Abstract
Purpose
This study was designed to investigate the potential effect of bergapten on lipopolysaccharide (LPS)-mediated osteoclast formation, bone resorption and osteoclast survival in vitro.
Methods
After osteoclast precursor RAW264.7 cells were treated with bergapten (5, 20, 40 μmol/L) for 72 hours in the presence of LPS (100 ng/ml), osteoclastogenesis was identified by tartrate-resistant acid phosphatase (TRAP) staining, and the number of TRAP-positive multinucleated cells [TRAP(+)MNCs] per well were counted. To investigate the effect of bergapten on osteoclastic bone resorption, RAW264.7 cells were treated with bergapten for six days in the presence of LPS, and the area of bone resorption was analyzed with Image Pro-Plus. Next, we examined apoptosis of RAW264.7 cells after bergapten incubation for 48 hours by flow cytometer using annexin V/propidium iodide (PI) double labeling. Finally, osteoclast survival was observed by Hoechst 33342 labeling and Western blotting after bergapten treatment for 24 hours.
Results
Data showed that bergapten (5–40 μmol/L) dose-dependently inhibited LPS-induced osteoclast formation and bone resorption. Treatment with bergapten triggered apoptotic death of osteoclast precursor RAW264.7 cells in a dose-dependent manner. Furthermore, bergapten significantly reduced the survival of mature osteoclast, as demonstrated by emergence of apoptotic nuclei and activation of apoptotic protein caspase 3/9.
Conclusions
These findings suggest that bergapten effectively prevents LPS-induced osteoclastogenesis, bone resorption and survival via apoptotic response of osteoclasts and their precursors. The study identifies bergapten as an inhibitor of osteoclast formation and bone resorption and provides evidence that bergapten might be beneficial as an alternative for prevention and treatment of inflammatory bone loss.
Keywords: Bergapten, LPS, Osteoclasts, Bone resorption, Apoptosis
Introduction
Bone is continually renewed throughout the skeleton in a process known as remodeling. The two major cell types responsible for bone remodeling are osteoblasts, which form new bone, and osteoclasts, which resorb bone. When the balance between bone resorption and formation breaks down and osteoclastic bone resorption overrides osteoblastic bone formation, osteoporosis or inflammatory bone-resorptive diseases will occurred [1]. Then, inhibition of osteoclast functions and bone resorption is an important approach preventing inflammatory bone-destructive diseases such as osteomyelitis, septic arthritis and periodontitis. Some evidence demonstrates that lipopolysaccharide (LPS), a major component of gram-negative bacterial cell wall, can trigger macrophages and other cells to release a multitude of cytokines, chemokines, metalloproteinases and other agents [2]. Ultimately, these substances activate osteoclasts by receptor activator of nuclear factor kappa-B ligand (RANKL) or macrophage colony-stimulating factor (M-CSF) pathway and induce bone loss [3]. In addition, Nakanishi-Matsui reported that LPS directly stimulates osteoclast precursor RAW264.7 cell differentiation into osteoclasts and enhances osteoclast survival [4]. Effective therapeutic treatments for LPS-induced bone destruction are limited to antibiotics and surgical repairs, which often lead to serious side effects and surgical trauma. Therefore, new drugs with fewer undesirable side effects are needed to prevent LPS-induced bone loss.
Bergapten (Fig. 1), a coumarin found in many medicinal plants, has been used for decades in combination with UV radiation in skin photochemotherapy. It has other biological functions, including anticancer [5, 6], antioxidative [7, 8] and anti-inflammatory [9] effects. Recently, Tang et al. and Zhang et al. reported that bergapten stimulates activity of osteoblast-like UMR106 cells and enhances bone morphogenetic protein-2 expression and bone formation via p38 and extracellular signal regulated kinase (ERK)-dependent pathway in osteoblasts of human osteosarcoma cell line (MG-63) and human fetal osteoblast (FOB) cell line [10, 11]. However, whether bergapten will affect LPS-induced bone loss as a possible treatment of inflammatory bone-destructive diseases remains unclear.
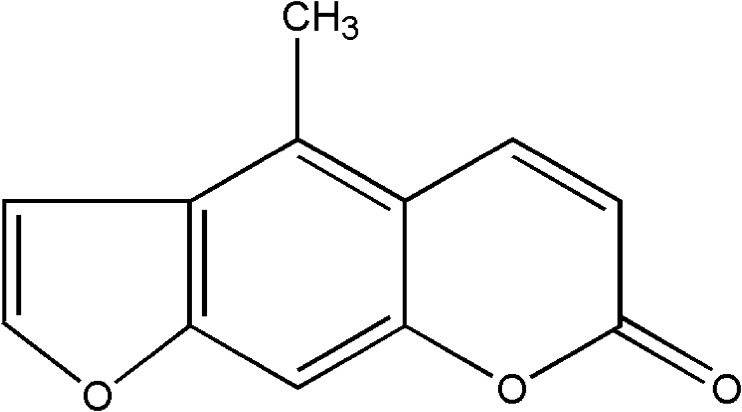
Fig. 1.
Chemical structure of bergapten (5-methoxypsoralen, C12H8O4, M = 216.19)
This study was designed to investigate the in vitro effects of bergapten on LPS-induced osteoclastogenesis and bone resorption, focusing on osteoclast differentiation from murine RAW264.7 cells and osteoclastic bone resorption. To explain its possible mechanism in this regard, we examined apoptosis of osteoclast precursor cells and osteoclast survival.
Materials and methods
Cell culture and osteoclast differentiation
RAW264.7 cells (ATCC, TIB-71) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) high glucose (Gibco) with 10 % fetal bovine serum (FBS) (Gibco) and 1 % (v/v) penicillin–streptomycin solution and incubated at 37 °C in 5 % carbon dioxide (CO2) humidified air. To induce osteoclast formation, RAW264.7 cells were seeded at 5 × 103/well in a 24-well plate and cultured in the presence of LPS (100 ng/ml) for 72 hours.
Cell viability assay
The effect of bergapten on the viability and/or cytotoxicity of RAW264.7 cells was examined by a standard thiazolyl blue tetrazolium bromide (MTT) assay, and half maximal inhibitory concentration (IC50) values were calculated using nonlinear regression analysis. Briefly, RAW264.7 cells were treated with or without bergapten (1, 5, 20, 40, 100 μmol/L) for 24 hours, 48 hours and 72 hours; 10 μl of MTT (5 mg/ml) in phosphate-buffered saline (PBS) was added per well, and cells were further incubated for 4 hours at 37 °C. MTT media were removed, and 200 μl dimethylsulfoxide (DMSO) was added. Absorbance was measured at 490 nm under Multiskan Spectrum Microplate Reader (Thermo Scientific, Waltham, USA).
Tartrate-resistant acid phosphatase (TRAP) staining
RAW264.7 cells were treated with or without bergapten (5, 20, 40 μmol/L) in the presence of LPS (100 ng/ml) for 72 hours. Cells were washed twice with PBS and fixed in 4 % paraformaldehyde (pH 7.4) at room temperature for 15 minutes. Then, cells were stained with tartrate-resistant acid phosphatase (TRAP) (Shanghai Rainbow Medical Reagent Research, Shanghai, China) according to Yu et al. [12]. TRAP-positive multinucleated cells [TRAP(+)MNCs] containing three or more nuclei were counted as osteoclast under IX70 light microscope microscopy (Olympus, Japan), and the number of TRAP(+)MNCs/well were expressed as means ± standard deviation (SD).
Resorption pit assay
RAW264.7 cells were cultured on 200-μm-thick bovine bone slices and treated with or without bergapten (5, 20, 40 μmol/L) for six days in the presence of LPS (100 ng/ml). After washing in PBS, slices were fixed in 2.5 % glutaraldehyde for seven minutes and dehydrated by gradient alcochol solution. Bone slices were incubated with TRAP [12], and resorption pits were observed under an IX70 light microscope. Photographs were taken under a light microscope, and the total areas of resorption pits were analyzed with Image Pro-Plus version 4.0 (Media Cybernetics).
Quantification of apoptosis using flow cytometry
To examine whether bergapten could induce apoptosis of osteoclast precursor cells, RAW264.7 cells were treated with or without bergapten (5, 20, 40 μmol/L) in the presence of LPS (100 ng/ml) for 48 h and collected by trypsinisation. The pallets were resuspended and stained with 5 μg/ml fluorescein isothiocyanate (FITC)-conjugated annexin V/propidium iodide (PI) for 15 minutes in the dark. Cells in each tube were added to 200 μl of PBS and analysed using a fluorescence-activated cell sorter (FACS) Calibur flow cytometer. At least 10,000 cells were collected for each sample, and data were analysed using the BD CellQuest software (Becton Dickinson, USA).
Hoechst 33342 staining
Fluorescent DNA-binding dye was commonly used to define nuclear chromatin morphology as a quantitative index of apoptosis within a cell culture system [13]. RAW264.7 cells were cultured on glass coverslips in 35-mm dishes in the presence of LPS (100 ng/ml) for 72 hours to obtain mature osteoclasts. Then, osteoclasts were treated with or without bergapten (5-40 μmol/L) for 24 hours. After being fixed with 4 % paraformaldehyde for 20 minutes, cells were washed twice in PBS and stained with Hoechst 33324 at 10 μg/ml for 10 minutes. Nuclear morphology was observed using Olympus IX70 fluorescence microscopy (Tokyo, Japan). Multinucleated cells with fragmented chromatin were considered as osteoclast apoptosis.
Protein extraction and Western blotting
RAW264.7 cells were starved in DMEM containing 0.2 % FBS for two hours and incubated with or without bergapten (5, 20, 40 μmol/L) in the presence or absence of LPS (100 ng/ml) for 48 hours. The cellular lysates were prepared as described previously [14]. The proteins were separated by 15 % sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred electrophoretically onto polyvinylidene fluoride (PVDF) membranes. Membranes were blocked with 2 % bovine serum albumin (BSA) overnight at 4 °C and probed with the primary antibodies for caspase 3/9. Finally, membranes were incubated with horseradish-peroxidase (HRP)-conjugated secondary antibody for 2 hours, and membranes were detected using a tetramethylbenzidine dihydrochloride (TMB) liquid substrate kit.
Statistical analysis
All results were expressed as means ± SD. Statistical comparisons were determined by one-way analysis of variance (ANOVA) to compare differences between experimental and control group. The least significant difference (LSD) t test was used for multiple comparison. p values <0.05 were considered statistically significant.
Results
Bergapten inhibited LPS-mediated osteoclast formation
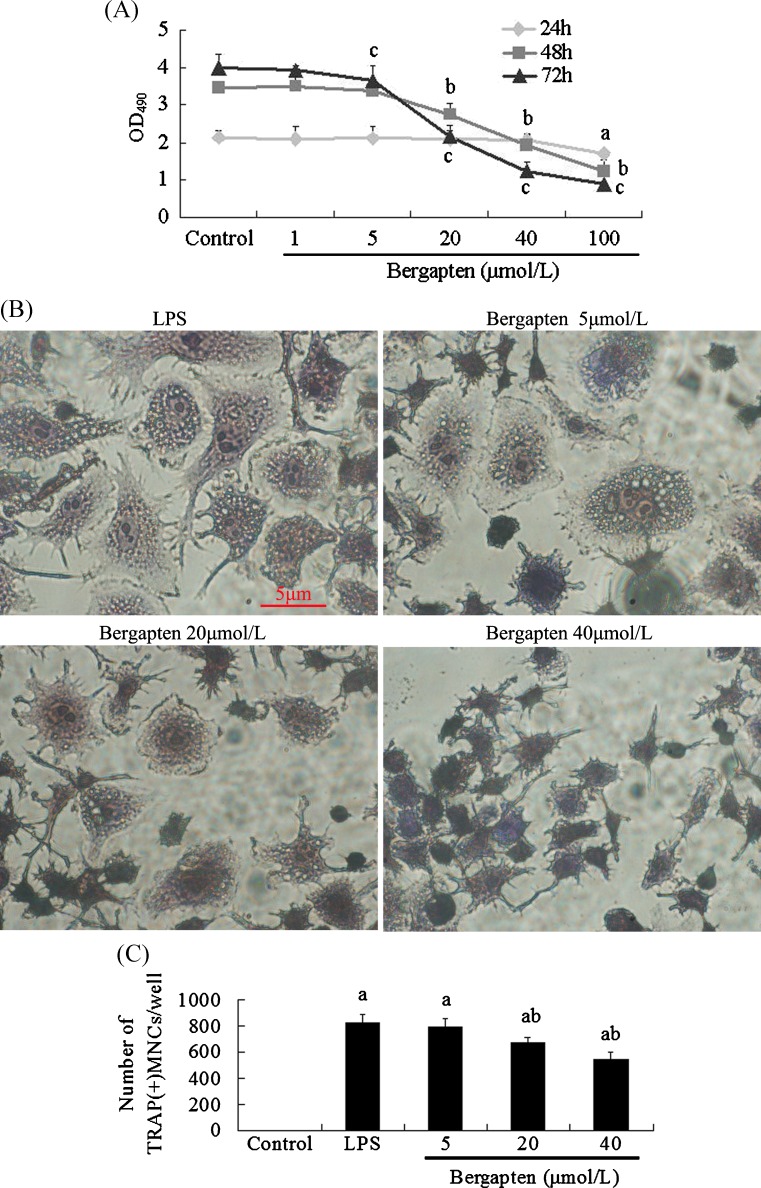
We first examined the effect of bergapten on the viability and/or cytotoxicity of osteoclast precursor RAW264.7 cells with MTT assay. Data showed that treatment with bergapten (1–100 μmol/L) for 24 hours did not affect cell viability (Fig. 2A). However, following treatment for 48 hours and 72 hours, bergapten significantly inhibited the viability of RAW264.7 cells in a dose-dependent manner (Fig. 2A), and the calculated IC50 value for bergapten was 25.6 ± 1.7 μmol/L. Then, bergapten concentrations of 5, 20 and 40 μmol/L were used for subsequent experiments.
Fig. 2.
Bergapten inhibited lipopolysaccharide (LPS)-induced osteoclast formation (n = 4). A RAW264.7 cells were treated with or without bergapten (1–100 μmol/L) for 24 h, 48 h and 72 h, and cell viability was measured by thiazolyl blue tetrazolium bromide (MTT) assay. B After RAW264.7 cells were treated with or without bergapten (5–40 μmol/L) in the presence of LPS (100 ng/ml) for 72 h, cells were stained for tartrate-resistant acid phosphatase (TRAP), and TRAP(+) multinucleated cells (MNCs) containing more than three nuclei were counted as osteoclasts under I X70 microscope. C Data are expressed as the percentage of TRAP(+)MNCs/well (means ± standard deviation). a p < 0.05 compared with control group; b p < 0.05 compared with LPS-treated group. Bar = 5 μm
Osteoclast exhibit elevated TRAP activity, which is correlated with high levels of enzyme expression. Then, we investigated the effect of bergapten treatment for 72 hours on osteoclast formation, and TRAP staining showed that bergapten inhibited LPS-induced osteoclast formation, caused osteoclast less-rounded and containing few nuclei at a concentration range of 5–40 μmol/L (Fig. 2B). Bergapten reduced the number of TRAP(+)MNCs with a dose-dependent pattern (Fig. 2C), with 18.1 % reduction at 20 μmol/L and with 34.14 % at 40 μmol/L; its strongest inhibitory effect occurs during the late stage of osteoclastogenesis (data not shown).
Bergapten significantly prevented LPS-induced osteoclastic bone resorption
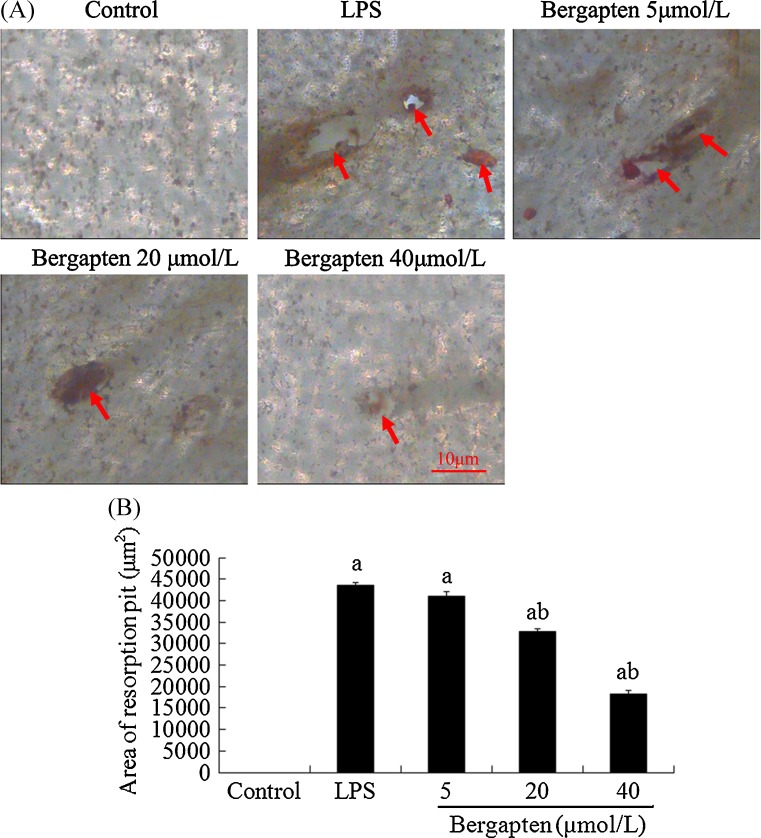
To investigate whether bergapten affected bone resorption, RAW264.7 cells were treated with or without bergapten (5–40 μmol/L) for six days in the presence of LPS (100 ng/ml). As shown in Fig. 3, bergapten remarkably prevented LPS-induced osteoclastic bone resorption and reduced the size of resorption pit area was reduced from 43,550 μm2 (LPS-treated group) to 32,883 μm2 at 20 μmol/L and to 18,347 μm2 at 40 μmol/L (Fig. 3). However, no resorption pit formed was in the control group during the incubation time. Furthermore, we found many multinucleated or mononuclear cells dead in higher concentrations of bergapten-treated groups, implying that bergapten exerts an inhibitory effect on osteoclastogenesis (Fig. 2) and bone resorption (Fig. 3), possibly by inducing apoptotic death of osteoclasts and their precursor cells.
Fig. 3.
Bergapten prevented lipopolysaccharide (LPS)-induced bone resorption (n = 4). RAW264.7 cells cultured on bone slices were treated with or without bergapten (5–40 μmol/L) for 6 days in the presence or absence of LPS (100 ng/ml). A The slices were stained with tartrate-resistant acid phosphatase (TRAP), and resorption pits were observed under an Olympus I × 70 light microscope. B Total areas of resorption pits were analysed with Image Pro-Plus version 4.0, and data are expressed as means ± standard deviation. a p < 0.05 compared with control group; b p < 0.05 compared with LPS-treated group. Red arrows resorption pits. Bar = 10 μm
Bergapten triggered apoptosis of osteoclast precursor RAW264.7 cells
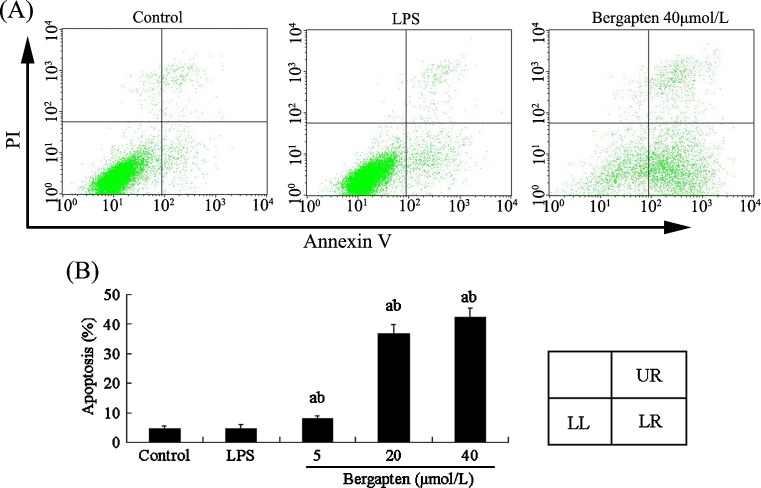
To examine the extent of bergapten-induced injuries in osteoclast precursor RAW264.7 cells, we detected apoptosis of bergapten-treated RAW264.7 cells using an annexin V/PI double-staining kit. Flow cytometric analysis showed bergapten treatment for 48 h triggered apoptotic death of RAW264.7 cells (Fig. 4A), and the percentages of apoptotic cells treated with bergapten increased from 4.57 % to 36.65 % at 20 μmol/L and to 42.17 % at 40 μmol/L (Fig. 4B). No significant difference was obvious between control and LPS-treated groups. These results suggest that bergapten can cause apoptotic death of osteoclast precursor cells and exerts direct proapoptotic effects in a dose-dependent manner. As shown in Fig. 4A, necrosis may be also involved in bergapten-induced damage to osteoclast precursor cells, except for apoptosis.
Fig. 4.
Bergapten triggered apoptotic death of osteoclast precursor RAW264.7 cells by flow cytometric analysis using annexin V/propidium iodide (PI) double staining (n = 3). A RAW264.7 cells were treated with or without bergapten (5–40 μmol/L) in the presence or absence of lipopolysaccharide (LPS) (100 ng/ml) for 48 h and stained with fluorescein isothiocyanate (FITC)-conjugated annexin V (5 μg/ml) and PI (10 μg/ml). Cell apoptosis or necrosis was analysed by flow cytometry. The units of the Y and X axes are fluorescence intensity. The assay identifies normal cells as PI negative and annexin V (FITC) negative, apoptotic cells as PI negative and annexin V positive and necrotic cells as PI positive and annexin V positive. Cells in the lower left (LL) region were PI negative and annexin V negative, in the lower right (LR) region PI negative and annexin V positive and in upper right (UR) region PI positive and annexin V positive. B Data obtained from a are expressed as apoptosis (%) or necrosis (%). a p < 0.05 compared with control group; b p < 0.05 compared with LPS-treated group
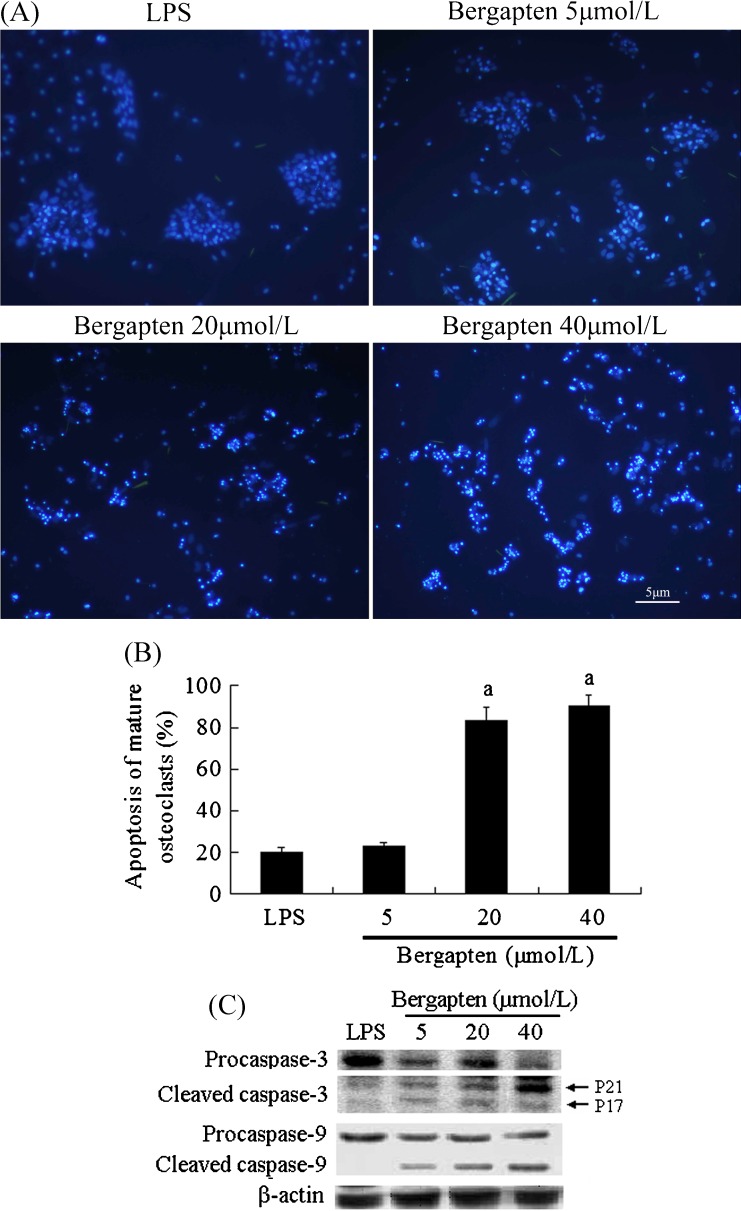
Bergapten reduced the survival rate of mature osteoclasts induced by LPS
Finally, we examined the effect of bergapten on osteoclast survival and found that mature osteoclast showed spontaneous cell death, maintaining ∼80 % survival rate within 24 hours without any treatment (Fig. 5). Interestingly, the addition of bergapten for 24 hours enhanced dose–response of apoptosis in mature osteoclasts, which displayed obviously morphological characteristic of apoptosis, such as cell shrinkage, chromatin compaction and cytoplasmic condensation in multinucleated osteoclasts by Hoechst 33342 staining (Fig. 5A). Only multinucleated cells with condensed and fragmented chromatin were regarded as apoptotic, with 83 % showing apoptosis at 20 μmol/L and with 90 % at 40 μmol/L (Fig. 5B). Moreover, bergapten induced apoptosis of mature osteoclasts, supported by assessment of cleaved caspase-3/9 (Fig. 5C), which is consistent with data from Hoechst 33342 staining. In contrast, no nuclear fragmentation activation of caspase-3/9 was observed in the untreated control group.
Fig. 5.
Bergapten reduced the survival rate of mature osteoclasts (n = 5). RAW264.7 cells were cultured on glass coverslips in 35-mm dishes in the presence of lipopolysaccharide (LPS) (100 ng/ml) for 72 h to obtain mature osteoclasts. Then, osteoclasts were treated with or without bergapten for 24 h and A stained with Hoechst 33342. B Data from A are shown as apoptosis of mature osteoclasts (means ± standard deviation). C After mature osteoclasts were treated with bergapten (5, 20, 40 μmol/L) for 24 h, total protein was extracted and analysed by Western blots with specific antibodies to detect the level of procaspase-3, cleaved caspase-3, procaspase-9 and cleaved caspase-9. a p < 0.05 compared with LPS-treated group
Discussion
Osteoclasts are present only in bone, where they play a central role in LPS-induced bone loss, such as osteomyelitis, septic arthritis and periodontitis [2, 3]. Inhibition of osteoclast function has been considered a protective treatment for inflammatory bone-resorption diseases [15]. However, to date, effective treatment is not available. We found bergapten could prevent LPS-induced osteoclast formation, bone resorption and osteoclast survival, perhaps mediated by triggering apoptosis of osteoclasts and their precursors, suggesting that this medicinal plant extract may be useful for preventing LPS-induced bone loss in infective diseases.
Bergapten is a natural coumarin found in many plants such as grasses, orchids, citrus fruits and legumes. Previous studies have shown that bergapten possesses anticarcinogenic, antiproliferative, vasorelaxation and antiallergic effects [5–9]. Moreover, it can enhance bone formation and possibly prevent osteoclastic bone resorption in vitro or in vivo [10, 11], suggesting that bergapten may prevent or treat osteoporosis and other bone-destruction diseases. However, the effect of bergapten on bacteria-induced inflammatory bone loss has not been reported. In this study, our results from TRAP staining showed that bergapten dose dependently inhibited LPS-induced TRAP(+)MNCs formation and bone resorption (Figs. 2 and 3). Interestingly, the optimal concentration for inhibiting osteoclast differentiation and bone resorption was 40 μmol/L (Figs. 2 and 3), which is four times higher than that of another coumarin derivative (osthole) on osteoclasts separated from long-limb bones of newborn rabbits (10−5 mol/L) [16]. These observations suggest that the inhibitory extent of bergapten on osteoclastogenesis and bone resorption is a little weaker than that of osthole at the same concentration. Furthermore, the effective concentration of bergapten (20–40 μmol/L) on LPS-induced bone loss in vitro was higher than that of it on osteoblast-like cells (such as UMR106, MG 63 and hFOB) (0.3-10 μmol/L) [10, 11], indicating that bergapten preventing LPS-induced bone loss requires stronger concentrations than that of its stimulation of bone formation required in vitro. However, Zhang et al. reported that bergapten had no effect on separated mature osteoclast and osteoclast resorption [17]. These different experimental results require further study. To observe the safety of bergapten 20–40 μmol/L on other kinds of cells, such as osteoblasts and MLO-Y4 osteocytes (which constitute 90–95 % of all bone cells), we used MTT assay to examine cell viability and found that bergapten at 20–40 μmol/L had no obvious cytotoxicity on calvarial osteoblasts and osteocytes (data not shown).
Besides the inhibitory effect of bergapten on LPS-induced osteoclasts and bone resorption, we observed many multinucleated or mononuclear cells dead in groups treated with higher concentrations of bergapten. Hence, we supposed that apoptosis of osteoclasts and their precursors may contribute to the above-described inhibition. New evidence demonstrates that triggering osteoclast apoptosis occurs via a variety of extracellular stimuli, such as estrogens [18–20], bisphosphonates [21] and bafilomycin A1 [22]. The consequences are diminished bone loss and reduced bone turnover. Our results confirm that bergapten significantly triggered apoptosis of osteoclast precursor RAW264.7 cells and reduced the survival of osteoclast, as demonstrated by exposure to phosphatidylserine (PS) (Fig. 4), the emergence of nuclear fragmentation and cleaved caspase-3/9 (Fig. 5). In addition, the same concentration of bergapten caused an obviously weaker apoptotic response of RAW264.7 cells than of mature osteoclast. For example, 40 μmol/L of bergapten treatment caused 42.17 % apoptosis of RAW264.7 cells but 90 % apoptosis of mature osteoclast, suggesting that bergapten prevents LPS-induced osteoclastic bone resorption mainly via inhibition of osteoclast function and survival.
In summary, bergapten can prevent LPS-mediated osteoclast formation, bone resorption and osteoclast survival in vitro, possibly by inducing apoptotic death of osteoclast and their precursor cells. Therefore, we believe that bergapten can inhibit LPS-induced osteoclastic differentiation and resorption activity of mature osteoclast. Future study will be carried out to explore the potential in vivo.
Acknowledgments
This work was supported by Applied Research Program of Public Good in Zhejiang Province (No.2011C33046), Science and Technology Project of Shaoxing City (No.2011A33004), Zhejiang Provincial Natural Science Foundation of China (No.LQ12H18001 and No.Y2111046), Undergraduate Scientific and Technological Innovation Project in Zhejiang Province (No.03160104) and Undergraduate Scientific and Technological Innovation Project in Shaoxing City (No.2012R426024).
Conflict of interest
None.
References
- 1.Goltzman D. Discoveries, drugs and skeletal disorders. Nat Rev Drug Discov. 2002;1:784–796. doi: 10.1038/nrd916. [DOI] [PubMed] [Google Scholar]
- 2.Wada N, Maeda H, Yoshimine Y, et al. Lipopolysaccharide stimulates expression of osteoprotegerin and receptor activator of NF-kappa B ligand in periodontal ligament fibroblasts through the induction of interleukin-1 beta and tumor necrosis factor-alpha. Bone. 2004;35:629–635. doi: 10.1016/j.bone.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 3.Soysa NS, Alles N, Takahashi M, et al. Defective nuclear factor-κB-inducing kinase in aly/aly mice prevents bone resorption induced by local injection of lipopolysaccharide. J Periodontal Res. 2011;46:280–284. doi: 10.1111/j.1600-0765.2010.01333.x. [DOI] [PubMed] [Google Scholar]
- 4.Nakanishi-Matsui M, Yano S, Matsumoto N, et al. Lipopolysaccharide induces multinuclear cell from RAW264.7 line with increased phagocytosis activity. Biochem Biophys Res Commun. 2012;425:144–149. doi: 10.1016/j.bbrc.2012.07.050. [DOI] [PubMed] [Google Scholar]
- 5.Panno ML, Giordano F, Mastroianni F, et al. Breast cancer cell survival signal is affected by bergapten combined with an ultraviolet irradiation. FEBS Lett. 2010;584:2321–2326. doi: 10.1016/j.febslet.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Sumiyoshi M, Sakanaka M, Taniguchi M et al (2013) Anti-tumor effects of various furocoumarins isolated from the roots, seeds and fruits of Angelica and Cnidium species under ultraviolet A irradiation. J Nat Med [DOI] [PubMed]
- 7.Yu J, Wang L, Walzem RL, et al. Antioxidant activity of citrus limonoids, flavonoids, and coumarins. J Agric Food Chem. 2005;53:2009–2014. doi: 10.1021/jf0484632. [DOI] [PubMed] [Google Scholar]
- 8.Liu W, Jia F, He Y, et al. Protective effects of 5-methoxypsoralen against acetaminophen-induced hepatotoxicity in mice. World J Gastroenterol. 2012;18:2197–2202. doi: 10.3748/wjg.v18.i18.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bose SK, Dewanjee S, Sahu R, et al. Effect of bergapten from Heracleum nepalense root on production of proinflammatory cytokines. Nat Prod Res. 2011;25:1444–1449. doi: 10.1080/14786410902800665. [DOI] [PubMed] [Google Scholar]
- 10.Meng F, Xiong Z, Sun Y, et al. Coumarins from Cnidium monnieri (L.) and their proliferation stimulating activity on osteoblast-like UMR106 cells. Pharmazie. 2004;59:643–645. [PubMed] [Google Scholar]
- 11.Tang CH, Yang RS, Chien MY, et al. Enhancement of bone morphogenetic protein-2 expression and bone formation by coumarin derivatives via p38 and ERK-dependent pathway in osteoblasts. Eur J Pharmacol. 2008;579:40–49. doi: 10.1016/j.ejphar.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 12.Yu X, Zhao X, Wu T, et al. Inhibiting wear particles-induced osteolysis with naringin. Int Orthop. 2013;37:137–143. doi: 10.1007/s00264-012-1668-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Telford WG, King LE, Fraker PJ. Comparative evaluation of several DNA binding dyes in the detection of apoptosis-associated chromatin degradation by flow cytometry. Cytometry. 1992;13:137–143. doi: 10.1002/cyto.990130205. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Yan M, Yu A, et al. Inhibitory effects of β-tricalciumphosphate wear particles on osteocytes via apoptotic response and Akt inactivation. Toxicology. 2012;297:57–67. doi: 10.1016/j.tox.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Wauquier F, Philippe C, Léotoing L, et al. The free fatty acid receptor G protein-coupled receptor 40 (GPR40) protects from bone loss through inhibition of osteoclast differentiation. J Biol Chem. 2013;288:6542–6551. doi: 10.1074/jbc.M112.429084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ming L, Wang M, Chen K, et al. Effect of osthol on apoptosis and bone resorption of osteoclasts cultured in vitro. Yao Xue Xue Bao. 2012;47:174–179. [PubMed] [Google Scholar]
- 17.Zhang Q, Qin L, He W, et al. Coumarins from Cnidium monnieri and their antiosteoporotic activity. Planta Med. 2007;73:13–19. doi: 10.1055/s-2006-951724. [DOI] [PubMed] [Google Scholar]
- 18.Faloni AP, Sasso-Cerri E, Katchburian E, et al. Decrease in the number and apoptosis of alveolar bone osteoclasts in estrogen-treated rats. J Periodontal Res. 2007;42:193–201. doi: 10.1111/j.1600-0765.2006.00932.x. [DOI] [PubMed] [Google Scholar]
- 19.Xie H, Sun M, Liao XB, et al. Estrogen receptor α36 mediates a bone-sparing effect of 17β-estrodiol in postmenopausal women. J Bone Miner Res. 2011;26:156–168. doi: 10.1002/jbmr.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faloni AP, Sasso-Cerri E, Rocha FR, et al. Structural and functional changes in the alveolar bone osteoclasts of estrogen-treated rats. J Anat. 2012;220:77–85. doi: 10.1111/j.1469-7580.2011.01449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsumoto T, Nagase Y, Iwasawa M, et al. Distinguishing the proapoptotic and antiresorptive functions of risedronate in murine osteoclasts: role of the Akt pathway and the ERK/Bim axis. Arthritis Rheum. 2011;63:3908–3917. doi: 10.1002/art.30646. [DOI] [PubMed] [Google Scholar]
- 22.Xu J, Feng H, Wang C, et al. Effects of Bafilomycin A1: an inhibitor of vacuolar H (+)-ATPases on endocytosis and apoptosis in RAW cells and RAW cell-derived osteoclasts. J Cell Biochem. 2003;88:1256–1264. doi: 10.1002/jcb.10477. [DOI] [PubMed] [Google Scholar]