Abstract
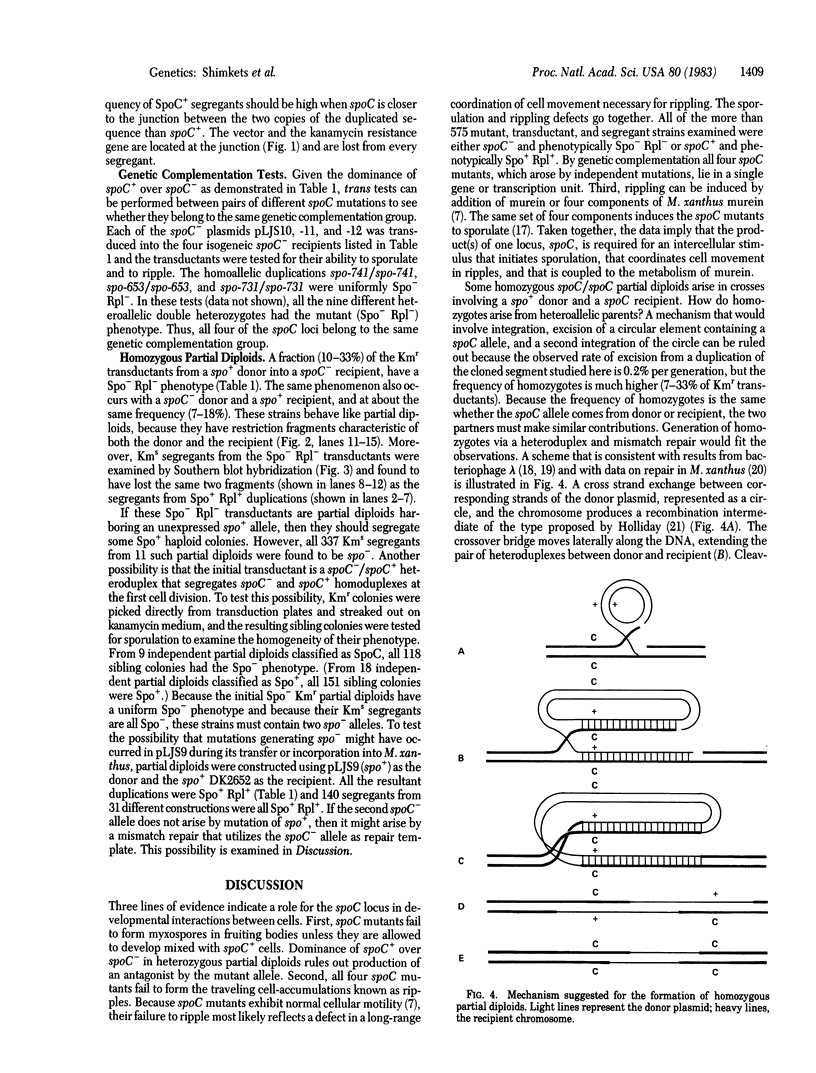
The product(s) of the Myxococcus xanthus spoC locus is required for two multicellular activities in fruiting body development, rippling and sporulation. Ripples, which are formed early in development, are spatially separated ridges of cells that move synchronously. Myxospores are heat-resistant resting cells that are formed near the end of the developmental process. To investigate the function of spoC, it was cloned in an Escherichia coli plasmid, then transferred to M. xanthus by specialized transduction with coliphage P1. The plasmid, which cannot replicate in M. xanthus, integrated into the M. xanthus chromosome, producing two copies of the spoC locus in tandem. Cells containing one copy of a mutant allele and one copy of the wild-type allele displayed the wild-type phenotype. Cells containing two different mutant alleles failed to ripple or sporulate, implying that all four independent spoC mutations are in the same gene or unit of transcription. Homozygous mutant duplications arose from constructions in which DNA from a spo+ donor was transduced into a spoC recipient, or vice versa, at an average frequency of 14%, indicating that gene conversion was a frequent event.
Keywords: tandem duplications, sporulation, plasmid integration, genetic complementation, gene conversion
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. P., Roth J. R. Tandem genetic duplications in phage and bacteria. Annu Rev Microbiol. 1977;31:473–505. doi: 10.1146/annurev.mi.31.100177.002353. [DOI] [PubMed] [Google Scholar]
- Fox M. S., Dudney C. S., Sodergren E. J. Heteroduplex regions in unduplicated bacteriophage lambda recombinants. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):999–1007. doi: 10.1101/sqb.1979.043.01.109. [DOI] [PubMed] [Google Scholar]
- Hagen D. C., Bretscher A. P., Kaiser D. Synergism between morphogenetic mutants of Myxococcus xanthus. Dev Biol. 1978 Jun;64(2):284–296. doi: 10.1016/0012-1606(78)90079-9. [DOI] [PubMed] [Google Scholar]
- Hodgkin J., Kaiser D. Cell-to-cell stimulation of movement in nonmotile mutants of Myxococcus. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2938–2942. doi: 10.1073/pnas.74.7.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen R. A., Rothstein S. J., Reznikoff W. S. A restriction enzyme cleavage map of Tn5 and location of a region encoding neomycin resistance. Mol Gen Genet. 1979;177(1):65–72. doi: 10.1007/BF00267254. [DOI] [PubMed] [Google Scholar]
- Kaiser D., Dworkin M. Gene transfer to myxobacterium by Escherichia coli phage P1. Science. 1975 Feb 21;187(4177):653–654. doi: 10.1126/science.803710. [DOI] [PubMed] [Google Scholar]
- Kaiser D., Manoil C., Dworkin M. Myxobacteria: cell interactions, genetics, and development. Annu Rev Microbiol. 1979;33:595–639. doi: 10.1146/annurev.mi.33.100179.003115. [DOI] [PubMed] [Google Scholar]
- Karn J., Brenner S., Barnett L., Cesareni G. Novel bacteriophage lambda cloning vector. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5172–5176. doi: 10.1073/pnas.77.9.5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuner J. M., Kaiser D. Introduction of transposon Tn5 into Myxococcus for analysis of developmental and other nonselectable mutants. Proc Natl Acad Sci U S A. 1981 Jan;78(1):425–429. doi: 10.1073/pnas.78.1.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner J. L. Formation, induction, and curing of bacteriophage P1 lysogens. Virology. 1972 Jun;48(3):679–689. doi: 10.1016/0042-6822(72)90152-3. [DOI] [PubMed] [Google Scholar]
- Shimkets L. J., Kaiser D. Induction of coordinated movement of Myxococcus xanthus cells. J Bacteriol. 1982 Oct;152(1):451–461. doi: 10.1128/jb.152.1.451-461.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimkets L. J., Kaiser D. Murein components rescue developmental sporulation of Myxococcus xanthus. J Bacteriol. 1982 Oct;152(1):462–470. doi: 10.1128/jb.152.1.462-470.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg N., Powers M., Yarmolinsky M., Austin S. Group Y incompatibility and copy control of P1 prophage. Plasmid. 1981 Mar;5(2):138–149. doi: 10.1016/0147-619x(81)90015-9. [DOI] [PubMed] [Google Scholar]
- Wagner R., Jr, Meselson M. Repair tracts in mismatched DNA heteroduplexes. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4135–4139. doi: 10.1073/pnas.73.11.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]