Abstract
Phytoestrogens exist in edible compounds commonly found in fruits or plants. For long times, phytoestrogens have been used for therapeutic treatments against human diseases, and they can be promising ingredients for future pharmacological industries. Kaempferol is a yellow compound found in grapes, broccoli and yellow fruits, which is one of flavonoid as phytoestrogens. Kaempferol has been suggested to have an antioxidant and anti-inflammatory effect. In past decades, many studies have been performed to examine anti-toxicological role(s) of kaempferol against human cancers. It has been shown that kaempferol may be involved in the regulations of cell cycle, metastasis, angiogenesis and apoptosis in various cancer cell types. Among them, there have been a few of the studies to examine a relationship between kaempferol and apoptosis. Thus, in this review, we highlight the effect(s) of kaempferol on the regulation of apoptosis in diverse cancer cell models. This could be a forecast in regard to use of kaempferol as promising treatment against human diseases.
Keywords: Phytoestrogen, Kaempferol, Apoptosis, Cancer models, Estrogen receptors
INTRODUCTION
Typically, cells are maintained through cell division, growth and arrest. However, abnormal cell regulatory processes could lead to excessive growth of cells and become cancers (1). Cancer growths unexpectedly invade into normal tissue or organ through more than required unlimited proliferation. And then they metastasize into many organs and interrupt normal cell function. In cancer progression, DNA repair system is disrupted, and inflammation, angiogenesis and apoptosis are altered in cancer cells (2). Main treatments to cure cancers include surgical removal, radiation treatment or chemotherapy, while other hormonal or biological gene therapy have been proposed for cancer treatments as well (3).
As a substitute, phytoestrogen as a natural substance has been proposed for cancer patient treatments. Phytoestrogens derived from fruits and vegetables reduced general risk of diverse human cancers (4). In particular, some endocrine cancers may be influenced by hormones, such as estrogen, androgen or cortisol etc. Because of the similar molecular structures between phytoestrogens and endogenous steroid hormones, these phytoestrogens may have similar functions in our body (5). Thus, phytoestrogens are used as hormone replacement therapy (HRT) for prolonged therapy, for instance, the prevention of breast cancer, postmenopause, and osteoporosis (6). Thus, phytoestrogens may be employed for potential therapy as proposed by substantial evidence.
In past decade, many studies have been done to clarify relationship between phytoestrogens and cancers for an alternative treatment. Risk of heart disease, osteoporosis and various cancers could be reduced by high consume of legumes and soy products (7). In vitro and in vivo studies suggest that isoflavonoid may inhibit cancer cell growth by competing estradiol to type 2 estrogen binding sites (8). Genistein derived from isoflavones could inhibit cancer progression induced by 17beta-estradiol or various endocrine disrupting chemicals by regulating cell cycle related or insulin-growth factor-1 (IGF-1) signaling pathway in BG-1 ovarian cancer cells expressing estrogen receptor (ER)s (9,10). However, all of phytoestrogens may not be applicable since detailed underlying mechanism needs to be uncovered in the inhibition of cancer cell growth by phytoestrogens (8).
Kaempferol is a yellow compound that belongs to the flavonoid (11). This is mostly included in fruits such as apple, grape and tomato and in plants such as green tea, pine, angelica decursiva and ginkgo leaf (12-14). Kaempferol has been shown to have an antioxidant activity and antiinflammatory function such as flavonoids (11). Kaempferol may also reduce the risk of cardiovascular and neuroinflammatory diseases (15). Taken together, this may have a therapeutic potential for cancer prevention. There are substantial studies to reveal the underlying mechanism(s) of kaempferol on the regulation of apoptosis (16-18). Among them, we will highlight the detailed effects of kaempferol on the regulation of apoptosis in this review.
Phytoestrogens. From 50 years ago, phytoestrogen attention comes from Western Australia, inspired by sheep which changed reproduction ability when fed red clover (19). Dietary intake of phytoestrogens, i.e., tobu and bean products, is quite different and diverse by each country and culture, while the uptake ratio of phytoestrogen-rich diet is much higher in Asian counties. Epidemiological studies suggest that prostate, breast and colorectal cancers called Western disease have occurred lower in Asian counties than Westerns (20). By the same reason, flushing of postmenopausal women is lower clinically in Asian counties (21). Estrogen deficiency due to postmenopausal affect occurrence of chronic diseases associated with aging (22). Phytoestrogen supplement could reduce or mitigate the number of symptoms that came from menopause. Isoflavones have a protective effect on bones in women, particularly in lumbar spine (23). They could act on cells or tissues by competitively binding to ERs with endogenous estrogens during developmental and reproductive stages. Actual research shows that phytoestrogens have been shown to have diverse effects depending on their concentrations (24). For example, kaempferol has been demonstrated to have a biphasic effect on the estrogenicity in MCF-7 breast cancer cells (25). At the high concentration of kaempferol, diverse cancer growth could be inhibited. Taken together, the current studies indicate that rich consumption of phytoestrogens may be beneficial for prevention of cancer formation based on in vitro and in vivo results.
This anti-cancer effect of phytoestrogens could be estimated from their chemical structures. Most of their chemical structures appear to be similar to that of an estrogen active form, estradiol. Thus, they may have high binding affinity for ERs, since phytoestrogen and estradiol have competitive binding affinity to ERs. Using a competitive binding assay to measure their binding capacity to ERs, phytroestrogens were examined compared to that of endogenous estradiol. In particular, ERbeta may play more critical roles rather than ERalpha to trigger biological responses caused by phytoestrogens (26). In molecular aspect, flavones and isoflavone could inhibit cytochrome P450 aromatase, which is an essential enzyme in the conversion of androgens to estrogens (27). In our previous study, resveratrol, a natural polyphenolic compound, was shown to inhibit the growth of BG-1 ovarian cancer cells by blocking the interaction of ERalpha signaling pathway and then suppressing cell cycle progression (28).
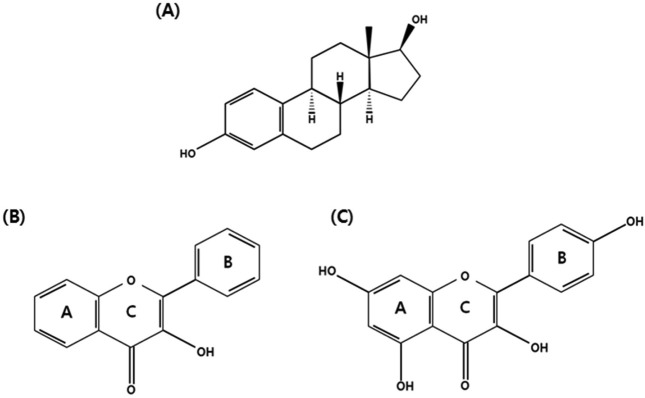
Anti-cancer effect of kaempferol. Phytoestrogens are classified into isoflavones, coumestans, lignans and coumstans (29). In most of flavonoid structure, the flavon nucleus exists. They have 15 carbon atoms arranged in three rings consist of 2-phenyl-3 hydroxyl-chromonesm. While ring A and B are benzene rings, ring C is a heterocyclic pyran or pyrone (30,31). Kaempferol is a flavonoid, but has different biological properties compared to other flavonoids. Distinct biological properties of kaempferol appear to be derived from its different structure, showing that one of hydroxyl groups in kaempferol is different to other flavonoids shown in Fig. 1 (32).
Fig. 1. Chemical structures of (A) estradiol, (B) flavane nucleus, (C) kaempferol.
Kaempferol has been shown to have antioxidant and antineoplastic activities. For instance, kaempferol may reduce proliferation and significantly decrease the expression of vascular endothelial growth factor (VEGF), a marker of angiogenesis, in ovarian cancer cells (33). Kaempferol has been involved to inhibit angiogenesis by suppressing extracellular signal-regulated kinase (ERK)-NFκB-cMyc-p21- VEGF pathway in a cancer cell model (34).
Cells are determined whether they can keep produce or stop through cell cycle, thus the regulation of cell cycle related genes and proteins may be a fundamental procedure for homeostasis (35). Kaempferol has been shown to inhibit cell proliferation in a dose dependent manner by regulating cyclin-dependent kinase 1 (CDK1) and cyclin B, a marker for transition of G2 to M phase, and by regulating a tumor suppressor gene which plays a key role in cell cycle arrest, p53 or PLK-1, in MCF-7 breast cancer and HeLa cervical cancer cells, respectively (36,37). In addition, kaempferol was demonstrated to suppress cell metastasis through ERKp38- JNK and AP-1 signaling pathways in U-2 OS human osteosarcoma cells (38). Src kinase has tyrosine kinase activity to regulate mitogen-activated protein kinase (MAPK) activity, which mediate cell cycle and angiogenesis via the inhibition of ultraviolet B (UVB)-induced cyclooxygenase- 2 (COX-2). Thus, kaempferol could be a potential therapeutic agent by suppressing these genes against a skin cancer model (38). Based on its molecular mechanism, further in vivo study warranties to be examined for a clinical trial to apply beneficial effect of kaempferol.
Physiological role of apoptosis. Human body consists of thirty-three billion of cells, and diverse cell functions maintain their balance. But dual external stimuli, stress and generation of mutant cells may break this balance (39). Program cell death, apoptosis, plays a critical role in maintaining cell functions by removing unnecessary cells, which is a basic procedure to maintain balance by deleting modified or infected cells from our body (40). Cell death may be derived from two major phenomena, necrosis and apoptosis. Necrosis occurs slowly for long period of time by accident, such as physical and chemical stimuli (41). Moisture is absorbed into cells, and then cell membranes are burst and die due to lack of oxygen and bloodstream. Unlike necrosis, apoptosis is a programmed death process against unwanted cells. Cell membrane is distorted shortly and nucleus is disintegrated followed by degradation products which can be disposed of macrophages (42).
In ischemic liver patients, apoptotic bodies appeared to be different from necrotic bodies in morphology. Thus, new form of death to shrinkage necrosis was found at first time, and it was named as apoptosis at the following year (43). Apoptosis was derived from Greek letter of falling off, implying overall natural metabolic process like fallen leaves. While necrosis always occur in pathological cells, apoptosis can be also observed in pathological and normal cells (44). In embryonic development, many unnecessary cells are removed through apoptosis, for example, when fingers are formed in embryonic development, intervals cells are removed to get entire function. A similar process happened when tadpole’s tail is missing because its function is no longer required after growing (45). In this situation, abnormal or unnecessary cells could be dead by apoptosis. When cells are exposed to stress by chemical substance and internal stimuli, normal cells turn into mutant and finally changed as cancer cells (45). In this case, our immune system could find out pathological or abnormal cells to remove, indicating that homeostasis is maintained and regulated by apoptosis (45).
To measure apoptosis, traditional cell-based methods have been developed, i.e., a DNA laddering assay. When programmed cell death begins, DNAs in nuclei are regularly fragmented to 80~200 bp, which can be visualized on agarose gel (46). Terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling of DNA fragments (TUNEL) and in situ end labeling (ISEL) staining methods have been developed to detect apoptosis in cells and tissues (47). These methods can be also used together with standard flow cytometric staining methods related with cell death using various cellular parameters, including cell cycle and phenotypes (48). TUNEL assay not only has a merit to maintain tissue structure but also observe apoptotic body in individual cells. In addition, we can use microscopy to observe cytological appearance changes, i.e., membrane blebbing and nuclear condensation. A MTT/XTT enzyme assay and caspase activity assay can be employed to measure apoptosis in cells or tissues (49).
Effect of kaempferol on apoptosis. In extrinsic pathway, there are death receptors in cell surface, and they may recognize substances which induce death and penetrate to inner cytoplasm. Death receptors include tumor necrosis factor (TNF), FAS and TRAIL (50). Kaempferol down-regulates TNF-α production in aged rat gingival tissues via the inhibition of transcription factor nuclear factor kappa B (NF-κB) (51). Unlikely quercetin, kaempferol appears to be effective in both osteoblasts and osteoclasts by antagonizing TNF-α and receptor activator of NF-κB ligand receptor activator of NF-κB ligand (RANKL) (52). TRAIL has been shown to induce apoptosis in SW480 human colon cancer cells (53). It was demonstrated that kaempferol significantly up-regulate TRAIL receptors, indicating that kaempferol may be an effective factor in the treatment for TRAIL relatedimmune deficient disease (54). In human glioma cells, kaempferol also sensitizes to TRAIL-mediated apoptosis by proteasomal degradation of survivin (55).
Caspases are a zymogen which is an element in the induction of apoptosis as a downstream signaling pathway. A difference of these caspase pathways is irreversible compared to other post-translational modifications (56). When procaspases convert into caspases, they have enzymatic activity in the induction of apoptosis (57). In apoptosis, kaempferol slightly induced caspase 3 activity in leukemia HL-60 cells and oral cavity cancer cells (36,58). An intrinsic pathway mainly occurred in mitochondria by intracellular factors, i.e., apoptotic genes; p53, PUMA, NOXA, Bax, BCL-2, Apaf-1, caspase 9 and cytochrome c etc. (59,60). In A549 lung cancer cells, kaempferol up-regulated pro-apoptotic bax and bad genes, while it down-regulated anti-apoptotic bcl-2 and bcl-xL expression (61).
Nitric oxide (NO) or reactive products modulate apoptotic signals. They regulate caspase 3-like proteases ultimately to control activity of protease (62). In addition, NO and reactive products have also influenced TNF- or FAS signals. NO has been shown to possess different functions depending on the type of cells and state (63). They kill cancer cells and protect normal cells by plotting between normal and cancer cells (64). NO synthesis is induced by isoforms of nitric oxide synthase (iNOS) and cyclooxygenase (COX- 2). In addition, NO production is regulated by reactive Cprotein (CRP) and NF-kB activation (65). In some diseases, iNOS and COX-2 can be overexpressed. In human hepatocyte-derived cell line, Chang liver cells, kaempferol has been shown to suppress the protein levels of iNOS, COX2, CRP and NF-κB at all concentrations of 5 to 200 μM (66). To measure NO synthesis by a drug in human and animals, it is more complicated in depending on various factors, thus real-time PCR can be used to directly measure NO synthesis products (67). In the previous study, kaempferol was shown to inhibit NO production in a rat model (68).
CONCLUSION
As an alternative method for HRT, the effects of phytoestrogens have been explored in the previous studies. However their effects appear to be diverse depending on their concentrations. A high concentration of 12 phytoestrogen mixture has been shown to have an estrogenic activity in Sprague-Dawley rats (69). As a phytoestrogen, kaempferol has also been determined to have a biphasic effect. For instance, kaempferol has an anti-cancer activity at higher concentrations, while it has a pro-cancer activity at lower concentrations (25). Thus, the concentration of its dosage should be considered for the application to human cancer treatments. In addition, the signaling pathways caused by kaempferol should be clarified in a cell model, for instance, kaempferol may be involved in the induction of apoptosis in many cancer cell types, implying that it may have an anticancer activity through intrinsic and extrinsic apoptotic pathways.
More detailed studies should be performed to confirm anti-cancer effect(s) of kaempferol for clinical use. An interaction caused by several factors, such as sex, concentration, and immune state, needs to be considered as well. Although many studies have been done to clarify the exact role(s) of kaempferol in in vitro and animal models until now, more detailed studies should be carried out to clarifying its underlying mechanism(s) for the clinical use.
CONFLICT OF INTEREST STATEMENT
The authors declare that there are no conflicts of interest.
Acknowledgments
This work was supported by the research grant of the Chungbuk National University in 2012. In addition, this study was also supported by the Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (MEST) of Korea government (2009- 0094035).
References
- 1.Danial N.N., Korsmeyer S.J. Cell death: critical control points. Cell. (2004);116:205–219. doi: 10.1016/S0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 2.Kastan M.B., Bartek J. Cell-cycle checkpoints and cancer. Nature. (2004);432:316–323. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- 3.Prehn R.T. Tumor progression and homeostasis. Adv. Cancer Res. (1976);23:203–236. doi: 10.1016/s0065-230x(08)60547-3. [DOI] [PubMed] [Google Scholar]
- 4.Branca F., Lorenzetti S. Health effects of phytoestrogens. Forum Nutr. (2005):100–111. doi: 10.1159/000083773. [DOI] [PubMed] [Google Scholar]
- 5.Dixon R.A. Phytoestrogens. Annu. Rev. Plant Biol. (2004);55:225–261. doi: 10.1146/annurev.arplant.55.031903.141729. [DOI] [PubMed] [Google Scholar]
- 6.Poluzzi E., Piccinni C., Raschi E., Rampa A., Recanatini M., De Ponti F. Phytoestrogens in postmenopause: the state of the art from a chemical, pharmacological and regulatory perspective. Curr. Med. Chem. In press; (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tham D.M., Gardner C.D., Haskell W.L. Clinical review 97: Potential health benefits of dietary phytoestrogens: a review of the clinical, epidemiological, and mechanistic evidence. J. Clin. Endocrinol. Metab. (1998);83:2223–2235. doi: 10.1210/jcem.83.7.4752. [DOI] [PubMed] [Google Scholar]
- 8.Adlercreutz H., Mousavi Y., Clark J., Höckerstedt K., Hämläinen E., Wähälä K., Mäkelä T., Hase T. Dietary phytoestrogens and cancer: in vitro and in vivo studies. J. Steroid Biochem. Mol. Biol. (1992);41:331–337. doi: 10.1016/0960-0760(92)90359-Q. [DOI] [PubMed] [Google Scholar]
- 9.Hwang K.A., Kang N.H., Yi B.R., Lee H.R., Park M.A., Choi K.C. Genistein, a soy phytoestrogen, prevents the growth of BG-1 ovarian cancer cells induced by 17beta-estradiol or bisphenol A via the inhibition of cell cycle progression. Int. J. Oncol. (2013);42:733–740. doi: 10.3892/ijo.2012.1719. [DOI] [PubMed] [Google Scholar]
- 10.Hwang K.A., Park M.A., Kang N.H., Yi B.R., Hyun S.H., Jeung E.B., Choi K.C. Anticancer effect of genistein on BG-1 ovarian cancer growth induced by 17 betaestradiol or bisphenol A via the suppression of the crosstalk between estrogen receptor alpha and insulin-like growth factor- 1 receptor signaling pathways. Toxicol. Appl. Pharmacol. (2013);272:637–646. doi: 10.1016/j.taap.2013.07.027. [DOI] [PubMed] [Google Scholar]
- 11.Rice-Evans C. Flavonoid antioxidants. Curr. Med.Chem. (2001);8:797–807. doi: 10.2174/0929867013373011. [DOI] [PubMed] [Google Scholar]
- 12.Kim S., Kim K.Y., Han C.S., Ki K.S., Min K.J., Zhang X., Whang W.K. Simultaneous analysis of six major compounds in Osterici Radix and Notopterygii Rhizoma et Radix by HPLC and discrimination of their origins from chemical fingerprint analysis. Arch. Pharmacal Res. (2012);35:691–699. doi: 10.1007/s12272-012-0413-3. [DOI] [PubMed] [Google Scholar]
- 13.Park J.S., Rho H.S., Kim D.H., Chan I.S. Enzymatic preparation of kaempferol from green tea seed and its antioxidant activity. J. Agric. Food Chem. (2006);54:2951–2956. doi: 10.1021/jf052900a. [DOI] [PubMed] [Google Scholar]
- 14.Yoshikawa T., Naito Y., Kondo M. Ginkgo biloba leaf extract: review of biological actions and clinical applications. Antioxid. Redox Signaling. (1999);1:469–480. doi: 10.1089/ars.1999.1.4-469. [DOI] [PubMed] [Google Scholar]
- 15.Kowalski J., Samojedny A., Paul M., Pietsz G., Wilczok T. Effect of apigenin, kaempferol and resveratrol on the expression of interleukin-1beta and tumor necrosis factor- alpha genes in J774.2 macrophages. Pharmacol. Rep. (2005);57:390–394. [PubMed] [Google Scholar]
- 16.Li R.J., Mei J.Z., Liu G.J. [Kaempferol-induced apoptosis of human esophageal squamous carcinoma Eca-109 cells and the mechanism]. Nanfang Yike Daxue Xuebao. (2011);31:1440–1442. [PubMed] [Google Scholar]
- 17.Marfe G., Tafani M., Indelicato M., Sinibaldi-Salimei P., Reali V., Pucci B., Fini M., Russo M.A. Kaempferol induces apoptosis in two different cell lines via Akt inactivation, Bax and SIRT3 activation, and mitochondrial dysfunction. J. Cell. Biochem. (2009);106:643–650. doi: 10.1002/jcb.22044. [DOI] [PubMed] [Google Scholar]
- 18.Xie F., Su M., Qiu W., Zhang M., Guo Z., Su B., Liu J., Li X., Zhou L. Kaempferol promotes apoptosis in human bladder cancer cells by inducing the tumor suppressor, PTEN. Int. J. Mol. Sci. (2013);14:21215–21226. doi: 10.3390/ijms141121215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bennetts H.W., Underwood E.J., Shier F.L. A specific breeding problem of sheep on subterranean clover pastures in Western Australia. Aust. Vet. J. (1946);22:2–12. doi: 10.1111/j.1751-0813.1946.tb15473.x. [DOI] [PubMed] [Google Scholar]
- 20.Adlercreutz H. Western diet and Western diseases: some hormonal and biochemical mechanisms and associations. Scand. Scand. J. Clin. Lab. Invest. Suppl. (1990);201:3–23. doi: 10.3109/00365519009085798. [DOI] [PubMed] [Google Scholar]
- 21.Murkies A.L., Lombard C., Strauss B.J., Wilcox G., Burger H.G., Morton M.S. Dietary flour supplementation decreases post-menopausal hot flushes: effect of soy and wheat. Maturitas. (1995);21:189–195. doi: 10.1016/0378-5122(95)00899-V. [DOI] [PubMed] [Google Scholar]
- 22.Simons L.A., von Konigsmark M., Simons J., Celermajer D.S. Phytoestrogens do not influence lipoprotein levels or endothelial function in healthy, postmenopausal women. Am. J. Cardiol. (2000);85:1297–1301. doi: 10.1016/S0002-9149(00)00759-1. [DOI] [PubMed] [Google Scholar]
- 23.Atkinson C., Compston J.E., Day N.E., Dowsett M., Bingham S.A. The effects of phytoestrogen isoflavones on bone density in women: a double-blind, randomized, placebo-controlled trial. Am. J. Clin. Nutr. (2004);79:326–333. doi: 10.1093/ajcn/79.2.326. [DOI] [PubMed] [Google Scholar]
- 24.Wang C., Kurzer M.S. Phytoestrogen concentration determines effects on DNA synthesis in human breast cancer cells. Nutr. Cancer. (1997);28:236–247. doi: 10.1080/01635589709514582. [DOI] [PubMed] [Google Scholar]
- 25.Oh S.M., Kim Y.P., Chung K.H. Biphasic effects of kaempferol on the estrogenicity in human breast cancer cells. Arch. Pharmacal Res. (2006);29:354–362. doi: 10.1007/BF02968584. [DOI] [PubMed] [Google Scholar]
- 26.Kuiper G.G., Lemmen J.G., Carlsson B., Corton J.C., Safe S.H., van der Saag P.T, van der Burg B., Gustafsson J.A. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. (1998);139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 27.Kao Y.C., Zhou C., Sherman M., Laughton C.A., Chen S. Molecular basis of the inhibition of human aromatase (estrogen synthetase) by flavone and isoflavone phytoestrogens: A site-directed mutagenesis study. Environ. Health Perspect. (1998);106:85–92. doi: 10.1289/ehp.9810685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang N.H., Hwang K.A., Lee H.R., Choi D.W., Choi K.C. Resveratrol regulates the cell viability promoted by 17beta-estradiol or bisphenol A via down-regulation of the cross-talk between estrogen receptor alpha and insulin growth factor-1 receptor in BG-1 ovarian cancer cells. Food Chem.Toxicol. (2013);59:373–379. doi: 10.1016/j.fct.2013.06.029. [DOI] [PubMed] [Google Scholar]
- 29.Murkies A.L., Wilcox G., Davis S.R. Clinical review 92: Phytoestrogens. J. Clin. Endocrinol. Metab. (1998);83:297–303. doi: 10.1210/jcem.83.2.4577. [DOI] [PubMed] [Google Scholar]
- 30.Choi E.J., Ahn W.S. Kaempferol induced the apoptosis via cell cycle arrest in human breast cancer MDA-MB- 453 cells. Nutr. Res. Pract. (2008);2:322–325. doi: 10.4162/nrp.2008.2.4.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Havsteen B.H. The biochemistry and medical significance of the flavonoids. Pharmacol. Ther. (2002);96:67–202. doi: 10.1016/S0163-7258(02)00298-X. [DOI] [PubMed] [Google Scholar]
- 32.Touillaud M.S., Pillow P.C., Jakovljevic J., Bondy M.L., Singletary S.E., Li D., Chang S. Effect of dietary intake of phytoestrogens on estrogen receptor status in premenopausal women with breast cancer. Nutr. Cancer. (2005);51:162–169. doi: 10.1207/s15327914nc5102_6. [DOI] [PubMed] [Google Scholar]
- 33.Luo H., Rankin G.O., Liu L., Daddysman M.K., Jiang B.H., Chen Y.C. Kaempferol inhibits angiogenesis and VEGF expression through both HIF dependent and independent pathways in human ovarian cancer cells. Nutr. Cancer. (2009);61:554–563. doi: 10.1080/01635580802666281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo H, Rankin G.O, Juliano N, Jiang B.H., Chen Y.C. Kaempferol inhibits VEGF expression and in vitro angiogenesis through a novel ERK-NFkappaB-cMyc-p21 pathway. Food Chem. (2012);130:321–328. doi: 10.1016/j.foodchem.2011.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vogelstein B., Kinzler K.W. Cancer genes and the pathways they control. Nat. Med. (2004);10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 36.Kang G.Y., Lee E.R., Kim J.H., Jung J.W., Lim J., Kim S.K., Cho S.G., Kim K.P. Downregulation of PLK-1 expression in kaempferol-induced apoptosis of MCF-7 cells. Eur. J. Pharmacol. (2009);611:17–21. doi: 10.1016/j.ejphar.2009.03.068. [DOI] [PubMed] [Google Scholar]
- 37.Xu W., Liu J., Li C., Wu H.Z., Liu Y.W. Kaempferol-7-O-beta-D-glucoside (KG) isolated from Smilax china L. rhizome induces G2/M phase arrest and apoptosis on HeLa cells in a p53-independent manner. Cancer Lett. (2008);264:229–240. doi: 10.1016/j.canlet.2008.01.044. [DOI] [PubMed] [Google Scholar]
- 38.Chen H.J., Lin C.M., Lee C.Y., Shih N.C., Peng S.F., Tsuzuki M., Amagaya S., Huang W.W., Yang J.S. Kaempferol suppresses cell metastasis via inhibition of the ERK-p38-JNK and AP-1 signaling pathways in U-2 OS human osteosarcoma cells. Oncol. Rep. (2013);30:925–932. doi: 10.3892/or.2013.2490. [DOI] [PubMed] [Google Scholar]
- 39.Martina M., Clerici M., Baldo V., Bonetti D., Lucchini G., Longhese M.P. A balance between Tel1 and Rif2 activities regulates nucleolytic processing and elongation at telomeres. Mol. Cell Biol. (2012);32:1604–1617. doi: 10.1128/MCB.06547-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Majno G., Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am. J. Pathol. (1995);146:3–15. [PMC free article] [PubMed] [Google Scholar]
- 41.Edinger A.L., Thompson C.B. Death by design: apoptosis, necrosis and autophagy. Curr. Opin. Cell Biol. (2004);16:663–669. doi: 10.1016/j.ceb.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 42.Fadok V.A., Voelker D.R., Campbell P.A., Cohen J.J., Bratton D.L., Henson P.M. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. (1992);148:2207–2216. [PubMed] [Google Scholar]
- 43.Kerr J.F. Shrinkage necrosis: a distinct mode of cellular death. J. Pathol. (1971);105:13–20. doi: 10.1002/path.1711050103. [DOI] [PubMed] [Google Scholar]
- 44.Kerr J.F., Winterford C.M., Harmon B.V. Apoptosis. Its significance in cancer and cancer therapy. Cancer. (1994);73:2013–2026. doi: 10.1002/1097-0142(19940415)73:8<2013::AID-CNCR2820730802>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 45.Kerr J.F., Wyllie A.H., Currie A.R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer. (1972);26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh N.P. A simple method for accurate estimation of apoptotic cells. Exp. Cell Res. (2000);256:328–337. doi: 10.1006/excr.2000.4810. [DOI] [PubMed] [Google Scholar]
- 47.Kelly K.J., Sandoval R.M., Dunn K.W., Molitoris B.A., Dagher P.C. A novel method to determine specificity and sensitivity of the TUNEL reaction in the quantitation of apoptosis. Am. J. Physiol. Cell Physiol. (2003);284:C1309–1318. doi: 10.1152/ajpcell.00353.2002. [DOI] [PubMed] [Google Scholar]
- 48.Riccardi C., Nicoletti I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat. Protoc. (2006);1:1458–1461. doi: 10.1038/nprot.2006.238. [DOI] [PubMed] [Google Scholar]
- 49.Loo D.T., Rillema J.R. Measurement of cell death. Methods Cell Biol. (1998);57:251–264. doi: 10.1016/s0091-679x(08)61583-6. [DOI] [PubMed] [Google Scholar]
- 50.Thorburn A. Death receptor-induced cell killing. Cell. Signalling. (2004);16:139–144. doi: 10.1016/j.cellsig.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 51.Kim H.K., Park H.R., Lee J.S., Chung T.S., Chung H.Y., Chung J. Down-regulation of iNOS and TNFalpha expression by kaempferol via NF-kappaB inactivation in aged rat gingival tissues. Biogerontology. (2007);8:399–408. doi: 10.1007/s10522-007-9083-9. [DOI] [PubMed] [Google Scholar]
- 52.Pang J.L., Ricupero D.A., Huang S., Fatma N., Singh D.P., Romero J.R., Chattopadhyay N. Differential activity of kaempferol and quercetin in attenuating tumor necrosis factor receptor family signaling in bone cells. Biochem. Pharmacol. (2006);71:818–826. doi: 10.1016/j.bcp.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 53.Jin Z., McDonald E.R. 3rd., Dicker D.T., El-Deiry W.S. Deficient tumor necrosis factor-related apoptosisinducing ligand (TRAIL) death receptor transport to the cell surface in human colon cancer cells selected for resistance to TRAIL-induced apoptosis. J. Biol. Chem. (2004);279:35829–35839. doi: 10.1074/jbc.M405538200. [DOI] [PubMed] [Google Scholar]
- 54.Yoshida T., Konishi M., Horinaka M., Yasuda T., Goda A.E., Taniguchi H., Yano K., Wakada M., Sakai T. Kaempferol sensitizes colon cancer cells to TRAILinduced apoptosis. Biochem. Biophys. Res. Commun. (2008);375:129–133. doi: 10.1016/j.bbrc.2008.07.131. [DOI] [PubMed] [Google Scholar]
- 55.Siegelin M.D., Reuss D.E., Habel A., Herold-Mende C., von Deimling A. The flavonoid kaempferol sensitizes human glioma cells to TRAIL-mediated apoptosis by proteasomal degradation of survivin. Mol. Cancer Ther. (2008);7:3566–3574. doi: 10.1158/1535-7163.MCT-08-0236. [DOI] [PubMed] [Google Scholar]
- 56.Deribe Y.L., Pawson T., Dikic I. Post-translational modifications in signal integration. Nat. Struct. Mol.Biol. (2010);17:666–672. doi: 10.1038/nsmb.1842. [DOI] [PubMed] [Google Scholar]
- 57.Alenzi F.Q., Lotfy M., Wyse R. Swords of cell death: caspase activation and regulation. Asian Pac. J. Cancer Prev. (2010);11:271–280. [PubMed] [Google Scholar]
- 58.Bestwick C.S., Milne L., Duthie S.J. Kaempferol induced inhibition of HL-60 cell growth results from a heterogeneous response, dominated by cell cycle alterations. Chem. Biol. Interact. (2007);170:76–85. doi: 10.1016/j.cbi.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 59.Engel T., Henshall D.C. Apoptosis, Bcl-2 family proteins and caspases: the ABCs of seizure-damage and epileptogenesis? Int. J. Physiol. Pathophysiol. Pharmacol. (2009);1:97–115. [PMC free article] [PubMed] [Google Scholar]
- 60.Indran I.R., Tufo G., Pervaiz S., Brenner C. Recent advances in apoptosis, mitochondria and drug resistance in cancer cells. Biochim. Biophys. Acta. (2011);1807:735–745. doi: 10.1016/j.bbabio.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 61.Nguyen T.T., Tran E., Ong C.K., Lee S.K., Do P.T., Huynh T.T., Nguyen T.H., Lee J.J., Tan Y., Ong C.S., Huynh H. Kaempferol-induced growth inhibition and apoptosis in A549 lung cancer cells is mediated by activation of MEK-MAPK. J. Cell. Physiol. (2003);197:110–121. doi: 10.1002/jcp.10340. [DOI] [PubMed] [Google Scholar]
- 62.Kim Y.M., Talanian R.V., Billiar T.R. Nitric oxide inhibits apoptosis by preventing increases in caspase-3-like activity via two distinct mechanisms. J. Biol. Chem. (1997);272:31138–31148. doi: 10.1074/jbc.272.49.31138. [DOI] [PubMed] [Google Scholar]
- 63.Cooper C.E. Nitric oxide and cytochrome oxidase: substrate, inhibitor or effector? Trends Biochem. Sci. (2002);27:33–39. doi: 10.1016/S0968-0004(01)02035-7. [DOI] [PubMed] [Google Scholar]
- 64.Cook J.A., Gius D., Wink D.A., Krishna M.C., Russo A., Mitchell J.B. Oxidative stress, redox, and the tumor microenvironment. Semin. Radiat. Oncol. (2004);14:259–266. doi: 10.1016/j.semradonc.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 65.Reade M.C., Millo J.L., Young J.D., Boyd C.A. Nitric oxide synthase is downregulated, while haem oxygenase is increased, in patients with septic shock. Br. J. Anaesth. (2005);94:468–473. doi: 10.1093/bja/aei082. [DOI] [PubMed] [Google Scholar]
- 66.García-Mediavilla V., Crespo I., Collado P.S., Esteller A., Sánchez-Campos S., Tuñón M.J., González-Gallego J. The anti-inflammatory flavones quercetin and kaempferol cause inhibition of inducible nitric oxide synthase, cyclooxygenase- 2 and reactive C-protein, and down-regulation of the nuclear factor kappaB pathway in Chang Liver cells. Eur. J. Pharmacol. (2007);557:221–229. doi: 10.1016/j.ejphar.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 67.Jiang S., Cheng R., Wang X., Xue T., Liu Y., Nel A., Huang Y., Duan X. Real-time electrical detection of nitric oxide in biological systems with sub-nanomolar sensitivity. Nat. Commun. (2013);4:2225. doi: 10.1038/ncomms3225. [DOI] [PubMed] [Google Scholar]
- 68.Rostoka E., Baumane L., Isajevs S., Line A., Dzintare M., Svirina D., Sharipova J., Silina K., Kalvinsh I., Sjakste N. Effects of kaempferol and myricetin on inducible nitric oxide synthase expression and nitric oxide production in rats. Basic Clin. Pharmacol. Toxicol. (2010);106:461–466. doi: 10.1111/j.1742-7843.2009.00526.x. [DOI] [PubMed] [Google Scholar]
- 69.Boberg J., Mandrup K.R., Jacobsen P.R., Isling L.K., Hadrup N., Berthelsen L., Elleby A., Kiersgaard M., Vinggaard A.M., Hass U., Nellemann C. Endocrine disrupting effects in rats perinatally exposed to a dietary relevant mixture of phytoestrogens. Reprod. Toxicol. (2013);40:41–51. doi: 10.1016/j.reprotox.2013.05.014. [DOI] [PubMed] [Google Scholar]


