Abstract
Objective To compare the survival outcomes of patients treated with surgery or radiotherapy for prostate cancer.
Design Observational study.
Setting Sweden, 1996-2010.
Participants 34 515 men primarily treated for prostate cancer with surgery (n=21 533) or radiotherapy (n=12 982). Patients were categorised by risk group (low, intermediate, high, and metastatic), age, and Charlson comorbidity score.
Main outcome measures Cumulative incidence of mortality from prostate cancer and other causes. Competing risks regression hazard ratios for radiotherapy versus surgery were computed without adjustment and after propensity score and traditional (multivariable) adjustments, as well as after propensity score matching. Several sensitivity analyses were performed.
Results Prostate cancer mortality became a larger proportion of overall mortality as risk group increased for both the surgery and the radiotherapy cohorts. Among patients with non-metastatic prostate cancer the adjusted subdistribution hazard ratio for prostate cancer mortality favoured surgery (1.76, 95% confidence interval 1.49 to 2.08, for radiotherapy v prostatectomy), whereas there was no discernible difference in treatment effect among men with metastatic disease. Subgroup analyses indicated more clear benefits of surgery among younger and fitter men with intermediate and high risk disease. Sensitivity analyses confirmed the main findings.
Conclusions This large observational study with follow-up to 15 years suggests that for most men with non-metastatic prostate cancer, surgery leads to better survival than does radiotherapy. Younger men and those with less comorbidity who have intermediate or high risk localised prostate cancer might have a greater benefit from surgery.
Introduction
Prostate cancer is the commonest non-dermatological cancer and the second leading cause of cancer related death in men in the Western world.1 In more than 90% of cases the cancer is localised, and radical prostatectomy, radiotherapy, and active surveillance represent the main treatment options.2 The landmark Scandinavian Prostate Cancer Group (SPCG)-4 trial3 has shown a definite survival advantage for surgery over watchful waiting, at a median follow-up of 12.8 years, although the more recent randomised controlled trial, the Prostate Intervention Versus Observation Trial (PIVOT),4 has indicated that the benefit from surgery might be confined to intermediate and high risk tumours. Only one recent randomised controlled trial examined the comparative effectiveness of different treatment modalities, the ProtecT study,5 the results of which will not be available for at least the next two years. Furthermore, the results of randomised controlled trials may have limited generalisability because of differences between the enrolled population and community populations, who are likely to be more heterogeneous for comorbidities and socioeconomic characteristics.6 Hence the importance of using observational data from actual medical practice in comparative effectiveness studies to complement the evidence from randomised controlled trials. In the specialty of prostate cancer, however, most such studies have evaluated biochemical recurrence as the endpoint, and have shown conflicting results.7 8 The definition of biochemical recurrence varies between surgical and radiotherapy cohorts, and even between individual radiotherapy series, and thus comparing biochemical recurrence across treatment modalities is problematic. Furthermore, the median time to death after biochemical recurrence has been shown to be as long as 13 years in a surgical series,9 and not all men who experience recurrence will develop clinical disease. Hence, death remains the most valid endpoint for comparative studies in prostate cancer.
In this nationwide population based cohort study we assessed prostate cancer related mortality in patients in the Swedish national prostate cancer registry, who underwent radical prostatectomy or radiotherapy as their primary treatment. Linkage with other healthcare and demographic databases enabled reduction of potential confounding through statistical techniques as well as adjustment for competing risks of mortality, as many men with prostate cancer are known to die of other causes.10 We hypothesised that survival differences would vary by treatment, and that age and the burden from comorbidities would have an impact on survival.
Methods
This study is based on the PCBaSe Sweden, which has been described previously.11 12 Briefly, it is a composite population based dataset of the National Prostate Cancer Registry of Sweden, the Swedish cancer register, the cause of death register, and six other national registers, using the unique 10 digit personal identity number assigned to every resident in Sweden. The dataset covers 98% of all cases of prostate cancer in Sweden diagnosed since 1998 (with coverage from 1996 and 1997 limited to certain regions), and has virtually complete data on year of diagnosis; age; clinical stage (tumour, node, metastases (TNM) classification); tumour grade (either Gleason sum or World Health Organization grade of differentiation); serum level of prostate specific antigen at the time of diagnosis; planned primary treatment within six months of diagnosis; county of residence; marital status; educational level; socioeconomic status; Charlson comorbidity index; and cancer related events during follow-up. The Charlson score was estimated from registrations in the inpatient register, which in a previous study based on the PCBaSe dataset has been shown to have an impact on management and survival.13
We identified a total of 109 333 men with a diagnosis of prostate cancer between 1996 and 2010 in PCBaSe Sweden. After exclusion of those whose treatment was unknown (n=4788) or who had died before treatment (n=512), we included all patients provided their primary treatment was listed as radical prostatectomy or radiotherapy (n=34 515). From this analysis we excluded patients who received androgen deprivation or surgical castration as their primary treatment (n=40 502), or watchful waiting (n=29 016). The median follow-up time for the included cohort was 5.37 years (interquartile range 3.00-7.81 years), for the radical prostatectomy group 5.26 (3.03-7.57) years, and for the radiotherapy group 5.60 (2.96-8.18) years. As in previous studies using this dataset,11 12 13 we categorised patients by clinical risk (low, intermediate, or high risk (collectively, non-metastatic prostate cancer), and metastatic disease, table 1), as well as by age (≤64, ≥65) and Charlson comorbidity index (0, ≥1). After stratification by risk group, the study cohort comprised 34 052 cases; 463/34 515 (1.3%) patients had missing data precluding risk categorisation. We merged WHO grade 1 tumours with Gleason scores 2-6, WHO 2 with Gleason score 7, and WHO 3 with Gleason scores 8-10.
Table 1.
Definition of prostate cancer risk groups used in this study (adapted from PCBaSe Sweden)11 12
| Risk categories | Clinical stage | Prostate specific antigen (ng/mL) | Biopsy Gleason |
|---|---|---|---|
| Group 1 (low risk) | T1-2 N0/Nx M0/Mx | ≤10 | ≤6 or WHO 1 |
| Group 2 (intermediate risk) | 10-20 | 7 or WHO 2 | |
| Group 3 (high risk) | T3 N0/Nx M0/Mx | 20-50 | ≥8 or WHO 3 |
| Group 4 (metastatic) | T4 N+ M+ | >50 | — |
The primary outcome of interest was death from prostate cancer. We defined survival time as the interval between date of diagnosis of prostate cancer and the date of death, emigration, or end of follow-up at 31 December 2010.
Statistical analysis
We used χ2 and Wilcoxon-Mann-Whitney tests to investigate differences in the distributions of patient characteristics by treatment groups. To visualise cause specific mortality, we plotted cumulative incidence curves for the treatment groups. We investigated differences in each cause of mortality (prostate cancer or other causes) using subdistribution hazard ratios estimated through Fine and Gray proportional hazards regression.14 To deal with any imbalances in the distribution of covariates among treatment groups, we produced both traditional multivariable model adjusted and propensity score adjusted estimates of subdistribution hazard ratios. We calculated propensity scores using logistic regression, with treatment group as outcome and all adjustment covariates as predictors; we made adjustments by including the resulting logit transformed propensities when modelling subdistribution hazard ratios. We tested heterogeneity of such ratios across risk groups using likelihood ratio tests of the relevant interaction terms. Furthermore, we used the propensity scores for matching, which we carried out within each risk group, using the larger of the two treatment groups and selecting a nearest neighbour 1-to-1 match for those in the smaller treatment group, with a caliper of 0.1 standard deviations for the propensity scores.
We carried out several sensitivity analyses. Assuming that the cancer treatment resulted in no difference in prostate cancer mortality, we assessed the effect required of a hypothetical unmeasured binary confounder to explain the propensity score adjusted subdistribution hazard ratios for prostate cancer mortality of radiotherapy versus radical prostatectomy for varying levels of confounder imbalance between treatment groups.15 16 To investigate possibly divergent developments in treatment efficiency we reassessed the comparisons after stratification by year of diagnosis (1996-99, 2000-04, and 2005-09). Furthermore, we used the propensity scores for inverse probability of treatment weight adjustments; we carried out these through weighting data from each individual with weights proportional to the estimated propensity of the treatment not received, using Cox proportional hazards regression.
All tests were performed two sided at the 5% significance level. Statistical analyses were performed with IBM SPSS Statistics (version 20.0, IBM, Armonk, NY), and R software (version 2.15, R Foundation for Statistical Computing, Vienna, Austria) using the cmprsk, survival, rmeta, and Matching packages.
Results
Table 2 shows the baseline clinical and tumour characteristics of the participants, stratified by treatment. Generally, the radiotherapy group had a greater proportion of high clinical stage and grade disease, with higher levels of prostate specific antigen than the radical prostatectomy group. This was also the case within each individual risk group. Patients who received radiotherapy were on average also older and had higher scores on the Charlson comorbidity index. Patients in the radical prostatectomy group on average received their diagnosis at a later date than patients in the radiotherapy group. Furthermore, the choice of treatment differed significantly by county of residence (data not shown), and the radiotherapy group had a greater proportion of patients of low educational level, low socioeconomic status, and unmarried status. In each non-metastatic risk group the demographic and clinical characteristics of the patients showed the same differences when stratified by treatment modality, albeit attenuated for the tumour covariates (as a result of the risk categorisation). In the metastatic risk group, some adverse prognostic criteria such as a higher Charlson comorbidity score, N+ stage, and M+ stage, were more common in the radical prostatectomy group, whereas others were more common in the radiotherapy cohort. By the end of the study, 339 prostate cancer related deaths and 1064 deaths from other causes occurred in the radical prostatectomy arm and 697 and 1127, respectively in the radiotherapy arm (table 3).
Table 2.
Baseline clinicopathological and follow-up data for cohort stratified by treatment type (radical prostatectomy or radiotherapy) and risk group. Values are numbers (percentages) unless otherwise stated
| Variables | All patients | Risk group 1 patients | Risk group 2 patients | Risk group 3 patients | Risk group 4 patients | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Surgery (n=215 33) | Radiotherapy (n=12 982) | Surgery (n=10 003) | Radiotherapy (n=3039) | Surgery (n=8125) | Radiotherapy (n=4030) | Surgery (n=2609) | Radiotherapy (n=5040) | Surgery (n=389) | Radiotherapy (n=817) | |||||||
| Median (IQR) follow-up time (years) | 5.26 (3.03-7.57) | 5.60 (2.96-8.18) | 5.64 (3.37-7.67) | 5.89 (3.52-8.21) | 4.97 (2.73-7.35) | 5.22 (2.70-7.95) | 5.16 (2.90-7.90) | 5.76 (3.06-8.38) | 4.97 (2.78-7.21) | 4.90 (2.51-8.18) | ||||||
| P value | <0.001 | <0.001 | 0.004 | <0.001 | 0.314 | |||||||||||
| Median (IQR) year of diagnosis | 2005 (2003-07) | 2004 (2001-07) | 2005 (2003-07) | 2004 (2002-07) | 2005 (2003-08) | 2005 (2002-08) | 2005 (2002-07) | 2004 (2001-07) | 2005 (2002-07) | 2004 (2000-08) | ||||||
| P value | <0.001 | <0.001 | <0.001 | <0.001 | 0.397 | |||||||||||
| Median (IQR) age at diagnosis | 62 (58-66) | 66 (62-70) | 62 (57-65) | 65 (60-69) | 63 (59-67) | 67 (63-71) | 64 (60-67) | 66 (62-70) | 64 (59-68) | 65 (60-69) | ||||||
| P value | <0.001 | <0.001 | <0.001 | <0.001 | 0.003 | |||||||||||
| Median (IQR) PSA (ng/mL) | 7.0 (5.0-11.0) | 10.9 (6.8-20.0) | 5.9 (4.4-7.5) | 6.4 (5.0-8.0) | 9.9 (6.0-13.0) | 11.0 (7.2-14.0) | 14.0 (7.2-24.0) | 19.0 (9.4-28.0) | 18.6 (8.9-59.0) | 60.0 (51.0-77.1) | ||||||
| P value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||||||||||
| Clinical stage: | ||||||||||||||||
| cT1 | 12 892 (60.7) | 4962 (38.6) | 6895 (69.7) | 1887 (62.9) | 4659 (57.8) | 1907 (47.9) | 996 (38.6) | 977 (19.5) | 129 (34.6) | 162 (19.9) | ||||||
| cT2 | 7613 (35.9) | 4928 (38.3) | 3000 (30.3) | 1112 (37.1) | 3399 (42.2) | 2076 (52.1) | 947 (36.7) | 1453 (29.0) | 157 (42.1) | 267 (32.8) | ||||||
| cT3 | 716 (3.4) | 2935 (22.8) | 0 | 0 | 0 | 0 | 640 (24.8) | 2583 (51.5) | 76 (20.4) | 352 (43.3) | ||||||
| cT4 | 11 (0.1) | 32 (0.2) | 0 | 0 | 0 | 0 | 0 | 0 | 11 (2.9) | 32 (3.9) | ||||||
| P value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||||||||||
| N stage: | ||||||||||||||||
| cN0/Nx | 21 315 (99.0) | 12 842 (98.9) | 10 003 (100.0) | 3039 (100.0) | 8125 (100.0) | 4030 (100.0) | 2609 (100.0) | 5040 (100.0) | 171 (44.0) | 677 (82.9) | ||||||
| cN1 | 218 (1.0) | 140 (1.1) | 0 | 0 | 0 | 0 | 0 | 0 | 218 (56.0) | 140 (17.1) | ||||||
| P value | 0.558 | <0.001 | ||||||||||||||
| M stage: | ||||||||||||||||
| cM0/MX | 21 486 (99.8) | 12933 (99.6) | 10 003 (100.0) | 3039 (100.0) | 8125 (100.0) | 4030 (100.0) | 2609 (100.0) | 5040 (100.0) | 342 (87.9) | 768 (94.0) | ||||||
| cM1 | 47 (0.2) | 49 (0.4) | 0 | 0 | 0 | 0 | 0 | 0 | 47 (12.1) | 49 (6.0) | ||||||
| P value | 0.007 | <0.001 | ||||||||||||||
| Gleason or WHO stage: | ||||||||||||||||
| ≤6 or WHO 1 | 13 393 (62.7) | 5700 (44.1) | 10 003 (100.0) | 3039 (100.0) | 2432 (29.9) | 1237 (30.7) | 651 (25.1) | 1253 (24.9) | 113 (29.4) | 158 (19.5) | ||||||
| 7 or WHO 2 | 6573 (30.8) | 5192 (40.2) | 0 | 0 | 5693 (70.1) | 2793 (69.3) | 667 (25.7) | 1978 (39.4) | 162 (42.1) | 407 (50.2) | ||||||
| ≥8 or WHO 3 | 1390 (6.5) | 2039 (15.8) | 0 | 0 | 0 | 0 | 1280 (49.3) | 1794 (35.7) | 110 (28.6) | 245 (30.2) | ||||||
| P value | <0.001 | 0.389 | <0.001 | 0.001 | ||||||||||||
| Charlson comorbidity index: | ||||||||||||||||
| 0 | 17 679 (82.1) | 9685 (74.6) | 8363 (83.6) | 2275 (74.9) | 6765 (83.3) | 2904 (72.1) | 2068 (79.3) | 3813 (75.7) | 293 (75.3) | 651 (79.7) | ||||||
| 1 | 2417 (11.2) | 2115 (16.3) | 1092 (10.9) | 512 (16.8) | 900 (11.1) | 719 (17.8) | 348 (13.3) | 771 (15.3) | 40 (10.3) | 106 (13.0) | ||||||
| 2 | 1070 (5.0) | 783 (6.0) | 420 (4.2) | 162 (5.3) | 347 (4.3) | 266 (6.6) | 152 (5.8) | 306 (6.1) | 33 (8.5) | 45 (5.5) | ||||||
| ≥3 | 367 (1.7) | 399 (3.1) | 128 (1.3) | 90 (3.0) | 113 (1.4) | 141 (3.5) | 41 (1.6) | 150 (3.0) | 23 (5.9) | 15 (1.8) | ||||||
| P value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||||||||||
| Marital status: | ||||||||||||||||
| Married | 16 033 (74.5) | 9329 (71.9) | 7515 (75.1) | 2177 (71.6) | 5989 (73.7) | 2870 (71.2) | 1964 (75.3) | 3669 (72.8) | 275 (70.7) | 578 (70.7) | ||||||
| Widower | 663 (3.1) | 563 (4.3) | 266 (2.7) | 120 (3.9) | 272 (3.3) | 186 (4.6) | 94 (3.6) | 214 (4.2) | 18 (4.6) | 36 (4.4) | ||||||
| Divorced | 2987 (13.9) | 1967 (15.2) | 1361 (13.6) | 470 (15.5) | 1178 (14.5) | 660 (16.4) | 325 (12.5) | 707 (14.0) | 59 (15.2) | 119 (14.6) | ||||||
| Single | 1847 (8.6) | 1123 (8.7) | 861 (8.6) | 272 (9.0) | 684 (8.4) | 314 (7.8) | 225 (8.6) | 450 (8.9) | 37 (9.5) | 84 (10.3) | ||||||
| P value | <0.001 | <0.001 | <0.001 | 0.089 | 0.969 | |||||||||||
| Educational level: | ||||||||||||||||
| High | 6457 (30.1) | 3093 (24.0) | 3155 (31.6) | 771 (25.5) | 2334 (28.9) | 972 (24.3) | 744 (28.7) | 1138 (22.7) | 109 (28.1) | 199 (24.5) | ||||||
| Intermediate | 8687 (40.5) | 4993 (38.7) | 4080 (40.9) | 1204 (39.8) | 3296 (40.8) | 1579 (39.5) | 1007 (38.9) | 1869 (37.3) | 149 (38.4) | 319 (39.2) | ||||||
| Low | 6296 (29.4) | 4815 (37.3) | 2735 (27.4) | 1047 (34.6) | 2458 (30.4) | 1451 (36.3) | 839 (32.4) | 2002 (40.0) | 130 (33.5) | 295 (36.3) | ||||||
| P value | <0.001 | <0.001 | <0.001 | <0.001 | 0.375 | |||||||||||
| Socioeconomic status: | ||||||||||||||||
| White collar | 12 023 (56.5) | 6689 (52.1) | 5570 (56.3) | 1579 (52.5) | 4557 (56.7) | 2101 (52.6) | 1450 (56.4) | 2574 (51.6) | 220 (57.1) | 404 (50.3) | ||||||
| Blue collar or unemployed | 9262 (43.5) | 6161 (47.9) | 4320 (43.7) | 1428 (47.5) | 3481 (43.3) | 1893 (47.4) | 1121 (43.6) | 2416 (48.4) | 165 (42.9) | 399 (49.7) | ||||||
| P value | <0.001 | <0.001 | <0.001 | <0.001 | 0.027 | |||||||||||
IQR=interquartile range; PSA=prostate specific antigen; WHO=World Health Organization.
Table 3.
Mortality figures and crude, propensity score adjusted and traditionally adjusted subdistribution hazard ratios (sHR) for radiotherapy versus radical prostatectomy for deaths from prostate cancer and other causes, stratified by risk group
| Cause of death by risk group | No of events/Total No (%) | Radiotherapy versus surgery | ||||||
|---|---|---|---|---|---|---|---|---|
| Crude sHR (95% CI) | P value | Propensity score adjusted sHR (95% CI) | P value | Traditional covariate adjusted sHR (95% CI) | P value | |||
| Radiotherapy | Surgery | |||||||
| Risk group 1: | ||||||||
| Prostate cancer | 32/3039 (1.1) | 44/10 003 (0.4) | 2.16 (1.37 to 3.42) | <0.001 | 1.91 (1.16 to 3.14) | 0.011 | 2.03 (1.22 to 3.40) | 0.007 |
| Other causes | 223/3039 (7.3) | 412/10 003 (4.1) | 1.67 (1.42 to 1.97) | <0.001 | 1.26 (1.05 to 1.51) | 0.013 | 1.24 (1.04 to 1.48) | 0.019 |
| Risk group 2: | ||||||||
| Prostate cancer | 142/4030 (3.5) | 131/8125 (1.6) | 1.95 (1.53 to 2.47) | <0.001 | 1.77 (1.37 to 2.29) | <0.001 | 1.77 (1.36 to 2.30) | <0.001 |
| Other causes | 376/4030 (9.3) | 365/8125 (4.5) | 1.94 (1.68 to 2.24) | <0.001 | 1.43 (1.23 to 1.68) | <0.001 | 1.40 (1.19 to 1.64) | <0.001 |
| Risk group 3: | ||||||||
| Prostate cancer | 418/5040 (8.3) | 118/2609 (4.5) | 1.69 (1.38 to 2.07) | <0.001 | 1.50 (1.19 to 1.88) | <0.001 | 1.63 (1.28 to 2.06) | <0.001 |
| Other causes | 458/5040 (9.1) | 154/2609 (5.9) | 1.42 (1.18 to 1.70) | <0.001 | 1.20 (0.98 to 1.48) | 0.081 | 1.20 (0.97 to 1.48) | 0.091 |
| Non-metastatic (risk groups 1-3): | ||||||||
| Prostate cancer | 592/12 109 (4.9) | 293/20 737 (1.4) | 3.09 (2.69 to 3.56) | <0.001 | 1.76 (1.49 to 2.08) | <0.001 | 1.77 (1.49 to 2.09) | <0.001 |
| Other causes | 1057/12 109 (8.7) | 931/20 737 (4.5) | 1.77 (1.62 to 1.93) | <0.001 | 1.32 (1.18 to 1.47) | <0.001 | 1.28 (1.16 to 1.42) | <0.001 |
| Risk group 4: | ||||||||
| Prostate cancer | 102/817 (12.5) | 43/389 (11.1) | 1.04 (0.73 to 1.48) | 0.835 | 0.76 (0.49 to 1.19) | 0.231 | 0.65 (0.40 to 1.05) | 0.081 |
| Other causes | 65/817 (8.0) | 40/389 (10.3) | 0.71 (0.48 to 1.05) | 0.089 | 0.77 (0.45 to 1.34) | 0.362 | 0.61 (0.35 to 1.07) | 0.087 |
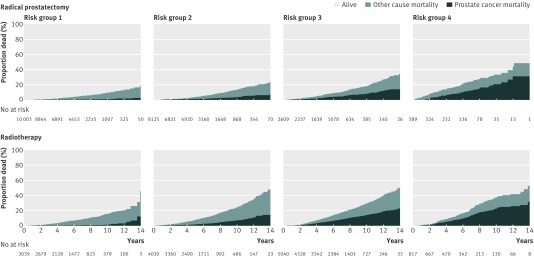
The mortality estimates in the radical prostatectomy group were lower for non-metastatic disease (risk groups 1-3) than in the radiotherapy group (fig 1 ). This was the case for mortality from both prostate cancer and other causes, although as clinical risk increased prostate cancer related mortality became a larger proportion of the overall mortality. In metastatic cases (risk group 4), roughly half of all the men had died by 15 years, and over half of those deaths were due to prostate cancer, regardless of treatment type.
Fig 1 Cumulative incidence function estimates of cancer specific and other cause mortality survival curves (n=34 515), stratified according to treatment type
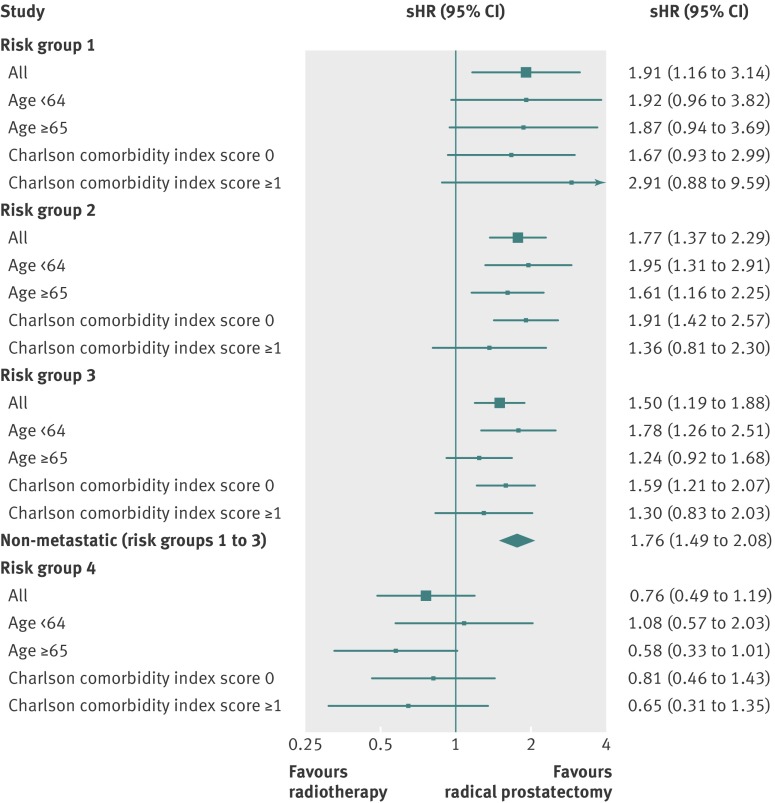
Among men with non-metastatic prostate cancer, treatment with radiotherapy was associated with a significantly higher crude prostate cancer mortality than surgery (subdistribution hazard ratio 3.09, 95% confidence interval 2.69 to 3.56; table 3). Both propensity score and traditional adjustment reduced the subdistribution hazard ratios consistently, but results remained in favour of surgery (1.76, 1.49 to 2.08 and 1.77, 1.49 to 2.09, respectively); the subdistribution hazard ratios for radiotherapy versus surgery did not differ significantly between risk groups (P for homogeneity, 0.17). Differences in survival outcomes were non-discernible between treatment modalities for patients with metastatic disease (crude subdistribution hazard ratio 1.04, 95% confidence interval 0.74 to 1.48; table 3); lack of difference persisted even after the main statistical adjustments. Figure 2 is a forest plot examining whether surgery or radiotherapy leads to improved survival with prostate cancer, based on risk group, age, and Charlson comorbidity index, and after adjustment for propensity score. Among men without metastatic disease, the subdistribution hazard ratios consistently favoured radical prostatectomy over radiotherapy, with point estimates for surgery trending to be better in younger men (<65 years) and fitter (Charlson score 0) men with intermediate (risk group 2) and high risk (risk group 3) prostate cancer; no discernible differences can be seen between treatment modalities for patients with metastatic disease. Similar results to those in figure 2 were obtained after traditional (multivariable) adjustment (data not shown). Propensity score matching also confirmed our main findings of cancer outcomes favouring surgery over radiotherapy for patients without metastatic prostate cancer with no significant differences for the metastatic group, albeit with attenuated point estimates (table 4).
Fig 2 Forest plot depicting propensity score adjusted subdistribution hazard ratios (sHR) for radiotherapy versus radical prostatectomy for cancer specific mortality stratified by risk group, and substratified by age and Charlson comorbidity index score
Table 4.
Mortality figures and subdistribution hazard ratios (sHR) for radiotherapy versus radical prostatectomy for deaths from prostate cancer and other causes after propensity score matching, stratified by risk group
| Risk categories | Initial group sizes* | No (%) of non-matches† | Type of mortality | No of events/Total No (%) | sHR (95% CI): radiotherapy v surgery | P value | ||
|---|---|---|---|---|---|---|---|---|
| Radiotherapy | Surgery | Radiotherapy | Surgery | |||||
| Group 1 | 2962 | 9765 | 271 (9.1) | Prostate cancer | 27/2691 (1.0) | 17/2691 (0.6) | 1.62 (0.88 to 3.01) | 0.123 |
| Other causes | 191/2691 (7.1) | 156/2691 (5.8) | 1.27 (1.03 to 1.57) | 0.026 | ||||
| Group 2 | 3929 | 7950 | 752 (19.1) | Prostate cancer | 103/3177 (3.2) | 57/3177 (1.8) | 1.78 (1.29 to 2.47) | <0.001 |
| Other causes | 264/3177 (8.3) | 188/3177 (5.9) | 1.41 (1.17 to 1.70) | <0.001 | ||||
| Group 3 | 4923 | 2508 | 688 (27.4) | Prostate cancer | 136/1820 (7.5) | 91/1820 (5.0) | 1.52 (1.17 to 1.98) | 0.002 |
| Other causes | 131/1820 (7.2) | 112/1820 (6.2) | 1.19 (0.93 to 1.54) | 0.165 | ||||
| Non-metastatic (groups 1-3) | 11 814 | 20 223 | 3987 (33.7) | Prostate cancer | 264/7827 (3.4) | 182/7827 (2.3) | 1.48 (1.22 to 1.78) | <0.001 |
| Other causes | 594/7827 (7.6) | 467/7827 (6.0) | 1.31 (1.16 to 1.48) | <0.001 | ||||
| Group 4 | 791 | 357 | 138 (38.7) | Prostate cancer | 22/219 (10.0) | 25/219 (11.4) | 0.85 (0.48 to 1.50) | 0.574 |
| Other causes | 17/219 (7.8) | 17/219 (7.8) | 1.00 (0.52 to 1.94) | 0.997 | ||||
*Treatment groups were matched 1:1in each risk group.
†Excluded from analysis.
Men without metastatic prostate cancer had lower survival from other causes of death after radiotherapy (crude subdistribution hazard ratio 1.77, 95% confidence interval 1.62 to 1.93, table 3). After propensity score and traditional adjustments the results moved in the direction of the null but remained worse after radiotherapy (subdistribution hazard ratio 1.32, 95% confidence interval 1.18 to 1.42 and 1.28, 1.16 to 1.42, respectively). In men with metastatic prostate cancer, no differences were seen in other cause mortality between treatments with and without the main statistical adjustments. Again, these results were confirmed in the propensity score matched analyses (table 4).
Sensitivity analysis on the non-metastatic groups showed that the required subdistribution hazard ratio effect of an unmeasured confounder would have to be large (and in some cases, infinite), given varying the differential expression of the unmeasured confounder between the surgery and radiotherapy groups (always assuming higher confounder levels in the radiotherapy group), to produce the observed subdistribution hazard ratios for radiotherapy versus radical prostatectomy (table 5). Analyses stratified by year of diagnosis showed no discernible temporal trend in point estimates for treatment comparisons for any risk groups (see appendix 1 on bmj.com). Furthermore, inverse probability of treatment weight adjustments showed that cancer outcomes favoured surgery over radiotherapy for patients without metastatic prostate cancer, with no significant differences for the metastatic group, but as with the propensity score matched sample, the point estimates were attenuated (see appendix 2 on bmj.com).
Table 5.
Required effect (subdivision hazard ratio, sHR) of hypothetical unmeasured confounder to give observed propensity score adjusted sHR of radiotherapy versus radical prostatectomy assuming no difference between cancer treatments
| Risk categories | Propensity score adjusted sHR (95% CI) | Assumed prevalence (%) of unmeasured confounder in surgery cohort | Assumed prevalence (%) of unmeasured confounder in radiotherapy cohort | |||
|---|---|---|---|---|---|---|
| 30 | 50 | 70 | 90 | |||
| Required confounder sHR to produce observed radiotherapy v surgery sHR | ||||||
| Group 1 | 1.91 (1.16 to 3.14) | 10 | 9.37 | 3.95 | 2.79 | 2.29 |
| 30 | — | Not possible | 8.19 | 3.79 | ||
| 50 | — | — | Not possible | Not possible | ||
| 70 | — | — | — | Not possible | ||
| Group 2 | 1.77 (1.37 to 2.29) | 10 | 7.30 | 3.40 | 2.48 | 2.07 |
| 30 | — | Not possible | 5.60 | 3.10 | ||
| 50 | — | — | Not possible | 58.15 | ||
| 70 | — | — | — | Not possible | ||
| Group 3 | 1.50 (1.19 to 1.88) | 10 | 4.32 | 2.43 | 1.91 | 1.67 |
| 30 | — | 10.92 | 2.99 | 2.11 | ||
| 50 | — | — | Not possible | 4.32 | ||
| 70 | — | — | — | Not possible | ||
| Non-metastatic (groups 1-3) | 1.76 (1.49 to 2.08) | 10 | 7.12 | 3.34 | 2.45 | 2.05 |
| 30 | — | Not possible | 5.41 | 3.04 | ||
| 50 | — | — | Not possible | 38.59 | ||
| 70 | — | — | — | Not possible | ||
Discussion
In this observational study in men treated primarily with either radical prostatectomy or radiotherapy, with follow-up to 15 years, we found that surgery was associated with better cancer specific survival than radiotherapy among men with non-metastatic prostate cancer. Younger and fitter men (those with fewer comorbidities) and those with higher risk disease might possibly have the greatest differential benefit with surgery. We found little if any difference in outcome between treatment modalities for men with metastatic prostate cancer in our main analyses, although sensitivity analyses indicated a possible benefit for radiotherapy. Deaths from other causes seemed to be worse after radiotherapy for men with non-metastatic prostate cancer, but no differences were seen for those with metastatic prostate cancer.
Comparison with other studies
Other investigators have also examined the comparative effectiveness of surgery versus radiotherapy in men with prostate cancer. Recently, the Prostate Cancer Results Study Group compared radical prostatectomy, radiotherapy, cryotherapy, and high intensity focused ultrasonography, but interpretation of their results is difficult owing to multiple study limitations, including the use of differing definitions of biochemical recurrence as the endpoint.7 One study used the American Society for Radiation Oncology definition of biochemical recurrence in 1872 patients treated by surgery, external beam radiotherapy, or brachytherapy, and found that surgery was superior in the group with Gleason 8-10 scores, but with no differences seen for low risk patients in whom follow-up was relatively short; thus this last cohort did well for cancer outcome regardless of treatment choice.8 Using clinical recurrence (metastases) as an endpoint, a study of 2380 patients again showed that low risk patients had few events and thus no differences between surgery and radiotherapy were observed, but for intermediate and high risk patients the data favoured radical prostatectomy (overall hazard ratio 0.35 for surgery v radiotherapy).17 Data from an observational study of more than 404 000 men treated at more than 1000 community hospitals in 44 states of the United States from the Nationwide Inpatient Sample making up more than 20% of all community hospital admissions, including Medicare, Medicaid, uninsured, and private patients, suggested that surgery had superior cancer specific and other cause mortality outcomes for men aged less than 80 years with low and intermediate risk disease.18 19 A multi-institutional US study of 10 429 men treated with surgery, external beam radiotherapy, or brachytherapy showed that external beam radiotherapy was associated with an adjusted 10 year prostate cancer specific mortality hazard ratio of 1.5 compared with surgery, but that brachytherapy and surgery were equivalent.20 Furthermore, a study of 6849 patients from the primary data source used in our study, the National Prostate Cancer Registry of Sweden, found that the difference between observed and expected mortality was greater for surgery compared with observation than for radiotherapy compared with observation.21 A study that analysed the Cancer of the prostate Strategic Urologic research Endeavor database on 7538 men with high risk prostate cancer found a roughly twofold increase in cancer specific mortality for radiotherapy compared with surgery.22 Furthermore, data on 6692 men without comorbidities treated from 1995 to 2007 at two academic centres in the United States showed that radiotherapy was associated with an increase in prostate cancer related deaths compared with radical prostatectomy (hazard ratio 1.66).23 Finally, a cohort of 68 665 men with localised prostate cancer treated by surgery or radiotherapy from 1992-2005 showed that the surgical group fared substantially better in terms of prostate cancer mortality, although this study, as with many of the others discussed here, was limited by the quality of the dataset (Surveillance, Epidemiology and End Results (SEER)/Medicare) used; the SEER registry represents only 14% of the US population before 2000 and 26% thereafter, and the Medicare insurance linked programme only captures those aged 65 or more; missing data and lack of important covariates is also prevalent.16
A comparative analysis of 1238 patients with high risk disease who underwent surgery—344 who had external beam radiotherapy plus hormones, and 265 who received external beam radiotherapy alone—again showed improved mortality outcomes in the surgical cohort.24 Our study further supports that men with high risk disease fared better after surgery, with statistically significant improvements over radiotherapy in men aged less than 65 and those without comorbidity. We found no significant differences between treatments in high risk cases in older patients or those with comorbidities, and the Nationwide Inpatient Sample study found that the only subgroup in which radiotherapy fared better was those aged more than 80 with high risk disease. Our study included only small numbers of older men and those with Charlson scores of 1 or more who had high risk disease, and thus lack of a statistically significant benefit for radiotherapy might be due to low power. In the absence of randomised head to head comparisons between surgery and radiotherapy, we would therefore conclude that for men with non-metastatic prostate cancer where radical treatment is indicated, current collective evidence supports surgery as initial treatment in improving mortality outcomes in younger and fitter men (those with fewer comorbidities) with intermediate or high risk disease who are, at the outset, more likely to die of prostate cancer; older men and those with comorbidities are likely to fare as well, if not better, with upfront radiotherapy.
We also evaluated mortality outcomes in men with metastatic prostate cancer who underwent surgery or radiotherapy as their initial treatment option. We found no statistically significant differences between the two treatment groups, regardless of age or Charlson comorbidity index subgrouping in our main analyses. Our inverse probability of treatment weight analysis compared two hypothetical groups of surgical and radiotherapy patients, both cohorts having characteristics with similar distributions as those of the combined group of all included surgical and radiotherapy patients. Hence, this analysis allows a comparison of the situation where all patients received surgery with the situation where all patients received radiotherapy. In this supplementary analysis, radiotherapy was associated with significantly decreased prostate cancer related mortality (hazard ratio 0.54, 95% confidence interval 0.39 to 0.74). Importantly, although this group contained the smallest sample sizes, especially after substratification, we have no knowledge of secondary or tertiary treatments that may have affected outcome in these patients. Plus, this grouping was heterogeneous, including men with limited nodal disease on the one hand and multimetastatic disease on the other. It is therefore hypothesis generating rather than hypothesis testing. Another analysis using the national prostate cancer registry showed that treatment with curative intent decreased cancer specific mortality noticeably compared with palliative only treatment in men with presenting prostate specific antigen concentrations of 51-100 ng/mL (hazard ratio 0.22).25 Hence, although radical treatment might have a role in this patient population, our data do not favour one primary treatment over the other.
Our study showed that the majority of men with low risk prostate cancer did not die of prostate cancer within 15 years of follow-up. The recent PIVOT study, which randomly assigned 731 men with localised prostate cancer to radical prostatectomy or to observation found no benefit for surgery in men with low risk disease at a median follow-up of 10 years.4 The SPCG-4 trial showed that the benefit of surgery over watchful waiting continued to be seen beyond nine years, with the recent update (median 12.8 years of follow-up) showing larger improvements in survival associated with surgery.3 Benefit was seen in those with low risk disease as well, although it was greater in those with higher risk and was confined to men aged less than 65 years. Cumulatively, these two randomised controlled trials suggest that radical treatment is more beneficial in younger men and in those with intermediate and high risk tumours, since these men are at the highest risk of dying from prostate cancer. Competing risks models based on the SPCG-4 trial suggested that surgery was unequivocally of benefit over watchful waiting in men with Gleason 8 or Gleason 7, T2 disease.26 These findings are consistent with our analyses showing more deaths from prostate cancer in those with intermediate and high risk disease (fig 1); in such men with longer life expectancies at the outset (younger men and those with fewer comorbidities) the differential benefit for surgery over radiotherapy might also be greater (fig 2). However, it can also be seen that the absolute numbers of men dying from prostate cancer is still substantial in the low risk cohort, suggesting that treatment may still be warranted, at least for some (table 3). Our study also shows, as in another recent study,27 that to show differential outcomes from prostate cancer mortality, follow-up of at least 10 years (and even longer in low risk cases) is required.
Strengths and limitations of this study
Our study has several strengths; it is based on a large, population based dataset obtained by linking nine national registries in Sweden, and provides more than 98% complete data collection of prostate cancer cases at the time of diagnosis and during follow-up, with their important and highly validated patient-tumour covariates including comorbidity status. The reliability of the Swedish cause of death register for correct assignment of cause of death among patients with prostate cancer has also been shown to be high.28 Follow-up in this study was also long, and thus differences in mortality beyond 10 years since diagnosis and treatment were captured. Furthermore, we utilised well described statistical methodology to adjust for differences in the distribution of covariates between surgical and radiotherapy cohorts and to account for competing risks of mortality. Both propensity score and traditional adjustments led to similar results (table 3), further confirmed by propensity score matching (table 4) and an inverse probability of treatment weight sensitivity analysis (see appendix 2 on bmj.com); none the less, even sophisticated statistical techniques cannot completely eliminate the biases associated with observational studies, such as confounding by indication,6 29 and our finding of differences in non-prostate cancer mortality between treatments after adjustment might suggest this, especially as the radiotherapy group had poorer prognosis at baseline. Although there is evidence that radiotherapy for prostate cancer increases the incidence of secondary cancers in the longer term,30 31 and that the accompanying androgen deprivation treatment increases the risk of diabetes and cardiovascular disease,32 a recent meta-analysis evaluating 4141 patients from eight randomised trials found that cardiovascular death rates were not significantly different between the androgen deprivation treatment and control groups33; hence, residual confounding is likely to be at least contributory to our main study findings, as it was in the National Prostate Cancer Registry Follow-up Study that used the same primary dataset.21 Hence, we performed a sensitivity analysis on the non-metastatic groups where differences in outcomes were seen, and showed that the effect of any unmeasured confounder would have to be large to infinite to account for our observed findings (table 5), making it highly unlikely that our results are completely due to residual confounding. As our study started before the Gleason system was fully introduced in Sweden, some of the earlier cases were graded according to the WHO system and were not regraded for this study; also, no information was available on tumour extent in core biopsy specimens, serum prostate specific antigen levels after the date of diagnosis, actual treatment received, or secondary or later treatments. Information on radiotherapy dosing or type, or surgical technique was not available and thus we do not know how comparable these evaluated treatments are to contemporary practice, especially with the advent of intensity modulated radiotherapy, dose escalation programmes, high dose brachytherapy, and robotic assisted radical prostatectomy. For instance, it is not known whether radiotherapy dosing levels over this period in Sweden reached the acceptable contemporary standard of 74 Gy or more for low risk disease and 78 Gy for intermediate and high risk cases.34 This might have affected the results, and is likely to be a much more important confounder than advances in surgical practice over the years captured in this study. However, we did a further sensitivity analysis evaluating comparisons stratified by year of diagnosis (see appendix 1 on bmj.com) and found no temporal trend in results, suggesting that a presumed increase in radiotherapy dosing with time did not significantly confound our findings. No information is also available from PCBaSe as to whether secondary treatments were applied, and it might be that a greater proportion of men in the surgery arm underwent multimodality therapy than in the radiotherapy arm, because giving radiotherapy after surgery is more often done than performing surgery after radiotherapy. This would again bias our findings in favour of surgery. Also, the Swedish data overwhelmingly constitute white men and represent a non-screen diagnosed population; hence, how the findings relate to screen diagnosed men of various ethnicities in other countries is uncertain. Finally, treatment choices for men with prostate cancer and their doctors must take account of toxicity profiles as well as cancer outcomes, and such data were not available in this study.
Conclusions and policy implications
Notwithstanding these limitations, and in the absence of prospective, randomised controlled trials, our study suggests that surgery might result in improved outcomes compared with radiotherapy in terms of survival for men with non-metastatic prostate cancer, and that radiotherapy seems at least equivalent, and may be superior, to surgery for men with metastatic disease. Given the important burden that prostate cancer poses on the National Health Service and healthcare systems worldwide, these findings could have important policy implications for the allocation of resources in the management of this disease.
What is already known on this topic
High quality evidence comparing cancer outcomes after surgery and radiotherapy in men with prostate cancer is lacking
Many observational datasets do not have accurate and complete data recording with sufficient follow-up making these comparative effectiveness studies subject to bias
What this study adds
The majority of men with clinically localised prostate cancer might benefit more from surgery than radiotherapy, whereas radiotherapy might be preferable in men with metastatic disease
Younger men and those with fewer comorbidities who have intermediate or high risk localised prostate cancer might have a greater benefit from surgery
This project was made possible by the continuous work of the National Prostate Cancer Registry of Sweden steering group: Pär Stattin (chairman), Anders Widmark, Camilla Thellenberg, Ove Andrén, Anna Bill-Axelsson, Ann-Sofi Fransson, Magnus Törnblom, Stefan Carlsson, Marie Hjälm-Eriksson, Bodil Westman, Bill Pettersson, David Robinson, Mats Andén, Jan-Erik Damber, Jonas Hugosson, Maria Nyberg, Göran Ahlgren, Ola Bratt, René Blom, Lars Egevad, Calle Walller, Olof Akre, Per Fransson, Eva Johansson, Fredrik Sandin, Hans Garmo, Mats Lambe, Karin Hellström, Annette Wigertz, and Erik Holmberg. PS is part funded by the National Institute for Health Research Oxford Biomedical Research Centre based at Oxford University Hospitals NHS Trust and the University of Oxford. The views expressed are those of the author and not necessarily those of the NHS, the National Institute for Health Research, or the Department of Health. PS was also a European urology scholarship fund fellow during part of the time this research was performed. OA is supported by a grant from the Swedish Cancer Society. PW is supported by a grant from the Swedish Research Council (K2013-99X-22283-01-3).
Contributors: PS was involved at every stage from the literature search, planning and design of the study, data abstraction, data analysis, data interpretation, and writing. TN was involved with data abstraction and data analysis. OA was involved with data interpretation and writing. LH was involved with the study plan and design and data abstraction. IH was involved with data abstraction and data analysis. MO was involved with study plan and design. SC was involved with the study plan and design. MR supervised data abstraction and data analysis, and was involved with data interpretation. GS was involved with data interpretation and editing the manuscript for important intellectual content. PW was involved at every stage but especially with data interpretation and editing the manuscript for important intellectual content. He is guarantor. All authors had full access to the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: This study received no funding. The PCBaSe database, however, is funded by the Swedish Research Council (25-2012-5047).
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare that: none of the authors have support for the submitted work; none of the authors have relationships with any companies that might have an interest in the submitted work in the previous three years; none of the authors’ spouses, partners, or children have any financial relationships that may be relevant to the submitted work; and none of the authors have non-financial interests that may be relevant to the submitted work.
Ethical approval: This study was approved by the central research ethics committee and the regional ethical review board in Stockholm.
Data sharing: No additional data available.
Transparency: The senior author (PW) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; no important aspects of the study have been omitted; and there were no discrepancies from the study as planned.
Cite this as: BMJ 2014;348:g1502
Web Extra. Extra material supplied by the author
Appendix 1: comparative oncological effectiveness for radiotherapy versus radical prostatectomy stratified by risk group and period of treatment
Appendix 2: comparative oncological effectiveness for radiotherapy versus radical prostatectomy stratified by risk group and also using inverse probability of treatment weights
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [DOI] [PubMed] [Google Scholar]
- 2.Makarov DV, Trock BJ, Humphreys EB, Mangold LA, Walsh PC, Epstein JI, et al. Updated nomogram to predict pathologic stage of prostate cancer given prostate-specific antigen level, clinical stage, and biopsy Gleason score (Partin tables) based on cases from 2000 to 2005. Urology 2007;69:1095-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bill-Axelson A, Holmberg L, Ruutu M, Garmo H, Stark JR, Busch C, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med 2011;364:1708-17. [DOI] [PubMed] [Google Scholar]
- 4.Wilt TJ, Brawer MK, Jones KM, Barry MJ, Aronson WJ, Fox S, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med 2012;367:203-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lane JA, Hamdy FC, Martin RM, Turner EL, Neal DE, Donovan JL. Latest results from the UK trials evaluating prostate cancer screening and treatment: the CAP and ProtecT studies. Eur J Cancer 2010;46:3095-101. [DOI] [PubMed] [Google Scholar]
- 6.Hadley J, Yabroff KR, Barrett MJ, Penson DF, Saigal CS, Potosky AL. Comparative effectiveness of prostate cancer treatments: evaluating statistical adjustments for confounding in observational data. J Natl Cancer Inst 2010;102:1780-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grimm P, Billiet I, Bostwick D, Dicker AP, Frank S, Immerzeel J, et al. Comparative analysis of prostate-specific antigen free survival outcomes for patients with low, intermediate and high risk prostate cancer treatment by radical therapy. Results from the Prostate Cancer Results Study Group. BJU Int 2012;109(Suppl 1):22-9. [DOI] [PubMed] [Google Scholar]
- 8.D’Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 1998;280:969-74. [DOI] [PubMed] [Google Scholar]
- 9.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA 1999;281:1591-7. [DOI] [PubMed] [Google Scholar]
- 10.Fouad MN, Mayo CP, Funkhouser EM, Irene Hall H, Urban DA, Kiefe CI. Comorbidity independently predicted death in older prostate cancer patients, more of whom died with than from their disease. J Clin Epidemiol 2004;57:721-9. [DOI] [PubMed] [Google Scholar]
- 11.Hagel E, Garmo H, Bill-Axelson A, Bratt O, Johansson JE, Adolfsson J, et al. PCBaSe Sweden: a register-based resource for prostate cancer research. Scand J Urol Nephrol 2009;43:342-9. [DOI] [PubMed] [Google Scholar]
- 12.Van Hemelrijck M, Wigertz A, Sandin F, Garmo H, Hellstrom K, Fransson P, et al. Cohort Profile: The National Prostate Cancer Register of Sweden and Prostate Cancer data Base Sweden 2.0. Int J Epidemiol 2013;42;956-67. [DOI] [PubMed]
- 13.Berglund A, Garmo H, Tishelman C, Holmberg L, Stattin P, Lambe M. Comorbidity, treatment and mortality: a population based cohort study of prostate cancer in PCBaSe Sweden. J Urol 2011;185:833-9. [DOI] [PubMed] [Google Scholar]
- 14.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Ass 1999;94:496-509. [Google Scholar]
- 15.Lin DY, Psaty BM, Kronmal RA. Assessing the sensitivity of regression results to unmeasured confounders in observational studies. Biometrics 1998;54:948-63. [PubMed] [Google Scholar]
- 16.Abdollah F, Schmitges J, Sun M, Jeldres C, Tian Z, Briganti A, et al. Comparison of mortality outcomes after radical prostatectomy versus radiotherapy in patients with localized prostate cancer: a population-based analysis. Int J Urol 2012;19:836-44; author reply 844-5. [DOI] [PubMed] [Google Scholar]
- 17.Zelefsky MJ, Eastham JA, Cronin AM, Fuks Z, Zhang Z, Yamada Y, et al. Metastasis after radical prostatectomy or external beam radiotherapy for patients with clinically localized prostate cancer: a comparison of clinical cohorts adjusted for case mix. J Clin Oncol 2010;28:1508-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trinh QD, Sammon J, Sun M, Ravi P, Ghani KR, Bianchi M, et al. Perioperative outcomes of robot-assisted radical prostatectomy compared with open radical prostatectomy: results from the nationwide inpatient sample. Eur Urol 2012;61:679-85. [DOI] [PubMed] [Google Scholar]
- 19.Abdollah F, Sun M, Thuret R, Jeldres C, Tian Z, Briganti A, et al. A competing-risks analysis of survival after alternative treatment modalities for prostate cancer patients: 1988-2006. Eur Urol 2011;59:88-95. [DOI] [PubMed] [Google Scholar]
- 20.Kibel AS, Ciezki JP, Klein EA, Reddy CA, Lubahn JD, Haslag-Minoff J, et al. Survival among men with clinically localized prostate cancer treated with radical prostatectomy or radiation therapy in the prostate specific antigen era. J Urol 2012;187:1259-65. [DOI] [PubMed] [Google Scholar]
- 21.Stattin P, Holmberg E, Johansson JE, Holmberg L, Adolfsson J, Hugosson J. Outcomes in localized prostate cancer: National Prostate Cancer Register of Sweden follow-up study. J Natl Cancer Inst 2010;102:950-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooperberg MR, Vickers AJ, Broering JM, Carroll PR. Comparative risk-adjusted mortality outcomes after primary surgery, radiotherapy, or androgen-deprivation therapy for localized prostate cancer. Cancer 2010;116:5226-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nepple KG, Stephenson AJ, Kallogjeri D, Michalski J, Grubb RL 3rd, Strope SA, et al. Mortality after prostate cancer treatment with radical prostatectomy, external-beam radiation therapy, or brachytherapy in men without comorbidity. Eur Urol 2013;64:372-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boorjian SA, Karnes RJ, Viterbo R, Rangel LJ, Bergstralh EJ, Horwitz EM, et al. Long-term survival after radical prostatectomy versus external-beam radiotherapy for patients with high-risk prostate cancer. Cancer 2011;117:2883-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ladjevardi S, Berglund A, Varenhorst E, Bratt O, Widmark A, Sandblom G. Treatment with curative intent and survival in men with high-risk prostate cancer. A population-based study of 11 380 men with serum PSA level 20-100 ng/mL. BJU Int 2013;111:381-8. [DOI] [PubMed] [Google Scholar]
- 26.Vickers A, Bennette C, Steineck G, Adami HO, Johansson JE, Bill-Axelson A, et al. Individualized estimation of the benefit of radical prostatectomy from the Scandinavian Prostate Cancer Group randomized trial. Eur Urol 2012;62:204-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boorjian SA, Eastham JA, Graefen M, Guillonneau B, Karnes RJ, Moul JW, et al. A critical analysis of the long-term impact of radical prostatectomy on cancer control and function outcomes. Eur Urol 2012;61:664-75. [DOI] [PubMed] [Google Scholar]
- 28.Fall K, Stromberg F, Rosell J, Andren O, Varenhorst E. Reliability of death certificates in prostate cancer patients. Scand J Urol Nephrol 2008;42:352-7. [DOI] [PubMed] [Google Scholar]
- 29.Vickers AJ. Re: comparative effectiveness of prostate cancer treatments: evaluating statistical adjustments for confounding in observational data. J Natl Cancer Inst 2011;103:1134; author reply 1134-5. [DOI] [PubMed] [Google Scholar]
- 30.Okajima K, Ishikawa K, Matsuura T, Tatebe H, Fujiwara K, Hiroi K, et al. Multiple primary malignancies in patients with prostate cancer: increased risk of secondary malignancies after radiotherapy. Int J Clin Oncol 2013;18:1078-84. [DOI] [PubMed] [Google Scholar]
- 31.Bostrom PJ, Soloway MS. Secondary cancer after radiotherapy for prostate cancer: should we be more aware of the risk? Eur Urol 2007;52:973-82. [DOI] [PubMed] [Google Scholar]
- 32.Keating NL, O’Malley AJ, Freedland SJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J Natl Cancer Inst 2010;102:39-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen PL, Je Y, Schutz FA, Hoffman KE, Hu JC, Parekh A, et al. Association of androgen deprivation therapy with cardiovascular death in patients with prostate cancer: a meta-analysis of randomized trials. JAMA 2011;306:2359-66. [DOI] [PubMed] [Google Scholar]
- 34.Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol 2014;65:124-37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1: comparative oncological effectiveness for radiotherapy versus radical prostatectomy stratified by risk group and period of treatment
Appendix 2: comparative oncological effectiveness for radiotherapy versus radical prostatectomy stratified by risk group and also using inverse probability of treatment weights