Abstract
Introduction
To date, only few studies focusing on the issue of host general and immune activity have been performed in localized prostate cancer (PCa). The aim of this study was to elucidate potent non tumor–related biomarkers that express aggressiveness of PCa treated by radical prostatectomy (RP).
Materials and methods
Data from 179 patients who underwent RP were analyzed. The correlations between various kinds of non tumor–related factors in addition to tumor–related factors and biochemical recurrence (BCR) were analyzed. The correlations between pre–, intra– and post–operative factors were also analyzed.
Results
Thirty–two cases (17.9%) had a BCR. The factors found to be significantly predictive of BCR using a Cox–proportional hazard model were the pre–operative serum prostate specific antigen (PSA) level and the existence of pathological lymph node metastasis (LNM). A low pre–operative serum albumin level (<4.0 g/dl) was significantly correlated with BCR univariately. Logistic regression analysis revealed that a low pre–operative serum albumin level, an American Society of Anesthesiologists (ASA) score above class 2, and a Gleason score above 8 in the biopsy specimens were significantly predictive of pathological LNM.
Conclusions
Tumor–related characteristics are more important for predicting BCR. However, our results suggest that low pre–operative serum albumin level may indicate extensive disease of clinically localized PCa and may ultimately be correlated with BCR. Although multiple reasons may account for the significance of the serum albumin level, it is noteworthy that delayed diagnostic and therapeutic procedures in comorbid patients with low serum albumin levels may lead to PCa progression.
Keywords: prostatic cancer, radical prostatectomy, prostate–specific antigen, recurrence, serum albumin
INTRODUCTION
Biochemical recurrence (BCR) in prostate cancer (PCa) has a unique definition in the field of solid malignancies. This definition has been achieved because of the discoveries related to the ultra–specific tumor marker, prostate–specific antigen (PSA). The BCR–free rate is often considered to be the primary endpoint for the oncological outcomes of a radical prostatectomy (RP); this endpoint is based on the low incidence of PCa–related mortality and long post–BCR survival that have been reported in studies of PCa progression after various types of treatment modalities after BCR [1, 2, 3]. Since the era of PSA, the analysis of factors influencing BCR after RP has been a major area for clinical research in the PCa field. To date, seminal vesicle involvement, lymph node metastasis (LNM), and extra–capsular extension of cancer are well–established predictors of BCR, all of which are obtained by detailed investigations of pathological findings in surgical specimens [4, 5]. Moreover, many physicians can estimate the BCR–free rate in a patient who underwent RP using various established nomograms. Kattan’s post–operative nomogram is one of the best predictive tools of BCR, which uses the pre–operative serum PSA levels in addition to the pathological findings in post–surgical specimens [6]. Although detailed investigations of the tumor–related factors that influence BCR have been performed, the utility of other non–tumor–related factors, such as the general characteristics of the patients and the host immune activity, are unknown.
Cancer progression is determined by a balance between cancer cell proliferation and cancer cell death induced by the host immune reaction, which is thought to be complexly related to peripheral blood examination results and the general characteristics of the patients. Tumor progression due to the deficiency of host’s immune activity and general characteristics should not be ignored, even though the patients with localized PCa treated by RP are relatively older (approximately of 60 to 75 years of age). Patients in this age range often have other complicating medical problems and diseases. To comprehensively characterize the various factors that influence BCR, we investigated the correlation of BCR with both tumor–related factors and the general and immune characteristics of patients who underwent RP, as obtained from demographic information and peripheral blood examinations. In addition, the correlations between pre– and intra–operative factors and post–operative pathological factors were also analyzed.
MATERIALS AND METHODS
Patients
Between 2002 and 2011, 242 patients with localized PCa underwent RP at our institution or one of our affiliated hospitals. Sixty–three patients received endocrine therapy before surgery. These patients were excluded from the study because of the difficulties in interpreting the pathological findings of radical prostatectomy specimens that were subjected to androgen deprivation. Therefore, 179 patients were eligible for the study. The tumor–related patients’ characteristics are summarized according to the International Union against Cancer standards (sixth edition). The demographic and non–tumor–related patients’ characteristics are summarized in Table 1. The surgery types were as follows: open radical prostatectomy in 129 patients, minimum incision endoscopic radical prostatectomy in 26 patients, and robot–assisted radical prostatectomy in 24 patients (Table 1).
Table 1.
Demographic and non-tumor-related patients’ characteristics
| Characteristics | Values or number of cases |
|---|---|
| Total number of cases | 179 |
| Mean age at surgery (years), range | 66.2, 48–81 |
| Pre-operative mean BMI (kg/m2) (SD) | 23.4 (3.7) |
| Pre-operative mean serum albumin (g/dl) | 4.3 (0.3) |
| ASA risk classification system at surgery | |
| Class 1 / Class 2 | 107 (59.8%) / 66 (36.9%) |
| / Class 3 | / 6 (3.3%) |
| Surgery type | |
| Open / Minimum incision endoscopic | 129 (72.1%) / 26 (14.5%) |
| / Robot-assisted | / 24 (13.4%) |
| Blood loss at surgery (ml) | |
| < 1000 / 1000–2000 | 115 (64.2%) / 49 (27.4%) |
| / >3000 | / 15 (8.4%) |
BMI – body mass index; SD – standard deviation; ASA – American Society of Anesthesiologists
Data collection
The demographic and clinical non–tumor–related factors analyzed were age, body mass index (BMI), surgery type, amount of blood loss at surgery, complicating diseases, and pre–operative serum albumin level, which is thought to be related to immune activity. Complicating diseases were categorized using the American Society of Anesthesiologists (ASA) risk classification system. The tumor–related factors analyzed were pre–operative serum PSA level, clinical stage, and the tumor pathological findings in biopsy and surgical specimens. All factors were also categorized as pre–, intra–, and post–operative factors. The pre–operative non–tumor–related factors were age, BMI, complicating diseases assessed by the ASA score, and serum albumin level, all of which were collected at a pre–operative visit by the anesthesiologists. The pre–operative tumor–related factors were serum PSA level, clinical stage, and pathological findings of biopsy specimens. Intra– and post–operative factors were surgery type, the amount of blood loss at surgery, and pathological findings of the surgical specimens.
PSA monitoring
PSA monitoring was performed in all patients until their death or last follow–up visit. Initial PSA monitoring was conducted by at the discretion of the attending physician; however, the follow–up PSA monitoring policy was set by consensus of the out–patients clinic physicians. Namely, PSA monitoring was to be performed every three months for two years after surgery and every six months thereafter for at least five years after surgery. None of the patients were lost to follow–up for unexpected reasons. BCR was defined as when the serum PSA level exceeded 0.2 ng/ml or when a radical prostatectomy was carried out and the PSA did not decrease below 0.2 ng/ml after surgery.
Statistical analysis
The BCR–free rate was determined by the Kaplan–Meier method, and comparisons of the rate among groups were analyzed by the log–rank test. Univariate and multivariate analyses of the significance of the demographic and clinico–pathological parameters associated with BCR were assessed by a Cox proportional hazards regression model. Variables in univariate analysis with P <0.05 were included in the multivariate analysis. The associations between pre– and intra–operative factors and post–operative factors were analyzed using a logistic regression model. P values <0.05 were considered to be statistically significant.
RESULTS
Tumor–related patients’ characteristics
The mean (SD) pre–operative PSA was 8.3 (5.3) ng/ml. The number of cases with a Gleason score of 2–6 / 7 / 8–10 in biopsy and surgical specimens was 96 (53.6%) / 52 (29.1%) / 31 (17.3%) and 85 (47.5%) / 62 (34.6%) / 32 (17.9%), respectively. The number of cases with pathological T2a / T2b / T2c / T3a / T3b were 43 (24.0%) / 2 (1.1%) / 78 (43.6%) / 47 (26.3%) / 9 (5.0%), respectively. A positive surgical margin and LNM were detected in 42 (23.5%) and 9 (5.0%) cases, respectively.
The analyses of tumor–related factors influencing BCR
The mean follow–up duration was 25.2 months (maximum 94). Thirty–two cases (17.9%) exhibited BCR, and the 5–year BCR–free rate for all patients was 67.1%. High pre–operative serum PSA levels (>10 ng/ml), high Gleason scores (≥8) in surgical specimen, positive surgical margin, extra–capsular extension, seminal vesicle involvement, peri–neural invasion, lympho–vascular invasion, and LNM were associated with BCR univariately (Table 2). In the multivariate analysis, high pre–operative PSA levels (>10 ng/mL), and LNM were significant predictors of BCR (Table 2).
Table 2.
Univariate and multivariate analyses of parameters predicting BCR in all cases (n = 179)
| Variables | Univariate P Value | Multivariate | |||
|---|---|---|---|---|---|
|
|
|||||
| Coefficient | Hazards Ratio (95% CI) | P value | |||
| *Pre-operative serum PSA (ng/ml): | |||||
| <10 | Reference | Reference | |||
| ≥10 | 0.0004 | 1.381 | 3.979 (1.873–8.451) | 0.0003 | |
| *Pre-operative serum albumin (g/dl): | |||||
| >4 | Reference | Reference | |||
| ≤4 | 0.0407 | 0.495 | 1.641 (0.707–3.804) | 0.2486 | |
| *Pathological Gleason Score: | ≤7 | Reference | Reference | ||
| ≥8 | 0.0281 | -0.187 | 0.829 (0.291–2.359) | 0.7256 | |
| *Positive surgical margin: | No | Reference | Reference | ||
| Yes | 0.0252 | 0.479 | 1.615 (0.65–4.015) | 0.3023 | |
| *Extra-capsular extension: | No | Reference | Reference | ||
| Yes | 0.0015 | 0.679 | 2.008 (0.862–4.68) | 0.1062 | |
| *Seminal vesicle involvement: | No | Reference | Reference | ||
| Yes | 0.0302 | -1.035 | 0.355 (0.076–1.649) | 0.1863 | |
| *Peri-neural invasion: | No | Reference | Reference | ||
| Yes | 0.0198 | -0.077 | 4.786 (1.37–16.72) | 0.8618 | |
| *Lympho-vascular invasion: | No | Reference | Reference | ||
| Yes | 0.006 | 0.518 | 1.679 (0.705–3.997) | 0.2416 | |
| *Lymph node metastasis: | No | Reference | Reference | ||
| Yes | <0.0001 | 1.566 | 4.786 (1.37–16.72) | 0.0141 | |
BMI, body mass index; SD, standard deviation; ASA, American Society of Anesthesiologists
The analyses of non–tumor–related factors influencing BCR
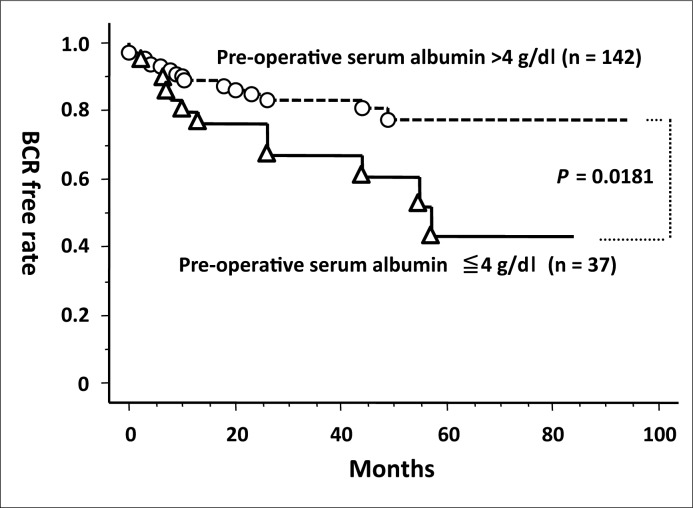
When the cutoff value for pre–operative serum albumin was defined as 4 g/dl, the BCR–free rate in the low–level group was significantly worse than that in high–level group (Figure 1). Univariate analysis by Cox proportional hazards regression model also demonstrated the significance of low–level of serum albumin associated with BCR (Table 2). None of the other non–tumor–related factors significantly influenced BCR.
Figure 1.
Comparison of the biochemical recurrence (BCR)–free rate between two groups according to their pre–operative serum albumin levels.
The analyses of potent pre– and intra–operative factors predictive of post–operative factors
For each post–operative factor, the associations with all pre– and intra–operative factors were analyzed using a logistic regression model. In case of a low R–squared value, the data were not used because they did not fit the logistic regression model. Only LNM was predictive of the pre– and intra–operative factors. Namely, a high ASA score (class 2 or 3 vs. 1), low pre–operative serum albumin levels (≤4 vs. >4 g/dl), and high Gleason scores (≥8 vs. ≤6) in the biopsy specimens were the significantly predictive for LNM (Table 3). In this analysis, the intra–operative factor of surgery type was excluded because there was no case that showed LNM in minimum incision endoscopic radical prostatectomy and robot–assisted radical prostatectomy. In the sub–group analysis of the characteristics of patients with a high ASA score (class 2 and 3), there was a significantly positive correlation of high ASA score with low serum albumin levels and high serum pre–operative PSA levels. Namely, 23 out of 72 patients with ASA class 2 and 3 (31.9%) had low pre–operative serum albumin levels (≤4 g/dl), whereas 19 out of 107 patients of ASA class 1 (17.8%) had low pre–operative serum albumin levels (P = 0.0293). The mean (SD) serum pre–operative PSA level in ASA class 2 and 3 patients was 10.3 (4.2), whereas it was 7.1 (5.9) ng/ml in ASA class 1 patients (P = 0.0022).
Table 3.
The analysis of potent pre- and intra-operative factors predictive of LNM
| Pre-operative variables | Odds ratio (95% CI) | P value |
|---|---|---|
| *Age | 1.016 (0.874–1.182) | 0.8346 |
| *BMI | 0.849 (0.637–1.132) | 0.2656 |
| *PSA | 1.013 (0.851–1.204) | 0.8875 |
| *ASA risk classification | ||
| (class 2, 3 vs. 1) | 11.311 (1.067–119.910) | 0.0440 |
| *Serum albumin | ||
| (≤4 vs. >4 g/dl) | 12.295 (1.633–92.545) | 0.0148 |
| *Biopsy Gleason Score | ||
| (7 vs. ≤6) | 1.977 (0.083–47.378) | 0.6740 |
| (≥8 vs. ≤6) | 48.585 (3.070–768.999) | 0.0058 |
| *Clinical T stage | ||
| (cT1, 2ab vs. cT2c, 3) | 0.628 (0.069–5.684) | 0.6791 |
| *Blood loss at surgery | 1.001 (0.999–1.002) | 0.4466 |
R2 = 0.47
LNM – lymph node metastasis; PSA – prostate specific antigen; BMI – body mass index; ASA – American Society of Anesthesiologists
DISCUSSION
Early PCa detection efforts have been substantially improved with the introduction of PSA. Patients’ characteristics treated by RP have shifted toward earlier stages and more localized disease. Additionally, the retropubic RP has been substantially refined in the past two decades, transforming it into a highly reliable, oncological safe, and anatomically accurate procedure [7, 8, 9]. Such trends and progress in RP provide patients with localized PCa notably high probabilities of a cancer–free survival. Moreover, the progress of other therapeutic modalities, such as radiation, endocrine therapy and chemotherapy, also provide patients after BCR a long survival time. Therefore, the oncological focus of surgeons is characterizing the factors that influence BCR. A recent review article [10] on predicting for survival and BCR in a large number of patients treated with RP demonstrated that the post–operative outcome could be predicted by PSA and some pathological factors, such as T stage, Gleason score, and positive surgical margin [11, 12 13]. In addition, extra–capsular extension, seminal vesicle involvement, and LNM were demonstrated as significant predictors for BCR [12, 13]. We have demonstrated the significance of the following predictors for BCR: 1) high pre–operative serum PSA levels (>10 ng/ml), high Gleason scores (≥8) in surgical specimens, positive surgical margin, extra–capsular extension, seminal vesicle involvement, peri–neural invasion, lympho–vascular invasion, and LNM in the univariate analysis and 2) high pre–operative PSA levels (>10 ng/mL) and LNM in the multivariate analysis. These results are generally in accord with the previous reports mentioned above. The strong impact of LNM on BCR, which is defined as the systemic expansion of PCa in our study, is in accord with previous studies that have demonstrated the order of prognostic impact to be LNM > seminal vesicle involvement > extra–capsular extension [4, 5].
To our knowledge, this is the first report demonstrating the significance of serum albumin level as a potential predictor for BCR. Albumin is frequently part of a standard preoperative evaluation and has also been shown important in predicting survival in various types of cancers, including urological malignancies, such as bladder carcinoma [14], renal cell carcinoma [15], and hormone refractory PCa [16]. Most studies cited that poor nutritional status of the host was reflected by low levels of serum albumin and consequently led to short survival. Other than nutritional status, inflammatory mediators, which are produced by tumor progression and host immune reaction to malignancies, lower serum albumin levels in patients with malignancies. First, inflammatory mediators increase the transcapillary escape rate of albumin such that albumin is lost into tissue spaces [17]. Second, inflammatory mediators, such as IL–6, TNF, and acute phase reactants, lead to decreased production of albumin [18]. Hypoalbuminemia is a poor prognostic indicator because it reflects malnutrition and existence of inflammation in the host, both of which are correlated with advanced stages of cancer.
Because of effective screening with PSA, PCa is detected at an earlier stage, and the deleterious effects of the disease are not as frequent in PCa as in other cancers with less effective screening methods, and metastases and resulting cachexia observed in these primary cancers may alter albumin levels. Moreover, candidates selected for RP as their treatment modality are generally healthy and without significant co–morbid diseases because other therapeutic modalities, such as radiation and endocrine therapies, preclude patients with extensive PCa, serious morbidities and short life expectancies. Therefore, the potential of serum albumin as a prognostic biomarker has not been identified in localized PCa treated by RP. The only report describing the value of the serum albumin level as a biomarker of PCa progression treated by RP demonstrated that it had no value as a predictive indicator for BCR [19]. This study compared BCR–free rates between two groups (≥3.8 vs. ≤3.7 g/dl). The mean value of serum albumin level was 4.3 g/dl in our study, and only ten patients (5.6%) had their serum albumin levels below 3.7 g/dl. Relatively low cut–off values in their study should be changed for the purpose of investigating if the pre–operative serum albumin level has the potential to be a predictive indicator for BCR.
PCa is known to be hormonally sensitive to testosterone. Bioactive testosterone is thought to be albumin–binding testosterone and free testosterone in circulating plasma. Free testosterone is thought to have an impact on hormonally sensitive PCa because of its active effect on the synthesis of dihydrotestosterone in prostate tissue. In normal human males, 57%, 40%, and less than 1% of testosterone in the plasma is bound to testosterone–binding globulin (TeBG), serum albumin, and corticosteroid–binding globulin, respectively, and only 2% of total testosterone is free [20]. The difference of testosterone–binding protein characteristics between TeBG and albumin is that the former is high–affinity and low capacity, whereas the latter is low–affinity and high capacity. Because a large percent of TeBG is saturated, low levels of serum albumin may decrease the level of albumin–binding testosterone and ultimately increase the level of free testosterone. Our results of pre–operative low serum albumin levels as a predictor for BCR and LNM may be related to increased free testosterone contributions. Richter et al. also demonstrated pre–operative low serum albumin levels to be a predictor for non–organ confined disease assessed by RP specimens and hypothesized the role of free testosterone [19]. The associations between pre–operative sex hormones, including testosterone and sex hormone–binding globulin, with BCR were recently demonstrated in a prospective patient cohort treated with RP [21]. The authors concluded that increased sex hormone–binding globulin was an independent predictor for BCR, whereas neither total nor free testosterone was associated with BCR. There are two conflicting reports in patient cohorts treated with therapies other than RP: one study demonstrated the positive association between high levels serum testosterone and worsening PCa disease [22, 23, 24], and the other study demonstrated the inverse association [25, 26, 27]. However, no studies have demonstrated the specific association between serum free testosterone in combination with albumin and PCa progression. It is suggested that multiple factors, including hormonal, nutrition, inflammatory, and immune–reactive aspects triggered by low levels of serum albumin, were reflected in our results.
Along with low levels of serum albumin, high Gleason scores in biopsy specimens and co–morbid diseases expressed by the ASA score were also significantly correlated with LNM. The Gleason score is known to be a predictive factor for extensive disease as assessed by pathological findings in surgical specimens; however, no previous report has demonstrated the significance of the ASA score as a predictor for the PCa disease burden. There were a significantly high percentage of patients with pre–operative low levels of serum albumin in ASA class 2 and 3 as compared with the levels in class 1, which may reflect the significance of our results. It is also suggested that significantly higher pre–operative serum PSA levels in ASA class 2 and 3 patients compared with those in class 1 patients had effects on the positive correlation between the high ASA score and LNM. The reason for higher pre–operative serum PSA levels in ASA class 2 and 3 patients was the hesitation by physicians to conduct the invasive diagnostic and therapeutic procedure of PCa; this led to an increased delay between the first detection of abnormal serum PSA levels and the initiation of therapy in these patients. However, the influence of co–morbid diseases on PCa progression remains unknown. Detailed studies focused on co–morbid diseases in a large number of patients with localized PCa are needed to discover new non–tumor–related potent biomarkers.
CONCLUSIONS
Tumor–related characteristics are more important than other factors for predicting BCR. However, our results suggest that a low pre–operative serum albumin level may indicate extensive disease of clinically localized PCa and may ultimately be correlated with BCR. Although multiple reasons, including general patient characteristics, suppressed immune activity and changes in the serum free testosterone level caused by hypoalbuminemia, may be responsible for the progression of PCa, it is noteworthy that delayed diagnostic and therapeutic procedures in comorbid patients with low serum albumin levels may lead to PCa progression. Additional investigations based on large numbers of patients are needed to fully understand the potent non–tumor–related biomarkers of clinically localized PCa.
References
- 1.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–1597. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 2.Roehl KA, Han M, Ramos CG, Antenor JA, Catalona WJ. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: long–term results. J Urol. 2004;172:910–914. doi: 10.1097/01.ju.0000134888.22332.bb. [DOI] [PubMed] [Google Scholar]
- 3.Makarov DV, Humphreys EB, Mangold LA, Carducci MA, Partin AW, Eisenberger MA, Walsh PC, Trock BJ. The natural history of men treated with deferred androgen deprivation therapy in whom metastatic prostate cancer developed following radical prostatectomy. J Urol. 2008;179:156–161. doi: 10.1016/j.juro.2007.08.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cagiannos I, Karakiewicz P, Eastham JA, Ohori M, Rabbani F, Gerigk C, et al. A preoperative nomogram identifying decreased risk of positive pelvic lymph nodes in patients with prostate cancer. J Urol. 2003;170:1798–1803. doi: 10.1097/01.ju.0000091805.98960.13. [DOI] [PubMed] [Google Scholar]
- 5.Chun FK, Graefen M, Zacharias M, Haese A, Steuber T, Schlomm T, Walz J, Karakiewicz PI, Huland H. Anatomic radical retropubic prostatectomy–long–term recurrence–free survival rates for localized prostate cancer. World J Urol. 2006;24:273–280. doi: 10.1007/s00345-006-0058-2. [DOI] [PubMed] [Google Scholar]
- 6.Kattan MW, Wheeler TM, Scardino PT. Postoperative nomogram for disease recurrence after radical prostatectomy for prostate cancer. J Clin Oncol. 1999;17:1499–1507. doi: 10.1200/JCO.1999.17.5.1499. [DOI] [PubMed] [Google Scholar]
- 7.Walsh PC, Donker PJ. Impotence following radical prostatectomy: insight into etiology and prevention. J. Urol. 2002;167:1005–1010. doi: 10.1016/s0022-5347(02)80325-1. [DOI] [PubMed] [Google Scholar]
- 8.Reiner WG, Walsh PC. An anatomical approach to the surgical management of the dorsal vein and Santorini’s plexus during radical retropubic surgery. J Urol. 1979;121:198–200. doi: 10.1016/s0022-5347(17)56718-x. [DOI] [PubMed] [Google Scholar]
- 9.Graefen M, Walz J, Huland H. Open retropubic nerve–sparing radical prostatectomy. Eur Urol. 2006;49:38–48. doi: 10.1016/j.eururo.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Lughezzani G, Briganti A, Karakiewicz PI, Kattan MW, Montorsi F, Shariat SF, Vickers AJ. Predictive and prognostic models in radical prostatectomy candidates: a critical analysis of the literature. Eur Urol. 2010;58:687–700. doi: 10.1016/j.eururo.2010.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Amico AV, Whittington R, Malkowicz SB, Fondurulia J, Chen MH, Tomaszewski JE, Wein A. The combination of preoperative prostate specific antigen and postoperative pathological findings to predict prostate specific antigen outcome in clinically localized prostate cancer. J Urol. 1998;160:2096–2101. doi: 10.1097/00005392-199812010-00041. [DOI] [PubMed] [Google Scholar]
- 12.Walz J, Chun FK, Klein EA, Rether A, Saad F, Graefen M, et al. Nomogram predicting the probability of early recurrence after radical prostatectomy for prostate cancer. J Urol. 2009;181:601–607h. doi: 10.1016/j.juro.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 13.Stephenson AJ, Scardino PT, Eastham JA, Bianco FJ, Jr, Dotan ZA, DiBlasio Ch, Reuther A, Klein AE, Katton MW. Postoperative nomogram predicting the 10–year probability of prostate cancer recurrence after radical prostatectomy. J Clin Oncol. 2005;23:7005–7012. doi: 10.1200/JCO.2005.01.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hannisdal E, Fosså SD, Høst H. Blood tests and prognosis in bladder carcinomas treated with definitive radiotherapy. Radiother Oncol. 1993;27:117–122. doi: 10.1016/0167-8140(93)90131-q. [DOI] [PubMed] [Google Scholar]
- 15.Citterio G, Bertuzzi A, Tresoldi M, Galli L, Lucca G, Scaglietti U, Rugarli C. Prognostic factors for survival in metastatic renal cell carcinoma: retrospective analysis from 109 consecutive patients. Eur Urol. 1997;31:286–291. doi: 10.1159/000474469. [DOI] [PubMed] [Google Scholar]
- 16.Berry WR, Laszlo J, Cox E, Walker A, Paulson D. Prognostic factors in metastatic and hormonally unresponsive carcinoma of the prostate. Cancer. 1979;44:763–775. doi: 10.1002/1097-0142(197908)44:2<763::aid-cncr2820440251>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 17.Fleck A, Raines G, Hawker F, Trotter J, Wallace PI, Ledingham IM, Calman KC. Increased vascular permeability: a major cause of hypoalbuminaemia in disease and injury. Lancet. 1985;325:781–784. doi: 10.1016/s0140-6736(85)91447-3. [DOI] [PubMed] [Google Scholar]
- 18.Deehan DJ, Heys SD, Simpson W, Herriot R, Broom J, Eremin O. Correlation of serum cytokine and acute phase reactant levels with alterations in weight and serum albumin in patients receiving immunotherapy with recombinant IL–2. Clin Exp Immunol. 1994;95:366–372. doi: 10.1111/j.1365-2249.1994.tb07005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richter E, Connelly RR, Moul JW. The role of pretreatment serum albumin to predict pathological stage and recurrence among radical prostatectomy cases. Prostate Cancer Prostatic Dis. 2000;3:186–190. doi: 10.1038/sj.pcan.4500418. [DOI] [PubMed] [Google Scholar]
- 20.Vermeulen A. The physical state of testosterone in plasm. In: James VHT, Serio M, Maratini L, editors. The Endocrine Function of the Human Testis. New York: Academic Press; 1973. pp. 157–170. [Google Scholar]
- 21.Waldert M, Schatzl G, Swietek N, Rom M, Klatte T. Sex hormone–binding globulin is an independent predictor of biochemical recurrence after radical prostatectomy. J Urol. 2012;188:792–797. doi: 10.1016/j.juro.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 22.Zagars GK, Pollack A, von Eschenbach AC. Serum testosterone – a significant determinant of metastatic relapse for irradiated localized prostate cancer. Urology. 1997;49:327–334. doi: 10.1016/S0090-4295(96)00619-X. [DOI] [PubMed] [Google Scholar]
- 23.Gann PH, Hennekens CH, Ma J, Longcope C, Stampfer MJ. Prospective study of sex hormone levels and risk of prostate cancer. J Natl Cancer Inst. 1996;88:1118–1126. doi: 10.1093/jnci/88.16.1118. [DOI] [PubMed] [Google Scholar]
- 24.Imamoto T, Suzuki H, Akakura K, Komiy A, Nakamachi H, Ichikawa T, Igarashi T, Ito H. Pretreatment serum level of testosterone as a prognostic factor in Japanese men with hormonally treated stage D2 prostate cancer. Endocr J. 2001;48:573–578. doi: 10.1507/endocrj.48.573. [DOI] [PubMed] [Google Scholar]
- 25.Ribeiro M, Ruff P, Falkson G. Low serum testosterone and a younger age predict for a poor outcome in metastatic prostate cancer. Am J Clin Oncol. 1997;20:605–608. doi: 10.1097/00000421-199712000-00015. [DOI] [PubMed] [Google Scholar]
- 26.Chen SS, Chen KK, Lin AT, Chang YH, Wu HH, Chang LS. The correlation between pretreatment serum hormone levels and treatment outcome for patients with prostatic cancer and bony metastasis. BJU Int. 2002;89:710–713. doi: 10.1046/j.1464-410x.2002.02733.x. [DOI] [PubMed] [Google Scholar]
- 27.Massengill JC, Sun L, Moul JW, Wu H, McLeodt DG, Amling Ch, et al. Pretreatment total testosterone level predicts pathological stage in patients with localized prostate cancer treated with radical prostatectomy. J Urol. 2003;169:1670–1675. doi: 10.1097/01.ju.0000062674.43964.d0. [DOI] [PubMed] [Google Scholar]