Abstract
Purpose
Fungi, rhinoviruses (RVs), and eosinophils are associated with upper respiratory diseases. We evaluated the effects of fungal stimulation and eosinophil co-culture on the expression of mucin genes in RV-infected nasal polyp epithelial cells.
Methods
Nasal polyp epithelial cells were obtained from chronic rhinosinusitis patients. Cultured epithelial cells were stimulated with Alternaria and Aspergillus with or without RV-16 infection. The epithelial cells were co-cultured with eosinophils for 16 h. MUC4, MUC5AC, MUC5B, and MUC8 mRNA expressions in the epithelial cells were quantified using real-time RT-PCR. To determine the underlying mechanism, nuclear factor-κB (NF-κB), activator protein-1 (AP-1), and mitogen-activated protein kinase (MAPK) inhibitors were used to inhibit mucin gene expression.
Results
Fungi and RV-16 induced mucin gene expression in nasal polyp epithelial cells. However, there was no synergistic increase in mucin gene expression, with the exception of MUC4 mRNA expression stimulated by 25 µg/mL Aspergillus. When RV-16-infected epithelial cells were stimulated with fungi and then co-cultured with eosinophils, MUC4, MUC5B, and MUC8 mRNA expressions increased. Mucin gene expression was inhibited by NF-κB inhibitors.
Conclusions
RV-16, airborne fungi, and eosinophils may exacerbate the inflammatory process in nasal mucosal diseases by enhancing mucin gene expression.
Keywords: Rhinovirus, nasal epithelial cell, mucin gene, fungus, eosinophil, nuclear factor-κB
INTRODUCTION
Fungi are ubiquitous and deposited continuously on the airway mucosa with inhalation. Airborne fungi are rarely pathogenic in healthy individuals. However, immunological reactions to fungi have been suggested as an etiologic factor in chronic rhinosinusitis (CRS) and asthma. Evidence acquired over the last decade supports a role of fungi as not only an etiology but a risk factor for respiratory diseases.1 Fungal proteins and enzymes induce immunological and inflammatory responses. Proteases from fungal extracts are potent inducers of epithelial cell desquamation and facilitate antigen access to target tissues that can cause direct activation of cells. Their proteolytic activity leads to the production of proinflammatory cytokines by direct activation of epithelial cells. These chemical mediators influence the migration and activation of inflammatory cells.2
Rhinoviruses (RVs) are the major cause of respiratory tract infections, which can be complicated by otitis media, sinusitis, and bronchitis. RVs infect nasal epithelial cells and cause local inflammatory responses that are associated with increased numbers of neutrophils, eosinophils, and lymphocytes in nasal secretions. The manifestations of RV-induced respiratory inflammatory responses are thought to be the result of virus-induced chemical mediators.3,4 Viral inflammation causes impaired mucociliary clearance and edematous ostiomeatal obstruction, leading to the development of CRS. Most bacterial rhinosinusitis cases are believed to result from a preceding viral respiratory infection.5
Nasal polyps are edematous projections that originate from the nasal mucosa and of which inflammation is a major feature. In contrast to acute rhinosinusitis, the etiology and pathogenesis of CRS and nasal polyps are not well understood. Eosinophils are inflammatory cells and play an important role in the pathogenesis of CRS with polyps via release of their cytotoxic granule proteins.6 Eosinophil toxic proteins can damage the respiratory mucosa and play a role in the immune defense against large pathogens, such as fungi and parasites.7
Mucus hypersecretion is one of the major symptoms of upper and low airway inflammation. An increased surface area, as a result of mucosal hypertrophy and nasal polyps, increases the production of mucus and inflammatory cytokines, such as IL-6, IL8, and TNF-α, increasing mucin expression at the level of transcription, translation, or both.8,9 Local inflammatory conditions trigger the upregulation of mucin genes and enhance the production of mucin. These alterations in mucus secretion can have negative effects on mucosal protection and predispose to disease development.
Fungi, RVs, and eosinophils are associated with upper respiratory diseases, such as allergic rhinitis and CRS with or without polyps, through the production of chemical mediators by direct or indirect activation of epithelial cells. However, to date, no study has assessed the effects of interactions among fungi, eosinophils, and RVs on the expression of mucin by nasal polyp epithelial cells, an important pathological process in CRS. The aim of this study was to evaluate the effects of fungal stimulation and eosinophil co-culture on the expression of mucin genes in RV-infected nasal polyp epithelial cells.
MATERIALS AND METHODS
Nasal polyp epithelial cell (NPEC) culture
Nasal polyps were obtained from 12 patients undergoing endoscopic sinus surgery. Patients were excluded if they had an allergy or asthma, had received systemic or topical steroids, or had taken antibiotics or antihistamine medications during the 4 weeks preceding the study. Allergy status was defined using the skin prick test or multiple allergen simultaneous test-chemiluminescent assay. Patient immunologic status, recent viral infection or vaccination, and asthma were determined on the basis of medical and clinical histories.
The study was approved by the Institutional Review Board of Daegu Catholic University Medical Center. Each patient signed a consent form that outlined the objectives of the research and experiments.
Specimens were placed in Ham's F-12 medium supplemented with 100 international units (IU) of penicillin, 100 µg/mL streptomycin, and 2 µg/mL amphotericin B, and were then transported to the laboratory. NPECs were isolated by the protease digestion method. The polyps were rinsed with Ham's F-12 medium supplemented with antibiotics and incubated with dispase (Life Technologies, Gaithersburg, MD, USA) in Ham's F-12 medium for 16 h at 4℃. After incubation, the nasal polyp epithelial cells were isolated by gentle rinsing with culture medium. Cell suspensions were filtered through a No. 60 mesh cell-dissociation sieve and centrifuged (1,000 rpm, 5 min, room temperature). The cell pellet was then resuspended in Ham's F-12 medium supplemented with antibiotics, 150 µg/mL glutamine, 5 µg/mL transferrin, 25 ng/mL epithelial growth factor, 15 µg/mL endothelial cell growth supplement, insulin 5 IU/mL, 200 pM triiodothyronine, 100 nM hydrocortisone, and 10% fetal bovine serum (FBS). Cell suspensions (106 cells/mL) were plated in 6-well culture plates and placed in a 5% CO2 humidified incubator at 37℃. The culture medium was changed after 24 h and every 2 days thereafter.
RV-16 infection of NPECs and activation with fungi
RV serotype 16 (RV-16) was purchased from the American Type Culture Collection (Manassas, VA, USA). Viral stocks were prepared by infection of sensitive cell monolayers (Ohio HeLa), as previously described.10 Second-passage NPECs were seeded in 24-well plates at a density of ~1×105/mL and incubated for 24 h at 37℃. In the RV-16 infection group, the cells were washed with phosphate-buffered saline (PBS) and infected with RV-16 at a multiplicity of infection (MOI) of 1 for 8 h in FBS-free culture medium. This MOI was adopted on the basis of previous observations that it resulted in the greatest cytokine release.11 In the control group, the cells were cultured without RV-16 infection.
After 8 h, supernatants were harvested, the cells were washed with PBS, and then RV 16 infected epithelial cells were activated with endotoxin-free Alternaria alternata (10 and 50 µg/mL) and Aspergillus fumigatus (10 and 25 µg/mL; Greer Lab, Lenoir, NC, USA). After a 24 h incubation, the supernatants and epithelial cells were harvested and stored at -70℃ until assayed. The Alternaria and Aspergillus concentrations used were based on a previous study and preliminary activation experiments; maximal cytokine production was found at 50 µg/mL Alternaria and 25 µg/mL Aspergillus; no toxicity to the epithelial cells or eosinophils occurred with use of these levels.2
Co-culture of RV-16-infected NPECs and eosinophils
Eosinophils were isolated from peripheral blood obtained from normal healthy volunteers after they provided informed consent. The procedure was approved by the Institutional Review Board of Daegu Catholic University Medical Center.
Isolation was performed according to a method described previously, with minor modifications.2 Briefly, heparinized venous blood was layered onto 1,085 g/mL Percoll prepared in piperazine-N,N'-bis(2-ethanesulfonic acid) (PIPES) buffer (Sigma-Aldrich, St. Louis, MO, USA) and centrifuged (1,000 g, 30 min). The supernatants and mononuclear cells at the interface were removed carefully, and the erythrocytes in the sediment were lysed by suspension in hypotonic sterile water. The remaining granulocyte pellet was mixed with anti CD 16 antibody conjugated with magnetic particles (Miltenyi Biotec, Sunnyvale, CA, USA) and incubated for 30 min. Resuspended cells were separated using a magnetic cell separation system. After the cells were eluted with PIPES buffer, eosinophil counts and purity were determined. The purity of the eosinophils, as determined by Randolph's staining, exceeded 95%.
To determine whether fungi had a synergistic effect on mucin gene expression in RV-16-infected NPECs, RV-16-infected NPECs were stimulated with fungi for 24 h, and then treated with 1% paraformaldehyde in PBS for 1 h at room temperature to inhibit the synthesis of the cytokines that maintain intercellular interactions. Before co-culturing, the NPECs were washed several times with PBS because fungi alone could induce the expression of mucin genes. The medium was then replaced with RPMI 1640 containing 10% FBS with or without eosinophils. After 16 h, the supernatants and epithelial cells were harvested and stored at -70℃ until assayed.
Mucin gene expression by NPECs and inhibition study with transcription factor inhibitors
After stimulation with fungi or co-culture with eosinophils, the cell pellets were placed in cryo-tubes, and 1 mL of TRIzol reagent was added. RNA was extracted according to the manufacturer's protocol (Roche Diagnostics, Mannheim, Germany). The purity and concentration of RNA were evaluated using a spectrophotometer (Beckman, Mountain View, CA, USA). From the amplified cDNA, quantitative polymerase chain reaction (PCR) of MUC4, MUC5AC, MUC5B, MUC8, and β-actin was performed in the same 96-well plate using the SYBR Green PCR core kit (PE Applied Biosystems, Foster City, CA, USA) with the GeneAmp 5700 system (PE Applied Biosystems). The primer sequences and amplified products were as follows: MUC4 sense 5'-CGC GGT GGT GGA GGC GTT CTT-3' and antisense 5'-GAA GAA TCC TGA CAG CCT TCA-3' (101 bp), MUC5AC sense 5'-TCA TCA TCC AGC AGC AGG GCT-3' and antisense 5'-CCG AGC TCA GAG GAC ATA TGG G-3' (103 bp), MUC5B sense 5'-TGC CCC TTG TTC TGT GAC TT-3' and antisense 5'-ACG CAC TTC ATC TGG TCC TC-3' (194 bp), MUC8 sense 5'-GAC AGG GTT TCT CCT CAT TG-3' and antisense 5'-CGT TTA TTC CAG CAC TGT TC-3' (240 bp) and β actin sense 5'-ACA GGA AGT CCC TTG CCA TC-3' and antisense 5'-AGG GAG ACC AAA AGC CTT CA-3' (248 bp). The annealing temperatures were 54℃ for MUC4 and MUC5B, 60℃ for MUC5AC, and 56℃ for MCU8. All samples were amplified in triplicate. Then, mRNA expression levels were normalized to the median value for β-actin. For relative quantitation of mRNA levels, the 2-ΔΔCT method was used.
For inhibition experiments, nuclear factor-κB (NF-κB) inhibitor (BAY 11-7082), activator protein-1 (AP-1) inhibitor (curcumin), and mitogen-activated protein kinase (MAPK) inhibitor (SB 203580) were purchased from Calbiochem (San Diego, CA, USA). Bay 11-7082 and curcumin were dissolved in dimethyl sulfoxide, and SB 203580 was dissolved in water. NPECs were pre-treated with transcription factor inhibitors for 1 h. Mucin gene expression in epithelial cells was determined after treatment with fungi.
Statistical analysis
All experiments were performed at least 5 times; results were comparable. Results are presented as means±SD. Differences in mucin gene expression were assessed by the Kruskal-Wallis test between the groups, and the effects of transcription factor inhibitors were compared by one-way ANOVA using SPSS software (ver. 19.0; SPSS Inc., Chicago, IL, USA). Differences were considered significant if p values were less than 0.05.
RESULTS
Mucin gene expression in RV-16-infected NPEC and fungal stimulation
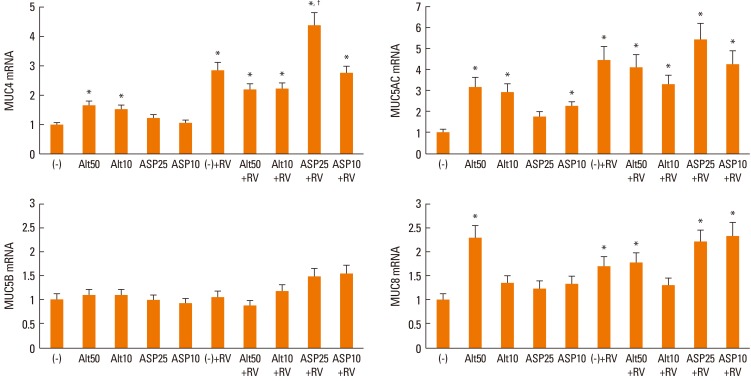
When the NPECs were stimulated with Alternaria, MUC4, MUC5AC, and MUC8 mRNA expressions were increased significantly compared with unstimulated cells. Aspergillus induced only MUC5AC mRNA expression. RV-16 infection alone also induced MUC4, MUC5AC, and MUC8 mRNA expression. However, when RV-16-infected epithelial cells were stimulated with fungi, there was no further (synergistic) increase in mucin gene expression, with the sole exception of MUC4 mRNA expression in the presence of 25 µg/mL Aspergillus (Fig. 1).
Fig. 1.
Quantification of mucin gene expression in nasal polyp epithelial cells stimulated with Alternaria and Aspergillus with or without rhinovirus-16 (RV-16) infection for 24 h. MUC4, MUC5AC, and MUC8 mRNA expression levels were increased significantly by Alternaria; however, only MUC5AC mRNA expression was increased by Aspergillus. RV-16 also induced MUC4, MUC5AC, and MUC8 mRNA expression. When RV-infected epithelial cells were stimulated by fungi, MUC4, MUC 5AC, and MUC8 mRNA expression levels were increased. Values are expressed as means±SD of 6 independent experiments. Alt: Alternaria (µg/mL), Asp: Aspergillus (µg/mL), RV: rhinovirus-16-infected, (-): unstimulated. *significantly higher than unstimulated cells, †significantly higher than RV-infected cells.
Mucin gene expression after co-culture of RV 16-infected NPECs and eosinophils
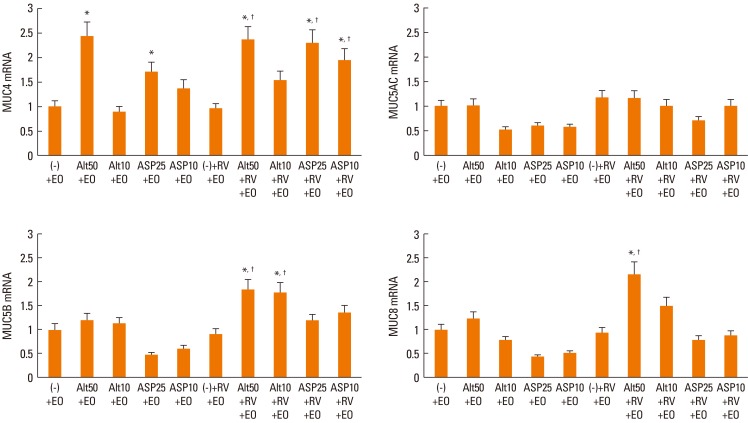
When NPECs were co-cultured with eosinophils with or without fungal stimulants, only MUC4 mRNA expression was significantly increased by Alternaria (50 µg/mL) and Aspergillus (25 µg/mL). When RV-16-infected NPECs were co-cultured with eosinophils, mucin gene expression was not affected. However, when these epithelial cells were pre-treated with fungi, MUC4 (Alternaria and Aspergillus), MUC5B and MUC8 (Alternaria), mRNA expressions were increased significantly. However, Alternaria-induced MUC4 mRNA expression was not synergistically increased with RV-16 infection of nasal polyp epithelial cells (Fig. 2).
Fig. 2.
Quantification of mucin gene expression in nasal polyp epithelial cells co-cultured with eosinophils and stimulated with Alternaria and Aspergillus with or without rhinovirus-16 (RV-16) infection for 16 h. Values are expressed as the means±SD of 6 independent experiments. Alt: Alternaria (µg/mL), Asp: Aspergillus (µg/mL), RV: rhinovirus-16-infected, EO: eosinophils, (-): unstimulated. *significantly higher than unstimulated cells, †significantly higher than RV-infected cells.
Mucin gene expression after pre-treatment with transcription factor inhibitors
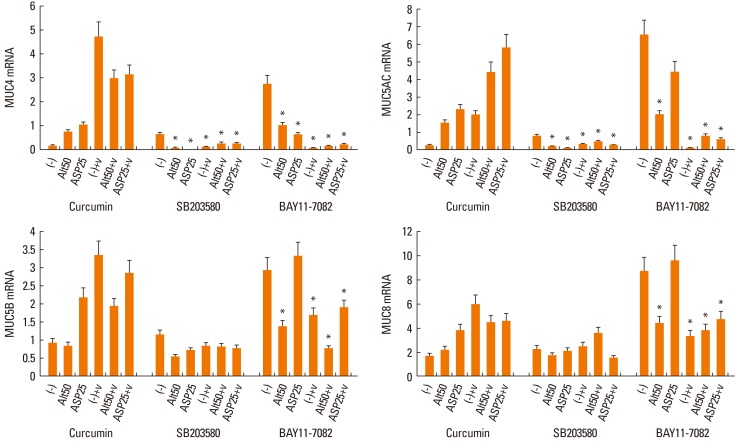
When RV-16-infected NPECs were pre-treated with transcription factor inhibitors, MUC4, MUC5AC, MUC5B, and MUC8 mRNA expressions were inhibited significantly by the NF-κB inhibitor. Furthermore, the NF-κB inhibitor inhibited Alternaria-induced mucin gene expression significantly. However, the NF-κB inhibitor inhibited only MUC4 mRNA expression in the presence of Aspergillus (Fig. 3).
Fig. 3.
Effects of curcumin, SB 203580 and BAY 11-7082 on the expression of mucin genes. SB 203580 (MAPK inhibitor) inhibited MUC4 and MUC5AC mRNA expression and BAY 11-7082 (NF-κB inhibitor) inhibited MUC4, MUC5AC, MUC5B, and MUC8 mRNA expression. Values are expressed as the means±SD of 5 independent experiments. Alt: Alternaria (µg/mL), Asp: Aspergillus (µg/mL), V: rhinovirus-16 infected, (-): non-stimulated. *P<0.05.
DISCUSSION
Respiratory epithelial cells play important roles in the initiation, maintenance, and regulation of innate and adaptive immune responses. Mucus maintains hydration and has important protective functions in mucosal defenses by entrapping particulates, such as bacteria and viruses. However, mucus hypersecretion is one of the major symptoms of upper and lower airway inflammation. Twenty-one human mucin genes have been identified. Normal sinus and nasal mucosa express MUC1, MUC2, MUC4, MUC5AC, MUC5B, MUC7, and MUC8.11 MUC4 and MUC5AC are the most highly expressed mucin genes in healthy nasal mucosa, and MUC4, MUC5AC, MUC5B, and MUC8 are upregulated in chronic sinusitis or nasal polyp tissues.12,13 In this study, we evaluated these 4 mucin genes. Fungal protein and enzymatic allergens react with specific receptors on respiratory epithelial cells, such as protease-activated receptors and Toll-like receptors. They induce the production of proinflammatory cytokines and chemokines, which may enhance mucin production and mucin gene expression by epithelial cells. In this study, Alternaria and Aspergillus were used to activate the nasal polyp epithelial cells because these are common pathogens in nasal secretions and respiratory tract diseases. MUC4, MUC5AC, and MUC8 mRNA expressions were increased by Alternaria; however, only MUC5AC mRNA expression was increased by Aspergillus. Thus, Alternaria may more strongly induce the production of mucus and be associated with the aggravation of respiratory symptoms. Our results are consistent with a previous report that Aspergillus fumigates induces MUC5AC expression in bronchial epithelial cells.16
Respiratory RV infection is the major cause of the common cold and is linked to the exacerbation of various airway diseases. RV infection of the nasal mucosa leads to an early transient neutrophil infiltration and causes a local inflammatory response that is associated with the production of cytokines and kinins.3 These chemical mediators may up-regulate the mRNA expression of mucin genes and RV infection may directly activate mucin gene expression even in the absence of inflammation.4 In this study, RV-16 infection of nasal polyp epithelial cells enhanced MUC4, MUC5AC, and MUC8 mRNA expression. Although we did not investigate mechanisms underlying the activation of mucin gene expression, RV-16-induced chemical mediator production and direct effects on nasal polyp epithelial cells may influence the expression of mucin genes. The production of IL-6, IL-8, and GM-CSF within NPECs induced by infection with RV-16 is significantly enhanced upon exposure to airborne fungi. When RV-16-infected NPECs were co-cultured with eosinophils, IL-6 and tumor necrosis factor-α production was increased by fungal exposure.17 In this study, only MUC4 mRNA expression was increased significantly when fungi-stimulated epithelial cells were co-cultured with eosinophils. There was some synergism between RV-16 infection and airborne fungal exposure in enhancing mucin gene expression in nasal polyp epithelial cells. Alternaria enhanced mucin gene expression in a dose-dependent manner. These effects may be associated with the increased production of chemical mediators from nasal polyp epithelial cells. When NPECs were stimulated by fungi, maximal cytokine production was found at 50 µg/mL Alternaria and 25 µg/mL Aspergillus. Alternaria and Aspergillus also enhanced the production of cytokines from RV-16 NPEC in a dose-dependent manner.17,18 Increased production of chemical mediators also may more strongly activate eosinophils or RV-16, and fungal allergens enhance the expression of intracellular adhesion molecule-1 (ICAM-1) of epithelial cells, which may interact with the ligands of eosinophils, such as leukocyte function-associated antigen-1. Takafuji et al. suggested that CD18-dependent adhesion of eosinophils to activated airway epithelial cells may be involved in enhancing eosinophil degranulation.19 Human nasal polyp-conditioned media activated eosinophils, with the production of superoxide and eosinophil-derived neurotoxin.20 When RV-16-infected nasal polyp epithelial cells were stimulated with airborne fungi and co cultured with eosinophils, MUC4, MUC5B, and MUC8 mRNA expression levels were increased. These results suggest that the clinical symptoms of eosinophilic inflammatory disease, such as bronchial asthma, allergic rhinitis, and CRS, aggravate RV infection and fungal exposure by enhancing the production of mucus by respiratory epithelial cells. However, fungi and RV-16-induced mucin gene expression patterns differed according to the presence of eosinophils.
To activate the transcription of mucin genes, each stimulant acts through a distinct cell-surface receptor, a distinct signaling pathway, and a distinct gene-regulatory element.19 Among these elements, MAPK, NF-κB, and AP-1 are key transcription factors that orchestrate the expression of many genes involved in inflammation. Alternaria enhances the production of chemical mediators through NF-κB-dependent pathways.21 Mucin gene transcription is generally regulated by the Sre/Ras/MAPK/pp90rsk cascade, which leads to the activation of the transcription factor NF-κB, the epidermal growth factor receptor, and the extracellular signal-regulated kinase pathway. In this study, fungi- and RV-16-induced MUC4, MUC5AC, MUC5B, and MUC8 mRNA expressions were significantly inhibited by the NF-κB inhibitor. The overexpression of these mucin genes might be associated with the various inflammatory mediators produced by epithelial cells upon fungal and RV-16 stimulation.
The results of this study demonstrate that fungi, RV-16, and eosinophils induce mucin gene expression in primary NPECs. Airborne fungi modulate the immunological reactions of virus-infected respiratory epithelial cells not only by influencing the production of chemical mediators but also by enhancing the expression of mucin genes. There was some synergism between RV-16 and airborne fungal exposure in terms of enhancing mucin gene expression. When RV-16-infected epithelial cells were stimulated with fungi then co-cultured with eosinophils, MUC4, MUC5B, and MUC8 mRNA expression levels were enhanced significantly. NF-κB seems to be one of the most important transcription factors controlling mucin gene expression in nasal polyp epithelial cells. Our results suggest that exposure of RV-infected nasal polyp epithelial cells to airborne fungi and eosinophils may be associated with mucin gene overexpression, which can enhance mucus production by upper respiratory epithelial cells.
ACKNOWLEDGMENTS
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology (2009-0073137).
Footnotes
There are no financial or other issues that might lead to conflict of interest.
References
- 1.Andersson M, Downs S, Mitakakis T, Leuppi J, Marks G. Natural exposure to Alternaria spores induces allergic rhinitis symptoms in sensitized children. Pediatr Allergy Immunol. 2003;14:100–105. doi: 10.1034/j.1399-3038.2003.00031.x. [DOI] [PubMed] [Google Scholar]
- 2.Shin SH, Lee YH, Jeon CH. Protease-dependent activation of nasal polyp epithelial cells by airborne fungi leads to migration of eosinophils and neutrophils. Acta Otolaryngol. 2006;126:1286–1294. doi: 10.1080/00016480500395179. [DOI] [PubMed] [Google Scholar]
- 3.Igarashi Y, Skoner DP, Doyle WJ, White MV, Fireman P, Kaliner MA. Analysis of nasal secretions during experimental rhinovirus upper respiratory infections. J Allergy Clin Immunol. 1993;92:722–731. doi: 10.1016/0091-6749(93)90016-9. [DOI] [PubMed] [Google Scholar]
- 4.Johnston SL, Papi A, Bates PJ, Mastronarde JG, Monick MM, Hunninghake GW. Low grade rhinovirus infection induces a prolonged release of IL-8 in pulmonary epithelium. J Immunol. 1998;160:6172–6181. [PubMed] [Google Scholar]
- 5.Jang YJ, Kwon HJ, Park HW, Lee BJ. Detection of rhinovirus in turbinate epithelial cells of chronic sinusitis. Am J Rhinol. 2006;20:634–636. doi: 10.2500/ajr.2006.20.2899. [DOI] [PubMed] [Google Scholar]
- 6.Stoop AE, van der Heijden HA, Biewenga J, van der Baan S. Eosinophils in nasal polyps and nasal mucosa: an immunohistochemical study. J Allergy Clin Immunol. 1993;91:616–622. doi: 10.1016/0091-6749(93)90267-j. [DOI] [PubMed] [Google Scholar]
- 7.Khan DA, Cody DT, 2nd, George TJ, Gleich GJ, Leiferman KM. Allergic fungal sinusitis: an immunohistologic analysis. J Allergy Clin Immunol. 2000;106:1096–1101. doi: 10.1067/mai.2000.110929. [DOI] [PubMed] [Google Scholar]
- 8.Ali MS, Wilson JA, Bennett M, Pearson JP. Mucin gene expression in nasal polyps. Acta Otolaryngol. 2005;125:618–624. doi: 10.1080/00016480510027538. [DOI] [PubMed] [Google Scholar]
- 9.Kim JY, Kim CH, Kim KS, Choi YS, Lee JG, Yoon JH. Extracellular signal-regulated kinase is involved in tumor necrosis factor-alpha-induced MUC5AC gene expression in cultured human nasal polyp epithelial cells. Acta Otolaryngol. 2004;124:953–957. doi: 10.1080/00016480310017054. [DOI] [PubMed] [Google Scholar]
- 10.Jang YJ, Wang JH, Kim JS, Kwon HJ, Yeo NK, Lee BJ. Levocetirizine inhibits rhinovirus-induced ICAM-1 and cytokine expression and viral replication in airway epithelial cells. Antiviral Res. 2009;81:226–233. doi: 10.1016/j.antiviral.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 12.Martínez-Antón A, Debolós C, Garrido M, Roca-Ferrer J, Barranco C, Alobid I, Xaubet A, Picado C, Mullol J. Mucin genes have different expression patterns in healthy and diseased upper airway mucosa. Clin Exp Allergy. 2006;36:448–457. doi: 10.1111/j.1365-2222.2006.02451.x. [DOI] [PubMed] [Google Scholar]
- 13.Ding GQ, Zheng CQ. The expression of MUC5AC and MUC5B mucin genes in the mucosa of chronic rhinosinusitis and nasal polyposis. Am J Rhinol. 2007;21:359–366. doi: 10.2500/ajr.2007.21.3037. [DOI] [PubMed] [Google Scholar]
- 14.Xaubet A, Mullol J, López E, Roca-Ferrer J, Rozman M, Carrión T, Fabra JM, Picado C. Comparison of the role of nasal polyp and normal nasal mucosal epithelial cells on in vitro eosinophil survival. Mediation by GM-CSF and inhibition by dexamethasone. Clin Exp Allergy. 1994;24:307–317. doi: 10.1111/j.1365-2222.1994.tb00240.x. [DOI] [PubMed] [Google Scholar]
- 15.Mullol J, Xaubet A, Gaya A, Roca-Ferrer J, López E, Fernàndez JC, Fernàndez MD, Picado C. Cytokine gene expression and release from epithelial cells. A comparison study between healthy nasal mucosa and nasal polyps. Clin Exp Allergy. 1995;25:607–615. doi: 10.1111/j.1365-2222.1995.tb01108.x. [DOI] [PubMed] [Google Scholar]
- 16.Kim SS, Kim KS, Lee JG, Park IY, Koo JS, Yoon JH. Levels of intracellular protein and messenger RNA of mucin and lysozyme in normal human nasal and polyp epithelium. Laryngoscope. 2000;110:276–280. doi: 10.1097/00005537-200002010-00017. [DOI] [PubMed] [Google Scholar]
- 17.Jang YJ, Lee YH, Shin SH. Rhinovirus-infected nasal polyp epithelial cells: effect on the activation and migration of eosinophils by airborne fungi. Ann Allergy Asthma Immunol. 2010;104:434–439. doi: 10.1016/j.anai.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Shin SH, Lee YH. Airborne fungi induce nasal polyp epithelial cell activation and toll-like receptor expression. Int Arch Allergy Immunol. 2010;153:46–52. doi: 10.1159/000301578. [DOI] [PubMed] [Google Scholar]
- 19.Basbaum C, Lemjabbar H, Longphre M, Li D, Gensch E, McNamara N. Control of mucin transcription by diverse injury-induced signaling pathways. Am J Respir Crit Care Med. 1999;160:S44–S48. doi: 10.1164/ajrccm.160.supplement_1.12. [DOI] [PubMed] [Google Scholar]
- 20.Shin SH, Lee SH, Jeong HS, Kita H. The effect of nasal polyp epithelial cells on eosinophil activation. Laryngoscope. 2003;113:1374–1377. doi: 10.1097/00005537-200308000-00020. [DOI] [PubMed] [Google Scholar]
- 21.Takafuji S, Ohtoshi T, Takizawa H, Tadokoro K, Ito K. Eosinophil degranulation in the presence of bronchial epithelial cells. Effect of cytokines and role of adhesion. J Immunol. 1996;156:3980–3985. [PubMed] [Google Scholar]