Abstract
Introduction
Treatment of cryptorchidism includes hormonal therapy and/or operative methods. To evaluate effectiveness of neoadjuvant hCG-therapy in cryptorchid boys regarding testicle position before and after treatment.
Material and methods
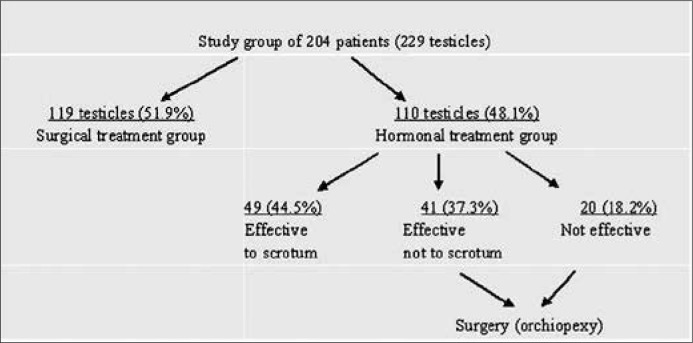
204 boys with 229 UDT, median age at presentation 6,6 years, SD ±3.4; 179 (87.7%) with unilateral and 25 (12.3%) with bilateral defect were treated between 1994 and 2008. 103 boys (119 gonads – 51.9%) underwent orchiopexy alone, while 101 boys (110 gonads – 48.1%) neoadjuvant hCG-therapy. The testicle position was evaluated before and one year after therapy. Every patient was seen in our outpatient department 2 to 16 years after the therapy.
Results
Out of 110 testes of 101 boys after hCG-therapy, 49 testicles (44.5%) descended to the scrotum and these 44 patients were not qualified for orchiopexy. Remaining 61 testes (55.5%) did not reach the scrotum after hormonal therapy and these 57 boys underwent orchiopexy. Gonadotropin induced the descent of 90 out of 110 testicles at least one level down, therefore overall effectiveness of hCG therapy was 81.8% (chi = 29.778, p = 0.000). 49 out of 110 UDT descended to the scrotum (44.5%). The efficacy of hormonal treatment did not depended on initial position of UDT (p = 0.43, p = 0.04, p = 0.97). We performed only 7 orchidectomies of disgenetic testes (3.1%). Neither type of treatment nor initial position of testicle influenced the future gonad atrophy (p = 0.5, p = 0.979).
Conclusions
Neoadjuvant hCG-therapy induced descent to the scrotum of 44.5% UDT and improved position of testis before orchidopexy in further 37.3% of patients.
Keywords: cryptorchidism, human Chorionic Gonadotropin, hormonal therapy effectiveness, testis position, orchiopexy
INTRODUCTION
Cryptorchidism is a developmental disorder in which one or both testicles fail to descend into the scrotum and are retained in the abdomen or inguinal canal. This is one of the most common urogenital anomalies of childhood affecting 1.8–4% of full term boys according to literature. The main causes of cryptochidism include: congenital anomalies of testis, invalid junctions between testicle, epididymis and vas deferens, hormonal disorders, and anatomic obstacles (e.g. inguinal hernia) [1–6].
Cryptorchidism treatment includes hormonal therapy and/or operative methods [7]. Hormonotherapy using gonadotropin–releasing hormone (GnRH), human chorionic gonadotropin (hCG), testosterone, and GnRH analogues precedes surgical intervention in number of cases, particularly in Europe [8]. It has been shown that preoperative and postoperative treatment with GnRH analogues improves fertility index [9, 10]. Human chorionic gonadotropin (hCG) has been shown to induce testicular descent, presumably by increasing weight and vascularity of testis and also by stimulating testosterone and/or dihydrotestosterone production. Hormonal therapy improves the histopathology of testis without harming germ cells and its effectiveness is estimated to be about 20–25% [11, 12].
However, more recent studies show that immediately after administration of hCG in boys with undescended testes (UDT) apoptosis of germ cells increases in hCG–treated testes, as compared with those that underwent surgical treatment alone. Furthermore, according to literature, when these patients were followed–up into adulthood, it was found that the hCG–treated testes were 50% smaller. In the opinion of some researchers, hCG treatment may damage future spermatogenesis [13, 14].
The purpose of this study was to evaluate effectiveness of therapy with chorionic gonadotropin (hCG) before surgery in boys with cryptorchidism regarding position of testis before and after treatment.
MATERIAL AND METHODS
Patients with cryptorchidism treated in our department between 1994 and 2008 were enrolled into the study. We studied records of 204 boys, median age at presentation 6.6 years, SD ±3.4 with cryptorchidism; 179 (87.7%) with unilateral and 25 (12.3%) with bilateral defect, with total of 229 treated gonads. Out of 179 patients with unilateral cryptorchidism, 79 (44.1%) had left and 100 (55.9%) had right testicle involved and the difference was not significant (p = 0.234). Patients were divided into two groups: surgical treatment group – 103 boys (119 gonads – 51.9%), median age 7.6 years, SD ±3.4; and hormonal treatment group – 101 boys (110 gonads – 48.1%), median age 5.7 years, SD ±3.2. To surgical treatment group were qualified patients whose parents did not give informed consent for hormonal therapy and boys older than 6 years (school age).
For the hormonal treatment we used hCG (Biogonadyl, BIOMED, Lublin, Poland) administrated intramuscularly 30–50 IU per kilogram of body weight, two times a week for five succeeding weeks. The retrospective analysis of the testicle position before and three, six, and 12 months after hormonal treatment was performed in the study group. The testicle position was evaluated based on clinical examination and ultrasound result. Our own classification of the testicle position was used for research purposes: A – for abdominal, UIC – for upper inguinal canal, LIC – for lower inguinal canal, and S – for scrotal testes.
The descent of testicle after hormonotherapy was noted if the gonad moved at least one level down, i.e. from A to UIC, from UIC to LIC or from LIC to S, and stayed in a new position while assessed six and 12 months after medication. The testicle position one year after therapy was considered as the final one. If the gonad retracted after initial descent or moved down less than one level, its position was recorded as a higher one and hormonal treatment as non–effective.
From 2008 to 2012, every patient of the study group was seen in our outpatient department two to 16 years after the therapy.
Statistical analysis was performed with Student t–test, chi–square test, and Fisher's exact test (Statistica 9.1PL software package).
RESULTS
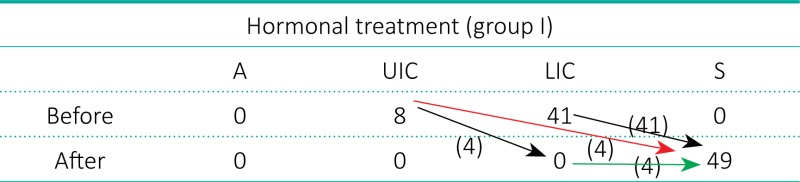
Out of 110 testes of 101 boys who underwent hormonal therapy, 49 testicles (44.5%) of 44 boys, median age 6.3 years, SD ±2.6 (Subgroup 1), descended to the scrotum and they were not qualified for orchiopexy. To reach the scrotum, 45 testes (91.8%) moved one level down and four gonads (8.2%) moved two levels down (Table 1.).
Table 1.
Subgroup 1 of hormonal treatment. Arrows indicate descent of testicles: black – one level, red – two levels down, green – staged descent. Descent from UIC to LIC: 4/8 – 50% (further to S after 12 months), from UIC to S: 4/8 – 50%, from LIC to S: 41/41 – 100%.
 |
The remaining 61 testes (55.5%) of 57 boys, median age 5.2 years, SD ±3.5 (Subgroup 2) did not reach the scrotum after hormonal therapy. Forty–one gonads (37.3%) moved one level down and 20 testes (18.2%) did not change position (Table 2). All 57 boys with 61 undescended testes of this subgroup underwent orchiopexy (Figure 1).
Table 2.
Subgroup 2 of hormonal treatment. Arrows indicate descent of testicles: black – one level down, red – no change in position, green – retracted one level back. Descent from A to UIC: 2/2 – 100%, from UIC to LIC: 39/44 – 88.6%, from LIC to S: 0/15 (7 testes descended to S but retracted to LIC before 12 months)
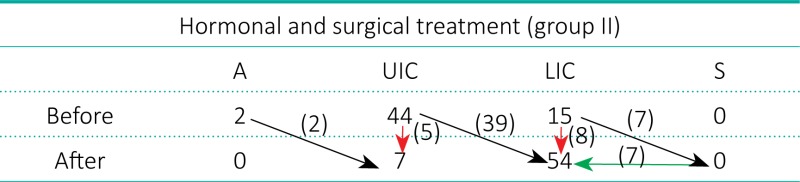 |
Figure 1.
Diagram of criptorchidism study group of boys with division to surgical and hormonal treatment groups.
In the hormonal treatment group, 90 out of 110 testicles (81.8%) moved at least one level down (chi = 72.391, p = 0.000). Forty–nine out of 110 undescended testes (44.5%) descended all the way down to the scrotum (chi = 42.423, p = 0.000). The efficacy of hormonal treatment did not depend on initial position of UDT (p = 0.43, p = 0.04, p = 0.97).
Boys of the hormonal therapy group were significantly younger than patients of the surgical treatment group (t = 4.346, p = 0.000).
Orchidectomies of dysgenetic gonads were performed in 7 boys (7/229 testes – 3.1%) from two to 10 years after completing therapy of cryptorchidism. There were five patients (5/119 gonads – 4.2%) of surgical treatment group at median age 8.4 years (SD ±5.0) and two boys (2/110 – 1.8%) after hormonal and surgical therapy at median age 11 years (SD ±1.4), with no statistical difference (p = 0.5). Out of these seven patients, five presented with unilateral and two with bilateral cryptorchidism, with no statistical difference (p = 0.979).
DISCUSSION
Cryptorchidism is the most common urogenital disorder of childhood affecting boys. The most serious problems that can arise from UDT are infertility and an increased risk of seminoma in situ [15, 16].
Despite the therapy used in cryptorchid boys (hormonal and/or surgical), the main goal is to situate the testes in the scrotum and improve future fertility index [17]. However, the treatment options should be reviewed according to gonad localization and presence of associated anomalies of the testis [5, 9, 18]. Although there is a common consensus about the surgical treatment of cryptorchidism and only operating techniques differ, there are controversies about the hormonal therapy regarding its influence on both testicle position and future function (fertility) [9, 10]. Review of the literature shows that hCG therapy is the most frequently used method of hormonal treatment, also in Poland.
Hormonal therapy efficacy is estimated in the literature at 20–25% [4, 11, 12, 19]. To reach our own opinion in this matter we decided to analyze a group of patients with cryptorchidism who underwent hormonal and/or surgical treatment in our department. A number of our patients were treated 10–20 years ago, therefore, their qualification did not meet currently accepted recommendations and guidelines. It is recommended now that surgical orchidolysis and orchidopexy should be performed at the latest by 12–18 months of age [20]. However, the age of a patient with UDT referred for surgery was usually beyond a pediatric surgeon's influence. Clinical observations show that even now, despite our efforts, a large number of patients arereferred by pediatricians at the age of 3–5 years.
This analysis revealed that hCG therapy induced descent of 81.8% of UDT in our series and the basic criterion used was position of the testicle before and after treatment. The successful descent to the scrotum was achieved in 44.5% of patients before surgery. Hormonal therapy proved to be efficient not only in LIC testicles, but also in gonads located in abdomen and UIC. Even if the testis did not descend to the scrotum it moved one level down in the majority of patients (41/61 gonads – 67.2%), which definitely made orchiopexy much easier.
We compared the results obtained in the hormonal treatment group with the results in the surgical treatment group. Patients who underwent surgery alone were older than boys treated with hCG (median age 7.6 years, t = 4.346, p = 0.000), therefore we assumed that it was a long enough time for the testis to descend to the scrotum spontaneously, but no such case was observed.
According to literature, preoperative hCG treatment increases testicular volume and improves its vascularity [12]. To our surprise we found only a few reports about the effect of hormonal therapy on the position of the testicle, which, in our opinion, seems to be crucial for such an evaluation. It mainly regarded non–palpable testes that became palpable after therapy [11]. Our observations on change of UDT volume after hCG administration in cryptorchid boys will be given in another report.
According to Comploj et al., hormonal therapy can be used as neoadjuvant therapy in UDT to improve testes histology and future fertility index [18].
Bukowski et al. demonstrated on 51 patients (64 testes) in a 15–month observation period that hCG may have a positive effect on non–palpable testes to become palpable because of the increase in size and blood supply, allowing better preoperative localization by physical examination and subsequent optimal planning of orchiopexy [11].
According to Nane et al., who enrolled 48 boys (70 testes) in a prospective study, hormonal treatment with the GnRH and hCG combination is an effective therapy for UDT located at and beyond the external inguinal ring and should be used as a treatment of choice because of its noninvasiveness [9]. Also Schwentner et al. reported recently that neoadjuvant GnRH therapy in a group of 21 prepubertal cryptorchid boys significantly improved the histopathological fertility index as compared with 21 boys after orchiopexy alone. In the authors opinion, consequently, preoperative hormone therapy should improve future fertility in adulthood [21].
Ritzen presented recently the consensus of a Nordic group of specialists in testicular physiology. In their opinion, treatment with hCG may be harmful to future spermatogenesis through increased apoptosis of germ cells. As orchiopexy results in about 95% anatomical success it is the preferred method, rather than hCG or GnRH treatment. The unanimous conclusion of the group was that orchiopexy should be performed between six and 12 months of age [13].
Ong et al. stated that although hormonal therapy may not stimulate transformation of neonatal gonocytes, it may trigger prepubertal mitosis of primary spermatocytes [22].
Dunkel at al. also demonstrated on group of 25 adult man after surgically treated cryptorchidism in which 15 had received an unsuccessful (hCG) therapy before orchiopexy that hormonal treatment of cryptorchidism was followed by an increased germ cell apoptosis and was correlated negatively with testes volume [14].
Marchetti et al., on behalf of the Italian Study Group on Undescended Testes, stated that the Consensus guidelines were not followed in a cohort of Italian children. Their study showed an important delay in orchiopexy – 70% of UDT cases underwent surgery at mean age of 22.8 months. Neoadjuvant hormonal therapy was applied in 23% of UDT cases with reported success rate of 25% [23].
Ludwikowski, after her review of the literature, stated that hormonal treatment should not be recommended for boys with unilateral UDT due to its disadvantages, such as: cost (182 Euro in Germany), the risk of postponing surgery beyond the recommended age of 18 months and potential adverse effects (discussed above in our article) [24].
We observed that the rate of gonad descent after hormonal therapy did not depended on the position of the UDT before therapy.
Only a few authors gave data about atrophy rate or orchidectomies after surgical and/or hormonal treatment of boys with UDT. We performed only seven orchidectomies of dysgenetic testes in the entire group of 204 boys with 229 UDT, which gives a very low rate of 3.1%. Neither type of treatment nor initial position of testicle influenced the future gonad atrophy (p = 0.5, p = 0.979). It clearly shows that gonad atrophy after completing UDT treatment in our series was connected with the disorder itself and was not therapy–dependent.
CONCLUSIONS
Our study proved that intramuscular injections of hCG given as neoadjuvant therapy in cryptorchid boys induced gonad descent to the scrotum in 44.5% of cases and improved position of testis before orchidopexy in further 37.3% of patients. In comparison with patients treated only surgically hCG therapy did not induce significantly more frequent testicular atrophy in cryptorchid boys in long-term observation.
Financial sources: the Medical University of Łódź grant No: 502–03/5–120–03/502–54–044
References
- 1.Barthold JS, González R. The epidemiology of congenital cryptorchidism, testicular ascent and orchiopexy. J Urol. 2003;170:2396–2401. doi: 10.1097/01.ju.0000095793.04232.d8. [DOI] [PubMed] [Google Scholar]
- 2.Berkowitz GS, Lapinski RH, Dolgin SE, Gazella JG, Bodian CA, Holzman IR. Prevalence and natural history of cryptorchidism. Pediatrics. 1993;92:44–49. [PubMed] [Google Scholar]
- 3.Thonneau PF, Gandia P, Mieusset R. Cryptorchidism: incidence, risk factors, and potential role of environment; an update. J Androl. 2003;24:155–162. doi: 10.1002/j.1939-4640.2003.tb02654.x. [DOI] [PubMed] [Google Scholar]
- 4.Niedzielski J, Przewratil P. Cryptorchidism in view of current literature. Przegl Pediatr. 2000;30:180–185. [Google Scholar]
- 5.Miodek M, Niedzielski J. Congenital anomalies of testis, epididymis and vas deferens in cryptorchid boys. Urol Pol. 2001;54:63–66. [Google Scholar]
- 6.Przewratil P, Kobos J, Niedzielski J. Expression of estrogen and progesterone receptors in paratesticular tissues of cryptorchid boys – preliminary report. Urol Pol. 1999;52:382–392. [Google Scholar]
- 7.Lala R, Matarazzo P, Chiabotto P, Gennari F, Cortese MG, Canavese F, de Sanctis C. Early hormonal and surgical treatment of cryptorchidism. J Urol. 1997;157:1898–1901. [PubMed] [Google Scholar]
- 8.Nane I, Ziylan O, Esen T, Kocak T, Ander H, Tellalgolu S. Primary gonadotropin releasing hormone and adjunctive human chorionic gonadotropin treatment in cryptorchidism: a clinical trial. Urology. 1997;49:108–111. doi: 10.1016/S0090-4295(96)00359-7. [DOI] [PubMed] [Google Scholar]
- 9.Hadziselimović F, Herzog B. Treatment with a luteinizing hormone–releasing hormone analogue after successful orchiopexy markedly improves the chance of fertility later in life. J Urol. 1997;158:1193–1195. doi: 10.1097/00005392-199709000-00130. [DOI] [PubMed] [Google Scholar]
- 10.Huff DS, Snyder HM, 3rd, Rusnack SL, Zedric SA, Carr MC, Canning DA. Hormonal therapy for the subfertility of cryptorchidism. Horm Res. 2001;55:38–40. doi: 10.1159/000049962. [DOI] [PubMed] [Google Scholar]
- 11.Bukowski TP, Sedberry S, Richardson B. Is human chorionic gonadotropin useful for identifying and treating nonpalpable testis? J Urol. 2001;165:221–223. doi: 10.1097/00005392-200101000-00063. [DOI] [PubMed] [Google Scholar]
- 12.Miller OF, Stock JA, Cilento BG, Cilento BG, McAleer IM, Kaplan W. Prospective evaluation of human chorionic gonadotropin in the differentiation of undescended testes from retractile testes. J Urol. 2003;169:2328–2331. doi: 10.1097/01.ju.0000065823.80051.bb. [DOI] [PubMed] [Google Scholar]
- 13.Ritzén EM. Undescended testes: a consensus on management. Eur J Endocrinol. 2008;159(Suppl 1):S87–90. doi: 10.1530/EJE-08-0181. [DOI] [PubMed] [Google Scholar]
- 14.Dunkel L, Taskinen S, Hovatta O, Tilly JL, Wikström S. Germ cell apoptosis after treatment of cryptorchidism with human chorionic gonadotropin is associated with impaired reproductive function in the adult. J Clin Invest. 1997;100:2341–2346. doi: 10.1172/JCI119773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jallouli M, Rebai T, Abid N, Kassis M, Mhiri R. Neoadjuvant gonadotropin–releasing hormone therapy before surgery and effect on fertility index in unilateral undescended testes: a prospective randomized trial. Urology. 2009;73:1251–1254. doi: 10.1016/j.urology.2008.10.078. [DOI] [PubMed] [Google Scholar]
- 16.Kollin C, Stukenborg JB, Nurmio M, Sundgvist E, Gustafsson T, Söder O. Boys with Undescended Testes: Endocrine, Volumetric and Morphometric Studies on Testicular Function before and after Orchidopexy at Nine Months or Three Years of Age. J Clin Endocrinol Metab. 2012;97:4588–4595. doi: 10.1210/jc.2012-2325. [DOI] [PubMed] [Google Scholar]
- 17.Taran I, Elder JS. Results of orchiopexy for the undescended testis. World J Urol. 2006;24:231–239. doi: 10.1007/s00345-006-0056-4. [DOI] [PubMed] [Google Scholar]
- 18.Comploj E, Pycha A. Diagnosis and management of cryptorchidism. Eur Urol Suppl. 2012;11:8. [Google Scholar]
- 19.Bertelloni S, Baroncelli GI, Ghirri P, Spinelli C, Saggese C. Hormonal treatment for unilateral inguinal testis: comparison of four different treatments. Horm Res. 2001;55:236–239. doi: 10.1159/000050002. [DOI] [PubMed] [Google Scholar]
- 20.Tekgül S, Riedmiller H, Gerharz E, Hoebeke P, Kocvara R, Nijman R, et al. Cryptorchidism in Guidance on Paediatric Urology. European Society for Paediatric Urology. 2012:11–13. Chapt 3. [Google Scholar]
- 21.Schwentner C, Oswald J, Kreczy A, Lunacek A, Bartsch G, Deibl M, Radmayr C. Neoadjuvant gonadotropin–releasing hormone therapy before surgery may improve the fertility index in undescended testes: a prospective randomized trial. J Urol. 2005;173:974–977. doi: 10.1097/01.ju.0000153562.07287.77. [DOI] [PubMed] [Google Scholar]
- 22.Ong C, Hasthorpe S, Hutson JM. Germ cell development in the descended and cryptorchid testis and the effects of hormonal manipulation. Pediatr Surg Int. 2005;21:240–254. doi: 10.1007/s00383-005-1382-0. [DOI] [PubMed] [Google Scholar]
- 23.Marchetti F, Bua J, Tornese G, Piras G, Toffol G, Ronfani L. Management of cryptorchidism: a survey of clinical practice in Italy. BMC Pediatrics. 2012;12:4. doi: 10.1186/1471-2431-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ludwikowski B, Gonzalez R. The controversy regarding the need for hormonal treatment in boys with unilateral cryptorchidism goes on: a review of the literature. Eur J Pediatr. 2013;172:5–8. doi: 10.1007/s00431-012-1711-y. [DOI] [PubMed] [Google Scholar]