Abstract
Purpose
The purpose of this study was to revise the clinical use of commercial BMP2 (Infuse) and BMP7 (Osigraft) based bone devices and explore the mechanism of action and efficacy of low BMP6 doses in a novel whole blood biocompatible device OSTEOGROW.
Methods
Complications from the clinical use of BMP2 and BMP7 have been systemically reviewed in light of their role in bone remodeling. BMP6 function has been assessed in Bmp6-/- mice by μCT and skeletal histology, and has also been examined in mesenchymal stem cells (MSC), hematopoietic stem cells (HSC) and osteoclasts. Safety and efficacy of OSTEOGROW have been assessed in rats and rabbits.
Results
Clinical use issues of BMP2 and BMP7 have been ascribed to the limited understanding of their role in bone remodeling at the time of device development for clinical trials. BMP2 and BMP7 in bone devices significantly promote bone resorption leading to osteolysis at the endosteal surfaces, while in parallel stimulating exuberant bone formation in surrounding tissues. Unbound BMP2 and BMP7 in bone devices precipitate on the bovine collagen and cause inflammation and swelling. OSTEOGROW required small amounts of BMP6, applied in a biocompatible blood coagulum carrier, for stimulating differentiation of MSCs and accelerated healing of critical size bone defects in animals, without bone resorption and inflammation. BMP6 decreased the number of osteoclasts derived from HSC, while BMP2 and BMP7 increased their number.
Conclusions
Current issues and challenges with commercial bone devices may be resolved by using novel BMP6 biocompatible device OSTEOGROW, which will be clinically tested in metaphyseal bone fractures, compartments where BMP2 and BMP7 have not been effective.
Keywords: Bone regeneration, Bone morphogenetic protein 6 (BMP6), Bone fracture, OSTEOGROW, Whole blood containing device, BMP2, BMP7, Commercial bone devices
Introduction
Trauma is the second most expensive medical condition in the European Union (EU) and the United States (US), after cardiovascular diseases, and costs the health care system 43 billion euros and 50 billion dollars per year, respectively, of which nearly half is used for the treatment of broken bones alone [1]. Approximately 2.5 million bone grafting operations are performed annually in the EU and US [2]. It is estimated that nearly six million fractures are persistent in the EU each year with 5–11 % resulting in delayed or impaired healing [3] and are associated with well-known complications and a socioeconomic burden [4]. As the population ages, it is predicted that more than 12 million bone fractures will occur yearly by 2050 in the EU. Present orthopaedic procedures supporting bone repair are dependent on our understanding of the molecular processes responsible for tissue repair [5], which is a prerequisite for the development of novel biological therapies for supporting bone repair when physiological mechanisms of regeneration fail.
Molecular mechanisms of bone remodeling
During bone remodeling, resorption by osteoclasts acts as a trigger to stimulate bone formation by osteoblasts, which is called coupling [6]. The molecular mechanisms by which osteoblasts control osteoclastogenesis have been extensively studied and elucidated to a great extent. Osteoblasts are functionally coupled to osteoclasts via the receptor activator of nuclear factor kappa-B (RANK)–receptor activator of nuclear factor kappa-B ligand (RANKL)–osteoprotegerin (OPG) system stimulating their formation and activity [7–9]. However, it is not well understood whether osteoclasts in turn influence bone formation by directing the osteoblast activity. In vitro bone marrow-derived mature osteoclasts express growth factors including vascular endothelial growth factor C (VEGFC), leukemia inhibitory factor (LIF), interleukin 1 receptor antagonist (IL1ra), chemokine (C-C motif) ligand 9 (CCL9), twisted gastrulation protein homolog 1 (TWGS1) and platelet derived growth factor-BB (PDGF-BB). Functional analysis demonstrated that PDGF-BB stimulated osteoblast chemotaxis that was reduced by assessing siRNAs of PDGFR-β in osteoblasts [10, 11]. Growth factors released during bone resorption that rebound to the extracellular matrix components also include transforming growth factor β1 (TGFβ1) and insulin growth factor 1 (IGF-1) [12], stimulating osteoblast differentiation from bone marrow mesenchymal stem cells (BMSCs) [13, 14]. A novel molecule named couplin has been recently identified in osteoclasts that also promotes osteoblast differentiation from BMSCs. Couplin is decreased during ageing and upon alendronate treatment, and increased by parathyroid hormone administration [15]. Under normal conditions, the homeostasis of both pathways proceeds in timely controlled cycles (Fig. 1). This multistage cascade controls fetal osteogenesis, bone volume maintenance in postnatal life, and recapitulates embryonic endochondral bone formation during fracture healing.
Fig. 1.
Osteoblast–osteoclast communication in bone remodeling. The basic RANKL-RANK communication system is influenced by hormones like PTH and 1,25(OH)2D3, Wnt signaling, growth factors, including BMPs, TGFβ1, IGF-1, PDGF-BB and others. Following bone resorption, growth factors released from the ECM influence differentiation of bone marrow stromal cells or directly act on osteoblasts. PTH can act via newly discovered couplin. In combination with 1,25(OH)2D3, BMPs upregulate cathepsin K and anhydrase II to promote bone resorption
BMPs
Following the identification of BMPs and their receptors [16–25] there have been important discoveries and clinical reports on BMP use [26–35]. The roles of individual BMPs have been studied through the identification of mutated genes in classic mouse mutants and through conventional gene targeting approaches, gene disruption and overexpression of genes encoding BMPs, BMPRs and Smads. Collectively, these studies have confirmed that BMPs have important roles in the development of the skeleton, nervous system, eye, kidney, heart and primordial germ cells [36–49]. Bone healing in mammals is however restricted compared to more complex embryonic events like limb development that remains active following limb amputation in amphibians.
The role of BMPs in bone remodeling has not been well established prior to the formulation of bone devices containing BMP2 and BMP7 (currently in use) which resulted in less than expected bone formation robustness in clinical trials, and subsequent complications in the post-marketing observation period (see below).
Issues related to bone devices containing BMP2 and BMP7
Currently, two therapeutic concepts have been introduced to the market in order to overcome non-healing bone or complicated bone fractures. The bone devices consist of a bovine collagen matrix soaked with BMP2 (Infuse Bone Graft, lumbar tampered fusion device) or BMP7 (Osigraft) [50, 51]. Randomized, blinded and controlled clinical trials have supported the use of recombinant BMP2 and BMP7 for long bone acute fractures, non-unions and spinal fusions [52–63]. BMP2 based Infuse has been confronted with major issues limiting its use in the post-marketing period [55, 56]. Although the human body contains about 2 mg of BMPs at any time, clinicians use up to 40 mg of BMP2 in patients with spinal fusion surgeries, including off-label use in the cervical spine, which was associated with swelling in the neck resulting in life-threatening complications [55, 56]. From this large BMP2 amount only 75 µg bind specifically to 1 g of collagen [64] and the remaining amount precipitates onto the bovine matrix. Moreover, mature recombinant BMP is not soluble at neutral pH and therefore forms large molecular weight (MW) agglomerates, which then, in combination with bovine collagen, induce significant inflammation, swelling and heterotopic ossification at adjacent and distant cell compartments. Both BMP2 and BMP7 may cause early osteolysis associated with implant dislodgment, subsidence and loss of alignment, specifically in patients following spinal fusion surgery [55, 56]. Similar events eventually occurred when BMP2 and BMP7 devices were used for long-bone fractures and non-unions. Inflammation and swelling were transient and not observed unless used under the skin, in patients operated for distal radial osteotomies and treated with BMP7 where metaphyseal bone is predominantly composed of bone marrow and mesenchymal stem cells, resulting in bone resorption and skin rash [65]. Also, when used in preclinical studies within the bone medullary canal, a pronounced bone resorption has been observed [66]. When a BMP2 device was used in patients for the lower lumbar spine fusion the complications included an autonomic plexus injury, retrograde ejaculation and heterotopic ossification [55–57, 59, 61]. In another prospective study with clinical and radiographic outcomes performed with a BMP2 device and autogenous bone in surgical treatment of a laparoscopic anterior lumbar interbody fusion, it has been demonstrated that the fusion occurred quickly and predictably with no adverse effects identified [58, 62]. Similar results were observed in patients undergoing posterior cervical fusion, as it does not produce complications at the rate previously seen in the anterior cervical spine where the surgery was associated with postoperative oedema, dysphagia and haematoma formation [60].
Further drawbacks include the use of the bovine collagen as a carrier and the use of non-injectable formulations for treating closed fractures, as well as a very high market price. Due to a potential problem with prions and bovine spongiform encephalopathy, bovine collagen has been subjected to strict new regulations if used as a medicinal product for human and veterinary applications [67].
Therefore, although orthopaedic surgeons would use an affordable bone inducing and enhancing agent in almost every osteoporotic fracture and/or a non-union, the side effects and the price are restrictive components in routine use of current BMP devices in patients with bone defects. This is especially the case in elderly patients with a high proportion of secondary interventions. Complications observed with clinical use of BMP2 and BMP7 bone devices were mainly due to the previously limited understanding of their molecular mechanisms in bone remodeling.
In the light of these issues, there is a medical need for the development of a new osteogenic device that will offer safe and cost-effective healing. Well designed and executed studies are necessary to better define the incidence of various complications relative to the type of BMP, form and region of fusion, surgical technique, dose and carrier, and importantly, the natural history and management of associated complications [63].
BMP induced bone formation at ectopic sites is not coupled to bone resorption
Growth factors expressed in the callus affecting fracture healing include TGFβ, FGF, PDGF, IGF and BMP [68]. However, at ectopic sites, e.g. under the skin or in the muscle, only BMPs can induce bone formation [69]. Progenitor cells around blood vessels and in the connective tissues cannot differentiate into the osteogenic pathway without a BMP [47]. In the absence of osteoclasts which are in the bone coupled to adjacent osteoblasts, a BMP is required for the formation of an ossicle which undergoes a cascade of events, including cartilage formation, blood vessel invasion and formation of bone. The bone marrow progenitors arrive later from the systemic circulation to the newly formed bone to organize the bone marrow and osteoclasts [70] (Fig. 1). Thus, at an ectopic site BMPs act on the differentiation of osteogenic precursor cells into osteoblasts and form bone in the absence of osteoclasts. This situation is fully reproducible in vitro when osteoprogenitor cells and osteoblast-like cells are treated with a BMP [46, 71]. However, when BMP acts in the bone microenvironment, the outcome dramatically changes due to bone remodeling, as shown below.
In the presence of coupled bone cells BMPs stimulate resorption
Contrary to the belief that BMPs primarily induce new bone at an endosteal bone site, inactivation of the BMP type I receptor signaling in mouse osteoblasts surprisingly resulted in a significantly increased bone volume [72]. Similarly, a conditional deletion of the Bmp receptor in differentiated mouse osteoclasts increased the osteoblastic bone formation and bone volume [73]. Accordingly, BMP4 overexpression in mouse osteoblasts resulted in bone loss [74]. Intramedullary use of BMP2 at the endosteal bone site resulted in the suppression of osteogenesis due to the downregulation of Runx2 and synthesis of collagen I, as well as inhibition of Wnt signaling [75] as a consequence of targeting Wnt inhibitors Dkk1 and Sost downstream of BMP signaling through the type IA receptor in osteoblasts [76]. These in vivo BMP effects on endosteal bone cells are a consequence of a pronounced stimulation and response of osteoclasts and their progenitors which express BMPR-IA and II receptors on their membranes [77, 78] and synthesize BMPs [39, 40, 79–82]. It is therefore suggested that in vivo the effect of BMPs on osteoclasts outweighs their effect on osteoblasts, resulting in a net bone loss. These surprising results are contradictory to the in vitro evidence showing that BMP2 and -7 promote differentiation of various osteoblast-like cells and in vivo induce new bone formation at ectopic sites in experimental animals (Fig. 2). When used at orthotopic bone sites for supporting bone repair the outcome depends on the bone microenvironment. Thus, in the presence of coupled bone cells, the net effect of BMP therapy on the bone volume will be a loss. However, when used in an uncoupled cell environment at an ectopic site, or in the vicinity of the periosteum or muscle, new bone will form and support the bone healing by extending the area of new bone from an uncoupled to a coupled bone surface (Fig. 3).
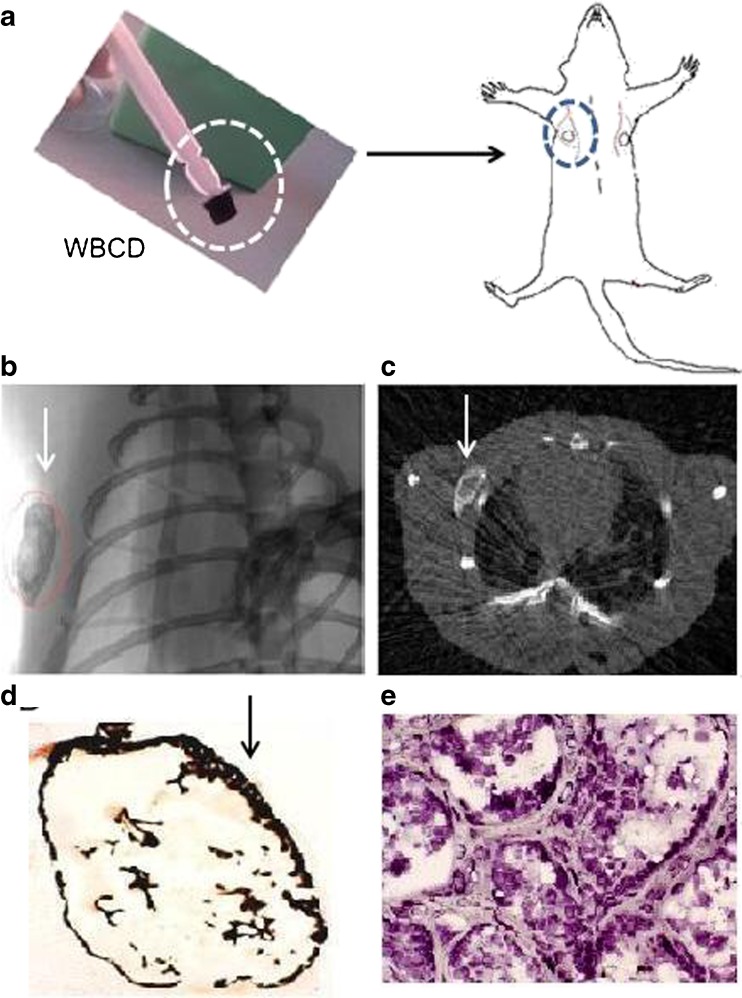
Fig. 2.
Ectopic bone formation assay after implanting whole blood coagulum device (WBCD) OSTEOGROW (white circle) subcutaneously in the axillary region (circles) of a rat (a). Two weeks later μCT analysis in axial (b) and longitudinal (c) planes showed a newly formed mineralized ossicle (white arrows). Histological sections stained by von Kossa (d) and toluidine blue (e) demonstrate mineralized bone with a bone marrow and absence of inflammation and fibrosis at the external surface of the ossicle (arrow in d), which is usually covered by a thick layer of fibrotic tissue in the form of a fibrous capsule when commercially available Osigraft (3.5 mg BMP7) or Infuse (1.5 mg/ml BMP2) are used on a bovine collagen carrier at a subcutaneous site in rats (results not shown)
Fig. 3.
In vivo effect of BMP2 and -7 on bone, periosteum and muscle compartment. (a) At the endosteal surface BMP2 affects both osteoclasts and osteoblasts with a net outcome of downregulation of Runx2, collagen I and Wnt signaling [75]; at the periosteum BMPs stimulate differentiation of precursor cells (green) into osteoblasts (orange); and in surrounding muscle cells (myoblasts, pericytes and vascular satellite cells) BMPs upregulate Id genes and form new osteoblasts and prechondrocytes to form cartilage and new bone around the cortical layer from which the new bone spreads into the medullar cavity. (b) As shown in patients with high tibial osteotomy and a fibular defect, BMP7 (Osigraft) stimulates bone formation from periosteum (P) and surrounding muscles (M) spreading endostealy at six and ten weeks to rebuild the cortical bone (CB) at one year following implantation (modified from [109])
There is also additional evidence that supports the role of BMPs in bone resorption. BMPs in vivo promote bone resorption in animal models of bone defect healing [83–86]. In culture, human monocytes chemotactically respond to BMP4 [87], while BMP7 stimulates the formation of TRAP positive mononuclear cells which, in the presence of 1,25(OH)2D3, mature and resorb bone [88, 89]. BMP2 also mediates the osteoblast–osteoclast interaction, and in the presence of bone marrow stromal cells supports the maturation of mononuclear cells and bone resorption [90, 91]. In the presence of inflammation, BMP2 enhances bone resorption via upregulation of COX-2 and RANKL in osteoblasts [92]. In line with this, Noggin, a BMP antagonist, prevents osteoclastogenesis in cultures of the bone marrow [93]. Osteoclasts isolated from long bones, in the presence of RANKL, increased resorption by BMP2 via cathepsin K and carbonic anhydrase II [77]. TGFβ1 and activin, in the presence of RANKL and granulocyte macrophage colony-stimulating factor (GM-CSF), also stimulate osteoclastogenesis [94]. In rats with removed thyroid and parathyroid glands systemic administration of BMP2 and -7 resulted in an increased bone resorption, indicating a direct effect on osteoclasts in vivo which is not mediated by calciotropic hormones [95]. In contrast, BMP5 and -6 activated osteoclasts in a biphasic mode, depending on the RANKL/OPG mRNA ratio and the BMP concentration [96]. Mechanistically, BMP2 transcriptionally regulates both RANKL [92, 93] and CSF-1 [97], which are critical factors for osteoblast-induced osteoclastogenesis. It is suggested that BMP2 and -7 regulate the osteoclast activity both via OPG-RANKL system and through upregulating the expression of GM-CSF, stromal cell-derived factor-1 (SCF1) and resorption enzymes.
Clinical testing of BMP2 and -7 revised
If BMPs in vivo promote bone resorption, how was it then that clinical trials demonstrated that BMP2 and -7 supported bone healing with an efficacy equal to an autologous bone graft? Moreover, in animal studies it has been documented that BMPs when implanted on a collagen carrier promote formation of a new endochondral bone in mice, rats, rabbits, dogs, goats, sheep, and baboons [23, 44, 98–102]. In long bone acute fracture clinical trials using BMP2 and -7, bone loss has not been recorded [52, 54]. The first evidence for a pronounced bone resorption came from patients in whom BMP7 was used for distal radial osteotomy [65] and BMP2 for the spinal fusion surgery [53, 55, 56, 58–63].
In patients receiving BMPs mainly for spinal fusions [103–106] it has been observed that the use of intracorporal BMP7 in unstable thoracolumbar fractures resulted in severe bone resorption, loss of reduction and segmental collapse [103]. Vertebrae consist mainly of trabecular bone with surfaces lined by coupled bone cells, and a recombinant BMP2 and BMP7 in large amounts caused bone resorption due to their pronounced effect on osteoclasts, at endosteal/trabecular surfaces (Fig. 3). In clinical studies the retrospective analyses suggested that the initial BMP induced resorption was transient and that bone formation and repair subsequently occurred [55, 56].
The role of soft tissues adjacent to bone in BMP-induced bone formation
The cortical bone is covered on the outside with periosteal cells that are not coupled to osteoclasts and respond in vitro to BMPs [107, 108]. In the work of Geesink et al. osteogenic activity of BMP7 with a collagen (Osigraft) as a carrier was tested in a human fibular defect in patients undergoing a high tibial osteotomy for prevention of osteoarthritis [109]. Patients treated with Osigraft had a spherical formation of bone which began at the external borders of the defect (Fig. 3). BMP7 primarily stimulated the differentiation and mobility of cells outside the bone cavity originating from the periosteum and surrounding muscles to rebridge the fracture outside the bone defect, which later occupied the endosteal bone space. It has been well established that BMP2 and BMP7 turn muscles into bone via upregulating the inhibition of differentiation (Id) genes [45, 110–112], and in parallel promote the differentiation of pericytes and myoblasts into osteoblasts (Fig. 3) [113]. Therefore, their collective effect on endosteal mesenchymal cells, periosteal cells, and the surrounding myoblasts/pericytes initiates bone formation and the formation of a bone callus. The initial direct stimulation of endosteal osteoclasts might be an important step for removing nonfunctional bone pieces following a fracture within the bone cavity. While resorption takes place endostealy, forming an intramedullar “halo” [66], in parallel, new bone tissue is formed outside the bone cavity to biomechanically support the broken bone ends (Fig. 3). The size of the new bone is, however, dose and carrier dependent. The effect of recombinant BMP2 and -7 in clinical trials for acute and chronic repair of long-bone defects was therefore not very effective, since the distal tibia is less encased with muscles, and due to open procedures it is not known whether the periosteum has been preserved [38]. Also, the amount of BMP2 and -7 in current commercial BMP devices significantly exceeds their biological need, containing an overdose of BMP2 and/or -7, which upon in vivo use is lost from the injury site, leading to increased bioavailability elsewhere, and eventually to uncontrolled immunological responses, including formation of antibodies, exuberant ectopic bone formation and other undesirable side effects [55, 56].
OSTEOGROW: A new bone device consisting of BMP6 and a biocompatible blood coagulum-derived carrier
As shown, a biological response to BMPs is dependent on the cell type and microenvironment that is present at the site of BMP delivery. In a situation in which pluripotential cells are abundant, BMPs are effective. In bone injuries skeletal progenitor cells arise from multiple tissue compartments including the injured periosteum, endosteum, bone marrow cavity, vascular tissue and the surrounding musculature. All these progenitor populations contribute cells to the healing skeletal injury, but whether they respond equivalently to BMPs is not yet fully understood (Fig. 3) [41, 42, 52, 75, 114].
The ability to enhance osteogenesis and uncouple it from bone resorption, through the use of BMPs, is a critically important step in advancing the skeletal tissue engineering. Periosteal bone formation in the vicinity of bone and the ectopic bone microenvironment outside the bone medullar cavity have a common denominator reflected by absence of osteoclasts and therefore uncoupled osteoblast precursor cells which would form bone with a BMP.
We have developed a novel osteogenic device called OSTEOGROW which is aimed to accelerate bone regeneration. It contains a biologically compatible autologous carrier made from the peripheral blood (whole blood containing device, WBCD), that significantly limits inflammatory processes common in commercial bone devices. Finally, BMP6 is added in small amounts to the carrier WBCD to accelerate and enhance bone formation, as evidenced in preclinical models of bone repair (Fig. 4) [115].
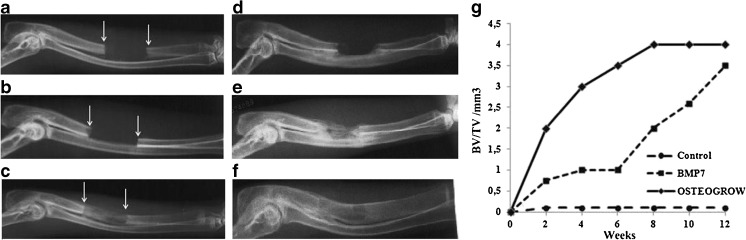
Fig. 4.
X-ray analysis of critical size defects of rabbit ulnae. An ulnar critical size defect model was used to evaluate the efficacy of BMP6 in bone healing of adult male New Zealand white rabbits. Blood was collected from rabbit marginal ear veins into tubes without any anticoagulant substance in which BMP6 or BMP7 were added. Animals were divided into three groups (n = 8) and defects were treated with: modified whole blood containing device as a carrier (WBCD) alone (a, d), a commercial device containing 1 g of bovine collagen and 3.5 mg of BMP7 (Osigraft) (b, e), and WBCD containing 100 μg BMP6 (c, f) in vivo eight weeks later. Healing was followed by X-rays and μCT (not shown) over a period of 12 weeks. Healing dynamics (g) was analysed by radiographic grading scores (0–6) [132] and BV/TV values determined by μCT [128]
Uniqueness and exceptionality of BMP6
BMP6 has specific effects in its ability to convert stem cells to cartilage and bone forming cells [13, 48, 116]. BMP6 and several other BMP family members are produced and released by BMSC and hematopoietic stem cells (HSC) which both constitute the stem cell niche of the bone marrow [117]. HSC express BMP type I receptors and can also synthesize BMP6, which in turn influences the differentiation of BMSC, and subsequent differentiation into osteoblast, chondrocyte, adipocyte and other cell types [118–120]. HSC-derived BMP6 is responsible for enhanced osteoblast differentiation and bone formation from BMSC. In the crystal structure of BMP6 the H3 pre-helix loop region (residues 65–73) shows the largest difference between the BMP6 and BMP7 structures [121]. Although highly homologous, BMP6 and BMP7 appear to have distinct type I receptor specificities with BMP6 displaying a 20-fold higher affinity to BMPR-IA than BMP7, but 20-fold lower than BMP2. This is certainly an unexpected finding given that BMP6 shares numerous receptor binding and signaling characteristics with BMP7 [28, 122]. Such a resistance of BMP6 to inactivation by BMP antagonist(s) might, in addition, be ascribed to BMP receptor type I binding affinity and explain why large amounts of BMP7 are needed in commercially used BMP-based bone devices for a successful bone formation and repair in monkeys and patients [116, 123, 124].
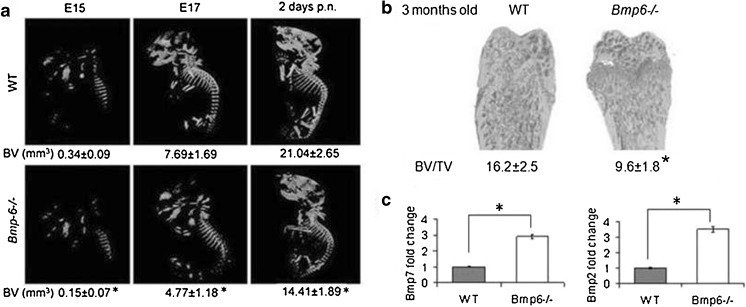
Unlike knockout mice for some other BMPs, such as BMP2 [125], Bmp6-/- mice are viable and fertile [43]. However, although bones of the wild type (WT) and Bmp6-/- littermates are of similar size and shape, the bone volume (BV) is reduced in the development and adult life. At three months of age, trabecular BV (tBV) was decreased by 32 % in Bmp6-/- animals due to the decreased bone forming capacity of bone marrow MSC and increased differentiation towards osteoclasts from bone marrow HSCs (Fig. 5).
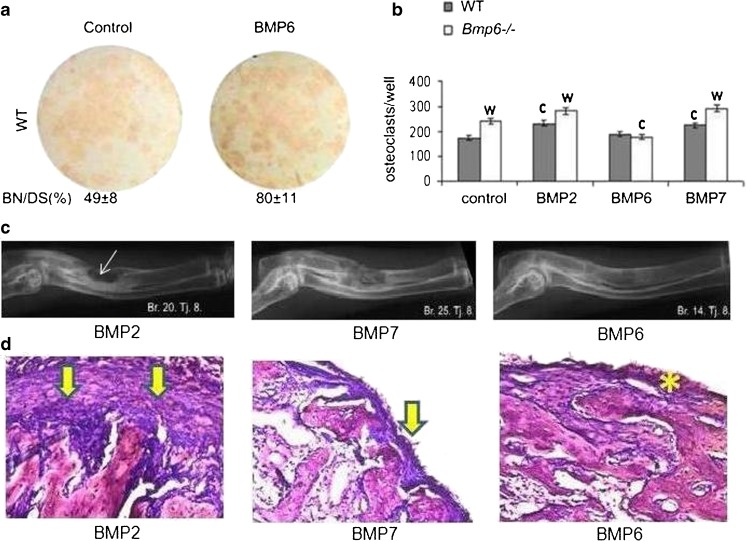
Fig. 5.
Resorption, inflammation/fibrosis and efficacy of BMP2, BMP6 and BMP7. (a) Mesenchymal progenitor cells stained for alkaline phosphatase positive bone nodules which were stimulated by BMP6. Differentiation medium for mesenchymal stem cells added on day 7 contained α-MEM, 10 % FCS, 8 mM β-glycerophosphate, 50 μg/mL ascorbic acid and 10-8 M dexamethasone. The medium was changed every two days until the culture was terminated. BMPs (200 ng/ml) were added to the medium at every feeding. Osteoblasts were identified by alkaline phosphatase stain using a commercially available kit (Sigma Aldrich). (b) Number of osteoclasts derived from HSCs of WT and Bmp6-/- mice. Bone marrow cells were harvested from femurs and tibiae of rats after 14 days of therapy. Differentiation media for osteoclasts contained α-MEM, 10 % FCS, macrophage colony-stimulating factor (M-CSF, 50 ng/mL; Sigma Aldrich), and recombinant human soluble receptor activator of nuclear factor-ĸB ligand (RANKL, 50 ng/mL; Sigma Aldrich). Equal amounts of 200 ng/mL BMP2, BMP6 and BMP7 were added on day 1 and replaced every second day until termination on day 6. The wells were fixed with 4 % paraformaldehyde, and adherent osteoclasts were identified by tartrate-resistant acid phosphatase (TRAP) staining using a commercially available kit (Sigma Aldrich). Significant difference from control (c), and WT (w) (P < 0.05, ANOVA Dunnett test). (c) Efficacy of BMP2, BMP7 and BMP6 in a rabbit critical size defect model. Equal amounts (200 μg) of BMPs were added to 1.5 mL of full rabbit blood, and formulated WBCD was implanted into radius defects of rabbits (n = 4/group. Fourteen weeks following surgery BMP2 implants had a pronounced periosteal rebridgement with endosteal resorption (arrow); BMP7 implants rebridged defects by a cortical union and showed advanced endosteal remodeling; BMP6 treated defects fully healed with advanced bone remodeling and graft incorporation into the radius. (d) Histology of implants (n = 3/group) following subcutaneous axillary implantation of 75 ng of BMP2, BMP7 and BMP6 in WBCD (1.0 mL). BMP2 implant had a thick fibrous capsule on the implant surface (arrows), the BMP7 implant was covered by a thinner fibrous capsule (arrow), while BMP6 in WBCD had no fibrous tissue outside the implant (asterisk)
Increased expression of Bmp2, -4 and -7 (2.5-6 fold), in femurs of Bmp6-/- mice suggested that the effects of the depletion of BMP6 could not be overcome by other BMPs and that their effects are specific and distinguishable (Fig. 6).
Fig. 6.
Bmp6-/- mice have reduced trabecular bone volume (BV). (a) μCT analyses of WT and Bmp6-/- embryos at days 15 (E15), 17 (E17) and at 2 days of postnatal (p.n.) life. (b) μCT of distal femurs. (c) Real time PCR expression of Bmp2 and -7 in the femur of WT and Bmp6-/- mice. BV/TV bone volume/tissue volume; significant difference from WT (P < 0.05, ANOVA Dunnett test) (*)
Exogenous BMP6 had the most powerful effect on inducing differentiation of MSCs towards osteoblasts and formation of bone nodules in vitro as compared to BMP2 and -7 in both WT and Bmp6-/- bone marrow cells (Fig. 5). At the same time, BMP6 decreased the number of osteoclasts derived from HSC, while BMP2 and -7 increased their number. Since endogenous BMP6 affected the bone volume in development, we further determined by liquid chromatography-mass spectrometry (LC-MS) and Western blotting [126] whether it was available in the circulation of adults. BMP6 was found in the plasma of WT mice and in healthy humans [127]. In rats with osteoporosis, systemically administered BMP6 increased the bone volume indicating that BMP6 has a systemic role in bone biology [128]. To elucidate the underlying mechanism, gene expression profiling experiments revealed enrichment of IGF-1 and EGF related pathways in rats treated with BMP6. Similar results were obtained on primary human osteoblasts, suggesting that BMP6 exerts its osteoinductive effect, at least in part, through IGF-1 and EGF pathways [13]. In C2C12 premyoblasts, alteration of the plasma membrane heparan-sulfate (HS) structures with chlorate or heparinase III treatment inhibited BMP6-mediated signaling and differentiation of the cells into osteoblasts, indicating that endogenous HS plays an important role in the BMP6 activity. Addition of exogenous heparin could not rescue BMP6 signaling in HS-disrupted cells, which leads to the conclusion that anchored HS is required on the plasma membrane for normal BMP6 signaling [129]. It has been recently demonstrated that mutations in Exostosin 1 and exostosin 2 genes, encoding glycosyltransferases involved in the biosynthesis of ubiquitously expressed heparan sulfate (HS) chains, are associated with multiple hereditary exostoses (MHE) [130].
At the cellular level, loss of the BMP6 signal in knock-out mice led to increased numbers of MSCs in the bone marrow, showing that they were primed to differentiate, but accumulated due to the lack of a specific signal. BMP6 therapy increased the differentiation of stem cells towards chondrocytes and osteocalcin positive osteoblasts [48, 116, 123] in the bone (Fig. 3). This unique role of BMP6 in bone healing and regeneration prompted us to use it in the OSTEOGROW device.
BMP6 dosing
The dosing regimen performed in pre-clinical experiments indicated that BMP6 is efficacious when used in a significantly lower amount than BMP2 and -7. We demonstrated that 50 μg BMP6 in a WBCD device is more efficacious than 3.5 mg of BMP7 in Osigraft on a bovine collagen carrier in in vivo rabbit ulna critical size defects (Fig. 4). Finally, when equal amounts of BMP2, BMP7 and BMP6 were used in biocompatible WBCD as a carrier in rabbits with a critical size ulna defect, BMP6-treated animals showed advanced healing and bone remodeling without endosteal resorption (Fig. 5) and lack of fibrosis and inflammation when implanted subcutaneously in the rat axillar region (Figs. 2 and 5). We proposed that the higher BMP6 potency is related to a lesser sensitivity for endogenous inhibitors. Unlike BMP7, BMP6 dissociates from the BMP antagonist noggin, following binding of the complex BMP-noggin to cell surface receptors. This suggested why more BMP7 was required in vivo for the local bone therapy in the presence of a large Noggin amount locally in the surrounding tissues [123]. We further explored potential structural differences between BMP2, BMP7 and BMP6 in binding to Noggin, and found that lysine in the position 60 of the mature BMP6 domain was essential for its reversible binding to noggin. When prolin and glutamic acid in BMP2 and BMP7 in the position 60 were replaced by lysine, their biological activity significantly increased at lower concentrations [123]. This might also prolong the BMP6 half-life time activity resulting in an enhanced osteoblast differentiation beneficial for the fracture healing [131].
New BMP6 biocompatible carrier
In parallel we discovered that blood coagulum from the patient’s own blood (WBCD) modified with calcium salts serves as an appropriate autologous carrier for BMPs [115] (Fig. 7). Moreover, the WBCD is non-immunogenic, non-inflammatory, and does not contain animal material, unlike commercial non-autologous devices. Modifications in its preparation resulted in a flexible, malleable, compact and injectable WBCD, thus preventing retraction and disassembling into smaller pieces within the period of at least seven days following injection. We then explored the use of WBCD as a carrier for BMP6 and showed that OSTEOGROW successfully rebridged critical size bone defects in animals (Fig. 5). BMP6 added to the blood remained active in the WBCD for approximately seven days and could not be recovered in the supernatant serum after the WBCD had been formed. It was bound to extracellular matrix molecules in the WBCD (Fig. 7) and eventually to membrane receptors of cells constituting the WBCD. This discovery served as a foundation for developing OSTEOGROW, a biocompatible new carrier device for BMP6. Multinational clinical studies will be performed to test the primary hypothesis that small amounts of BMP6 in a biocompatible carrier accelerate and enhance bone healing of the metaphyseal bone, the compartment where BMP2 and BMP7 are not effective.
Fig. 7.
Whole blood-derived coagulum binds BMP6. (a) In vivo blood samples from a rat injected with BMP6 (100 μg/kg) were collected into tubes with EDTA or citrate after one hour (lanes 1 and 2) and showed better recovery of BMP6 than blood samples collected without anticoagulants (lane 3). The same result was obtained in vitro when blood samples were spiked with BMP6 (1 μg) (lanes 4 and 5). In addition, good recovery was obtained when BMP6 was added to the serum after coagulum formation (lane 6). BMP6 standard (1 μg) was applied directly into the gel lane (lane 7). (b) BMP6 binds to extracellular matrix molecules as determined by dot blot analysis
Acknowledgments
The research leading to these results has received funding from the European Community’s Seventh Framework Programme [FP7/2007-2013] under grant agreement n°HEALTH-F4-2011-279239.
We thank Durdica Car and Mirjana Marija Renic for providing animal care and experimentation in mice and rat studies.
Conflict of interest
HO, M. Jankolija, IP, M. Jurin and MK are employees of Genera Research.
SV is a founder and acts voluntarily as CEO of Genera Research.
DV, JC, JB, M. Pauk, IE, IF, IDC, M. Jelic, DD, TV, RN, VK, TBN, ZBT, JBS, SVT, M. Peric, M. Pecina and LG declare that they have no conflict of interest and certify that they have no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing, arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
Footnotes
Mladenka Jurin contributed equally to this paper.
References
- 1.The American College of Surgeons (2002) National trauma data bank report. The American College of Surgeons, Chicago, IL
- 2.Deutsche Bank Alex Brown (2001) Estimates and company information. Deutsche Bank AG, Frankfurt am Main
- 3.American Academy of Orthopaedic Surgeons (2000) Musculoskeletal injuries report: incidence, risk factors and prevention. AAOS
- 4.Giannoudis PV, Kanakaris NK, Einhorn TA. Interaction of bone morphogenetic proteins with cells of the osteoclast lineage: review of the existing evidence. Osteoporos Int. 2007;18:1565–1581. doi: 10.1007/s00198-007-0441-x. [DOI] [PubMed] [Google Scholar]
- 5.Pecina M, Vukicevic S. Biological aspects of bone, cartilage and tendon regeneration. Int Orthop. 2007;31:719–720. doi: 10.1007/s00264-007-0425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parfitt AM. The coupling of bone formation to bone resorption: a critical analysis of the concept and of its relevance to the pathogenesis of osteoporosis. Metab Bone Dis Relat Res. 1982;4:1–6. doi: 10.1016/0221-8747(82)90002-9. [DOI] [PubMed] [Google Scholar]
- 7.Lacey DL, Timms E, Tan HL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 8.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 9.Borovecki F, Pecina-Slaus N, Vukicevic S. Biological mechanisms of bone and cartilage remodelling–genomic perspective. Int Orthop. 2007;31:799–805. doi: 10.1007/s00264-007-0408-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanchez-Fernandez MA, Gallois A, Riedl T, Jurdic P, Hoflack B. Osteoclasts control osteoblast chemotaxis via PDGF-BB/PDGF receptor beta signaling. PLoS One. 2008;3:e3537. doi: 10.1371/journal.pone.0003537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kubota K, Sakikawa C, Katsumata M, Nakamura T, Wakabayashi K. PDGF BB purified from osteoclasts acts as osteoblastogenesis inhibitory factor (OBIF) J Biomol Tech. 2002;13:62–71. [PMC free article] [PubMed] [Google Scholar]
- 12.Vukicevic S, Luyten FP, Kleinman HK, Reddi AH. Differentiation of canalicular cell processes in bone cells by basement membrane matrix components: regulation by discrete domains of laminin. Cell. 1990;63:437–445. doi: 10.1016/0092-8674(90)90176-f. [DOI] [PubMed] [Google Scholar]
- 13.Grasser WA, Orlic I, Borovecki F, Riccardi KA, Simic P, Vukicevic S, Paralkar VM. BMP-6 exerts its osteoinductive effect through activation of IGF-I and EGF pathways. Int Orthop. 2007;31:759–765. doi: 10.1007/s00264-007-0407-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mundy GR. The effects of TGF-beta on bone. Ciba Found Symp. 1991;157:137–143. [PubMed] [Google Scholar]
- 15.Arai S, Amizuka N, Azuma Y, Takeshita S, Kudo A. Osteoclastogenesis-related antigen, a novel molecule on mouse stromal cells, regulates osteoclastogenesis. J Bone Miner Res. 2003;18:686–695. doi: 10.1359/jbmr.2003.18.4.686. [DOI] [PubMed] [Google Scholar]
- 16.Chang SC, Hoang B, Thomas JT, Vukicevic S, Luyten FP, Ryba NJ, Kozak CA, Reddi AH, Moos M., Jr Cartilage-derived morphogenetic proteins. New members of the transforming growth factor-beta superfamily predominantly expressed in long bones during human embryonic development. J Biol Chem. 1994;269:28227–28234. [PubMed] [Google Scholar]
- 17.Ozkaynak E, Rueger DC, Drier EA, Corbett C, Ridge RJ, Sampath TK, Oppermann H. OP-1 cDNA encodes an osteogenic protein in the TGF-beta family. EMBO J. 1990;9:2085–2093. doi: 10.1002/j.1460-2075.1990.tb07376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sampath TK, Reddi AH. Dissociative extraction and reconstitution of extracellular matrix components involved in local bone differentiation. Proc Natl Acad Sci USA. 1981;78:7599–7603. doi: 10.1073/pnas.78.12.7599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ten Dijke P, Yamashita H, Sampath TK, Reddi AH, Estevez M, Riddle DL, Ichijo H, Heldin CH, Miyazono K. Identification of type I receptors for osteogenic protein-1 and bone morphogenetic protein-4. J Biol Chem. 1994;269:16985–16988. [PubMed] [Google Scholar]
- 20.Urist MR. Bone: formation by autoinduction. Science. 1965;150:893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 21.Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- 22.Paralkar VM, Vail AL, Grasser WA, Brown TA, Xu H, Vukicevic S, Ke HZ, Qi H, Owen TA, Thompson DD. Cloning and characterization of a novel member of the transforming growth factor-beta/bone morphogenetic protein family. J Biol Chem. 1998;273:13760–13767. doi: 10.1074/jbc.273.22.13760. [DOI] [PubMed] [Google Scholar]
- 23.Vukicevic S, Stavljenic A, Pecina M. Discovery and clinical applications of bone morphogenetic proteins. Eur J Clin Chem Clin Biochem. 1995;33:661–671. doi: 10.1515/cclm.1995.33.10.661. [DOI] [PubMed] [Google Scholar]
- 24.Vukicevic S, Luyten FP, Reddi AH. Stimulation of the expression of osteogenic and chondrogenic phenotypes in vitro by osteogenin. Proc Natl Acad Sci USA. 1989;86:8793–8797. doi: 10.1073/pnas.86.22.8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lissenberg-Thunnissen SN, de Gorter DJ, Sier CF, Schipper IB. Use and efficacy of bone morphogenetic proteins in fracture healing. Int Orthop. 2011;35:1271–1280. doi: 10.1007/s00264-011-1301-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graf D, Economides AN. Dissection of bone morphogenetic protein signalling using genome engineering tools. In: Vukicevic S, Sampath TK, editors. Bone Morphogenetic Proteins: From Local to Systemic Therapeutics. Basel: Birkhauser Verlag; 2008. pp. 115–140. [Google Scholar]
- 27.Aspenberg P, Basic N, Tagil M, Vukicevic S. Reduced expression of BMP-3 due to mechanical loading: a link between mechanical stimuli and tissue differentiation. Acta Orthop Scand. 2000;71:558–562. doi: 10.1080/000164700317362172. [DOI] [PubMed] [Google Scholar]
- 28.Sieber C, Schwaerzer GK, Knaus P. Bone morphogenetic protein signalling is fine-tuned on multiple levels. In: Vukicevic S, Sampath TK, editors. Bone Morphogenetic Proteins: From Local to Systemic Therapeutics. Basel: Birkhauser Verlag; 2008. pp. 81–114. [Google Scholar]
- 29.Anticevic D, Jelic M, Vukicevic S. Treatment of a congenital pseudarthrosis of the tibia by osteogenic protein-1 (bone morphogenetic protein-7): a case report. J Pediatr Orthop B. 2006;15:220–221. doi: 10.1097/01.bpb.0000194439.75378.ac. [DOI] [PubMed] [Google Scholar]
- 30.Bilic R, Simic P, Jelic M, Stern-Padovan R, Dodig D, van Meerdervoort HP, Martinovic S, Ivankovic D, Pecina M, Vukicevic S. Osteogenic protein-1 (BMP-7) accelerates healing of scaphoid non-union with proximal pole sclerosis. Int Orthop. 2006;30:128–134. doi: 10.1007/s00264-005-0045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dorai H, Vukicevic S, Sampath TK. Bone morphogenetic protein-7 (osteogenic protein-1) inhibits smooth muscle cell proliferation and stimulates the expression of markers that are characteristic of SMC phenotype in vitro. J Cell Physiol. 2000;184:37–45. doi: 10.1002/(SICI)1097-4652(200007)184:1<37::AID-JCP4>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 32.Grgic M, Jelic M, Basic V, Basic N, Pecina M, Vukicevic S. Regeneration of articular cartilage defects in rabbits by osteogenic protein-1 (bone morphogenetic protein-7) Acta Med Croatica. 1997;51:23–27. [PubMed] [Google Scholar]
- 33.Jelic M, Pecina M, Haspl M, Kos J, Taylor K, Maticic D, McCartney J, Yin S, Rueger D, Vukicevic S. Regeneration of articular cartilage chondral defects by osteogenic protein-1 (bone morphogenetic protein-7) in sheep. Growth Factors. 2001;19:101–113. doi: 10.3109/08977190109001079. [DOI] [PubMed] [Google Scholar]
- 34.Mihelic R, Pecina M, Jelic M, Zoricic S, Kusec V, Simic P, Bobinac D, Lah B, Legovic D, Vukicevic S. Bone morphogenetic protein-7 (osteogenic protein-1) promotes tendon graft integration in anterior cruciate ligament reconstruction in sheep. Am J Sports Med. 2004;32:1619–1625. doi: 10.1177/0363546504263703. [DOI] [PubMed] [Google Scholar]
- 35.de Gorter DJ, Krause C, Lowik CWGM, Bezooijen RL, ten Dijke P. Control of bone mass by sclerostin: Inhibiting BMP- and WNT-induced bone formation. In: Vukicevic S, Sampath TK, editors. Bone Morphogenetic Proteins: From Local to Systemic Therapeutics. Basel: Birkhauser Verlag; 2008. pp. 257–276. [Google Scholar]
- 36.Vukicevic S, Kopp JB, Luyten FP, Sampath TK. Induction of nephrogenic mesenchyme by osteogenic protein 1 (bone morphogenetic protein 7) Proc Natl Acad Sci USA. 1996;93:9021–9026. doi: 10.1073/pnas.93.17.9021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katagiri T, Boorla S, Frendo JL, Hogan BL, Karsenty G. Skeletal abnormalities in doubly heterozygous Bmp4 and Bmp7 mice. Dev Genet. 1998;22:340–348. doi: 10.1002/(SICI)1520-6408(1998)22:4<340::AID-DVG4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 38.Friedlander C, Nerubay J, Katznelson A, Nebel L. Osteogenesis by periosteal transplant. Experimental study of spinal fusion in rats. Isr J Med Sci. 1979;15:38–42. [PubMed] [Google Scholar]
- 39.Helder MN, Ozkaynak E, Sampath KT, Luyten FP, Latin V, Oppermann H, Vukicevic S. Expression pattern of osteogenic protein-1 (bone morphogenetic protein-7) in human and mouse development. J Histochem Cytochem. 1995;43:1035–1044. doi: 10.1177/43.10.7560881. [DOI] [PubMed] [Google Scholar]
- 40.Helder MN, Karg H, Bervoets TJ, Vukicevic S, Burger EH, D'Souza RN, Woltgens JH, Karsenty G, Bronckers AL. Bone morphogenetic protein-7 (osteogenic protein-1, OP-1) and tooth development. J Dent Res. 1998;77:545–554. doi: 10.1177/00220345980770040701. [DOI] [PubMed] [Google Scholar]
- 41.Pecina M, Giltaij LR, Vukicevic S. Orthopaedic applications of osteogenic protein-1 (BMP-7) Int Orthop. 2001;25:203–208. doi: 10.1007/s002640100262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pecina M, Haspl M, Jelic M, Vukicevic S. Repair of a resistant tibial non-union with a recombinant bone morphogenetic protein-7 (rhBMP-7) Int Orthop. 2003;27:320–321. doi: 10.1007/s00264-003-0475-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perry MJ, McDougall KE, Hou SC, Tobias JH. Impaired growth plate function in bmp-6 null mice. Bone. 2008;42:216–225. doi: 10.1016/j.bone.2007.09.053. [DOI] [PubMed] [Google Scholar]
- 44.Ripamonti U, Vukicevic S. Bone morphogenetic proteins: from developmental biology to molecular therapeutics. S Afr J Sci. 1995;91:277–280. [Google Scholar]
- 45.Simic P, Vukicevic S. Bone morphogenetic proteins: from developmental signals to tissue regeneration. Conference on bone morphogenetic proteins. EMBO Rep. 2007;8:327–331. doi: 10.1038/sj.embor.7400943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.ten Dijke P. Bone morphogenetic protein signal transduction in bone. Curr Med Res Opin. 2006;22(Suppl 1):S7–S11. doi: 10.1185/030079906X80576. [DOI] [PubMed] [Google Scholar]
- 47.Vukicevic S, Sampath TK. Bone Morphogenetic Proteins: From Laboratory to Clinical Practice. Basel: Birkhauser Verlag; 2002. [Google Scholar]
- 48.Vukicevic S, Grgurevic L. BMP-6 and mesenchymal stem cell differentiation. Cytokine Growth Factor Rev. 2009;20:441–448. doi: 10.1016/j.cytogfr.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 49.Simic P, Vukicevic S. BMPs in development. In: Vukicevic S, Pecina M, editors. Bone Morphogenetic Proteins: Regeneration of Bone and Beyond. Basel: Birkhauser Verlag; 2004. pp. 73–108. [Google Scholar]
- 50.Bishop GB, Einhorn TA. Current and future clinical applications of bone morphogenetic proteins in orthopaedic trauma surgery. Int Orthop. 2007;31:721–727. doi: 10.1007/s00264-007-0424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.White AP, Vaccaro AR, Hall JA, Whang PG, Friel BC, McKee MD. Clinical applications of BMP-7/OP-1 in fractures, nonunions and spinal fusion. Int Orthop. 2007;31:735–741. doi: 10.1007/s00264-007-0422-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Govender S, Csimma C, Genant HK, et al. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am. 2002;84-A:2123–2134. doi: 10.2106/00004623-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 53.Burkus JK, Transfeldt EE, Kitchel SH, Watkins RG, Balderston RA. Clinical and radiographic outcomes of anterior lumbar interbody fusion using recombinant human bone morphogenetic protein-2. Spine. 2002;27:2396–2408. doi: 10.1097/00007632-200211010-00015. [DOI] [PubMed] [Google Scholar]
- 54.Friedlaender GE, Perry CR, Cole JD, Cook SD, Cierny G, Muschler GF, Zych GA, Calhoun JH, LaForte AJ, Yin S. Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial nonunions. J Bone Joint Surg Am. 2001;83-A(Suppl 1):S151–S158. [PMC free article] [PubMed] [Google Scholar]
- 55.Fu R, Selph S, McDonagh M, Peterson K, Tiwari A, Chou R, Helfand M. Effectiveness and harms of recombinant human bone morphogenetic protein-2 in spine fusion: a systematic review and meta-analysis. Ann Intern Med. 2013;158:890–902. doi: 10.7326/0003-4819-158-12-201306180-00006. [DOI] [PubMed] [Google Scholar]
- 56.Simmonds MC, Brown JV, Heirs MK, Higgins JP, Mannion RJ, Rodgers MA, Stewart LA. Safety and effectiveness of recombinant human bone morphogenetic protein-2 for spinal fusion: a meta-analysis of individual-participant data. Ann Intern Med. 2013;158:877–889. doi: 10.7326/0003-4819-158-12-201306180-00005. [DOI] [PubMed] [Google Scholar]
- 57.Axelrad TW, Steen B, Lowenberg DW, Creevy WR, Einhorn TA. Heterotopic ossification after the use of commercially available recombinant human bone morphogenetic proteins in four patients. J Bone Joint Surg Br. 2008;90:1617–1622. doi: 10.1302/0301-620X.90B12.20975. [DOI] [PubMed] [Google Scholar]
- 58.Boden SD, Zdeblick TA, Sandhu HS, Heim SE. The use of rhBMP-2 in interbody fusion cages. Definitive evidence of osteoinduction in humans: a preliminary report. Spine. 2000;25:376–381. doi: 10.1097/00007632-200002010-00020. [DOI] [PubMed] [Google Scholar]
- 59.Carragee EJ, Mitsunaga KA, Hurwitz EL, Scuderi GJ. Retrograde ejaculation after anterior lumbar interbody fusion using rhBMP-2: a cohort controlled study. Spine J. 2011;11:511–516. doi: 10.1016/j.spinee.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 60.Hiremath GK, Steinmetz MP, Krishnaney AA. Is it safe to use recombinant human bone morphogenetic protein in posterior cervical fusion? Spine. 2009;34:885–889. doi: 10.1097/BRS.0b013e31819e334a. [DOI] [PubMed] [Google Scholar]
- 61.Kang JD. Another complication associated with rhBMP-2? Spine J. 2011;11:517–519. doi: 10.1016/j.spinee.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 62.Kleeman TJ, Ahn UM, Talbot-Kleeman A. Laparoscopic anterior lumbar interbody fusion with rhBMP-2: a prospective study of clinical and radiographic outcomes. Spine. 2001;26:2751–2756. doi: 10.1097/00007632-200112150-00026. [DOI] [PubMed] [Google Scholar]
- 63.Mroz TE, Wang JC, Hashimoto R, Norvell DC. Complications related to osteobiologics use in spine surgery: a systematic review. Spine. 2010;35:S86–S104. doi: 10.1097/BRS.0b013e3181d81ef2. [DOI] [PubMed] [Google Scholar]
- 64.Chatzinikolaidou M, Lichtinger TK, Muller RT, Jennissen HP. Peri-implant reactivity and osteoinductive potential of immobilized rhBMP-2 on titanium carriers. Acta Biomater. 2010;6:4405–4421. doi: 10.1016/j.actbio.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 65.Ekrol I, Hajducka C, Court-Brown C, McQueen MM. A comparison of rhBMP-7 (OP-1) and autogenous graft for metaphyseal defects after osteotomy of the distal radius. Injury. 2008;39(Suppl 2):S73–S82. doi: 10.1016/S0020-1383(08)70018-4. [DOI] [PubMed] [Google Scholar]
- 66.McGee MA, Findlay DM, Howie DW, Carbone A, Ward P, Stamenkov R, Page TT, Bruce WJ, Wildenauer CI, Toth C. The use of OP-1 in femoral impaction grafting in a sheep model. J Orthop Res. 2004;22:1008–1015. doi: 10.1016/j.orthres.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 67.Notices from European Union Institutions Bodies Offices and Agencies (2011) Note for guidance on minimising the risk of transmitting animal spongiform encephalopathy agents via human and veterinary medicinal products (EMA/410/01 rev.3). Official Journal of the European Union (2011/C 73/01)
- 68.Lieberman JR, Daluiski A, Einhorn TA. The role of growth factors in the repair of bone. Biology and clinical applications. J Bone Joint Surg Am. 2002;84-A:1032–1044. doi: 10.2106/00004623-200206000-00022. [DOI] [PubMed] [Google Scholar]
- 69.Reddi AH, Huggins C. Biochemical sequences in the transformation of normal fibroblasts in adolescent rats. Proc Natl Acad Sci USA. 1972;69:1601–1605. doi: 10.1073/pnas.69.6.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eghbali-Fatourechi GZ, Lamsam J, Fraser D, Nagel D, Riggs BL, Khosla S. Circulating osteoblast-lineage cells in humans. N Engl J Med. 2005;352:1959–1966. doi: 10.1056/NEJMoa044264. [DOI] [PubMed] [Google Scholar]
- 71.Vukicevic S, Luyten FP, Reddi AH. Osteogenin inhibits proliferation and stimulates differentiation in mouse osteoblast-like cells (MC3T3-E1) Biochem Biophys Res Commun. 1990;166:750–756. doi: 10.1016/0006-291x(90)90873-l. [DOI] [PubMed] [Google Scholar]
- 72.Kamiya N, Ye L, Kobayashi T, Lucas DJ, Mochida Y, Yamauchi M, Kronenberg HM, Feng JQ, Mishina Y. Disruption of BMP signaling in osteoblasts through type IA receptor (BMPRIA) increases bone mass. J Bone Miner Res. 2008;23:2007–2017. doi: 10.1359/JBMR.080809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Okamoto M, Murai J, Imai Y, Ikegami D, Kamiya N, Kato S, Mishina Y, Yoshikawa H, Tsumaki N. Conditional deletion of Bmpr1a in differentiated osteoclasts increases osteoblastic bone formation, increasing volume of remodeling bone in mice. J Bone Miner Res. 2011;26:2511–2522. doi: 10.1002/jbmr.477. [DOI] [PubMed] [Google Scholar]
- 74.Okamoto M, Murai J, Yoshikawa H, Tsumaki N. Bone morphogenetic proteins in bone stimulate osteoclasts and osteoblasts during bone development. J Bone Miner Res. 2006;21:1022–1033. doi: 10.1359/jbmr.060411. [DOI] [PubMed] [Google Scholar]
- 75.Minear S, Leucht P, Miller S, Helms JA. rBMP represses Wnt signaling and influences skeletal progenitor cell fate specification during bone repair. J Bone Miner Res. 2010;25:1196–1207. doi: 10.1002/jbmr.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kamiya N, Kobayashi T, Mochida Y, Yu PB, Yamauchi M, Kronenberg HM, Mishina Y. Wnt inhibitors Dkk1 and Sost are downstream targets of BMP signaling through the type IA receptor (BMPRIA) in osteoblasts. J Bone Miner Res. 2010;25:200–210. doi: 10.1359/jbmr.090806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kaneko H, Arakawa T, Mano H, Kaneda T, Ogasawara A, Nakagawa M, Toyama Y, Yabe Y, Kumegawa M, Hakeda Y. Direct stimulation of osteoclastic bone resorption by bone morphogenetic protein (BMP)-2 and expression of BMP receptors in mature osteoclasts. Bone. 2000;27:479–486. doi: 10.1016/s8756-3282(00)00358-6. [DOI] [PubMed] [Google Scholar]
- 78.Otsuka E, Notoya M, Hagiwara H. Treatment of myoblastic C2C12 cells with BMP-2 stimulates vitamin D-induced formation of osteoclasts. Calcif Tissue Int. 2003;73:72–77. doi: 10.1007/s00223-002-1071-0. [DOI] [PubMed] [Google Scholar]
- 79.Onishi T, Ishidou Y, Nagamine T, Yone K, Imamura T, Kato M, Sampath TK, ten Dijke P, Sakou T. Distinct and overlapping patterns of localization of bone morphogenetic protein (BMP) family members and a BMP type II receptor during fracture healing in rats. Bone. 1998;22:605–612. doi: 10.1016/s8756-3282(98)00056-8. [DOI] [PubMed] [Google Scholar]
- 80.Garimella R, Tague SE, Zhang J, Belibi F, Nahar N, Sun BH, Insogna K, Wang J, Anderson HC. Expression and synthesis of bone morphogenetic proteins by osteoclasts: a possible path to anabolic bone remodeling. J Histochem Cytochem. 2008;56:569–577. doi: 10.1369/jhc.2008.950394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Anderson HC, Hodges PT, Aguilera XM, Missana L, Moylan PE. Bone morphogenetic protein (BMP) localization in developing human and rat growth plate, metaphysis, epiphysis, and articular cartilage. J Histochem Cytochem. 2000;48:1493–1502. doi: 10.1177/002215540004801106. [DOI] [PubMed] [Google Scholar]
- 82.Nishimura T, Simmons DJ, Mainous EG. The origin of bone formed by heterotopic periosteal autografts. J Oral Maxillofac Surg. 1997;55:1265–1268. doi: 10.1016/s0278-2391(97)90182-8. [DOI] [PubMed] [Google Scholar]
- 83.Little DG, McDonald M, Bransford R, Godfrey CB, Amanat N. Manipulation of the anabolic and catabolic responses with OP-1 and zoledronic acid in a rat critical defect model. J Bone Miner Res. 2005;20:2044–2052. doi: 10.1359/JBMR.050712. [DOI] [PubMed] [Google Scholar]
- 84.Cowan CM, Aalami OO, Shi YY, Chou YF, Mari C, Thomas R, Quarto N, Nacamuli RP, Contag CH, Wu B, Longaker MT. Bone morphogenetic protein 2 and retinoic acid accelerate in vivo bone formation, osteoclast recruitment, and bone turnover. Tissue Eng. 2005;11:645–658. doi: 10.1089/ten.2005.11.645. [DOI] [PubMed] [Google Scholar]
- 85.Jeppsson C, Astrand J, Tagil M, Aspenberg P. A combination of bisphosphonate and BMP additives in impacted bone allografts. Acta Orthop Scand. 2003;74:483–489. doi: 10.1080/00016470310017839. [DOI] [PubMed] [Google Scholar]
- 86.Miyaji H, Sugaya T, Kato K, Kawamura N, Tsuji H, Kawanami M. Dentin resorption and cementum-like tissue formation by bone morphogenetic protein application. J Periodontal Res. 2006;41:311–315. doi: 10.1111/j.1600-0765.2006.00878.x. [DOI] [PubMed] [Google Scholar]
- 87.Cunningham NS, Paralkar V, Reddi AH. Osteogenin and recombinant bone morphogenetic protein 2B are chemotactic for human monocytes and stimulate transforming growth factor beta 1 mRNA expression. Proc Natl Acad Sci USA. 1992;89:11740–11744. doi: 10.1073/pnas.89.24.11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hentunen TA, Cunningham NS, Vuolteenaho O, Reddi AH, Vaananen HK. Osteoclast recruiting activity in bone matrix. Bone Miner. 1994;25:183–198. doi: 10.1016/s0169-6009(08)80238-3. [DOI] [PubMed] [Google Scholar]
- 89.Hentunen TA, Lakkakorpi PT, Tuukkanen J, Lehenkari PP, Sampath TK, Vaananen HK. Effects of recombinant human osteogenic protein-1 on the differentiation of osteoclast-like cells and bone resorption. Biochem Biophys Res Commun. 1995;209:433–443. doi: 10.1006/bbrc.1995.1521. [DOI] [PubMed] [Google Scholar]
- 90.Kanatani M, Sugimoto T, Kaji H, Kobayashi T, Nishiyama K, Fukase M, Kumegawa M, Chihara K. Stimulatory effect of bone morphogenetic protein-2 on osteoclast-like cell formation and bone-resorbing activity. J Bone Miner Res. 1995;10:1681–1690. doi: 10.1002/jbmr.5650101110. [DOI] [PubMed] [Google Scholar]
- 91.Hofbauer LC, Dunstan CR, Spelsberg TC, Riggs BL, Khosla S. Osteoprotegerin production by human osteoblast lineage cells is stimulated by vitamin D, bone morphogenetic protein-2, and cytokines. Biochem Biophys Res Commun. 1998;250:776–781. doi: 10.1006/bbrc.1998.9394. [DOI] [PubMed] [Google Scholar]
- 92.Koide M, Murase Y, Yamato K, Noguchi T, Okahashi N, Nishihara T. Bone morphogenetic protein-2 enhances osteoclast formation mediated by interleukin-1 alpha through upregulation of osteoclast differentiation factor and cyclooxygenase-2. Biochem Biophys Res Commun. 1999;259:97–102. doi: 10.1006/bbrc.1999.0715. [DOI] [PubMed] [Google Scholar]
- 93.Abe E, Yamamoto M, Taguchi Y, Lecka-Czernik B, O'Brien CA, Economides AN, Stahl N, Jilka RL, Manolagas SC. Essential requirement of BMPs-2/4 for both osteoblast and osteoclast formation in murine bone marrow cultures from adult mice: antagonism by noggin. J Bone Miner Res. 2000;15:663–673. doi: 10.1359/jbmr.2000.15.4.663. [DOI] [PubMed] [Google Scholar]
- 94.Koseki T, Gao Y, Okahashi N, Murase Y, Tsujisawa T, Sato T, Yamato K, Nishihara T. Role of TGF-beta family in osteoclastogenesis induced by RANKL. Cell Signal. 2002;14:31–36. doi: 10.1016/s0898-6568(01)00221-2. [DOI] [PubMed] [Google Scholar]
- 95.Dumic-Cule I, Grcevic D, Draca N, Tikvica A, Rogic D, Grgurevic L, Vukicevic S (2013) Effect of BMP2, BMP6 and BMP7 on bone in rats with removed thyroid and parathyroid glands. Int Orthop, in press
- 96.Wutzl A, Brozek W, Lernbass I, Rauner M, Hofbauer G, Schopper C, Watzinger F, Peterlik M, Pietschmann P. Bone morphogenetic proteins 5 and 6 stimulate osteoclast generation. J Biomed Mater Res A. 2006;77:75–83. doi: 10.1002/jbm.a.30615. [DOI] [PubMed] [Google Scholar]
- 97.Ghosh-Choudhury N, Singha PK, Woodruff K, St Clair P, Bsoul S, Werner SL, Choudhury GG. Concerted action of Smad and CREB-binding protein regulates bone morphogenetic protein-2-stimulated osteoblastic colony-stimulating factor-1 expression. J Biol Chem. 2006;281:20160–20170. doi: 10.1074/jbc.M511071200. [DOI] [PubMed] [Google Scholar]
- 98.Ripamonti U, Heliotis M, Ferretti C. Bone morphogenetic proteins and the induction of bone formation: from laboratory to patients. Oral Maxillofac Surg Clin North Am. 2007;19:575–589. doi: 10.1016/j.coms.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 99.Cook SD. Preclinical and clinical evaluation of osteogenic protein-1 (BMP-7) in bony sites. Orthopedics. 1999;22:669–671. [PubMed] [Google Scholar]
- 100.Seeherman H, Wozney JM. Delivery of bone morphogenetic proteins for orthopedic tissue regeneration. Cytokine Growth Factor Rev. 2005;16:329–345. doi: 10.1016/j.cytogfr.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 101.Cook SD, Rueger DC. Preclinical models of recombinant BMP induced healing of orthopedic defects. In: Vukicevic S, Sampath TK, editors. Bone Morphogenetic Proteins From Laboratory to Clinical Practice. Basel: Birkhauser Verlag; 2002. pp. 121–144. [Google Scholar]
- 102.Blokhuis TJ, Patka P, Haarman HJTM, Giltaij LR. Osteogenic protein-1 (OP-1, BMP-7) for stimulation of healing of closed fractures: Evidence based medicine and pre-clinical experience. In: Vukicevic S, Sampath TK, editors. Bone Morphogenetic Proteins From Laboratory to Clinical Practice. Basel: Birkhauser Verlag; 2002. pp. 145–156. [Google Scholar]
- 103.Laursen M, Hoy K, Hansen ES, Gelineck J, Christensen FB, Bunger CE. Recombinant bone morphogenetic protein-7 as an intracorporal bone growth stimulator in unstable thoracolumbar burst fractures in humans: preliminary results. Eur Spine J. 1999;8:485–490. doi: 10.1007/s005860050210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hansen SM, Sasso RC. Resorptive response of rhBMP2 simulating infection in an anterior lumbar interbody fusion with a femoral ring. J Spinal Disord Tech. 2006;19:130–134. doi: 10.1097/01.bsd.0000168512.61351.3a. [DOI] [PubMed] [Google Scholar]
- 105.Pradhan BB, Bae HW, Dawson EG, Patel VV, Delamarter RB. Graft resorption with the use of bone morphogenetic protein: lessons from anterior lumbar interbody fusion using femoral ring allografts and recombinant human bone morphogenetic protein-2. Spine. 2006;31:E277–E284. doi: 10.1097/01.brs.0000216442.12092.01. [DOI] [PubMed] [Google Scholar]
- 106.Poynton AR, Lane JM. Safety profile for the clinical use of bone morphogenetic proteins in the spine. Spine. 2002;27:S40–S48. doi: 10.1097/00007632-200208151-00010. [DOI] [PubMed] [Google Scholar]
- 107.Roberts SJ, Geris L, Kerckhofs G, Desmet E, Schrooten J, Luyten FP. The combined bone forming capacity of human periosteal derived cells and calcium phosphates. Biomaterials. 2011;32:4393–4405. doi: 10.1016/j.biomaterials.2011.02.047. [DOI] [PubMed] [Google Scholar]
- 108.De Bari C, Dell'Accio F, Vanlauwe J, Eyckmans J, Khan IM, Archer CW, Jones EA, McGonagle D, Mitsiadis TA, Pitzalis C, Luyten FP. Mesenchymal multipotency of adult human periosteal cells demonstrated by single-cell lineage analysis. Arthritis Rheum. 2006;54:1209–1221. doi: 10.1002/art.21753. [DOI] [PubMed] [Google Scholar]
- 109.Geesink RG, Hoefnagels NH, Bulstra SK. Osteogenic activity of OP-1 bone morphogenetic protein (BMP-7) in a human fibular defect. J Bone Joint Surg Br. 1999;81:710–718. doi: 10.1302/0301-620x.81b4.9311. [DOI] [PubMed] [Google Scholar]
- 110.Katagiri T, Imada M, Yanai T, Suda T, Takahashi N, Kamijo R. Identification of a BMP-responsive element in Id1, the gene for inhibition of myogenesis. Genes Cells. 2002;7:949–960. doi: 10.1046/j.1365-2443.2002.00573.x. [DOI] [PubMed] [Google Scholar]
- 111.Katagiri T, Akiyama S, Namiki M, Komaki M, Yamaguchi A, Rosen V, Wozney JM, Fujisawa-Sehara A, Suda T. Bone morphogenetic protein-2 inhibits terminal differentiation of myogenic cells by suppressing the transcriptional activity of MyoD and myogenin. Exp Cell Res. 1997;230:342–351. doi: 10.1006/excr.1996.3432. [DOI] [PubMed] [Google Scholar]
- 112.Katagiri T, Yamaguchi A, Komaki M, Abe E, Takahashi N, Ikeda T, Rosen V, Wozney JM, Fujisawa-Sehara A, Suda T. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol. 1994;127:1755–1766. doi: 10.1083/jcb.127.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nojima J, Kanomata K, Takada Y, et al. Dual roles of smad proteins in the conversion from myoblasts to osteoblastic cells by bone morphogenetic proteins. J Biol Chem. 2010;285:15577–15586. doi: 10.1074/jbc.M109.028019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Swiontkowski MF, Aro HT, Donell S, Esterhai JL, Goulet J, Jones A, Kregor PJ, Nordsletten L, Paiement G, Patel A. Recombinant human bone morphogenetic protein-2 in open tibial fractures. A subgroup analysis of data combined from two prospective randomized studies. J Bone Joint Surg Am. 2006;88:1258–1265. doi: 10.2106/JBJS.E.00499. [DOI] [PubMed] [Google Scholar]
- 115.Vukicevic S, Grgurevic L, Oppermann H (2012) Whole blood-derived coagulum device for treating bone defects. US 8197840
- 116.Bernardo ME, Emons JA, Karperien M, Nauta AJ, Willemze R, Roelofs H, Romeo S, Marchini A, Rappold GA, Vukicevic S, Locatelli F, Fibbe WE (2007) Hum.an mesenchymal stem cells derived from bone marrow display a better chondrogenic differentiation compared with other sources. Connect Tissue Res 48:132–140 [DOI] [PubMed]
- 117.Sammons J, Ahmed N, El-Sheemy M, Hassan HT. The role of BMP-6, IL-6, and BMP-4 in mesenchymal stem cell-dependent bone development: effects on osteoblastic differentiation induced by parathyroid hormone and vitamin D(3) Stem Cells Dev. 2004;13:273–280. doi: 10.1089/154732804323099208. [DOI] [PubMed] [Google Scholar]
- 118.Martinovic S, Mazic S, Kisic V, Basic N, Jakic-Razumovic J, Borovecki F, Batinic D, Simic P, Grgurevic L, Labar B, Vukicevic S. Expression of bone morphogenetic proteins in stromal cells from human bone marrow long-term culture. J Histochem Cytochem. 2004;52:1159–1167. doi: 10.1369/jhc.4A6263.2004. [DOI] [PubMed] [Google Scholar]
- 119.Jung Y, Song J, Shiozawa Y, Wang J, Wang Z, Williams B, Havens A, Schneider A, Ge C, Franceschi RT, McCauley LK, Krebsbach PH, Taichman RS. Hematopoietic stem cells regulate mesenchymal stromal cell induction into osteoblasts thereby participating in the formation of the stem cell niche. Stem Cells. 2008;26:2042–2051. doi: 10.1634/stemcells.2008-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Martinovic S, Borovecki F, Miljavac V, Kisic V, Maticic D, Francetic I, Vukicevic S. Requirement of a bone morphogenetic protein for the maintenance and stimulation of osteoblast differentiation. Arch Histol Cytol. 2006;69:23–36. doi: 10.1679/aohc.69.23. [DOI] [PubMed] [Google Scholar]
- 121.Allendorph GP, Isaacs MJ, Kawakami Y, Izpisua Belmonte JC, Choe S. BMP-3 and BMP-6 structures illuminate the nature of binding specificity with receptors. Biochemistry. 2007;46:12238–12247. doi: 10.1021/bi700907k. [DOI] [PubMed] [Google Scholar]
- 122.Korchynskyi O, van Bezooijen RL, Lowik CWGM, ten Dijke P. Bone morphogenetic protein receptors and their nuclear effectors in bone formation. In: Vukicevic S, Sampath TK, editors. Bone morphogenetic proteins: Regeneration of bone and beyond. Basel: Birkauser Verlag AG; 2004. pp. 9–114. [Google Scholar]
- 123.Song K, Krause C, Shi S, Patterson M, Suto R, Grgurevic L, Vukicevic S, van Dinther M, Falb D, ten Dijke P, Alaoui-Ismaili MH. Identification of a key residue mediating bone morphogenetic protein (BMP)-6 resistance to noggin inhibition allows for engineered BMPs with superior agonist activity. J Biol Chem. 2010;285:12169–12180. doi: 10.1074/jbc.M109.087197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vukicevic S, Sampath TK. Bone morphogenetic proteins: from local to systemic therapeutics. Basel: Birkauser Verlag AG; 2008. [Google Scholar]
- 125.Zhang H, Bradley A. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development. 1996;122:2977–2986. doi: 10.1242/dev.122.10.2977. [DOI] [PubMed] [Google Scholar]
- 126.Grgurevic L, Macek B, Erjavec I, Mann M, Vukicevic S. Urine release of systemically administered bone morphogenetic protein hybrid molecule. J Nephrol. 2007;20:311–319. [PubMed] [Google Scholar]
- 127.Grgurevic L, Macek B, Healy DR, Brault AL, Erjavec I, Cipcic A, Grgurevic I, Rogic D, Galesic K, Brkljacic J, Stern-Padovan R, Paralkar VM, Vukicevic S. Circulating bone morphogenetic protein 1-3 isoform increases renal fibrosis. J Am Soc Nephrol. 2011;22:681–692. doi: 10.1681/ASN.2010070722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Simic P, Culej JB, Orlic I, Grgurevic L, Draca N, Spaventi R, Vukicevic S. Systemically administered bone morphogenetic protein-6 restores bone in aged ovariectomized rats by increasing bone formation and suppressing bone resorption. J Biol Chem. 2006;281:25509–25521. doi: 10.1074/jbc.M513276200. [DOI] [PubMed] [Google Scholar]
- 129.Brkljacic J, Pauk M, Erjavec I, Cipcic A, Grgurevic L, Zadro R, Inman GJ, Vukicevic S. Exogenous heparin binds and inhibits bone morphogenetic protein 6 biological activity. Int Orthop. 2013;37:529–541. doi: 10.1007/s00264-012-1714-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cuellar A, Reddi AH. Cell biology of osteochondromas: bone morphogenic protein signalling and heparan sulphates. Int Orthop. 2013;37:1591–1596. doi: 10.1007/s00264-013-1906-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.de Gorter DJ, van Dinther M, Korchynskyi O, ten Dijke P. Biphasic effects of transforming growth factor beta on bone morphogenetic protein-induced osteoblast differentiation. J Bone Miner Res. 2011;26:1178–1187. doi: 10.1002/jbmr.313. [DOI] [PubMed] [Google Scholar]
- 132.Cook SD, Baffes GC, Wolfe MW, Sampath TK, Rueger DC (1994) Recombinant human bone morphogenetic protein-7 induces healing in a canine long-bone segmental defect model. Clin Orthop Relat Res 302–312 [PubMed]