoroscopic cervical interlaminar epidural injections in managing chronic pain of cervical post-surgery syndrome: Preliminary results of a randomized, double-blind active control trial. Pain Physician. 2012;15:13–26. [PubMed] [Google Scholar]
- 14.Manchikanti L, Cash KA, Pampati V, Wargo BW, Malla Y. A randomized, double-blind, active control trial of fluoroscopic cervical interlaminar epidural injections in chronic pain of cervical disc herniation: Results of a 2-year follow-up. Pain Physician. 2013;16:465–78. [PubMed] [Google Scholar]
- 15.Manchikanti L, Cash KA, Pampati V, Malla Y. Fluoroscopic cervical epidural injections in chronic axial or disc-related neck pain without disc herniation, facet joint pain, or radiculitis. J Pain Res. 2012;5:227–36. doi: 10.2147/JPR.S32692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manchikanti L, Pampati V, Falco FJE, Hirsch JA. Growth of spinal interventional pain management techniques: Analysis of utilization trends and Medicare expenditures 2000 to 2008. Spine (Phila Pa 1976) 2013;38:157–68. doi: 10.1097/BRS.0b013e318267f463. [DOI] [PubMed] [Google Scholar]
- 17.Manchikanti L, Falco FJE, Singh V. et al. Utilization of interventional techniques in managing chronic pain in the Medicare population: Analysis of growth patterns from 2000 to 2011. Pain Physician. 2012;15:E969–82. [PubMed] [Google Scholar]
- 18.Manchikanti L, Pampati V, Falco FJE, Hirsch JA. Assessment of the growth of epidural injections in the Medicare population from 2000 to 2011. Pain Physician. 2013;16:E349–64. [PubMed] [Google Scholar]
- 19.Patil PG, Turner DA, Pietrobon R. National trends in surgical procedures for degenerative cervical spine disease: 1990-2000. Neurosurgery. 2005;57:753–8. [PubMed] [Google Scholar]
- 20.Manchikanti L, Pampati V, Singh V, Falco FJE. Assessment of the escalating growth of facet joint interventions in the Medicare population in the United States from 2000 to 2011. Pain Physician. 2013;16:E365–78. [PubMed] [Google Scholar]
- 21.Onyewu O, Manchikanti L, Falco FJE. et al. An update of the appraisal of the accuracy and utility of cervical discography in chronic neck pain. Pain Physician. 2012;15:E777–E806. [PubMed] [Google Scholar]
- 22.Verhagen AP, van Middelkoop M, Rubinstein SM. et al. Effect of various kinds of cervical spinal surgery on clinical outcomes: A systematic review and meta-analysis. Pain. 2013 doi: 10.1016/j.pain.2013.07.022. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23.Oglesby M, Fineberg SJ, Patel AA, Pelton MA, Singh K. Epidemiological trends in cervical spine surgery for degenerative diseases between 2002 and 2009. Spine (Phila Pa 1976) 2013;38:1226–32. doi: 10.1097/BRS.0b013e31828be75d. [DOI] [PubMed] [Google Scholar]
- 24.Falco FJE, Datta S, Manchikanti L. et al. An updated review of diagnostic utility of cervical facet joint injections. Pain Physician. 2012;15:E807–38. [PubMed] [Google Scholar]
- 25.Falco FJE, Manchikanti L, Datta S. et al. Systematic review of therapeutic effectiveness of cervical facet joint interventions: An update. Pain Physician. 2012;15:E839–68. [PubMed] [Google Scholar]
- 26.Boselie TF, Willems PC, van Mameren H, de Bie RA, Benzel EC, van Santbrink H. Arthroplasty versus fusion in single-level cervical degenerative disc disease: a Cochrane review. Spine (Phila Pa 1976) 2013;38:E1096–107. doi: 10.1097/BRS.0b013e3182994a32. [DOI] [PubMed] [Google Scholar]
- 27.Wang TY, Lubelski D, Abdullah KG, Steinmetz MP, Benzel EC, Mroz TE. Rates of anterior cervical discectomy and fusion after initial posterior cervical foraminotomy. Spine J. 2013 doi: 10.1016/j.spinee.2013.05.042. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28.Fineberg SJ, Oglesby M, Patel AA, Pelton MA, Singh K. Outcomes of cervical spine surgery in teaching and non-teaching hospitals. Spine (Phila Pa 1976) 2013;38:1089–96. doi: 10.1097/BRS.0b013e31828da26d. [DOI] [PubMed] [Google Scholar]
- 29.Shamji MF, Cook C, Pietrobon R, Tackett S, Brown C, Isaacs RE. Impact of surgical approach on complications and resource utilization of cervical spine fusion: A nationwide perspective to the surgical treatment of diffuse cervical spondylosis. Spine J. 2009;9:31–8. doi: 10.1016/j.spinee.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Cunningham MR, Hershman S, Bendo J. Systematic review of cohort studies comparing surgical treatments for cervical spondylotic myelopathy. Spine (Phila Pa 1976) 2010;35:537–43. doi: 10.1097/BRS.0b013e3181b204cc. [DOI] [PubMed] [Google Scholar]
- 31.Shields LB, Raque GH, Glassman SD, Campbell M, Vitaz T, Harpring J, Shields CB. Adverse effects associated with high-dose recombinant human bone morphogenetic protein-2 use in anterior cervical spine fusion. Spine (Phila Pa 1976) 2006;31:542–7. doi: 10.1097/01.brs.0000201424.27509.72. [DOI] [PubMed] [Google Scholar]
- 32.Ong KL, Villarraga ML, Lau E, Carreon LY, Kurtz SM, Glassman SD. Off-label use of bone morphogenetic proteins in the United States using administrative data. Spine (Phila Pa 1976) 2010;35:1794–800. doi: 10.1097/BRS.0b013e3181ecf6e4. [DOI] [PubMed] [Google Scholar]
- 33.Singh K, Ahmadinia K, Park D, Nandyala SV, Marquez-Lara A, Patel AA, Fineberg SJ. Complications of spinal fusion with utilization of bone morphogenetic protein: a systematic review of the literature. Spine (Phila Pa 1976) 2013. [Epub ahead of print] [DOI] [PubMed]
- 34.Vaidya R, Carp J, Sethi A, Bartol S, Craig J, Les CM. Complications of anterior cervical discectomy and fusion using recombinant human bone morphogenetic protein-2. Eur Spine J. 2007;16:1257–65. doi: 10.1007/s00586-007-0351-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castagnera L, Maurette P, Pointillart V, Vital JM, Erny P, Senegas J. Long-term results of cervical epidural steroid injection with and without morphine in chronic cervical radicular pain. Pain. 1994;58:239–43. doi: 10.1016/0304-3959(94)90204-6. [DOI] [PubMed] [Google Scholar]
- 36.Stav A, Ovadia L, Sternberg A, Kaadan M, Weksler N. Cervical epidural steroid injection for cervicobrachialgia. Acta Anaesthesiol Scand. 1993;37:562–6. doi: 10.1111/j.1399-6576.1993.tb03765.x. [DOI] [PubMed] [Google Scholar]
- 37.Pasqualucci A, Varrassi G, Braschi A. et al. Epidural local anesthetic plus corticosteroid for the treatment of cervical brachial radicular pain: Single injection verus continuous infusion. Clin J Pain. 2007;23:551–7. doi: 10.1097/AJP.0b013e318074c95c. [DOI] [PubMed] [Google Scholar]
- 38.Manchikanti L, Singh V, Cash KA, Pampati V, Damron KS, Boswell MV. Effect of fluoroscopically guided caudal epidural steroid or local anesthetic injections in the treatment of lumbar disc herniation and radiculitis: A randomized, controlled, double blind trial with a two-year follow-up. Pain Physician. 2012;15:273–86. [PubMed] [Google Scholar]
- 39.Manchikanti L, Singh V, Cash KA, Pampati V, Falco FJE. The role of fluoroscopic interlaminar epidural injections in managing chronic pain of lumbar disc herniation or radiculitis: A randomized, double-blind trial. Pain Pract. 2013;13:547–558. doi: 10.1111/papr.12023. [DOI] [PubMed] [Google Scholar]
- 40.Manchikanti L, Cash KA, McManus CD, Pampati V. Fluoroscopic caudal epidural injections in managing chronic axial low back pain without disc herniation, radiculitis or facet joint pain. J Pain Res. 2012;5:381–90. doi: 10.2147/JPR.S35924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manchikanti L, Cash KA, McManus CD, Pampati V, Benyamin R. Fluoroscopic lumbar interlaminar epidural injections in managing chronic lumbar axial or discogenic pain. J Pain Res. 2012;5:301–11. doi: 10.2147/JPR.S32699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manchikanti L, Cash KA, McManus CD, Pampati V, Benyamin RM. A randomized, double-blind, active-controlled trial of fluoroscopic lumbar interlaminar epidural injections in chronic axial or discogenic low back pain: Results of a 2-year follow-up. Pain Physician. 2013;16:E491–504. [PubMed] [Google Scholar]
- 43.Manchikanti L, Cash KA, McManus CD, Pampati V, Fellows B. Results of 2-year follow-up of a randomized, double-blind, controlled trial of fluoroscopic caudal epidural injections in central spinal stenosis. Pain Physician. 2012;15:371–84. [PubMed] [Google Scholar]
- 44.Manchikanti L, Cash KA, McManus CD, Damron KS, Pampati V, Falco FJE. Lumbar interlaminar epidural injections in central spinal stenosis: Preliminary results of a randomized, double-blind, active control trial. Pain Physician. 2012;15:51–63. [PubMed] [Google Scholar]
- 45.Manchikanti L, Cash KA, McManus CD, Pampati V, Fellows B. Fluoroscopic caudal epidural injections with or without steroids in managing pain of lumbar spinal stenosis: One year results of randomized, double-blind, active-controlled trial. J Spinal Disord Tech. 2012;25:226–34. doi: 10.1097/BSD.0b013e3182160068. [DOI] [PubMed] [Google Scholar]
- 46.Manchikanti L, Singh V, Cash KA, Pampati V, Datta S. Fluoroscopic caudal epidural injections in managing post lumbar surgery syndrome: Two-year results of a randomized, double-blind, active-control trial. Int J Med Sci. 2012;9:582–91. doi: 10.7150/ijms.4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Altman DG, Schulz KF, Moher D, et al; CONSORT GROUP (Consolidated Standards of Reporting Trials). The revised CONSORT statement for reporting randomized trials: Explanation and elaboration. Ann Intern Med. 2001;134:663–94. doi: 10.7326/0003-4819-134-8-200104170-00012. [DOI] [PubMed] [Google Scholar]
- 48.Moher D, Hopewell S, Schulz KF. et al. CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869. doi: 10.1136/bmj.c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Manchukonda R, Manchikanti KN, Cash KA, Pampati V, Manchikanti L. Facet joint pain in chronic spinal pain: An evaluation of prevalence and false-positive rate of diagnostic blocks. J Spinal Disord Tech. 2007;20:539–45. doi: 10.1097/BSD.0b013e3180577812. [DOI] [PubMed] [Google Scholar]
- 50.Manchikanti L, Boswell MV, Singh V, Pampati V, Damron KS, Beyer CD. Prevalence of facet joint pain in chronic spinal pain of cervical, thoracic, and lumbar regions. BMC Musculoskelet Disord. 2004;5:15. doi: 10.1186/1471-2474-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.National Institutes of Health. Warren Grant Magnuson Clinical Center. Pain Intensity Instruments, Numeric Rating Scale, July. 2003. http://www.mvltca.net/Presentations/mvltca.pdf.
- 52.Cleland JA, Childs JD, Whitman JM. Psychometric properties of the Neck Disability Index and Numeric Pain Rating Scale in patients with mechanical neck pain. Arch Phys Med Rehabil. 2008;89:69–74. doi: 10.1016/j.apmr.2007.08.126. [DOI] [PubMed] [Google Scholar]
- 53.Pietrobon R, Coeytaux RR, Carey TS, Richardson WJ, DeVellis RF. Standard scales for measurement of functional outcome for cervical pain or dysfunction: A systematic review. Spine (Phila Pa 1976) 2002;27:515–22. doi: 10.1097/00007632-200203010-00012. [DOI] [PubMed] [Google Scholar]
- 54.Vernon H, Mior S. The Neck Disability Index: A study of reliability and validity. J Manipulative Physiol Ther. 1991;14:409–15. [PubMed] [Google Scholar]
- 55.Manchikanti L, Cash KA, Malla Y, Pampati V, Fellows B. A prospective evaluation of psychotherapeutic and illicit drug use in patients presenting with chronic pain at the time of initial evaluation. Pain Physician. 2013;16:E1–13. [PubMed] [Google Scholar]
- 56.Carragee EJ. The rise and fall of the “minimum clinically important difference”. Spine J. 2010;10:283–4. doi: 10.1016/j.spinee.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 57.Carragee EJ, Chen I. Minimum acceptable outcomes after lumbar spinal fusion. Spine J. 2010;10:313–20. doi: 10.1016/j.spinee.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 58.Gatchel RJ, Mayer TG, Choi Y, Chou R. Validation of a consensus-based minimal clinically important difference (MCID) threshold using an objective functional external anchor. Spine J. 2013;13:889–93. doi: 10.1016/j.spinee.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 59.Pereira J, Lawlor P, Vigano A, Dorgan M, Bruera E. Equianalgesic dose ratios for opioids. A critical review and proposals for long-term dosing. J Pain Symptom Manage. 2001;22:672–87. doi: 10.1016/s0885-3924(01)00294-9. [DOI] [PubMed] [Google Scholar]
- 60.Browner WS, Newman TB, Cummings SR, Hulley SB. Estimating sample size and power. In: Hulley SB, Cummings SR, Browner WS, Grady D, Hearst N, Newman TB, editors. Designing Clinical Research: An Epidemiologic Approach. 2nd ed. Philadelphia: Lippincott, Williams & Wilkins; 2001. pp. 65–84. [Google Scholar]
- 61.Cluff R, Mehio AK, Cohen SP, Chang Y, Sang CN, Stojanovic MP. The technical aspects of epidural steroid injections: A national survey. Anesth Analg. 2002;95:403–8. doi: 10.1097/00000539-200208000-00031. [DOI] [PubMed] [Google Scholar]
- 62.Stojanovic MP, Vu TN, Caneris O, Slezak J, Cohen SP, Sang CN. The role of fluoroscopy in cervical epidural steroid injections: An analysis of contrast dispersal patterns. Spine (Phila Pa 1976) 2002;27:509–14. doi: 10.1097/00007632-200203010-00011. [DOI] [PubMed] [Google Scholar]
- 63.Kim KS, Shin SS, Kim TS, Jeong CY, Yoon MH, Choi JI. Fluoroscopically guided cervical interlaminar epidural injections using the midline approach: An analysis of epidurography contrast patterns. Anesth Analg. 2009;108:1658–61. doi: 10.1213/ane.0b013e31819d107b. [DOI] [PubMed] [Google Scholar]
- 64.Goel A, Pollan JJ. Contrast flow characteristics in the cervical epidural space: An analysis of cervical epidurograms. Spine (Phila Pa 1976) 2006;31:1576–9. doi: 10.1097/01.brs.0000222020.45794.ac. [DOI] [PubMed] [Google Scholar]
- 65.Thangamuthu A, Russell IF, Purva M. Epidural failure rate using a standardised definition. Int J Obstet Anesth. 2013 doi: 10.1016/j.ijoa.2013.04.013. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 66.Lee SE, Joe HB, Park JH, Yi IK, Choi YH, Han KR, Kim C. Distribution range of cervical interlaminar epidural injections: a comparative study with 2.5 mL, 5 mL, and 10 mL of contrast. Pain Physician. 2013;16:155–64. [PubMed] [Google Scholar]
- 67.Manchikanti L, Malla Y, Wargo BW, Cash KA, Pampati V, Fellows B. A prospective evaluation of complications of 10,000 fluoroscopically directed epidural injections. Pain Physician. 2012;15:131–40. [PubMed] [Google Scholar]
- 68.Kaplan MS, Cunniff J, Cooke J, Collins JG. Intravascular uptake during fluoroscopically guided cervical interlaminar steroid injection at C6-7: A case report. Arch Phys Med Rehabil. 2008;89:553–8. doi: 10.1016/j.apmr.2007.08.165. [DOI] [PubMed] [Google Scholar]
- 69.Ho KY. Vascular uptake of contrast despite negative aspiration in interlaminar cervical epidural injection. Pain Physician. 2006;9:267–8. [PubMed] [Google Scholar]
- 70.Stout A. Epidural steroid injections for cervical radiculopathy. Phys Med Rehabil Clin N Am. 2011;22:149–59. doi: 10.1016/j.pmr.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 71.Abbasi A, Malhotra G, Malanga G, Elovic EP, Kahn S. Complications of interlaminar cervical epidural steroid injections: A review of the literature. Spine (Phila Pa 1976) 2007;32:2144–51. doi: 10.1097/BRS.0b013e318145a360. [DOI] [PubMed] [Google Scholar]
- 72.Hodges SD, Castleberg RL, Miller T, Ward R, Thornburg C. Cervical epidural steroid injection with intrinsic spinal cord damage. Two case reports. Spine (Phila Pa 1976) 1998;23:2137–42. doi: 10.1097/00007632-199810010-00020. [DOI] [PubMed] [Google Scholar]
- 73.McGrath JM, Schaefer MP, Malkamaki DM. Incidence and characteristics of complications from epidural steroid injections. Pain Med. 2011;12:726–31. doi: 10.1111/j.1526-4637.2011.01077.x. [DOI] [PubMed] [Google Scholar]
- 74.Shanthanna H, Park J. Acute epidural haematoma following epidural steroid injection in a patient with spinal stenosis. Anaesthesia. 2011;66:837–9. doi: 10.1111/j.1365-2044.2011.06770.x. [DOI] [PubMed] [Google Scholar]
- 75.Manchikanti L, Benyamin RM, Swicegood JR. et al. Assessment of practice patterns of perioperative management of antiplatelet and anticoagulant therapy in interventional pain management. Pain Physician. 2012;15:E955–68. [PubMed] [Google Scholar]
- 76.Scanlon GC, Moeller-Bertram T, Romanowsky SM, Wallace MS. Cervical transforaminal epidural steroid injections: More dangerous than we think? Spine (Phila Pa 1976) 2007;32:1249–56. doi: 10.1097/BRS.0b013e318053ec50. [DOI] [PubMed] [Google Scholar]
- 77.Singh R, Panagos A. Quadriparesis following cervical epidural steroid injections. Spine J. 2006;6:349. doi: 10.1016/j.spinee.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 78.Rathmel JP, Benzon HT. Transforaminal injection of steroids: Should we continue? Reg Anesth Pain Med. 2004;29:297–9. doi: 10.1016/j.rapm.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 79.Tiso RL, Cutler T, Catania JA, Whalen K. Adverse central nervous system sequelae after selective transforaminal block: the role of corticosteroids. Spine J. 2004;4:468–74. doi: 10.1016/j.spinee.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 80.Brouwers PJ, Kottink EJ, Simon MA, Prevo RL. A cervical anterior spinal artery syndrome after diagnostic blockade of the right C6-nerve root. Pain. 2001;91:397–9. doi: 10.1016/S0304-3959(00)00437-1. [DOI] [PubMed] [Google Scholar]
- 81.Wallace MA, Fukui MB, William RL, Ku A, Baghai P. Complications of cervical selective nerve root blocks performed with fluoroscopic guidance. AJR Am J Roentgenol. 2007;188:1218–21. doi: 10.2214/AJR.04.1541. [DOI] [PubMed] [Google Scholar]
- 82.Rathmell JP, Aprill C, Bogduk N. Cervical transforaminal injection of steroids. Anesthesiology. 2004;100:1595–1600. doi: 10.1097/00000542-200406000-00035. [DOI] [PubMed] [Google Scholar]
- 83.Provenzano DA, Fanciullo G. Cervical transforaminal epidural steroid injections: should we be performing them? Reg Anesth Pain Med. 2007;32:168. doi: 10.1016/j.rapm.2006.06.205. author reply 169-70. [DOI] [PubMed] [Google Scholar]
- 84.Datta S, Manchikanti L, Falco FJE. et al. Diagnostic utility of selective nerve root blocks in the diagnosis of lumbosacral radicular pain: Systematic review and update of current evidence. Pain Physician. 2013;16:SE97–SE124. [PubMed] [Google Scholar]
- 85.Manchikanti L, Buenaventura RM, Manchikanti KN. et al. Effectiveness of therapeutic lumbar transforaminal epidural steroid injections in managing lumbar spinal pain. Pain Physician. 2012;15:E199–245. [PubMed] [Google Scholar]
- 86.Lirk P, Kolbitsch C, Putz G. et al. Cervical and high thoracic ligamentum flavum frequently fails to fuse in the midline. Anesthesiology. 2003;99:1387–90. doi: 10.1097/00000542-200312000-00023. [DOI] [PubMed] [Google Scholar]
- 87.Ho PS, Yu SW, Sether LA, Wagner M, Ho KC, Haughton VM. Ligamentum flavum: appearance on sagittal and coronal MR images. Radiology. 1988;168:469–72. doi: 10.1148/radiology.168.2.3393666. [DOI] [PubMed] [Google Scholar]
- 88.Byrod G, Otani K, Brisby H, Rydevik B, Olmarker K. Methylprednisolone reduces the early vascular permeability increase in spinal nerve roots induced by epidural nucleus pulposus application. J Orthop Res. 2000;18:983–7. doi: 10.1002/jor.1100180619. [DOI] [PubMed] [Google Scholar]
- 89.Lee HM, Weinstein JN, Meller ST, Hayashi N, Spratt KF, Gebhart GF. The role of steroids and their effects on phospholipase A2. An animal model of radiculopathy. Spine (Phila Pa 1976) 1998;23:1191–6. doi: 10.1097/00007632-199806010-00001. [DOI] [PubMed] [Google Scholar]
- 90.Olmarker K, Byröd G, Cornefjord M, Nordborg C, Rydevik B. Effects of methylprednisolone on nucleus pulposus-induced nerve root injury. Spine (Phila Pa 1976) 1994;19:1803–8. doi: 10.1097/00007632-199408150-00003. [DOI] [PubMed] [Google Scholar]
- 91.Hayashi N, Weinstein JN, Meller ST, Lee HM, Spratt KF, Gebhart GF. The effect of epidural injection of betamethasone or bupivacaine in a rat model of lumbar radiculopathy. Spine (Phila Pa 1976) 1998;23:877–85. doi: 10.1097/00007632-199804150-00008. [DOI] [PubMed] [Google Scholar]
- 92.Alimasi W, Sawaji Y, Endo K. et al. Regulation of nerve growth factor by anti-inflammatory drugs, a steroid, and a selective cyclooxygenase 2 inhibitor in human intervertebral disc cells stimulated with interleukin-1. Spine (Phila Pa 1976) 2013;38:1466–72. doi: 10.1097/BRS.0b013e318294edb1. [DOI] [PubMed] [Google Scholar]
- 93.Minamide A, Tamaki T, Hashizume H, Yoshida M, Kawakami M, Hayashi N. Effects of steroids and lipopolysaccharide on spontaneous resorption of herniated intervertebral discs: An experimental study in the rabbit. Spine (Phila Pa 1976) 1998;23:870–6. doi: 10.1097/00007632-199804150-00007. [DOI] [PubMed] [Google Scholar]
- 94.Pasqualucci A. Experimental and clinical studies about the preemptive analgesia with local anesthetics. Possible reasons of the failure. Minerva Anestesiol. 1998;64:445–57. [PubMed] [Google Scholar]
- 95.Bisby MA. Inhibition of axonal transport in nerves chronically treated with local anesthetics. Exp Neurol. 1975;47:481–9. doi: 10.1016/0014-4886(75)90080-1. [DOI] [PubMed] [Google Scholar]
- 96.Cassuto J, Sinclair R, Bonderovic M. Anti-inflammatory properties of local anesthetics and their present and potential clinical implications. Acta Anaesthesiol Scand. 2006;50:265–82. doi: 10.1111/j.1399-6576.2006.00936.x. [DOI] [PubMed] [Google Scholar]
- 97.Tachihara H, Sekiguchi M, Kikuchi S, Konno S. Do corticosteroids produce additional benefit in nerve root infiltration for lumbar disc herniation? Spine (Phila Pa 1976) 2008;33:743–7. doi: 10.1097/BRS.0b013e3181696132. [DOI] [PubMed] [Google Scholar]
- 98.Sato C, Sakai A, Ikeda Y, Suzuki H, Sakamoto A. The prolonged analgesic effect of epidural ropivacaine in a rat model of neuropathic pain. Anesth Analg. 2008;106:313–20. doi: 10.1213/01.ane.0000296460.91012.51. [DOI] [PubMed] [Google Scholar]
- 99.Parr AT, Manchikanti L, Hameed H. et al. Caudal epidural injections in the management of chronic low back pain: A systematic appraisal of the literature. Pain Physician. 2012;15:E159–98. [PubMed] [Google Scholar]
- 100.Benyamin RM, Manchikanti L, Parr AT. et al. The effectiveness of lumbar interlaminar epidural injections in managing chronic low back and lower extremity pain. Pain Physician. 2012;15:E363–404. [PubMed] [Google Scholar]
- 101.Falco FJE, Manchikanti L, Datta S. et al. An update of the effectiveness of therapeutic lumbar facet joint interventions. Pain Physician. 2012;15:E909–53. [PubMed] [Google Scholar]
- 102.Manchikanti L, Singh V, Falco FJE, Cash KA, Pampati V. Evaluation of lumbar facet joint nerve blocks in managing chronic low back pain: A randomized, double-blind, controlled trial with a 2-year follow-up. Int J Med Sci. 2010;7:124–35. doi: 10.7150/ijms.7.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Manchikanti L, Singh V, Falco FJE, Cash KA, Fellows B. Comparative outcomes of a 2-year follow-up of cervical medial branch blocks in management of chronic neck pain: A randomized, double-blind controlled trial. Pain Physician. 2010;13:437–50. [PubMed] [Google Scholar]
- 104.Manchikanti L, Singh V, Falco FJE, Cash KA, Pampati V, Fellows B. The role of thoracic medial branch blocks in managing chronic mid and upper back pain: A randomized, double-blind, active-control trial with a 2-year follow-up. Anesthesiol Res Pract. 2012;2012:585806. doi: 10.1155/2012/585806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Manchikanti L, Falco FJE, Benyamin RM, Helm II S, Singh V, Hirsch JA. Value-based interventional pain management: A review of Medicare national and local coverage determination policies. Pain Physician. 2013;16:E145–80. [PubMed] [Google Scholar]
- 106.Manchikanti L, Falco FJE, Pampati V, Cash KA, Benyamin RM, Hirsch JA. Cost utility analysis of caudal epidural injections in the treatment of lumbar disc herniation, axial or discogenic low back pain, central spinal stenosis, and post lumbar surgery syndrome. Pain Physician. 2013;16:E129–43. [PubMed] [Google Scholar]
- 107.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline. Choice of Control Group and Related Issues in Clinical Trials E10. 2000.
- 108.Iversen T, Solberg T, Romner B. et al. Effect of caudal epidural steroid or saline injection in chronic lumbar radiculopathy: multicentre, blinded, randomised controlled trial. BMJ. 2011;343:d5278. doi: 10.1136/bmj.d5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Carette S, Leclaire R, Marcoux S. et al. Epidural corticosteroid injections for sciatica due to herniated nucleus pulposus. N Engl J Med. 1997;336:1634–40. doi: 10.1056/NEJM199706053362303. [DOI] [PubMed] [Google Scholar]
- 110.Karppinen J, Malmivaara A, Kurunlahti M. et al. Periradicular infiltration for sciatica: a randomized controlled trial. Spine (Phila Pa 1976) 2001;26:1059–67. doi: 10.1097/00007632-200105010-00015. [DOI] [PubMed] [Google Scholar]
- 111.Ghahreman A, Ferch R, Bogduk N. The efficacy of transforaminal injection of steroids for the treatment of lumbar radicular pain. Pain Med. 2010;11:1149–68. doi: 10.1111/j.1526-4637.2010.00908.x. [DOI] [PubMed] [Google Scholar]
- 112.Chou R, Atlas SJ, Loeser JD, Rosenquist RW, Stanos SP. Guideline warfare over interventional therapies for low back pain: can we raise the level of discourse? J Pain. 2011;12:833–9. doi: 10.1016/j.jpain.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 113.Manchikanti L, Benyamin RM, Falco FJE, Caraway DL, Datta S, Hirsch JA. Guidelines warfare over interventional techniques: Is there a lack of discourse or straw man? Pain Physician. 2012;15:E1–26. [PubMed] [Google Scholar]
- 114.Pinto RZ, Maher CG, Ferreira ML. et al. Epidural corticosteroid injections in the management of sciatica: A systematic review and meta-analysis. Ann Intern Med. 2012;157:865–77. doi: 10.7326/0003-4819-157-12-201212180-00564. [DOI] [PubMed] [Google Scholar]
- 115.Manchikanti L, Falco FJE, Hirsch JA. Epidural corticosteroid injections in the management of sciatica. Ann Intern Med. 2012;157:865–77. doi: 10.7326/0003-4819-157-12-201212180-00564. [DOI] [PubMed] [Google Scholar]
- 116.Indahl A, Kaigle AM, Reikeräs O, Holm SH. Interaction between the porcine lumbar intervertebral disc, zygapophysial joints, and paraspinal muscles. Spine (Phila Pa 1976) 1997;22:2834–40. doi: 10.1097/00007632-199712150-00006. [DOI] [PubMed] [Google Scholar]
- 117.Indahl A, Kaigle A, Reikeräs O, Holm S. Electromyographic response of the porcine multifidus musculature after nerve stimulation. Spine (Phila Pa 1976) 1995;20:2652–8. doi: 10.1097/00007632-199512150-00006. [DOI] [PubMed] [Google Scholar]
- 118.Pham Dang C, Lelong A, Guilley J. et al. Effect on neurostimulation of injectates used for perineural space expansion before placement of a stimulating catheter: normal saline versus dextrose 5% in water. Reg Anesth Pain Med. 2009;34:398–403. doi: 10.1097/AAP.0b013e3181b48648. [DOI] [PubMed] [Google Scholar]
- 119.Tsui BC, Kropelin B, Ganapathy S, Finucane B. Dextrose 5% in water: Fluid medium maintaining electrical stimulation of peripheral nerve during stimulating catheter placement. Acta Anaesthesiol Scand. 2005;49:1562–5. doi: 10.1111/j.1399-6576.2005.00736.x. [DOI] [PubMed] [Google Scholar]
- 120.Kaptchuk TJ, Friedlander E, Kelley JM. et al. Placebos without deception: A randomized controlled trial in irritable bowel syndrome. PLoS One. 2010;5:e15591. doi: 10.1371/journal.pone.0015591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Howick J, Friedemann C, Tsakok M. et al. Are treatments more effective than placebos? A systematic review and meta-analysis. PLoS One. 2013;8:e62599. doi: 10.1371/journal.pone.0062599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Howick J, Bishop FL, Heneghan. et al. Placebo use in the United Kingdom: Results from a national survey of primary care practitioners. PLoS One. 2013;8:e58247. doi: 10.1371/journal.pone.0058247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hróbjartsson A, Gøtzsche PC. Placebo interventions for all clinical conditions. Cochrane Database Syst Rev. 2010;1:CD003974. doi: 10.1002/14651858.CD003974.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gerdesmeyer L, Wagenpfeil S, Birkenmaier C. et al. Percutaneous epidural lysis of adhesions in chronic lumbar radicular pain: A randomized double-blind placebo controlled trial. Pain Physician. 2013;16:185–96. [PubMed] [Google Scholar]