Abstract
Purpose
After clinical introduction of the Fitmore® stem (Zimmer), we noticed the formation of cortical hypertrophies in a few cases. We questioned whether (1) the primary stability or (2) load transfer of the Fitmore® stem differs from other stems unassociated with the formation of hypertrophies. We compared the Fitmore® stem to the well-established CLS® stem.
Methods
Four Fitmore® and four CLS® stems were implanted in eight synthetic femurs. A cyclic torque around the stem axis and a mediolateral cyclic torque were applied. Micromotions between stems and femurs were measured to classify the specific rotational implant stability and to analyse the bending behaviour of the stem.
Results
No statistical differences were found between the two stem designs with respect to their rotational stability (p = 0.82). For both stems, a proximal fixation was found. However, for the mediolateral bending behavior, we observed a significantly (p < 0.01) higher flexibility of the CLS® stem compared to the Fitmore® stem.
Conclusion
Hip stem implantation may induce remodelling of the periprosthetic bone structure. Considering the proximal fixation of both stems, rotational stability of the Fitmore® stem might not be a plausible explanation for clinically observed formation of hypertrophies. However, bending results support our hypothesis that the CLS® stem presumably closely follows the bending of the bone, whereas the shorter Fitmore® stem acts more rigidly. Stem rigidity and flexibility needs to be considered, as they may influence the load transfer at the implant–bone interface and thus possibly affect bone remodelling processes.
Keywords: Cortical hypertrophy, Short stem, Fitmore®, CLS®, Primary stability
Introduction
Cementless total hip arthroplasty (THA) shows excellent long-term implant survival, with a mean of 94.7 % after 16 years [1]. However, the main reasons for revision are based on aseptic loosening [1], caused by factors such as missing primary stability, stress shielding and wear-particle-induced osteolysis [2]. An implanted hip stem may change bone structure in the proximal femur [3]. High intrafemoral stresses and nonphysiological loading pattern may determine the bone remodelling process. A reduced load transfer to the femur may induce a reduction of bone density (atrophy) around the stem [4]. Consequently, increased loading may increase bone density (hypertrophy) [5]. These remodelling processes are influenced by many implant-related parameters, such as implant material properties [6] or design [7]. The aim of a modern cementless hip stem is to generate a metaphyseal fixation to reach a load transfer in the subtrochanteric area closely comparable with physiological conditions. Proximal load transfer therefore reduces proximal stress shielding [8, 9], which can lead to implant loosening [10]. Another essential condition in cementless THA is an optimal press fit (primary fixation) with low micromotions at the implant–bone interface for sufficient bone ingrowth (secondary fixation) [11]. Long-term implant fixation requires a balance between decreasing primary and increasing secondary fixation. Consequently, an imbalance between the two may result in implant loosening [12].
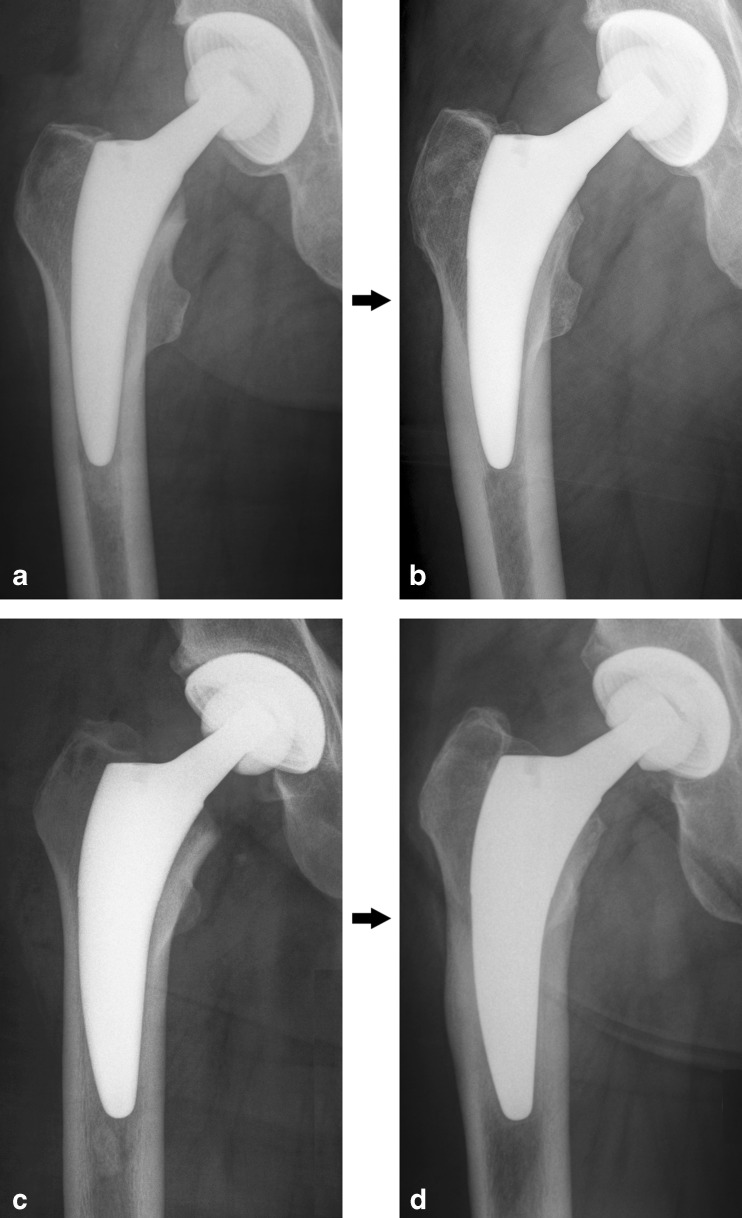
In our ongoing radiological follow-up investigation after THA using the Fitmore® stem (Zimmer), we sometimes noticed the formation of cortical hypertrophy in the distal part of the stem after one year of follow-up (Fig. 1). Comparable cases of cortical hypertrophies could be found [13]. Although we have yet to confirm whether or not the observed hypertrophies of the Fitmore® stem affect long-term stem stability, we analysed possible biomechanical and implant-related reasons for the formation of these distal hypertrophies.We hypothesised that (1) the rotational stability and (2) bending behaviour of the Fitmore® stem is different to that of the established CLS® stem, which is known for excellent clinical long-term results [14].
Fig. 1.
a–d Fitmore® stem, size 8 B Ext. Offset, in a 68-year-old man immediately postoperatively (a) and 1 year after surgery (b) . Fitmore® stem, size 11 B Ext. Offset, in a 63-year-old man immediately postoperatively (c) and 1 year after surgery (d)
Materials and methods
Eight synthetic femora (composite bone fourth generation (3406), Sawbones® Europe, Malmö, Sweden) were osteotomised using a standardised femoral-neck resection. Four Fitmore® stems (size B10 extended, Zimmer GmbH, Winterthur, Switzerland) and four CLS® stems (size 12.5, Zimmer) were chosen based on X-ray template planning. An experienced surgeon reamed and rasped the synthetic femora. After preparation, the stems were implanted into the femora with 25 axial load cycles of 2,000 N and an additional 25 load cycles of 4,000 N using a material testing device (Frank- Universalprüfmaschine 81816/B, Karl Frank GmbH, Weinheim-Birkenau, Germany) to achieve a press-fit situation similar to the fit achieved in vivo during hip surgery [15]. The implant fit was monitored by X-ray examination.
Using a well-established implant-stability measuring device [16–18], different load situations were applied on the stem-bone compound using two linear actuators. A rope system was used to minimise counteractions from the actuators and therefore to eliminate actuator-guided motions of the implant. During load application, six linear variable displacement transducers (LVDTs) (differential transducers type P2010, Mahr GmbH, Göttingen, Germany) with a resolution of 0.1 μm were attached to each measuring point (Fig. 2) to determine the micromotions in all degrees of freedom [17].
Fig. 2.
Defined measuring points of the implant (1, 2) and of the bone (0, 3–6) at different measuring levels
Two different cyclic load applications were performed to characterise loading behaviour in vitro:
Setup A: To classify rotational implant stability, a cyclic torque of ±7 Nm was applied around the stem axis. Micromotions were measured at different levels along the stem axis between bone and implant, as shown in Fig. 2. Two measuring points were defined at the stem: proximal shoulder (1) and distal tip of the stem (2). Five measuring points were located at the femur: two femoral points (4, 6) were located directly corresponding to the stem points (1, 2), and the level of the lesser trochanter (0) served as a global reference. Additionally, two points, located eight centimetres (3) and 20 cm (5) distal to the lesser trochanter, were placed to achieve a higher resolution of femoral motion. Comparing the motions of stem and femur at different levels allowed the relative micromotions at the bone–implant interface to be calculated.
Setup B: To evaluate bending behaviour, we rearranged the measuring system to apply a varus–valgus torque of ±3.5 Nm. In this case, the absolute micromotions of the implants (measuring points 1 and 2) were compared with respect to magnitude and direction (medial or lateral) for both designs.
Statistics
An analysis of variance (ANOVA) was used to compare implant designs regarding location of their main fixation (Setup A). A second ANOVA determined whether there were differences between implant measuring levels. A least significant difference test (LSD) was calculated as a post hoc test. For Setup B, Student’s t test was used to demonstrate whether differences between measuring levels appeared to compare both designs in terms of flexibility. A p value <0.05 was considered significant. Data are expressed as means ± standard deviation (SD).
Results
Setup A: rotational torque around the stem axis: rotational primary stability
In Fig. 3 micromotions are compared between implant designs during rotational torque application and showed no significant difference (p = 0.82). However, within each design, significant differences between measuring levels were found (p < 0.01). The largest micromotions were measured at the distal tip of the stems (Fitmore® 11.91 ± 2.58 mdeg/Nm vs. CLS® 12.32 ± 3.68 mdeg/Nm; with no difference between designs p = 0.77), whereas the smallest micromotions were measured more proximally at the lesser trochanter region (Fitmore® 7.18 ± 0.79 mdeg/Nm vs. CLS® 6.49 ± 0.87 mdeg/Nm; with no difference between designs p = 0.63). Micromotions significantly differed from the distal tip to the trochanter minor region (Fitmore® p < 0.01 and CLS® p < 0.01) and the distal tip to the proximal shoulder (Fitmore® p < 0.01 and CLS® p < 0.01) within each implant design, indicating a proximal fixation of both stems (Fig. 4). Comparison of relative micromotions of the proximal shoulder (Fitmore® 7.50 ± 0.65 mdeg/Nm vs. CLS® 7.21 ± 1.21 mdeg/Nm; p = 0.84) with the lesser trochanter level revealed no significant differences within either stems (Fitmore® p = 0.82 and CLS® p = 0.62).
Fig. 3.
Rotational stability of the Fitmore® and CLS® stems in mdeg/Nm
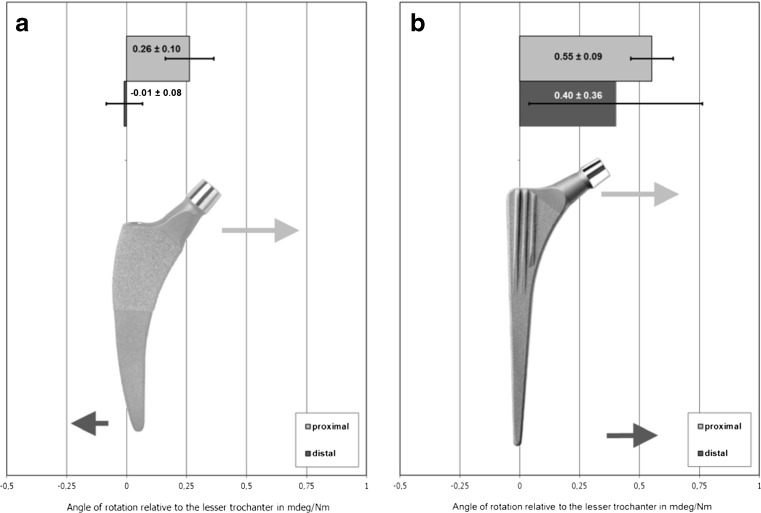
Fig. 4.
a, b Mediolateral bending behavior of the Fitmore® stem (a) compared with the CLS® stem (b)
Setup B: varus–valgus torque: bending behavior of the stem
The Fitmore® stem showed absolute micromotions of 0.26 ± 0.10 mdeg/Nm at the proximal shoulder and −0.01 ± 0.08 mdeg/Nm at the distal tip of the stem (Fig. 4). Proximal motion of the Fitmore® stem was directed medially but changed to lateral at the distal part of the stem. In contrast, the CLS® stem reacted with medial motions at both measuring points (proximally 0.55 ± 0.09 mdeg/Nm and distally 0.40 ± 0.36 mdeg/Nm). Based on these differences in micromotions, the CLS® stem showed significantly higher flexibility than the Fitmore® stem (CLS® 0.95 ± 0.31 mdeg/Nm vs. Fitmore®0.25 ± 0.03 mdeg/Nm; p < 0.01).
Discussion
Bone remodelling, as with clinically observed formations of cortical hypertrophies when using the Fitmore® stem, is already well known in THA. In the nineteenth century, Julius Wolff precisely described remodelling processes in bone tissue [19]. Wolff’s law states that depending on the applied load, the bone structure remodels itself over time to become more resistant. This is followed by secondary changes to the external cortical portion of the bone. In simple terms, the law is “load it or lose it”. Implantation of a hip stem will fundamentally change the load transfer to the bone [20]. One evident example of altered bone remodelling after implantation is the Zweymüller stem (e.g. Zimmer Alloclassic®), which has been clinically shown to be hypertrophic distally and osteolytic metaphyseally [21]. One study using the same experimental setup as used in this study showed that the Zweymüller stem has a distal fixation (location of load transfer) [16]. As expected from this distal fixation, parts of the proximal metaphysis will be isolated from loading, which may explain the proximal atrophy found in clinical cases [16].
A major limitation of our study is the use of synthetic femora rather than human bones. Similar measurements in living humans are obviously not practicable. Human specimens would offer more realistic in in vitro conditions. However, synthetic femora were used to create standardised and comparable conditions. The use of primary stability measurements to perform secondary stability observations is another study limitation. Similar measurements, including bone ingrowth, are not yet possible to perform in vitro. However, it has been shown that a causal relationship between initial implant fit and osseointegration seems to exist [14].
The CLS® stem was chosen as a reference due to its excellent clinical long-term survival [14, 22]. Compared with the relatively new Fitmore® stem, the CLS® stem has a 28-year history, and cortical hypertrophies are uncommon [14, 23]. Both stems showed low micromotions within the proximal part of the stem during rotational torque application (Setup A), similar to other stems [16, 24]. Comparable rotational stability results might indicate a similar proximal anchoring behaviour for both designs. Considering the proximal fixation of both stem types and the explained correlations of distal fixation and hypertrophy, rotational stability of the Fitmore® stem might not be a plausible explanation for clinically observed formations of hypertrophies. Under mediolateral torque application (Setup B), the CLS® stem acted more flexibly than the shorter Fitmore® stem. Presumably, the CLS® closely follows bending of the bone, whereas the shorter Fitmore® stem is more rigid. This may be due to an enlarged axial cross section of the stem and therefore an enlarged implant stiffness of the shorter Fitmore®.
Many clinical studies have observed cortical hypertrophy (mainly diaphyseal within Gruen zones 3, 5 and 6) [21] and atrophy (mainly metaphyseal within Gruen zones 1 and 7) [20, 21]. Furthermore, stem stiffness is one important design variable for bone remodelling processes (e.g. rigid cobalt-chromium alloy vs. flexible Titanium alloy) [6, 8, 25, 26]. Stems with greater flexibility reduce proximal bone loss and prevent cortical hypertrophies at the distal part of the stem [26]. Other aspects are fixation type (cemented vs. cementless) [27] and location (diaphyseal vs. metaphyseal vs. hybrid) [25] and implant surface modifications (e.g. porous-coated, hydroxyapatite layers, etc.) [28]. Short stems were introduced to facilitate small incision surgery as well as to achieve proximal load transfer within the metaphysis and therefore reduce distal hypertrophies and stress shielding [9, 25, 29] compared with traditional stems [30]. However, distal hypertrophies and proximal atrophy might also occur within short stems [31]. We therefore believe that the rigid bending behaviour of the Fitmore® stem may induce higher intrafemoral stresses, which might lead to the formation of cortical hypertrophies.
In conclusion, both stems showed low relative micromotions at each site and a similar metaphyseal fixation within the rotational torque test in vitro. Therefore, both the Fitmore® and CLS® stems may be adequate with respect to primary fixation. However, the stems exhibit different biomechanical behaviour under varus–valgus torque. Stem rigidity or flexibility needs to be considered, as this may influence load transfer at the implant–bone interface and so possibly affect long-term clinical bone-remodelling processes. Shorter and thicker stems, in particular, are generally confronted with increased rigidity. Further clinical studies may be necessary to follow the Fitmore® stem with respect to patient- or surgery-related parameters, such as frequency and severity of cortical hypertrophies, and the clinical long- term outcome of this stem.
Acknowledgments
Conflict of interest
None.
Footnotes
W. Pepke and J. Nadorf shared first-authorship: these authors contributed equally to the study.
References
- 1.Garellick G (2012) Swedish Hip Arthroplasty Register. Annual Report 2011. Department of Ortopaedics, Sahlgrenska University Hospital
- 2.Rubash HE, Sinha RK, Shanbhag AS, Kim SY. Pathogenesis of bone loss after total hip arthroplasty. Orthop Clin North Am. 1998;29(2):173–186. doi: 10.1016/S0030-5898(05)70316-3. [DOI] [PubMed] [Google Scholar]
- 3.Scannell PT, Prendergast PJ. Cortical and interfacial bone changes around a non- cemented hip implant: simulations using a combined strain/damage remodelling algorithm. Med Eng Phys. 2009;31(4):477–488. doi: 10.1016/j.medengphy.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Bobyn JD, Mortimer ES, Glassman AH, Engh CA, Miller JE, Brooks CE. Producing and avoiding stress shielding. Laboratory and clinical observations of noncemented total hip arthroplasty. Clin Orthop Relat Res. 1992;274:79–96. [PubMed] [Google Scholar]
- 5.Van Rietbergen B, Huiskes R, Weinans H, Sumner DR, Turner TM, Galante JO. ESB Research Award 1992. The mechanism of bone remodeling and resorption around press- fitted THA stems. J Biomech. 1992;26(4–5):369–382. doi: 10.1016/0021-9290(93)90001-u. [DOI] [PubMed] [Google Scholar]
- 6.Sumner DR, Galante JO. Determinants of stress shielding: design versus materials versus interface. Clin Orthop Relat Res. 1992;274:202–212. [PubMed] [Google Scholar]
- 7.Bougherara H, Bureau MN, Yahia L. Bone remodeling in a new biomimetic polymer-composite hip stem. J Biomed Mater Res A. 2010;92(1):164–174. doi: 10.1002/jbm.a.32346. [DOI] [PubMed] [Google Scholar]
- 8.Glassman AH, Bobyn JD, Tanzer M. New femoral designs: do they influence stress shielding? Clin Orthop Relat Res. 2006;453:64–74. doi: 10.1097/01.blo.0000246541.41951.20. [DOI] [PubMed] [Google Scholar]
- 9.Morrey BF, Adams RA, Kessler M. A conservative femoral replacement for total hip arthroplasty. A prospective study. J Bone Joint Surg Br. 2000;82(7):952–958. doi: 10.1302/0301-620X.82B7.10420. [DOI] [PubMed] [Google Scholar]
- 10.Wilkinson JM, Hamer AJ, Rogers A, Stockley I, Eastell R. Bone mineral density and biochemical markers of bone turnover in aseptic loosening after total hip arthroplasty. J Orthop Res. 2003;21(4):691–696. doi: 10.1016/S0736-0266(02)00237-1. [DOI] [PubMed] [Google Scholar]
- 11.Steinhauser E. Biomechanical principles of implant anchoring. In: Gradinger R, Gollwitzer H, editors. Ossear integration. Heidelberg: Springer Medizin Verlag; 2006. pp. 16–23. [Google Scholar]
- 12.Bensmann G. [Cementless fixation of endoprostheses] Biomed Tech (Berl) 1990;35(Suppl 3):44–47. doi: 10.1515/bmte.1990.35.s3.44. [DOI] [PubMed] [Google Scholar]
- 13.Gustke K. Short stems for total hip arthroplasty: initial experience with the Fitmore stem. J Bone Joint Surg Br. 2012;94(11 Suppl A):47–51. doi: 10.1302/0301-620X.94B11.30677. [DOI] [PubMed] [Google Scholar]
- 14.Streit MR, Schroder K, Korber M, Merle C, Gotterbarm T, Ewerbeck V, Aldinger PR. High survival in young patients using a second generation uncemented total hip replacement. Int Orthop. 2012;36(6):1129–1136. doi: 10.1007/s00264-011-1399-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidbauer U, Brendel T, Kunze KG, Nietert M, Ecke H. Dynamic force measurement in implantation of total endoprostheses of the hip joint. Unfallchirurgie. 1993;19(1):11–15. doi: 10.1007/BF02588222. [DOI] [PubMed] [Google Scholar]
- 16.Gortz W, Nagerl UV, Nagerl H, Thomsen M. Spatial micromovements of uncemented femoral components after torsional loads. J Biomech Eng. 2002;124(6):706–713. doi: 10.1115/1.1517565. [DOI] [PubMed] [Google Scholar]
- 17.Jakubowitz E, Bitsch RG, Heisel C, Lee C, Kretzer JP, Thomsen MN. Primary rotational stability of cylindrical and conical revision hip stems as a function of femoral bone defects: an in vitro comparison. J Biomech. 2008;41(14):3078–3084. doi: 10.1016/j.jbiomech.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Jakubowitz E, Kinkel S, Nadorf J, Heisel C, Kretzer JP, Thomsen MN. The effect of multifilaments and monofilaments on cementless femoral revision hip components: an experimental study. Clin Biomech (Bristol, Avon) 2011;26(3):257–261. doi: 10.1016/j.clinbiomech.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Wolff J. Law of bone remodeling. Law of bone remodeling. Hirschwald: Julius Wolf Institut, Charité - Universitätsmedizin Berlin; 1892. [Google Scholar]
- 20.Jasty M, O’Connor DO, Henshaw RM, Harrigan TP, Harris WH. Fit of the uncemented femoral component and the use of cement influence the strain transfer the femoral cortex. J Orthop Res. 1994;12(5):648–656. doi: 10.1002/jor.1100120507. [DOI] [PubMed] [Google Scholar]
- 21.Brodner W, Bitzan P, Lomoschitz F, Krepler P, Jankovsky R, Lehr S, Kainberger F, Gottsauner-Wolf F. Changes in bone mineral density in the proximal femur after cementless total hip arthroplasty. A five-year longitudinal study. J Bone Joint Surg Br. 2004;86(1):20–26. [PubMed] [Google Scholar]
- 22.Aldinger PR, Jung AW, Breusch SJ, Ewerbeck V, Parsch D. Survival of the cementless Spotorno stem in the second decade. Clin Orthop Relat Res. 2009;467(9):2297–2304. doi: 10.1007/s11999-009-0906-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Merle C, Streit MR, Volz C, Pritsch M, Gotterbarm T, Aldinger PR. Bone remodeling around stable uncemented titanium stems during the second decade after total hip arthroplasty: a DXA study at 12 and 17 years. Osteoporos Int. 2011;22(11):2879–2886. doi: 10.1007/s00198-010-1483-z. [DOI] [PubMed] [Google Scholar]
- 24.Bieger R, Ignatius A, Decking R, Claes L, Reichel H, Durselen L. Primary stability and strain distribution of cementless hip stems as a function of implant design. Clin Biomech (Bristol, Avon) 2012;27(2):158–164. doi: 10.1016/j.clinbiomech.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Huiskes R, Weinans H, van Rietbergen B. The relationship between stress shielding and bone resorption around total hip stems and the effects of flexible materials. Clin Orthop Relat Res. 1992;274:124–134. [PubMed] [Google Scholar]
- 26.Saito J, Aslam N, Tokunaga K, Schemitsch EH, Waddell JP. Bone remodeling is different in metaphyseal and diaphyseal-fit uncemented hip stems. Clin Orthop Relat Res. 2006;451:128–133. doi: 10.1097/01.blo.0000224045.63754.a3. [DOI] [PubMed] [Google Scholar]
- 27.Mulier M, Jaecques SV, Raaijmaakers M, Nijs J, Van der Perre G, Jonkers I. Early periprosthetic bone remodelling around cemented and uncemented custom-made femoral components and their uncemented acetabular cups. Arch Orthop Trauma Surg. 2011;131(7):941–948. doi: 10.1007/s00402-010-1239-4. [DOI] [PubMed] [Google Scholar]
- 28.Yamaguchi K, Masuhara K, Ohzono K, Sugano N, Nishii T, Ochi T. Evaluation of periprosthetic bone-remodeling after cementless total hip arthroplasty. The influence of the extent of porous coating. J Bone Joint Surg Am. 2000;82-A(10):1426–1431. doi: 10.2106/00004623-200010000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Falez F, Casella F, Panegrossi G, Favetti F, Barresi C. Perspectives on metaphyseal conservative stems. J Orthop Traumatol. 2008;9(1):49–54. doi: 10.1007/s10195-008-0105-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen HH, Morrey BF, An KN, Luo ZP. Bone remodeling characteristics of a short- stemmed total hip replacement. J Arthroplasty. 2009;24(6):945–950. doi: 10.1016/j.arth.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 31.Santori FS, Santori N. Mid-term results of a custom-made short proximal loading femoral component. J Bone Joint Surg Br. 2010;92(9):1231–1237. doi: 10.1302/0301-620X.92B9.24605. [DOI] [PubMed] [Google Scholar]