Abstract
Purpose
Patients undergoing total knee arthroplasty (TKA) are at high risk of venous thromboembolism, manifesting as deep vein thrombosis (DVT) or pulmonary embolism. The purpose of this study is to evaluate the efficacy and safety of edoxaban 15 mg once daily (o.d.) for preventing DVT in patients undergoing TKA.
Methods
Three hundred patients undergoing primary TKA under general anaesthesia for osteoarthritis were enrolled in this study: 100 treated with enoxaparin 2,000 IU twice daily (b.i.d.), 100 treated with fondaparinux 1.5 mg o.d. and 100 treated with edoxaban 15 mg o.d.. All treatments were scheduled to continue for 14 days.
Results
The incidence of DVT in patients treated with edoxaban 15 mg o.d. was lower than in patients with enoxaparin 2,000 IU b.i.d. and fondaparinux 1.5 mg o.d.. D-dimer levels were significantly lower in patients with edoxaban than in patients with enoxaparin and fondaparinux 1.5 mg o.d. on the first postoperative day; ΔHb levels were lower in patients with edoxaban than in patients with enoxaparin and fondaparinux on postoperative days, However, the difference was not statistically significant. Finally, the incidence of hepatic dysfunction was lower in patients with edoxaban than in patients with enoxaparin and fondaparinux.
Conclusions
Edoxaban 15 mg o.d. was more efficient than enoxaparin 2,000 IU b.i.d. and fondaparinux 1.5 mg o.d.. Furthermore, edoxaban was safe compared with enoxaparin and fondaparinux. Edoxaban, an orally administered direct factor Xa (FXa) inhibitor, may offer a new option for preventing DVT, with a level of evidence III.
Keywords: Edoxaban, Total knee arthroplasty, Deep vein thrombosis, Thromboprophylaxis, Direct factor Xa inhibitor
Introduction
Patients undergoing total knee arthroplasty (TKA) are at high risk of venous thromboembolism (VTE), manifesting as deep vein thrombosis (DVT) or pulmonary embolism (PE) [1]. As manifestations of VTE are often fatal, routine thromboprophylaxis is recommended in TKA [2–4]. Without thromboprophylaxis, DVT occurs in approximately 40–60 % of patients [5–7]. Parenteral agents, such as low-molecular-weight heparin (LMWH) enoxaparin and indirect and selective factor Xa (FXa) inhibitor fondaparinux, have been the standard therapy for VTE prophylaxis in patients undergoing TKA for several decades [8, 9]. However, innovation to develop new drugs continued to respond to the need to reduce such potentially fatal complications. Recently, a new anticoagulant for oral administration—edoxaban—a direct and selective FXa inhibitor [9] was developed and subsequently approved in July 2011, a long time following the approval of warfarin in the 1950s. Edoxaban is expected to be more efficient and convenient than warfarin for preventing VTE after TKA. A phase IIb study assessed the clinical utility of edoxaban for preventing DVT compared with the LMWH dalteparin in patients undergoing total hip arthroplasty (THA) [10]. Moreover, another phase III study reported the efficacy and safety of edoxaban 30 mg for preventing DVT compared with enoxaparin 2,000 IU b.i.d. after TKA [11] However, there are no reports comparing the effect of the orally direct FXa inhibitor edoxaban and the indirect FXa inhibitor fondaparinux, nor has a report investigated the efficacy and safety of edoxaban 15 mg once daily (o.d.) compared with enoxaparin. The purpose of this study was to evaluate the efficacy and safety of edoxaban 15 mg o.d. and compare it with enoxaparin 2,000 IU b.i.d. and fondaparinux 1.5 mg o.d. for preventing DVT in patients undergoing TKA.
Materials and methods
Patients
This retrospective comparative study was approved by the institutional review board at Kobe Kaisei Hospital. From April 2010 to September 2012, 486 patients underwent primary TKA under general anaesthesia for osteoarthritis at Kobe Kaisei Hospital. Patients with (1) planned bilateral knee or multiple joint replacements, (2) chronic or acute preoperative DVT diagnosed on colour Doppler ultrasonography, (3) posttraumatic osteoarthritis, and (4) history of thromboembolic disease were excluded: 300 patients were enrolled. Depending upon the time of surgery, patients were divided into three groups: 100 patients (April 2010 through December 2010) received subcutaneous injections of enoxaparin 2,000 IU b.i.d. beginning 24–36 hours after surgery; 100 patients (January 2011 through November 2011) received subcutaneous injections of fondaparinux 1.5 mg o.d. beginning within 24–36 hours after surgery; 100 patients (December 2011 through September 2012) received edoxaban 15 mg o.d. orally beginning within 12–24 hours after surgery. All treatments were scheduled to continue for 14 days. Demography in each group is presented in Table 1. All patients were operated with the standard medial parapatellar approach and implanted with posterior-stabilised prosthesis or posterior-retained prosthesis.
Table 1.
Patient characteristics
| Enoxaparin (n = 100) | Fondaparinux (n = 100) | Edoxaban (n = 100) | |
|---|---|---|---|
| Gender F/M | 92/8 | 89/11 | 92/8 |
| Age | 74.5 (0.78) | 74.6 (0.80) | 74.6 (0.63) |
| Height (cm) | 150.0 (0.74) | 150.5 (0.72) | 150.7 (0.68) |
| Body weight (kg) | 58.7 (1.12) | 56.5 (1.14) | 59.5 (0.99) |
| Body mass index (kg/m2) | 26.1 (0.41) | 24.9 (0.41) | 26.2 (0.39) |
DVT diagnosis
B-mode ultrasonography, an LOGIQ7 (GE Healthcare, Tokyo, Japan), with compression and colour Doppler imaging, were performed for bilateral common femoral veins, superficial veins, popliteal veins and calf veins by skilled physicians before surgery and seven days after surgery. Augmentation by calf squeezing or Valsalva maneuver were included, as needed. Criteria for diagnosing DVT were: loss of vein compressibility, presence of intraluminal echogenicity and absence of venous flow determined using a sonographic scanner with a linear transducer (6 MHz). DVT was classified as proximal if the thrombus involved the iliac, femoral or popliteal veins and as distal if limited to calf veins.
D-dimer levels
D-dimer, a degradation product of plasmin-cleaved cross-linked fibrin, has been considered a useful biochemical marker for diagnosing VTE [12]; thus, plasma D-dimer levels were measured before surgery and on days three, seven and 14 after surgery.
Safety assessments
Complications after anticoagulant agents, such as bleeding and hepatic dysfunction, have been reported. To detect such complications, blood tests were performed before surgery and on postoperative days one, three, seven, 14, 21 and 28. Bleeding was evaluated by hemoglobin (Hb) and ΔHb (decrease from postoperative day one, ΔHb = Hb postoperative day one Hb) levels, and subjective that needs transfusion was recorded. Hepatic function was evaluated by aspartate aminotransferase (AST), alanine aminotransferase (ALT) and gamma-glutamyl transpeptidase (γ-GTP).
Statistical analysis
Statistical analysis was performed using SPSS software (SPSS, Chicago, IL, USA). All data are expressed as mean ± standard error of mean (SE). We compared patients’ baseline characteristics using the Mann–Whitney U test, analysis of variance (ANOVA) and the chi-square test. The statistical significance level was p < 0.05.
Results
Patient details are listed in Table 1. There was no significant difference among the three groups. Table 2 shows the incidence of DVT. Twenty-eight (28 %), 28 (28 %) and 22 of 100 patients (22 %) showed DVT in the enoxaparin, fondaparinux and edoxaban groups, respectively. Of the 100 patients, proximal DVT was diagnosed in four (4 %) with enoxaparin, four (4 %) with fondaparinux and one (1 %) with edoxaban. Distal DVT was diagnosed in 28 (24 %) with enoxaparin, 23 (24 %) with fondaparinux and 21 (21 %) with edoxaban.
Table 2.
Incidence of deep vein thrombosis (DVT)
| Enoxaparin (n = 100) | Fondaparinux (n = 100) | Edoxaban (n = 100) | |
|---|---|---|---|
| Total DVT | 28 | 28 | 22 |
| Proximal DVT | 4 | 4 | 1 |
| Distal DVT | 24 | 24 | 21 |
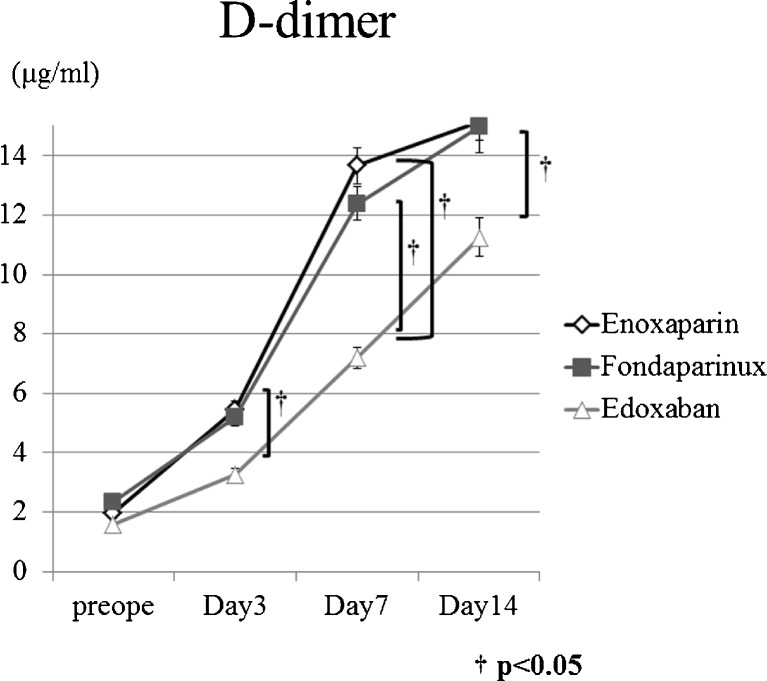
The incidence of DVT in patients treated with edoxaban was lower than in patients treated with enoxaparin and fondaparinux, but there was not statistically significant difference between groups. Symptomatic pulmonary embolism events were not noted. Figure 1 presents D-dimer results for DVT. The D-dimer value increased gradually until postoperative day 14 (Fig. 1) and were significantly lower in patients treated with edoxaban than those treated with enoxaparin and fondaparinux on postoperative days three, seven and 14.
Fig. 1.
Longitudal changes in D-dimer levels. D-dimer value increased gradually until postoperative day 14 and were significantly lower in patients on edoxaban than in patients on enoxaparin and fondaparinux on postoperative days 3, 7 and 14. † p < 0.05
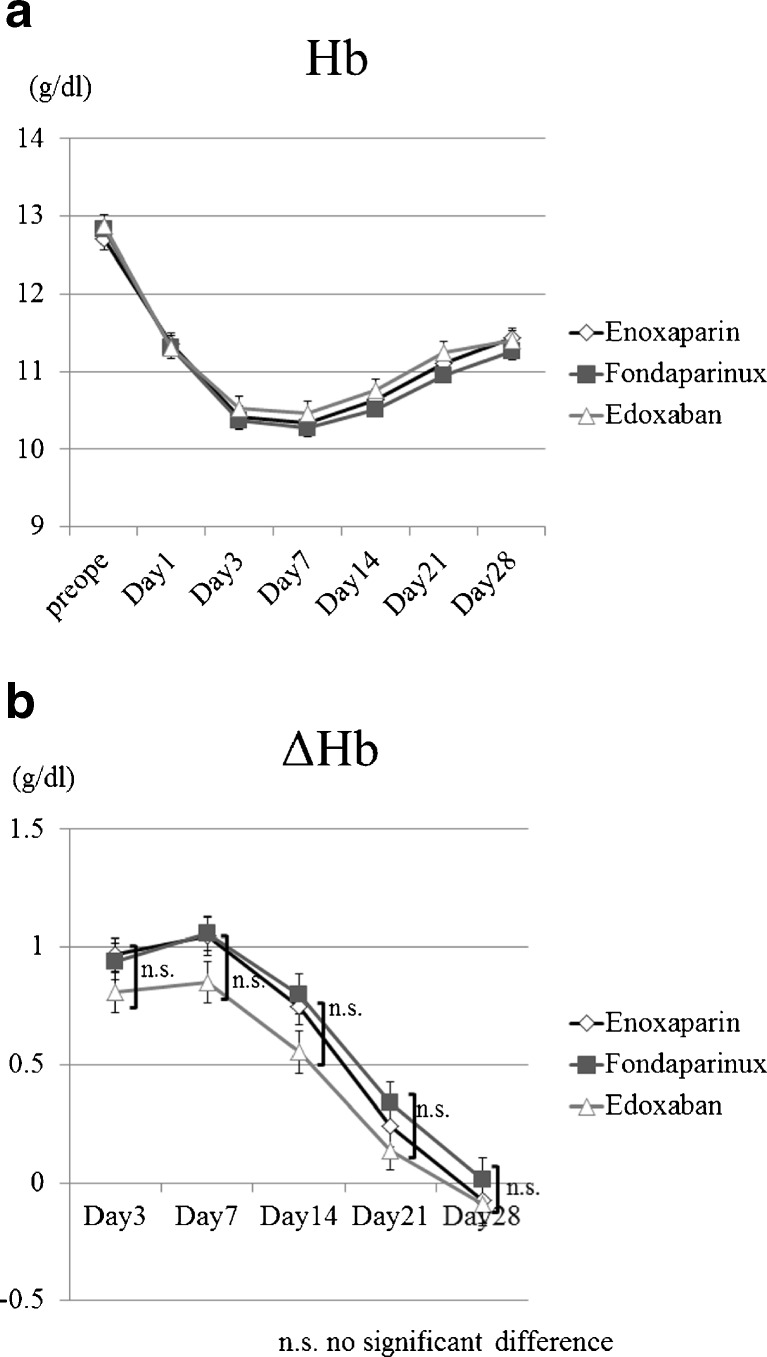
Figure 2 shows Hb and ΔHb results for bleeding. Hb values decreased gradually until postoperative day seven (Fig. 2a). Hb levels were lower in patients on edoxaban than in patients on enoxaparin and fondaparinux on postoperative days three, seven, 14 and 21. However, there was no significant difference between groups.
Fig. 2.
aHemoglobin value decreased gradually until postoperative day 7. , b ΔHb levels were lower in patients on edoxaban than in patients on enoxaparin and fondaparinux on postoperative days 3, 7, 14 and 21
Table 3 shows results for hepatic function. The incidence of ALT values above upper limits of normal (ULN) was 31 %, 23 % and 12 % in patients on enoxaparin , fondaparinux and edoxaban., respectively The incidence of AST values above ULN was 34 %, 18 % and 14 % in patients on enoxaparin, fondaparinux and edoxaban. The incidence of values of γ-GTP above ULN was 27 %, 16 % and 13 % in patients on enoxaparin, on fondaparinux and on edoxaban, respectively. Statistically, the incidence of values in patients on edoxaban was significantly lower for ALT, AST and γ-GTP than in patients on in enoxaparin, and significantly lower for ALT than in patients on fondaparinux. The incidence of values in patients on fondaparinux was significantly lower for AST than in patients on enoxaparin. The incidence of hepatic dysfunction was lower in patients on edoxaban than in patients on fondaparinux. Accordingly, the incidence of hepatic dysfunction was lower in patients on fondaparinux than in patients on enoxaparin. All abnormalities regarding these data resolved spontaneously.
Table 3.
Incidence of abnormal changes in hepatic function test values: Incidence of hepatic dysfunction was lower in patients on edoxaban than in patients on fondaparinux. Accordingly, the incidence of hepatic dysfunction was lower in patients on fondaparinux than in patients on enoxaparin
| Enoxaparin (n = 100) | Fondaparinux (n = 100) | Edoxaban (n = 100) | |
|---|---|---|---|
| ALT >ULN | 31 | 23 | 12 |
| AST >ULN | 34 | 18 | 14 |
| γ-GTP >ULN | 27 | 16 | 13 |
ALT alanine aminotransferase, AST aspartate aminotransferase, γ- GTP γ-glutamyl transpeptidase, ULN upper limit of normal
Discussion
The most important findings in this study are that a small volume of the novel orally administered direct FXa inhibitor edoxaban 15 mg o.d. could sufficiently and safely prevent the incidence of DVT compared with subcutaneously injection of enoxaparin 2000 IU b.i.d. and fondaparinux 1.5 mg o.d. in patients undergoing TKA. This finding was demonstrated in both distal and proximal DVT by ultrasonography, and D-dimer results also supported the efficacy of edoxaban 15 mg o.d.. Only a few studies report the efficacy of edoxaban. A study conducted in Japan for preventing thromboembolic events following orthopaedic surgery demonstrated that edoxaban was superior to enoxaparin for preventing DVT [13]. Another study found that edoxaban inhibited DVT comparably with warfarin and enoxaparin in rats [14]. The results of our study support these studies. In addition, no report other than this study found that edoxaban 15 mg o.d. inhibited DVT more efficiently than fondaparinux 1.5 mg o.d..
Edoxaban may be superior to enoxaparin and fondaparinux in coagulation pathways. FXa is a serine protease that plays a key role in coagulation pathways and binds to factor Va (FVa) on the surface of activated platelets to form the prothrombinase complex, which in turn converts prothrombin to thrombin. Indeed, FXa is the primary site of amplification in the coagulation cascade, and one molecule of FXa can activate approximately 1,000 prothrombin molecules [15]. Therefore, FXa is an attractive target for anticoagulant treatment as it is an important and rare-limiting source of amplification in the coagulation cascade [16]. Enoxaparin inhibits FXa indirectly and nonselectively, whereas edoxaban and fondaparinux inhibit FXa selectively only. The small molecular direct FXa inhibitor, edoxaban directly inhibits both free FXa and FXa bound to FVa in the prothrombinase complex. In contrast, the indirect FXa inhibitor, fondaparinux, forms a complex with antithrombin, blocking free FXa only and does not affect FXa in the prothrombinase complex. Free FXa is responsible for generating the first trace amounts of thrombin, and FXa in the prothrombinase complex induces the propagation phase of thrombin generation from prothrombin [17–19]. Thus, edoxaban may be more efficient for preventing DVT than enoxaparin and fondaparinux.
The incidence of all bleeding with edoxaban was decreased relative to with enoxaparin and fondaparinux, suggesting that edoxaban may demonstrate a clinical benefit relative to enoxaparin and fondaparinux in terms of a lower bleeding risk. A previous study conducted for preventing thromboembolic events following orthopaedic surgery demonstrated that edoxaban 30 mg o.d. was superior to enoxaparin 2,000 IU b.i.d. in efficacy and comparable regarding the incidence of bleeding [11]. Another study concluded that edoxaban inhibited DVT comparably to warfarin and enoxaparin, and the attendant bleeding risk of edoxaban was lower than that of warfarin and enoxaparin in rats [14]. Thus, it appears that the safety margin of edoxaban is greater than that of enoxaparin. Hence, there was no previous study comparing edoxaban and fondaparinux. This study may indicate that edoxaban 15 mg o.d. inhibits DVT with lower risk compared with fondaparinux 1.5 mg o.d.. Moreover, hepatic dysfunction with 15 mg of edoxaban was decreased relative to 2,000 IU of enoxaparin and 1.5 mg of fondaparinux. These results also support the safety of edoxaban for preventing DVT in TKA.
There are some limitations in this study. Firstly, this is a retrospective comparative study. A prospective blinded study will enhance the evidence level, although this study is one of the largest ever reported on this subject. Secondly, this study did not compare edoxaban 15 mg o.d. with edoxaban 30 mg o.d.. Therefore, we could not concluded that edoxaban 15 mg o.d. could efficiently prevent the incidence of DVT compared with edoxaban 30 mg o.d.. Further prospective studies that reveal the appropriate dose of edoxaban to prevent the incidence of DVT in a population such as ours are needed in this respect. Finally, only a single dose of each drug was compared; thus, results may differ depending on dosage variation.
Conclusions
Edoxaban 15 mg o.d. efficiently and safely prevented the incidence of DVT compared with enoxaparin 2,000 IU b.i.d. and fondaparinux 1.5 mg o.d.. Edoxaban, an orally administered direct FXa inhibitor, may offer a new option for preventing DVT.
References
- 1.Cohen AT. Long-term benefits of preventing venous thromboembolic events. Curr Med Res Opin. 2012;28(6):877–889. doi: 10.1185/03007995.2012.688737. [DOI] [PubMed] [Google Scholar]
- 2.McRae SJ, Ginsberg JS. Initial treatment of venous thromboembolism. Circulation. 2004;110(9 Suppl 1):I3–I9. doi: 10.1161/01.CIR.0000140904.52752.0c. [DOI] [PubMed] [Google Scholar]
- 3.Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, Colwell CW. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133(6 Suppl):381S–453S. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 4.Weinmann EE, Salzman EW. Deep-vein thrombosis. N Engl J Med. 1994;331(24):1630–1641. doi: 10.1056/NEJM199412153312407. [DOI] [PubMed] [Google Scholar]
- 5.Geerts WH, Pineo GF, Heit JA, Bergqvist D, Lassen MR, Colwell CW, Ray JG. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 Suppl):338S–400S. doi: 10.1378/chest.126.3_suppl.338S. [DOI] [PubMed] [Google Scholar]
- 6.Stulberg BN, Insall JN, Williams GW, Ghelman B. Deep-vein thrombosis following total knee replacement. An analysis of six hundred and thirty-eight arthroplasties. J Bone Joint Surg Am. 1984;66(2):194–201. [PubMed] [Google Scholar]
- 7.Westrich GH, Sculco TP. Prophylaxis against deep venous thrombosis after total knee arthroplasty. Pneumatic plantar compression and aspirin compared with aspirin alone. J Bone Joint Surg Am. 1996;78(6):826–834. doi: 10.2106/00004623-199606000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Beyer-Westendorf J, Lützner J, Donath L, Radke OC, Kuhlisch E, Hartmann A, Weiss N, Werth S. Efficacy and safety of rivaroxaban or fondaparinux thromboprophylaxis in major orthopedic surgery: findings from the ORTHO-TEP registry. J Thromb Haemost. 2012;10(10):2045–2052. doi: 10.1111/j.1538-7836.2012.04877.x. [DOI] [PubMed] [Google Scholar]
- 9.Falck-Ytter Y (2012) Prevention of VTE in Orthopedic Surgery Patients<alt-title alt-title-type=“short”>Prevention of VTE in Orthopedic Surgery Patients</alt-title><subtitle>Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines</subtitle>. CHEST Journal 141 (2_suppl):e278S. doi:10.1378/chest.11-2404 [DOI] [PMC free article] [PubMed]
- 10.Raskob G, Cohen AT, Eriksson BI, Puskas D, Shi M, Bocanegra T, Weitz JI. Oral direct factor Xa inhibition with edoxaban for thromboprophylaxis after elective total hip replacement. A randomised double-blind dose–response study. Thromb Haemost. 2010;104(3):642–649. doi: 10.1160/TH10-02-0142. [DOI] [PubMed] [Google Scholar]
- 11.Fuji T (2010) Edoxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty: the STARES E-3 trialls (abstrct). Paper presented at the Pathophysiol Haemost Thromb
- 12.Bounameaux H, Cirafici P, de Moerloose P, Schneider PA, Slosman D, Reber G, Unger PF. Measurement of D-dimer in plasma as diagnostic aid in suspected pulmonary embolism. Lancet. 1991;337(8735):196–200. doi: 10.1016/0140-6736(91)92158-X. [DOI] [PubMed] [Google Scholar]
- 13.Fuji T, Fujita S, Tachibana S, Kawai Y. A dose-ranging study evaluating the oral factor Xa inhibitor edoxaban for the prevention of venous thromboembolism in patients undergoing total knee arthroplasty. J Thromb Haemost. 2010;8(11):2458–2468. doi: 10.1111/j.1538-7836.2010.04021.x. [DOI] [PubMed] [Google Scholar]
- 14.Morishima Y, Honda Y, Kamisato C, Tsuji N, Kita A, Edo N, Shibano T. Comparison of antithrombotic and haemorrhagic effects of edoxaban, an oral direct factor Xa inhibitor, with warfarin and enoxaparin in rats. Thromb Res. 2012;130(3):514–519. doi: 10.1016/j.thromres.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 15.Turpie AG. Oral, direct factor Xa inhibitors in development for the prevention and treatment of thromboembolic diseases. Arterioscler Thromb Vasc Biol. 2007;27(6):1238–1247. doi: 10.1161/ATVBAHA.107.139402. [DOI] [PubMed] [Google Scholar]
- 16.Piccini JP, Lopes RD, Mahaffey KW. Oral factor Xa inhibitors for the prevention of stroke in atrial fibrillation. Curr Opin Cardiol. 2010;25(4):312–320. doi: 10.1097/HCO.0b013e32833a524f. [DOI] [PubMed] [Google Scholar]
- 17.Karnicki K, McBane RD, 2nd, Miller RS, Leadley RJ, Jr, Morser J, Owen WG, Chesebro JH. Inhibition and reversal of platelet-rich arterial thrombus in vivo: direct vs. indirect factor Xa inhibition. J Thromb Haemost. 2004;2(12):2162–2169. doi: 10.1111/j.1538-7836.2004.01040.x. [DOI] [PubMed] [Google Scholar]
- 18.Rezaie AR. Prothrombin protects factor Xa in the prothrombinase complex from inhibition by the heparin-antithrombin complex. Blood. 2001;97(8):2308–2313. doi: 10.1182/blood.V97.8.2308. [DOI] [PubMed] [Google Scholar]
- 19.Turpie AG. New oral anticoagulants in atrial fibrillation. Eur Heart J. 2008;29(2):155–165. doi: 10.1093/eurheartj/ehm575. [DOI] [PubMed] [Google Scholar]