Abstract
The silkworm extract powder contain 1-deoxynojirimycin (DNJ), a potent α-glycosidase inhibitor, has therapeutic potency against diabetes mellitus. Therefore, natural products containing DNJ from mulberry leaves and silkworm are consumed as health functional food. The present study was performed to evaluate the safety of the silkworm extract powder, a health food which containing the DNJ. The repeated toxicity studies and gentic toxicity studies of the silkworm extract powder were performed to obtain the data for new functional food approval in MFDS. The safety was evaluated by a single-dose oral toxicity study and a 90 day repeated-dose oral toxicity study in Sprague-Dawley rats. The silkworm extract powder was also evaluated for its mutagenic potential in a battery of genetic toxicity test: in vitro bacterial reverse mutation assay, in vitro chromosomal aberration test, and in vivo mouse bone marrow micronucleus assay. The results of the genetic toxicology assays were negative in all of the assays. The approximate lethal dose in single oral dose toxicity study was considered to be higher than 5000 mg/kg in rats. In the 90 day study, the dose levels were wet at 0, 500, 1000, 2000 mg/kg/day, and 10 animals/sex/dose were treated with oral gavage. The parameters that were monitored were clinical signs, body weights, food and water consumptions, ophthalmic examination, urinalysis, hematology, serum biochemistry, necropsy findings, organ weights, and histopathological examination. No adverse effects were observed after the 90 day administration of the silkworm extract powder. The No-Observed-Adverse-Effect-Level (NOAEL) of silkworm extract powder in the 90 day study was 2000 mg/kg/day in both sexes, and no target organ was identified.
Keywords: Single oral dose toxicity, 90 day repeated dose toxicity, Genotoxicity, Silkworm extract powder
INTRODUCTION
Recently, the World Health Organization (WHO) estimated that 80% of people worldwide rely on herbal medicines for some part of their primary health care (1). A piperidine alkaloid, 1-deoxynojirimycin (DNJ), is known as one of the most potent α-glycosidase inhibitor (2-4). DNJ binds to α-glycosidase active site in small intestine and suppresses elevation of glucose absorption and blood sugar level (5-7). The major functional component of silkworm extract powder (SEP) is DNJ which exerts blood sugar-lowering effect. Due to its potential in hyperglycemia inhibition, the preventive effect of DNJ on diabetes has been widely studied (8,9).
Natural DNJ was first isolated from mulberry roots (10), and known to be highly synthesized in the mulberry (11). Recently, more than 20 kinds of polyhydroxylated alkaloids have been identified from mulberry and silkworm (12- 14). Mulberry leaves are widely cultivated in sericulture and have ecological importance being sole food source of the silkworms. Silkworms have more DNJ than mulberry, suggesting that larvae can accumulate and enrich DNJ from mulberry leaves (15,16). Silkworms and mulberry leaves are consumed extensively in Korea, China, and Japan as functional food and pharmaceutical for diabetes mellitus (DM). Although numerous studies have reported the potential and production of DNJ (17-20) few toxicology studies investigated the possible adverse effects. In order to make DNJ a widely used consumer product, the safety should be secured at first time. In this study, the safety of the SEP was evaluated by a single-dose oral toxicity study and a 90 day repeated-dose oral toxicity study in Sprague-Dawley rats. Mutagenic potential of the silkworm extract powder was evaluated by the three genetic toxicity tests.
This study was conducted in accordance with the current Good Laboratory Practice (GLP), and Test Guidelines of the Organization for Economic Cooperation and Development (OECD) and the Ministry of Food and Drug Safety (MFDS).
All animal experiments were approved by Institutional Animal Care and Use Committee of Chemon Nonclinical Research Institute. Chemon Inc. has received full accreditation from the AAALAC International in 2010.
MATERIALS AND METHODS
Silkworm extract powder. The SEP (Lot number: NAAS-SILK-1) was provided by the Department of Agricultural Biology, National Academy of Agricultural Science, Rural Development Administration (Suwon, Korea) and stored under refrigerated condition before use. Briefly, larvae of the silkworm strain YeonNokJam (Bombyx mori) were reared by feeding mulberry leaves in spring season at 2012 in National Academy of Agricultural Science.
The 5th instar 3rd day larvae were quickly frozen with the liquid nitrogen and lyophilized. The lyophilized silkworm was extracted with ethanol and lyophilized again to produce the SEP. Quantitation of DNJ in SEP was performed by Global Health Care (Seoul, Korea) by doubleextraction method (21). The content of DNJ in SEP used in this study was 1.25%.
Experimental animals and husbandry. Specific pathogen- free (SPF) Sprague-Dawley rats (Hsd:Sprague Dawley ®TMSD®TM) were used for general toxicity studies. SPF ICR mice (Hsd:ICR CD-1®) were used for the bone marrow micronucleus assay. The animals were obtained from Koatech Co., Ltd. (Pyeongtake-si, Gyeonggi-do, Korea).
Animals were kept in the laboratory animal facility with temperature and relative humidity maintained at 23 ± 3℃ and 55 ± 15%, respectively, 12 hr-light-dark cycle and 10~ 20 air changes per hour. The animals for the single-dose toxicity study were housed in stainless steel cages with mesh bottom. The animals for the repeated-dose studies and the micronucleus test were housed in solid bottom polycarbonate cages with bedding. Animals were offered irradiation-sterilized pellet feed (Teklad Certified Irradiated Global 18% Protein Rodent Diet, 2918C, Harlan Co., Hayward, CA, USA) and groundwater disinfected by an ultraviolet sterilizer and ultra-filtration ad libitum. All animals were acclimated for 7 days before the start of administration.
Preparation of dose formulations. SEP for oral administration or treatment was prepared in sterile distilled water, except the in vitro chromosome aberration test (CAT). For the CAT, SEP was dissolved in the complete culture medium. The sterile distilled water (Dailhan Pharm.co., Seoul, Korea) or the culture medium (Invitrogen, USA) served as vehicle.
Single oral dose toxicity study. Five male and five female rats (8 weeks old) per dose were administered with SEP at 0, 1250, 2500, and 5000 mg/kg. Observations of clinical signs and mortality were checked continuously for the first one hour, and then every hour until six hours after administration and daily for 14 days thereafter. Body weight was observed throughout the 15 day experimental period, and gross findings were observed at necropsy.
90 day repeated-dose oral toxicity. The dose range of 90 day study was selected based on the results of the 4 week study. Five male and five female rats (6 weeks old) per dose were administered with SEP at 0, 250, 500, 1000, and 2000 mg/kg/day. No SEP-related changes were observed. Therefore, the high dose of 90 day study was set at 2000 mg/kg/day.
Ten male and ten female rats (6 weeks old) per dose were administered with SEP at 0, 500, 1000, and 2000 mg/kg/day. Additional five animals/sex were assigned for recovery study for the control and the high dose group.
Clinical signs and mortalities were checked once a day, and the type, date of occurrence, and severity of signs recorded individually. Body weights were measured before initiation of dosing (day 1) and once weekly throughout the experiment. Animals were fasted overnight before necropsy and body weights were recorded at necropsy. Food and water consumption were measured on the scheduled body weight weighing days.
Ophthalmological examination was observed five animals/ sex/dose of main group in the last week of observation. A mydriatic (Mydriacyl Eye Drops 1%, Lot No.: 11J20F, MydriacylTM, Alkon Korea) was dropped into the both eyeballs to facilitate mydriasis. The anterior parts of the eye, optic media and fundus were observed with an ophthalmoscope and fundus camera (Vantage Plus Digital LED, Keeler Instruments Inc., USA). No photographs were taken since there were no abnormal signs in any of the animals. Animals for recovery study were subjected to ophthalmological examination in the last week of recovery.
Urinalysis was done for main groups in the last week of administration. Five animals/sex/dose were housed individual in metabolic cages and urine samples were collected 3 hr for urinalysis and urine sediment test. Total urine volume, collected for 24 hr, was also measured. Animals for recovery study were subjected to urinalysis in the last week of recovery.
Urine samples were analyzed for protein (PRO), specific gravity (SG), pH, urobilinogen (URO), occult blood (BLO), ketone body (KET), glucose (GLU), bilirubin (BIL), white blood cell (WBC), nitrite (NIT), Erythrocytes (RBC), cast, epithelial cells, urine color and clarity using a CliniTek-100 urine chemistry analyzer (Ames Division, Miles Laboratory, USA).
At necropsy, all over-night fasted animals were anesthetized by isoflurane (Ifran liquid, Hana Pharm,Co., Ltd, Korea) inhalation. Blood samples for hematological and serum biochemical test were collected from posterior vena cava at necropsy.
Hematology parameters (ADVIA 2120, Siemens, USA) were detected red blood cell count (RBC), hemoglobin distribution width (HDW), hemoglobin concentration (HGB), hematocrit (HCT), platelet count (PLT), mean corpuscular volume (MCV), mean cell hemoglobin (MCH), mean cell hemoglobin concentration (MCHC), red cell distribution width (RDW), mean platelet volume (MPV), reticulocytes, white blood cell count (WBC) and WBC differential count [neutrophils, basophils, monocytes, lymphocytes, eosinophils and large unstained cells (LUC)].
Serum biochemical parameters were measured by a serum biochemistry analyzer (AU680, Beckman coulter, Japan). The parameters examined were aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), creatine phosphokinase (CPK), Reticulocyte (RET), total bilirubin (TBil), glucose, total cholesterol (TCho), triglyceride, total protein (TP), albumin, albumin/globulin ratio (A/G ratio), blood urea nitrogen (BUN), creatinine, inorganic phosphorus (IP), calcium (Ca), sodium (Na), potassium ion (K) and chloride ion (Cl).
After collecting the blood samples, animals were sacrificed by bloodletting from the abdominal aorta and posterior vena cava. Body surface, the subcutis, head, and all of the organs in abdominal and thoracic cavity were observed grossly and the findings were recorded. Organs weights were measured with an electronic balance (Satorius AG, Germany): the ovary, uterus, prostate gland, testis, epididymis, adrenal gland, kidney (both), spleen, liver, thymus, heart, lung, brain and pituitary gland. Relative organ weights (%) to the terminal body weights were also calculated.
Bacterial reverse mutation test. The histidine auxotroph strains of Salmonella typhimurium TA100, TA1535, TA98, TA1537 (22) and a tryptophan auxotroph strain of Escherichia coli WP2 uvrA (23) were used. Test strains were treated with SEP with or without metabolic activation system. Test strains and S9 fraction were obtained from Molecular Toxicology Inc. (USA) (Table 1).
Table 1.
Positive control articles used in Bacterial reverse mutation test
| Activation | Positive control article (Abbrev.) | CAS No. | Strains | Conc. (μg/plate) |
|---|---|---|---|---|
|
| ||||
| + | 2-Aminoanthracene (2-AA) | 613-13-8 | TA100 | 1 |
| TA1535 | 2 | |||
| TA1537 | 1 | |||
| WP2 uvrA | 6 | |||
| Benzo[a]pyrene (B[a]P) | 50-32-8 | TA98 | 1 | |
|
| ||||
| - | Sodium azide (SA) | 26628-22-8 | TA100 | 0.5 |
| TA1535 | 0.5 | |||
| 2-Nitrofluorene (2-NF) | 607-57-8 | TA98 | 2 | |
| 4-Nitroquinoline-1-oxide (4NQO) | 56-57-5 | WP2 uvrA | 0.5 | |
| Acridine Mutagen ICR 191 (ICR-191) | 17070-45-0 | TA1537 | 0.5 | |
Dose ranges were selected based on the results of a range-finding test conducted on the test article using the five test strains in both the presence and absence of metabolic activation system with one plate per dose. In the range-finding test, precipitation was observed on the bottom agar at 1500 μg/plate and higher doses. A decrease of revertants per plate was observed at dose of 5000 μg/plate. Therefore, the highest dose of this study was set at 5000 mg/plate for all test strains and a six concentrations (5000, 1500, 500, 150, 50 and 10 mg/plate) were tested in this study.
Chromosomal aberration assay. Chinese hamster lung cells (CHL/IU) were obtained from American Type Culture Collection (Manassas, VA, USA). The Minimum Essential Medium (Gibco, #41500-034), containing 10% fetal bovine serum was used for the routine subculture and chemical treatment. The cells were maintained at 37 ± 1℃ in a 5% CO2- 95% air humidified incubator (Forma 311 and 3111) with cell culture flask (culture surface 75 cm2, Falcon). Cells were subcultured every 2~3 days using 0.1% trypsin solution. Cells were dissociated by trypsin and counted by counter model Z2 (Beckman Coulter) to determine the number of cells per 1 ml.
Dose ranges were selected based on the cytotoxicity (relative cell count, RCC) observed in a range-finding test. The high doses of this main test aimed to produce less than 50% RCC (Table 2).
Table 2.
Dose ranges of Chromosomal aberration assay
| Treatment series | Metabolic activation | Treatment time - recovery time (hr) | Dose of SEP (μg/ml) | Positive control and dose (μg/ml) |
|---|---|---|---|---|
|
| ||||
| 1 | + | 6-18 | 0, 275, 550, 900, 1100 | B[a]P 20 |
| 2 | − | 6-18 | 0, 150, 300, 600, 700 | EMS 800 |
| 3 | − | 24-0 | 0, 150, 300, 600, 700 | EMS 600 |
Rapidly growing cells were trypsinized and three series of 25 cm2 culture flasks were seeded with 6 × 104 cells, each in 5 ml of medium, and incubated for about 3 days before the chemical treatment. After 24 hr from the start of treatment, the mitotic cells of each flask were harvested and slides were prepared. A hundred metaphases per culture (200 metaphases per dose) were evaluated for chromosome aberrations. The results were expressed as mean frequency of metaphases with structural or numerical aberrations per 100 metaphases (24).
Mouse bone marrow micronucleus assay. Six male ICR mice (8 weeks old) per dose were administrated SEP orally at doses of 1250, 2500 and 5000 mg/kg for 2 consecutive days. Cyclophosphamide monohydrate (CPA, Sigma- Aldrich Co.) was used as a positive control article. Positive control had been given intraperitonealy once in the second day. Animals were sacrificed by cervical dislocation at 24 hr after the final administration. Preparation of bone marrow smears and counting of micronuclei was done according to the method of Schmid (25).
Statistical analysis. Data were statistically analyzed with the commercial program SPSS 10.1 K, and the significance level was set at p < 0.05.
Body weights, food and water consumption, hematological and serum biochemistry data, organ weights, and PCE:RBC ratio were assumed to be normally distributed and analyzed by one-way ANOVA. If the overall ANOVA was significant and the assumption of homogeneity of variance was met, then Duncan’s multiple range test was used as a post hoc test to find out which group was significantly different from negative control group. If the sample size was not equal for each group Scheffe test was used instead. If the assumption of homogeneity of variance was not met, Dunnett T3 test was used as a post hoc test.
For the urinalysis data, the rank transformation was performed and analyzed by the Mann-Whitney U-test, in order to find out which group is significantly different from control group.
For the frequency of micronucleus the rank transformation was performed and analyzed by the non-parametric Kruskal-Wallis’ H-test. If there was a statistically significant difference between groups, then the Mann-Whitney Utest was used to find out which group is significantly different from negative control group.
For the chromosome aberration test, Fisher’s exact test was used to compare the frequency of aberrant cell between negative control and treated groups.
In this study, unless specified, the term “significant” in the sentence with P-value implies that the inter-group differences have attained statistical significance compared to the control group.
RESULTS
Single oral dose toxicity study. No unscheduled deaths were observed at any of the doses tested. Soft stool was observed in males (3 cases at 2500 mg/kg, 5 cases at 5000 mg/kg) and females (2 cases at 5000 mg/kg) on day 2. No treatment-related changes were observed in body weight and no gross findings were recorded at necropsy (data not shown). The approximate lethal dose of the SEP was greater than 5000 mg/kg in male and female rats.
90 day Repeated-dose oral toxicity. No SEP-related deaths or abnormal clinical signs were observed at all dose levels (data not shown). Loss of fur was observed from day 5 to 14 and reddish tear was observed from day19 to 21 in one male at 500 mg/kg/day.
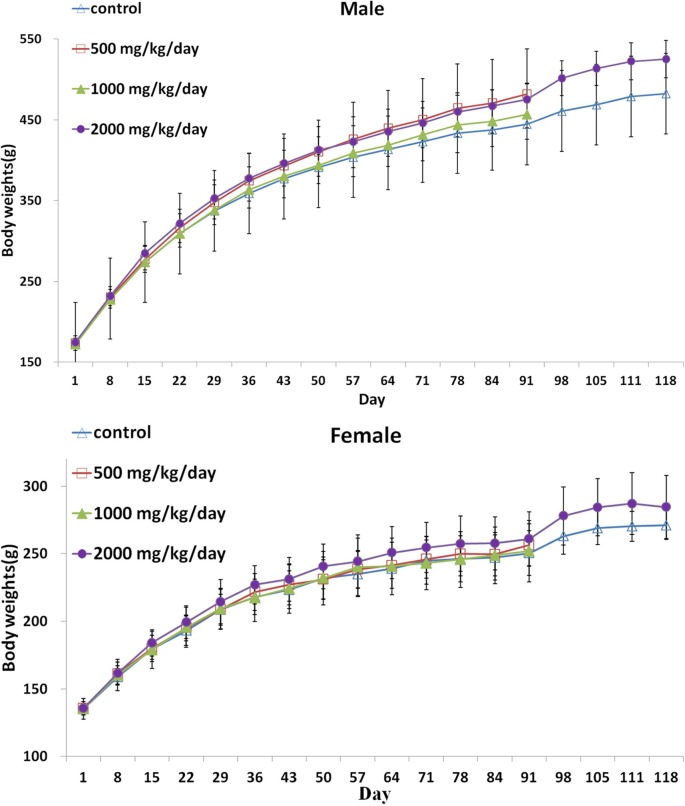
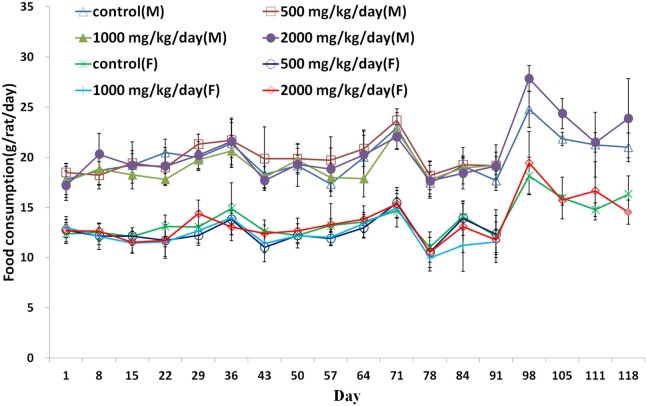
No SEP-related significant changes in body weights, food and water consumption were observed (Fig. 1,Fig. 2 and Fig. 3). The body weight of male at 2000 mg/kg/day on day 84 and 91 and weight gain on day 91 were significantly higher (p < 0.05). During the recovery period, the body weight on day 91, 105, 105, 111, and 118 in male were significantly higher (p < 0.05 or (p < 0.01). A significant increase in food consumption was observed in male at 1000 mg/kg/ day on day 22 (p < 0.05).
Fig. 1. Body weight (Mean ± S.D) of male and female rats treated to 0 (△), 500 (□), 1000 (▲), 2000 (●) mg/kg/day in the 90 day repeated oral dose toxicity study of the Silkworm extract powder with 28 day recovery period. * Significantly different from control at p < 0.05.
Fig. 2. Food consumption (Mean ± S.D) of male rats treated to 0 (△), 500 (□), 1000 (▲), 2000 (●) mg/kg/day and female rats treated to 0 (×), 500 (○), 1000 (|), 2000 (◇) mg/kg/day in the 90 day repeated oral dose toxicity study of the Silkworm extract powder with 28 day recovery period. * Significantly different from control at p<0.05.
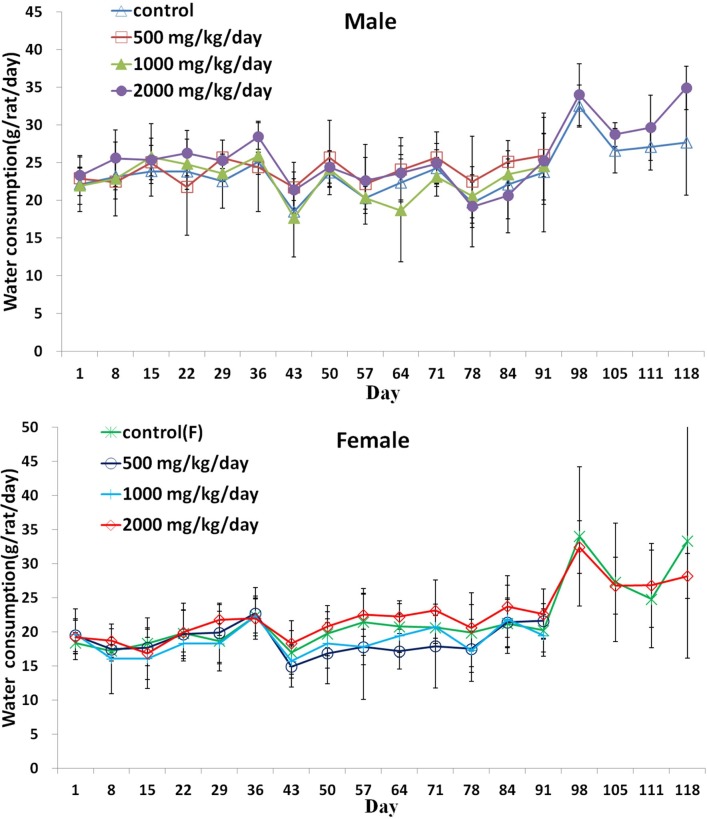
Fig. 3. Water consumption (Mean ± S.D) of of male rats treated to 0 (△), 500 (□), 1000 (▲), 2000 (●) mg/kg/day and female rats treated to (×), 500 (○), 1000 (|), 2000 (◇) mg/kg/day in the 90 day repeated oral dose toxicity study of the Silkworm extract powder with 28 day recovery period.
There were no associations between treatment and the findings recorded in the ophtalmological examinations.
In urinalysis, significant increases were observed in KET level (male at 2000 mg/kg/day), SG (in all male groups), and pH (females at 500 and 2000 mg/kg/day) (p < 0.05 or p < 0.01) (Table 3).
Table 3.
Urinalysis results of the silkworm extract powder
| Main group | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Parameter | Result | Dose (mg/kg/day) | |||||||
|
| |||||||||
| Male | Female | ||||||||
|
|
|
||||||||
| 0 | 500 | 1000 | 2000 | 0 | 500 | 1000 | 2000 | ||
|
| |||||||||
| Glucose | Negative | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Bilirubin | Negative | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Ketone body | Negative | 5 | 5 | 3 | 1 | 5 | 5 | 5 | 5 |
| Trace | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | |
| 15 mg/dl | 0 | 0 | 1 | 3* | 0 | 0 | 0 | 0 | |
| Specific gravity | ≤ 1.005 | 3 | 0* | 0* | 0 | 1 | 1 | 0 | 1 |
| 1.010 | 2 | 4 | 2 | 0 | 3 | 3 | 4 | 3 | |
| 1.015 | 0 | 1* | 1 | 1 | 1 | 0 | 1 | 1 | |
| 1.020 | 0 | 0 | 2* | 4** | 0 | 1 | 0 | 0 | |
| pH | 6.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 7.5 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | |
| 8.0 | 4 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | |
| 8.5 | 1 | 5 | 3 | 4 | 2 | 4 | 5 | 2 | |
| ≥ 9.0 | 0 | 0 | 0 | 0 | 0 | 1* | 0 | 3* | |
| Protein | Negative | 2 | 0 | 0 | 0 | 3 | 3 | 4 | 2 |
| Trace | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 3 | |
| 30 mg/dl | 1 | 2 | 1 | 1 | 1 | 1 | 0 | 0 | |
| 100 mg/dl | 1 | 3 | 2 | 3 | 0 | 0 | 1 | 0 | |
| ≥ 300 mg/dl | 0 | 0 | 1 | 1 | 3 | 3 | 4 | 2 | |
| Urobilinogen Nitrite | 0.2 EU/dl | 5 | 5 | 4 | 4 | 5 | 5 | 5 | 5 |
| 1.0 EU/dl | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | |
| Negative | 4 | 5 | 5 | 4 | 5 | 4 | 5 | 4 | |
| Positive | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | |
| Occult blood | Negative | 2 | 0 | 4 | 4 | 5 | 4 | 5 | 5 |
| Trace | 3 | 4 | 1 | 1 | 0 | 0 | 0 | 0 | |
| Small | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Moderate | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | |
| Clarity | Clear | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| RBC | 0 | 3 | 1 | 2 | 2 | 2 | 1 | 4 | 4 |
| ≤ 4 mean/field | 2 | 4 | 2 | 3 | 2 | 3 | 1 | 1 | |
| 5~8 mean/field | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | |
| WBC | 0 | 0 | 1 | 1 | 0 | 2 | 1 | 3 | 1 |
| ≤ 5 mean/field | 4 | 3 | 2 | 1 | 1 | 3 | 1 | 4 | |
| 6~20 mean/field | 1 | 1 | 0 | 2 | 2 | 1 | 1 | 0 | |
| 21~50 mean/field | 0 | 0 | 2 | 2 | 2 | 0 | 0 | 0 | |
| Epithelial cell | 0/20 field | 1 | 0 | 0 | 2 | 2 | 2 | 3 | 1 |
| Few/20 field | 2 | 1 | 2 | 3 | 1 | 3 | 1 | 4 | |
| Around 1/few field | 1 | 2 | 2 | 0 | 2 | 0 | 1 | 0 | |
| Few/field | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | |
| Casts | 0 | 1 | 4 | 0 | 0 | 2 | 2 | 2 | 1 |
| 1 mean/field | 3 | 1 | 2 | 2 | 2 | 2 | 2 | 4 | |
| 2~5 mean/field | 1 | 0 | 1 | 2 | 1 | 1 | 0 | 0 | |
| 6~10 mean/field | 0 | 0 | 2 | 1 | 0 | 0 | 1 | 0 | |
| Volume (ml/24 hr)a) | 12.2 ± 5.2 | 16.6 ± 3.6 | 14.2 ± 2.3 | 15.4 ± 5.0 | 21.4 ± 8.3 | 17.0 ± 5.1 | 21.4 ± 5.4 | 11.4 ± 3.8 | |
| Color-Yellow | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |
| No. of animals | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |
*/** Significantly different from control at p < 0.05/p<0.01.
a)Values are mean ± S.D.
No SEP-related significant changes in hematological values. A significant decrease in RDW in male at 1000 mg/kg/ day and a significant increase in WBC count in female at 2000 mg/kg/day were observed (p < 0.05 or p < 0.01) (Table 4 and Table 5).
Table 4.
Hematological values of the silkworm extract powder in male rats
| Parameter | Main group (mg/kg/day) | Recovery group (mg/kg/day) | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| 0 | 500 | 1000 | 2000 | 0 | 2000 | ||
|
| |||||||
| RBC | (106/μl) | 8.91 ± 0.43 | 8.68 ± 0.23 | 8.67 ± 0.22 | 8.91 ± 0.32 | 8.87 ± 0.32 | 8.75 ± 0.34 |
| HGB | (g/dl) | 16.1 ± 0.7 | 15.9 ± 0.4 | 15.9 ± 0.3 | 16.1 ± 0.6 | 16.5 ± 0.5 | 16.5 ± 0.3 |
| HCT | (%) | 46.5 ± 1.7 | 46.3 ± 1.2 | 46.1 ± 1.0 | 47.0 ± 1.8 | 45.6 ± 1.4 | 46.0 ± 1.2 |
| MCV | (fl) | 52.3 ± 1.5 | 53.3 ± 0.8 | 53.2 ± 0.8 | 52.8 ± 1.1 | 51.5 ± 0.8 | 52.6 ± 1.3 |
| MCH | (pg) | 18.1 ± 0.6 | 18.4 ± 0.4 | 18.4 ± 0.3 | 18.0 ± 0.4 | 18.6 ± 0.6 | 18.9 ± 0.5 |
| MCHC | (g/dl) | 34.5 ± 0.4 | 34.4 ± 0.5 | 34.6 ± 0.2 | 34.2 ± 0.5 | 36.2 ± 0.6 | 35.9 ± 0.4 |
| RDW | (%) | 12.7 ± 0.3 | 12.8 ± 0.3 | 12.5 ± 0.2* | 12.5 ± 0.2 | 12.8 ± 0.1 | 12.6 ± 0.3 |
| HDW | (g/dl) | 2.73 ± 0.14 | 2.70 ± 0.09 | 2.67 ± 0.12 | 2.60 ± 0.10 | 2.78 ± 0.11 | 2.73 ± 0.16 |
| RET | (%) | 2.56 ± 0.24 | 2.69 ± 0.28 | 2.55 ± 0.22 | 2.60 ± 0.12 | 2.46 ± 0.32 | 2.50 ± 0.19 |
| PLT | (103/μl) | 935.4 ± 80.4 | 948.4 ± 90.6 | 882.1 ± 195.1 | 966.7 ± 88.1 | 922.8 ± 70.4 | 954.0 ± 93.1 |
| MPV | (fl) | 4.8 ± 0.2 | 4.9 ± 0.3 | 5.0 ± 0.5 | 5.0 ± 0.3 | 5.7 ± 0.1 | 5.6 ± 0.4 |
| WBC | (103/μl) | 8.59 ± 1.50 | 9.98 ± 2.24 | 8.71 ± 1.34 | 9.95 ± 1.69 | 8.98 ± 1.64 | 8.46 ± 1.67 |
| NEU | (%) | 23.9 ± 9.6 | 23.8 ± 6.2 | 19.1 ± 6.6 | 23.0 ± 10.0 | 19.7 ± 7.0 | 19.1 ± 4.7 |
| LYM | (%) | 69.5 ± 9.6 | 70.2 ± 6.7 | 73.8 ± 7.5 | 70.1 ± 10.4 | 73.7 ± 6.3 | 74.6 ± 4.7 |
| MONO | (%) | 4.2 ± 1.4 | 3.8 ± 0.7 | 4.4 ± 0.9 | 4.4 ± 0.9 | 4.3 ± 1.7 | 3.9 ± 0.8 |
| EOS | (%) | 1.3 ± 0.1 | 1.2 ± 0.3 | 1.7 ± 0.4 | 1.4 ± 0.2 | 1.4 ± 0.3 | 1.4 ± 0.2 |
| BASO | (%) | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.5 ± 0.2 | 0.5 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 |
| LUC | (%) | 0.6 ± 0.3 | 0.5 ± 0.2 | 0.6 ± 0.2 | 0.6 ± 0.2 | 0.7 ± 0.3 | 0.8 ± 0.2 |
| PT | (sec) | 8.63 ± 0.48 | 8.73 ± 0.50 | 8.94 ± 0.61 | 8.67 ± 0.43 | 9.09 ± 0.17 | 9.00 ± 0.28 |
| APTT | (sec) | 19.3 ± 0.6 | 19.0 ± 1.0 | 18.5 ± 2.6 | 18.9 ± 1.5 | 20.2 ± 0.4 | 19.7 ± 1.3 |
| No. of animals | 10 | 10 | 10 | 10 | 5 | 5 | |
RBC: red blood cell count, HGB: hemoglobin, HCT: hematocrit, MCV: mean corpuscular volume, MCH: mean corpuscular hemoglobin, MCHC: mean corpuscular hemoglobin concentration, RDW: red cell distribution width, HDW: hemoglobin distribution width, RET: reticulocyte, PLT: platelet, MPV: mean platelet volume, WBC: white blood cell count, NEU: neutrophils, LYM: lymphocytes, MONO: monocytes, EOS: eosinophils, BOS: basophils, LUC: large unstained cells, PT: prothrombin time, APTT: active partial thromboplastin time.
Values are mean ± S.D.
**: Significantly different from control at p < 0.01.
Table 5.
Hematological values of the silkworm extract powder in female rats
| Parameter | Main group (mg/kg/day) | Recovery group (mg/kg/day) | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| 0 | 500 | 1000 | 2000 | 0 | 2000 | ||
|
| |||||||
| RBC | (106/μl ) | 7.71 ± 0.23 | 7.86 ± 0.28 | 7.79 ± 0.25 | 7.72 ± 0.18 | 7.81 ± 0.35 | 7.72 ± 0.16 |
| HGB | (g/dl) | 14.4 ± 0.5 | 14.4 ± 0.4 | 14.3 ± 0.4 | 14.3 ± 0.6 | 14.7 ± 0.4 | 15.0 ± 0.3 |
| HCT | (%) | 41.7 ± 1.5 | 42.1 ± 1.2 | 41.9 ± 1.2 | 41.5 ± 1.6 | 40.8 ± 0.7 | 41.1 ± 1.4 |
| MCV | (fl) | 54.1 ± 1.2 | 53.6 ± 2.7 | 53.9 ± 2.1 | 53.7 ± 1.4 | 52.4 ± 2.8 | 53.3 ± 0.9 |
| MCH | (pg) | 18.7 ± 0.3 | 18.4 ± 1.0 | 18.4 ± 0.6 | 18.5 ± 0.5 | 18.9 ± 1.2 | 19.4 ± 0.3 |
| MCHC | (g/dl) | 34.5 ± 0.6 | 34.3 ± 0.4 | 34.1 ± 0.6 | 34.5 ± 0.4 | 36.0 ± 0.8 | 36.4 ± 0.8 |
| RDW | (%) | 11.4 ± 0.3 | 11.6 ± 0.4 | 11.5 ± 0.2 | 11.3 ± 0.4 | 11.7 ± 0.6 | 11.5 ± 0.5 |
| HDW | (g/dl) | 2.41 ± 0.14 | 2.44 ± 0.12 | 2.45 ± 0.12 | 2.41 ± 0.17 | 2.46 ± 0.19 | 2.44 ± 0.18 |
| RET | (%) | 2.56 ± 0.56 | 2.86 ± 0.68 | 2.67 ± 0.55 | 2.86 ± 0.53 | 2.69 ± 0.72 | 2.41 ± 0.56 |
| PLT | (103/μl) | 1053.4 ± 64.0 | 992.7 ± 241.3 | 1131.1 ± 120.8 | 1060.0 ± 70.7 | 1041.2 ± 69.5 | 1012.0 ± 84.8 |
| MPV | (fl) | 5.8 ± 0.6 | 6.1 ± 0.5 | 5.8 ± 0.5 | 6.0 ± 0.5 | 5.3 ± 0.3 | 5.2 ± 0.1 |
| WBC | (103/μl) | 4.41 ± 1.37 | 4.71 ± 0.87 | 5.24 ± 1.03 | 6.10 ± 1.51** | 4.33 ± 1.09 | 3.88 ± 0.58 |
| NEU | (%) | 15.9 ± 6.5 | 14.2 ± 4.3 | 16.6 ± 6.9 | 16.0 ± 5.3 | 16.8 ± 5.2 | 11.6 ± 2.6 |
| LYM | (%) | 78.0 ± 6.7 | 78.4 ± 5.4 | 76.5 ± 7.3 | 76.8 ± 5.8 | 75.2 ± 5.0 | 80.1 ± 2.2 |
| MONO | (%) | 3.4 ± 1.0 | 4.5 ± 1.6 | 4.2 ± 1.4 | 4.4 ± 1.2 | 4.5 ± 0.1 | 5.0 ± 1.2 |
| EOS | (%) | 1.8 ± 0.3 | 1.9 ± 0.3 | 1.8 ± 0.4 | 1.9 ± 0.3 | 2.3 ± 0.6 | 2.4 ± 0.6 |
| BASO | (%) | 0.3 ± 0.1 | 0.4 ± 0.2 | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.2 ± 0.0 | 0.1 ± 0.1 |
| LUC | (%) | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.6 ± 0.1 | 0.9 ± 0.2 | 0.8 ± 0.2 |
| PT | (sec) | 8.81 ± 0.37 | 9.00 ± 0.26 | 8.97 ± 0.34 | 9.05 ± 0.34 | 8.82 ± 0.33 | 8.97 ± 0.29 |
| APTT | (sec) | 17.5 ± 1.4 | 17.7 ± 1.6 | 18.0 ± 0.7 | 17.6 ± 0.8 | 19.3 ± 0.4 | 19.4 ± 0.5 |
| No. of animals | 10 | 10 | 10 | 10 | 5 | 5 | |
RBC: red blood cell count, HGB: hemoglobin, HCT: hematocrit, MCV: mean corpuscular volume, MCH: mean corpuscular hemoglobin, MCHC: mean corpuscular hemoglobin concentration, RDW: red cell distribution width, HDW: hemoglobin distribution width, RET: reticulocyte, PLT: platelet, MPV: mean platelet volume, WBC: white blood cell count, NEU: neutrophils, LYM: lymphocytes, MONO: monocytes, EOS: eosinophils, BOS: basophils, LUC: large unstained cells, PT: prothrombin time, APTT: active partial thromboplastin time. Values are mean ± S.D.
*: Significantly different from vehicle control at p<0.05.
Serum biochemistry data were shown in Table 6 and Table 7.
Table 6.
Serum biochemical values of the silkworm extract powder in male rats
| Parameter | Main group (mg/kg/day) | Recovery group (mg/kg/day) | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| 0 | 500 | 1000 | 2000 | 0 | 2000 | ||
|
| |||||||
| AST | (U/L) | 83.4 ± 15.1 | 75.3 ± 9.0 | 86.2 ± 13.2 | 79.5 ± 8.0 | 106.6 ± 28.3 | 94.2 ± 9.1 |
| ALT | (U/L) | 41.0 ± 5.8 | 36.8 ± 5.7 | 42.6 ± 5.1 | 41.0 ± 5.0 | 52.7 ± 14.6 | 44.0 ± 5.4 |
| ALP | (U/L) | 101.6 ± 20.0 | 89.1 ± 16.4 | 100.3 ± 25.0 | 108.0 ± 19.6 | 97.8 ± 27.8 | 90.1 ± 18.2 |
| CPK | (U/L) | 172.5 ± 83.1 | 164.0 ± 76.1 | 166.3 ± 91.1 | 139.0 ± 71.8 | 210.8 ± 48.9 | 319.0 ± 48.5** |
| TBil | (mg/dl) | 0.15 ± 0.02 | 0.15 ± 0.01 | 0.15 ± 0.02 | 0.15 ± 0.01 | 0.19 ± 0.08 | 0.16 ± 0.01 |
| GLU | (mg/dl) | 117.7 ± 7.8 | 126.5 ± 11.1 | 126.4 ± 10.1 | 126.7 ± 7.0 | 112.3 ± 9.3 | 120.4 ± 7.3 |
| TCho | (mg/dl) | 110.9 ± 13.6 | 106.4 ± 14.8 | 92.8 ± 15.5* | 100.9 ± 11.7 | 111.2 ± 12.9 | 111.4 ± 10.9 |
| TG | (mg/dl) | 40.3 ± 11.1 | 51.7 ± 24.7 | 42.7 ± 12.3 | 41.1 ± 5.7 | 52.2 ± 10.1 | 59.2 ± 9.8 |
| TP | (g/dl) | 6.13 ± 0.20 | 6.12 ± 0.15 | 5.94 ± 0.19 | 6.03 ± 0.27 | 6.03 ± 0.23 | 5.94 ± 0.19 |
| ALB | (g/dl) | 2.94 ± 0.12 | 2.94 ± 0.07 | 2.91 ± 0.12 | 2.94 ± 0.09 | 2.88 ± 0.11 | 2.90 ± 0.10 |
| A/G | (ratio) | 0.92 ± 0.04 | 0.92 ± 0.04 | 0.96 ± 0.05 | 0.95 ± 0.07 | 0.92 ± 0.02 | 0.95 ± 0.05 |
| BUN | (mg/dl) | 15.8 ± 1.3 | 14.6 ± 1.3 | 14.8 ± 2.2 | 16.1 ± 1.5 | 15.5 ± 2.7 | 15.9 ± 1.8 |
| CRE | (mg/dl) | 0.47 ± 0.03 | 0.48 ± 0.03 | 0.47 ± 0.03 | 0.47 ± 0.03 | 0.52 ± 0.05 | 0.50 ± 0.03 |
| IP | (mg/dl) | 6.35 ± 0.21 | 6.28 ± 0.19 | 6.18 ± 0.31 | 6.46 ± 0.33 | 5.96 ± 0.65 | 5.97 ± 0.31 |
| Ca2+ | (mg/dl) | 9.73 ± 0.22 | 9.82 ± 0.20 | 9.61 ± 0.15 | 9.74 ± 0.27 | 9.59 ± 0.27 | 9.61 ± 0.15 |
| Na+ | (mmol/L) | 139.78 ± 1.01 | 139.44 ± 0.71 | 139.63 ± 1.08 | 139.35 ± 0.63 | 139.79 ± 0.84 | 139.15 ± 0.93 |
| K+ | (mmol/L) | 4.74 ± 0.18 | 4.82 ± 0.18 | 4.65 ± 0.26 | 4.84 ± 0.15 | 4.57 ± 0.19 | 4.88 ± 0.13* |
| Cl- | (mmol/L) | 102.43 ± 1.03 | 101.70 ± 0.73 | 102.28 ± 1.23 | 101.67 ± 1.30 | 101.30 ± 0.61 | 101.73 ± 0.43 |
| No. of animals | 10 | 10 | 10 | 10 | 5 | 5 | |
AST; aspartate aminotransferase, ALT; alanine aminotransferase, ALP; alkaline phosphatase, CPK; creatine phosphokinase, TBil; total bilirubin, GLU: glucose, TCho; total cholesterol, TG: triglyceride, TP; total protein, ALB: albumin, A/G; albumin/globulin, BUN; blood urea nitrogen, CRE: creatinine, IP: inorganic phosphorus. Ca2 : calcium, Na : sodium, K : potassium, Cl−: chloride. Values are mean ± S.D.
*/** Significantly different from control at p<0.05/p<0.01.
Table 7.
Serum biochemical values of the silkworm extract powder in female rats
| Parameter | Main group (mg/kg/day) | Recovery group (mg/kg/day) | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| 0 | 500 | 1000 | 2000 | 0 | 2000 | ||
|
| |||||||
| AST | (U/L) | 92.1 ± 12.6 | 85.2 ± 13.4 | 89.0 ± 13.9 | 80.6 ± 13.6 | 112.8 ± 32.0 | 89.5 ± 16.8 |
| ALT | (U/L) | 36.2 ± 5.2 | 34.2 ± 7.5 | 34.7 ± 6.4 | 36.6 ± 3.8 | 42.3 ± 7.1 | 38.9 ± 6.4 |
| ALP | (U/L) | 76.1 ± 11.0 | 81.1 ± 13.8 | 75.2 ± 15.3 | 94.8 ± 19.9* | 73.4 ± 19.7 | 61.5 ± 7.3 |
| CPK | (U/L) | 159.8 ± 88.8 | 135.6 ± 54.2 | 145.5 ± 66.7 | 100.9 ± 26.6 | 182.4 ± 42.5 | 151.8 ± 56.8 |
| TBil | (mg/dl) | 0.19 ± 0.03 | 0.19 ± 0.02 | 0.19 ± 0.02 | 0.20 ± 0.03 | 0.24 ± 0.04 | 0.19 ± 0.02* |
| GLU | (mg/dl) | 109.4 ± 9.1 | 114.3 ± 7.6 | 113.0 ± 9.5 | 113.9 ± 15.2 | 113.7 ± 7.0 | 121.3 ± 7.9 |
| TCho | (mg/dl) | 95.4 ± 14.5 | 96.2 ± 19.5 | 105.7 ± 15.7 | 99.3 ± 16.7 | 111.2 ± 25.0 | 105.6 ± 27.3 |
| TG | (mg/dl) | 26.1 ± 3.9 | 24.2 ± 3.5 | 29.0 ± 6.3 | 25.1 ± 5.1 | 28.2 ± 7.3 | 33.8 ± 6.3 |
| TP | (g/dl) | 5.79 ± 0.20 | 5.91 ± 0.22 | 5.90 ± 0.16 | 5.89 ± 0.19 | 5.99 ± 0.37 | 5.93 ± 0.14 |
| ALB | (g/dl) | 3.04 ± 0.07 | 3.06 ± 0.09 | 3.05 ± 0.13 | 3.00 ± 0.13 | 3.17 ± 0.25 | 3.14 ± 0.15 |
| A/G | (ratio) | 1.11 ± 0.05 | 1.07 ± 0.05 | 1.07 ± 0.07 | 1.04 ± 0.08 | 1.13 ± 0.08 | 1.13 ± 0.07 |
| BUN | (mg/dl) | 19.9 ± 2.3 | 19.6 ± 3.6 | 18.9 ± 2.6 | 19.9 ± 2.9 | 18.2 ± 3.1 | 16.7 ± 1.0 |
| CRE | (mg/dl) | 0.55 ± 0.05 | 0.54 ± 0.06 | 0.54 ± 0.05 | 0.52 ± 0.03 | 0.56 ± 0.01 | 0.55 ± 0.03 |
| IP | (mg/dl) | 5.32 ± 0.51 | 5.54 ± 0.47 | 5.57 ± 0.56 | 5.55 ± 0.42 | 4.85 ± 0.34 | 4.71 ± 0.40 |
| Ca2+ | (mg/dl) | 9.41 ± 0.16 | 9.52 ± 0.09 | 9.51 ± 0.17 | 9.49 ± 0.23 | 9.47 ± 0.20 | 9.42 ± 0.24 |
| Na+ | (mmol/L) | 138.45 ± 0.87 | 138.92 ± 0.78 | 139.13 ± 0.76 | 138.74 ± 0.70 | 138.29 ± 0.72 | 138.68 ± 0.78 |
| K+ | (mmol/L) | 4.44 ± 0.27 | 4.35 ± 0.36 | 4.46 ± 0.29 | 4.38 ± 0.15 | 4.49 ± 0.12 | 4.48 ± 0.09 |
| Cl- | (mmol/L) | 103.69 ± 1.19 | 103.78 ± 0.88 | 103.91 ± 0.78 | 104.57 ± 1.15 | 102.98 ± 1.29 | 103.01 ± 1.17 |
| No. of animals | 10 | 10 | 10 | 10 | 5 | 5 | |
AST; aspartate aminotransferase, ALT; alanine aminotransferase, ALP; alkaline phosphatase, CPK; creatine phosphokinase, TBil; total bilirubin, GLU: glucose, TCho; total cholesterol, TG: triglyceride, TP; total protein, ALB: albumin, A/G; albumin/globulin, BUN; blood urea nitrogen, CRE: creatinine, IP: inorganic phosphorus. Ca2+: calcium, Na+: sodium, K+: potassium, Cl−: chloride.
Values are mean ± S.D.
*Significantly different from control at p<0.05.
TCho in male at 1000 mg/kg was significantly decreased (p < 0.05) but ALP in female at 2000 mg/kg/day was significantly increased (p < 0.05). At the end of recovery period, levels of CPK and K+ in male at 2000 mg/kg/day were significantly increased (p < 0.05 or p < 0.01) but the level of TBil in female at 2000 mg/kg/day was significantly decreased (p < 0.05).
There were no SEP-related significant changes in absolute and relative organ weights (Table 8 and Table 9). There were significant increases in absolute weights of both adrenal gland and absolute weight of left kidney in male at 500 and 2000 mg/kg/day (p < 0.05 or p < 0.01). There was a significant increase in relative weight of liver in male at 2000 mg/kg/day (p < 0.05).
Table 8.
Absolute and relative organ weights of the silkworm extract powder in male rats
| Parameters | Main group (mg/kg/day) | Recovery group (mg/kg/day) | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| 0 | 500 | 1000 | 2000 | 0 | 2000 | |
|
| ||||||
| Body weights (g)a) | 419.93 ± 15.09 | 459.35 ± 53.46 | 434.28 ± 23.35 | 439.99 ± 31.47 | 461.03 ± 23.01 | 503.19 ± 25.81* |
| Adrenal gland-left | 0.024 ± 0.002 | 0.028 ± 0.003** | 0.025 ± 0.003 | 0.028 ± 0.004* | 0.025 ± 0.002 | 0.023 ± 0.009 |
| % to body weight | 0.006 ± 0.001 | 0.006 ± 0.001 | 0.006 ± 0.001 | 0.006 ± 0.001 | 0.006 ± 0.000 | 0.005 ± 0.002 |
| Adrenal gland-right | 0.023 ± 0.003 | 0.028 ± 0.004** | 0.024 ± 0.003 | 0.027 ± 0.003* | 0.026 ± 0.002 | 0.027 ± 0.003 |
| % to body weight | 0.006 ± 0.001 | 0.006 ± 0.000 | 0.006 ± 0.001 | 0.006 ± 0.001 | 0.006 ± 0.000 | 0.005 ± 0.001 |
| Pituitary gland | 0.011 ± 0.001 | 0.012 ± 0.002 | 0.012 ± 0.001 | 0.012 ± 0.002 | 0.012 ± 0.001 | 0.013 ± 0.001 |
| % to body weight | 0.003 ± 0.000 | 0.003 ± 0.000 | 0.003 ± 0.000 | 0.003 ± 0.000 | 0.003 ± 0.000 | 0.003 ± 0.000 |
| Thymus | 0.307 ± 0.070 | 0.316 ± 0.061 | 0.285 ± 0.057 | 0.285 ± 0.064 | 0.235 ± 0.061 | 0.265 ± 0.041 |
| % to body weight | 0.073 ± 0.016 | 0.069 ± 0.013 | 0.066 ± 0.015 | 0.065 ± 0.015 | 0.051 ± 0.011 | 0.053 ± 0.009 |
| Prostate gland | 0.595 ± 0.094 | 0.651 ± 0.1070 | 0.644 ± 0.094 | 0.583 ± 0.116 | 0.658 ± 0.051 | 0.691 ± 0.210 |
| % to body weight | 0.142 ± 0.022 | 0.144 ± 0.029 | 0.149 ± 0.023 | 0.133 ± 0.028 | 0.142 ± 0.017 | 0.139 ± 0.045 |
| Testis-left | 1.921 ± 0.352 | 1.995 ± 0.176 | 2.007 ± 0.107 | 2.105 ± 0.163 | 2.125 ± 0.140 | 2.158 ± 0.169 |
| % to body weight | 0.458 ± 0.085 | 0.438 ± 0.053 | 0.463 ± 0.030 | 0.480 ± 0.044 | 0.462 ± 0.040 | 0.429 ± 0.032 |
| Testis-right | 2.026 ± 0.157 | 2.047 ± 0.151 | 2.026 ± 0.087 | 2.108 ± 0.160 | 2.125 ± 0.094 | 2.196 ± 0.163 |
| % to body weight | 0.483 ± 0.037 | 0.449 ± 0.043 | 0.468 ± 0.027 | 0.481 ± 0.044 | 0.462 ± 0.035 | 0.438 ± 0.043 |
| Epididymis-left | 0.631 ± 0.100 | 0.689 ± 0.075 | 0.672 ± 0.037 | 0.704 ± 0.048 | 0.726 ± 0.018 | 0.749 ± 0.048 |
| % to body weight | 0.150 ± 0.023 | 0.151 ± 0.016 | 0.155 ± 0.007 | 0.161 ± 0.014 | 0.158 ± 0.010 | 0.149 ± 0.015 |
| Epididymis-right | 0.687 ± 0.058 | 0.720 ± 0.055 | 0.700 ± 0.053 | 0.706 ± 0.041 | 0.742 ± 0.035 | 0.771 ± 0.026 |
| % to body weight | 0.164 ± 0.011 | 0.158 ± 0.015 | 0.161 ± 0.010 | 0.161 ± 0.012 | 0.161 ± 0.012 | 0.154 ± 0.012 |
| Spleen | 0.828 ± 0.106 | 0.912 ± 0.158 | 0.833 ± 0.122 | 0.856 ± 0.091 | 0.830 ± 0.122 | 0.894 ± 0.074 |
| % to body weight | 0.197 ± 0.023 | 0.198 ± 0.021 | 0.192 ± 0.023 | 0.195 ± 0.014 | 0.180 ± 0.012 | 0.178 ± 0.020 |
| Kidney-left | 1.245 ± 0.081 | 1.395 ± 0.149* | 1.286 ± 0.077 | 1.359 ± 0.143* | 1.323 ± 0.054 | 1.406 ± 0.063 |
| % to body weight | 0.297 ± 0.020 | 0.304 ± 0.017 | 0.296 ± 0.012 | 0.309 ± 0.018 | 0.287 ± 0.013 | 0.280 ± 0.021 |
| Kidney-right | 1.298 ± 0.102 | 1.390 ± 0.122 | 1.294 ± 0.066 | 1.376 ± 0.134 | 1.342 ± 0.060 | 1.456 ± 0.075* |
| % to body weight | 0.309 ± 0.027 | 0.304 ± 0.019 | 0.298 ± 0.012 | 0.312 ± 0.012 | 0.292 ± 0.017 | 0.290 ± 0.021 |
| Heart | 1.413 ± 0.095 | 1.516 ± 0.205 | 1.406 ± 0.076 | 1.466 ± 0.118 | 1.488 ± 0.149 | 1.616 ± 0.099 |
| % to body weight | 0.337 ± 0.024 | 0.330 ± 0.016 | 0.324 ± 0.017 | 0.333 ± 0.019 | 0.322 ± 0.018 | 0.321 ± 0.019 |
| Lung | 1.727 ± 0.130 | 1.849 ± 0.177 | 1.746 ± 0.153 | 1.724 ± 0.127 | 1.793 ± 0.107 | 1.963 ± 0.111* |
| % to body weight | 0.411 ± 0.026 | 0.404 ± 0.028 | 0.402 ± 0.028 | 0.392 ± 0.009 | 0.390 ± 0.035 | 0.391 ± 0.035 |
| Brain | 1.945 ± 0.077 | 2.007 ± 0.102 | 1.973 ± 0.090 | 1.992 ± 0.069 | 1.915 ± 0.071 | 2.066 ± 0.098* |
| % to body weight | 0.464 ± 0.028 | 0.440 ± 0.033 | 0.455 ± 0.026 | 0.454 ± 0.025 | 0.416 ± 0.030 | 0.411 ± 0.024 |
| Liver | 10.580 ± 0.578 | 12.072 ± 1.729 | 11.034 ± 0.800 | 11.860 ± 1.453 | 10.970 ± 0.629 | 12.379 ± 0.803* |
| % to body weight | 2.521 ± 0.135 | 2.622 ± 0.119 | 2.540 ± 0.108 | 2.690 ± 0.189* | 2.380 ± 0.087 | 2.460 ± 0.0791 |
| No. of animals | 10 | 10 | 10 | 10 | 5 | 5 |
*/** Significantly different from control at p < 0.05/ p < 0.01. Values are mean ± S.D.
a)Body weights were measured right before necropsy after an overnight fast.
Table 9.
Absolute and relative organ weights of the silkworm extract powder in female rats
| Parameters | Main group (mg/kg/day) | Recovery group (mg/kg/day) | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| 0 | 500 | 1000 | 2000 | 0 | 2000 | |
|
| ||||||
| Body weights (g)a) | 233.90 ± 14.76 | 241.94 ± 15.52 | 238.02 ± 14.08 | 240.89 ± 12.47 | 256.70 ± 11.81 | 271.58 ± 21.28 |
| Adrenal gland-left | 0.032 ± 0.005 | 0.034 ± 0.002 | 0.030 ± 0.003 | 0.033 ± 0.002 | 0.045 ± 0.007 | 0.047 ± 0.008 |
| % to body weight | 0.014 ± 0.001 | 0.014 ± 0.001 | 0.013 ± 0.001 | 0.013 ± 0.001 | 0.017 ± 0.002 | 0.017 ± 0.003 |
| Adrenal gland-right | 0.031 ± 0.004 | 0.033 ± 0.003 | 0.030 ± 0.002 | 0.031 ± 0.002 | 0.040 ± 0.010 | 0.050 ± 0.008 |
| % to body weight | 0.013 ± 0.001 | 0.014 ± 0.002 | 0.012 ± 0.001 | 0.013 ± 0.001 | 0.016 ± 0.004 | 0.018 ± 0.003 |
| Ovary-left | 0.040 ± 0.007 | 0.043 ± 0.005 | 0.042 ± 0.011 | 0.039 ± 0.009 | 0.031 ± 0.002 | 0.027 ± 0.008 |
| % to body weight | 0.017 ± 0.003 | 0.018 ± 0.002 | 0.018 ± 0.004 | 0.016 ± 0.004 | 0.012 ± 0.001 | 0.010 ± 0.004 |
| Ovary-right | 0.037 ± 0.006 | 0.044 ± 0.007 | 0.045 ± 0.011 | 0.040 ± 0.009 | 0.030 ± 0.002 | 0.029 ± 0.003 |
| % to body weight | 0.016 ± 0.003 | 0.018 ± 0.003 | 0.019 ± 0.004 | 0.017 ± 0.004 | 0.012 ± 0.001 | 0.011 ± 0.002 |
| Pituitary gland | 0.012 ± 0.002 | 0.012 ± 0.002 | 0.012 ± 0.002 | 0.013 ± 0.003 | 0.015 ± 0.003 | 0.014 ± 0.001 |
| % to body weight | 0.005 ± 0.001 | 0.005 ± 0.001 | 0.005 ± 0.001 | 0.005 ± 0.001 | 0.006 ± 0.001 | 0.005 ± 0.000 |
| Thymus | 0.212 ± 0.028 | 0.252 ± 0.050 | 0.219 ± 0.035 | 0.225 ± 0.028 | 0.200 ± 0.014 | 0.198 ± 0.051 |
| % to body weight | 0.091 ± 0.015 | 0.104 ± 0.018 | 0.092 ± 0.014 | 0.093 ± 0.012 | 0.078 ± 0.005 | 0.072 ± 0.015 |
| uterus | 0.686 ± 0.463 | 0.683 ± 0.283 | 0.715 ± 0.332 | 0.827 ± 0.342 | 0.677 ± 0.127 | 0.831 ± 0.430 |
| % to body weight | 0.291 ± 0.189 | 0.284 ± 0.123 | 0.298 ± 0.128 | 0.341 ± 0.135 | 0.266 ± 0.059 | 0.302 ± 0.147 |
| Spleen | 0.542 ± 0.072 | 0.589 ± 0.054 | 0.578 ± 0.071 | 0.616 ± 0.065 | 0.591 ± 0.074 | 0.587 ± 0.096 |
| % to body weight | 0.232 ± 0.024 | 0.244 ± 0.017 | 0.243 ± 0.025 | 0.256 ± 0.026 | 0.230 ± 0.023 | 0.215 ± 0.021 |
| Kidney-left | 0.721 ± 0.040 | 0.750 ± 0.0540 | 0.742 ± 0.068 | 0.752 ± 0.042 | 0.765 ± 0.038 | 0.767 ± 0.028 |
| % to body weight | 0.309 ± 0.013 | 0.310 ± 0.015 | 0.312 ± 0.017 | 0.313 ± 0.012 | 0.298 ± 0.002 | 0.283 ± 0.016 |
| Kidney-right | 0.732 ± 0.042 | 0.786 ± 0.057 | 0.760 ± 0.066 | 0.778 ± 0.044 | 0.783 ± 0.016 | 0.809 ± 0.054 |
| % to body weight | 0.314 ± 0.018 | 0.325 ± 0.0170 | 0.319 ± 0.019 | 0.323 ± 0.012 | 0.305 ± 0.012 | 0.299 ± 0.019 |
| Heart | 0.893 ± 0.077 | 0.887 ± 0.065 | 0.905 ± 0.064 | 0.928 ± 0.070 | 0.954 ± 0.069 | 0.953 ± 0.081 |
| % to body weight | 0.382 ± 0.029 | 0.367 ± 0.0250 | 0.381 ± 0.028 | 0.386 ± 0.032 | 0.372 ± 0.017 | 0.352 ± 0.024 |
| Lung | 1.286 ± 0.135 | 1.256 ± 0.066 | 1.299 ± 0.097 | 1.263 ± 0.064 | 1.420 ± 0.132 | 1.382 ± 0.094 |
| % to body weight | 0.549 ± 0.039 | 0.521 ± 0.047 | 0.546 ± 0.0381 | 0.525 ± 0.017 | 0.553 ± 0.044 | 0.511 ± 0.042 |
| Brain | 1.771 ± 0.081 | 1.750 ± 0.076 | 1.7971 ± 0.098 | 1.762 ± 0.057 | 1.809 ± 0.092 | 1.805 ± 0.058 |
| % to body weight | 0.760 ± 0.055 | 0.727 ± 0.073 | 0.758 ± 0.062 | 0.733 ± 0.049 | 0.705 ± 0.038 | 0.667 ± 0.046 |
| Liver | 5.482 ± 0.592 | 5.757 ± 0.459 | 5.594 ± 0.348 | 5.655 ± 0.463 | 05.864 ± 0.553 | 6.043 ± 0.540 |
| % to body weight | 2.342 ± 0.184 | 2.379 ± 0.094 | 2.354 ± 0.136 | 2.345 ± 0.104 | 2.285 ± 0.190 | 2.227 ± 0.148 |
| No. of animals | 10 | 10 | 10 | 10 | 5 | 5 |
*/** Significantly different from control at p < 0.05/ p < 0.01. Values are mean±S.D.
a)Body weights were measured right before necropsy after an overnight fast.
Also, the absolute weight of liver, kidney, lung, brain, and fasting weight were significantly higher in both sexes at 2000 mg/kg/day in the recovery group (p < 0.05).
Decreased size of testis was observed in 1 male in control. Hydrometra in two females of each group, and adhesion of liver to diaphragm, and protuberance of white spots in the kidney at 2000 mg/kg/day were observed in one female.
At the end of recovery period, one case of protuberance of median lobe in the liver was observed in male of the control group. Decreased size of adrenal gland and yellowish discoloration of caudate lobe in the liver were observed in each two males at 2000 mg/kg/day. In female, the retention of clear fluid in the uterus was observed in one female of each group in the control and 2000 mg/kg/day groups.
No findings attributable to the administration of SEP were recorded in the histopatholgical examination (data not shown).
Bacterial reverse mutation test. There were no increases of the means of revertant per plate for all doses of SEP in all of test strains both in the presence and absence of metabolic activation (Table 10). Precipitation was observed on the bottom agar at 1500 and 5000 μg/plate. Clear positive responses were observed in all of the positive control plates.
Table 10.
Reverse mutagenicity assay results
| Test | Chemical | Dose | Colonies/plate [factor]a) | ||
|---|---|---|---|---|---|
|
| |||||
| Strain | Treated | (μg/plate) | With S9 mix | Without S9 mix | |
|
| |||||
| TA100 | SEP | 0 | 154 ± 3 [1.0] | 143 ± 6 [1.0] | |
| 15 | 139 ± 1 [0.9] | 141 ± 8 [1.0] | |||
| 50 | 134 ± 10 [0.9] | 152 ± 8 [1.1] | |||
| 150 | 122 ± 7 [0.8] | 131 ± 11 [0.9] | |||
| 500 | 103 ± 4 [0.7] | 139 ± 10 [1.0] | |||
| 1500 | # | 96 ± 6 [0.6] | 109 ± 7 [0.8] | ||
| 5000 | # | 84 ± 6 [0.5] | 91 ± 8 [0.6] | ||
| 0 | 12 ± 2 [1.0] | 12 ± 3 [1.0] | |||
| 15 | 10 ± 2 [0.8] | 13 ± 4 [1.1] | |||
| 50 | 12 ± 1 [1.1] | 13 ± 3 [1.1] | |||
| TA1535 | SEP | 150 | 14 ± 2 [1.2] | 12 ± 3 [1.0] | |
| 500 | 9 ± 2 [0.8] | 9 ± 3 [0.8] | |||
| 1500 | # | 8 ± 2 [0.7] | 12 ± 4 [1.0] | ||
| 5000 | # | 11 ± 2 [0.9] | 12 ± 1 [1.0] | ||
| 0 | 35 ± 3 [1.0] | 23 ± 3 [1.0] | |||
| 15 | 25 ± 4 [0.7] | 21 ± 4 [0.9] | |||
| 50 | 37 ± 3 [1.0] | 30 ± 3 [1.3] | |||
| TA98 | SEP | 150 | 33 ± 1 [0.9] | 21 ± 4 [0.9] | |
| 500 | 25 ± 3 [0.7] | 20 ± 3 [0.9] | |||
| 1500 | # | 29 ± 1 [0.8] | 22 ± 3 [1.0] | ||
| 5000 | # | 33 ± 4 [1.0] | 28 ± 5 [1.3] | ||
| 0 | 10 ± 1 [1.0] | 9 ± 2 [1.0] | |||
| 15 | 9 ± 1 [0.9] | 5 ± 2 [0.6] | |||
| 50 | 9 ± 2 [0.9] | 6 ± 2 [0.7] | |||
| TA1537 | SEP | 150 | 9 ± 3 [0.9] | 6 ± 3 [0.7] | |
| 500 | 7 ± 3 [0.7] | 8 ± 2 [0.9] | |||
| 1500 | # | 6 ± 1 [0.6] | 5 ± 1 [0.6] | ||
| 5000 | # | 6 ± 1 [0.6] | 6 ± 1 [0.6] | ||
| 0 | 20 ± 2 [1.0] | 17 ± 1 [1.0] | |||
| 15 | 23 ± 2 [1.1] | 15 ± 2 [0.9] | |||
| 50 | 22 ± 3 [1.1] | 12 ± 2 [0.7] | |||
| E. coli | SEP | 150 | 17 ± 2 [0.9] | 18 ± 3 [1.1] | |
| 500 | 20 ± 5 [1.0] | 15 ± 2 [0.9] | |||
| 1500 | # | 18 ± 4 [0.9] | 20 ± 4 [1.2] | ||
| 5000 | # | 26 ± 3 [1.3] | 22 ± 4 [1.3] | ||
| Positive | controls | ||||
| TA100 | 2-AA | 1.0 | 728 ± 29 [4.7] | ||
| TA1535 | 2-AA | 2.0 | 118 ± 8 [10.1] | ||
| TA98 | B[a]P | 1.0 | 272 ± 30 [7.8] | ||
| TA1537 | 2-AA | 1.0 | 133 ± 21 [13.3] | ||
| E. coli | 2-AA | 6.0 | 225 ± 20 [11.1] | ||
| TA100 | SA | 0.5 | 464 ± 19 [3.3] | ||
| TA1535 | SA | 0.5 | 318 ± 18 [25.8] | ||
| TA98 | 2-NF | 2.0 | 456 ± 56 [20.1] | ||
| TA1537 | ICR-191 | 0.5 | 290 ± 35 [32.2] | ||
| E. coli | 4NQO | 0.5 | 177 ± 20 [10.6] | ||
2-AA: 2-Aminoanthracene, B[a]P: Benzo[a]pyrene, Benzo[a]pyrene, SA: Sodium azide, 2-NF: 2-Nitrofluorene, 4NQO: 4-Nitroquinoline-1-oxide, ICR-191: Acridine Mutagen ICR 191.
#: Turbid in the treatment mixture.
a)Three plates/dose were used. No. of colonies of treated plate/No. of colonies of negative control plate.
Chromosomal aberration assay. No significant increases were observed in the number of aberrant metaphases in all of the treatment series both in the presence and absence of metabolic activation system. Clear positive responses were observed in all of the positive controls (p < 0.01) (Table 11, Table 12 and Table 13).
Table 11.
Chromosome aberration test in the absence of S9 mix - summary (6 hr treatment)a)
| Dose(μg/ml) | No. cells examined (mean) | Aberrations | PP+ER | No. aberrant metaphaseb) | RCC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Chromosome type | Chromatid type | Others (mean) | Gaps (mean) | No. (mean) | Decision | +Gaps | –Gaps | ||||||
|
|
|
||||||||||||
| csb(mean) | cse(mean) | ctb(mean) | cte(mean) | No.(mean) | No.(mean) | Decision | |||||||
|
| |||||||||||||
| 0 | 100 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | Negative | 1 | 0 | Negative | 100 |
| 100 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | ||||
| (100) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | (1.0) | (0.0) | (1.0) | (0.0) | ||||
| 275 | 100 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | Negative | 1 | 0 | Negative | 100 |
| 100 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | ||||
| (100) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | (1.0) | (0.0) | (1.0) | (0.0) | ||||
| 550 | 100 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | Negative | 2 | 2 | Negative | 96 |
| 100 | 0 | 0 | 0 | 2 | 0 | 3 | 0 | 5 | 2 | ||||
| (100) | (0.0) | (0.0) | (0.0) | (2.0) | (0.0) | (1.5) | (0.0) | (3.5) | (2.0) | ||||
| 900 | 100 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | Negative | 1 | 0 | Negative | 74 |
| 100 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 3 | 2 | ||||
| (100) | (0.0) | (0.0) | (1.0) | (0.0) | (0.0) | (1.0) | (0.0) | (2.0) | (1.0) | ||||
| 1100 | 100 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | Negative | 2 | 1 | Negative | 48 |
| 100 | 0 | 0 | 1 | 1 | 0 | 3 | 0 | 5 | 2 | ||||
| (100) | (0.0) | (0.0) | (0.5) | (1.0) | (0.0) | (2.0) | (0.0) | (3.5) | (1.5) | ||||
| 20 B[a]P | 100 | 0 | 0 | 2 | 27 | 0 | 2 | 0 | Negative | 20 | 18 | Positive | 73 |
| 100 | 0 | 0 | 3 | 25 | 0 | 4 | 0 | 20 | 18 | ||||
| (100) | (0.0) | (0.0) | (2.5) | (26.0) | (0.0) | (3.0) | (0.0) | (20.0) | (18.0)** | ||||
** Significantly different from the negative control at p<0.01 (Fisher’s exact test).
a)6 hr treatment - 18 hr recovery.
b)Inclusive/exclusive gaps, means of duplicate cultures. 100 metaphases were examined per culture.
-: uncountable.
PP: Polyploid, ER: Endoreduplication, B[a]P: Benzo[a]pyrene (positive control article).
RCC: Relative Cell Counts = (Cell count of treated flask/Cell count of control flask) × 100 (%).
Gaps: Chromosome type + Chromatid type gaps.
csb: Chromosome type deletions.
cse: Chromosome type exchanges.
ctb: Chromatid type deletions.
cte: Chromatid type exchanges.
Other: Metaphases with more than 10 aberrations (including gaps) or with chromosome fragmentation.
Table 12.
Chromosome aberration test in the absence of S9 mix - summary (6 hr treatment)a)
| Dose(μg/ml) | No. cells examined(mean) | Aberrations | PP+ER | No. aberrant metaphaseb) | RCC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Chromosome type | Chromatid type | Others(mean) | Gaps(mean) | No.(mean) | Decision | +Gaps | –Gaps | ||||||
|
|
|
||||||||||||
| csb(mean) | cse(mean) | ctb(mean) | cte(mean) | No.(mean) | No.(mean) | Decision | |||||||
|
| |||||||||||||
| 0 | 100 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | Negative | 1 | 1 | Negative | 100 |
| 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| (100) | (0.5) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | (0.5) | (0.5) | ||||
| 150 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Negative | 0 | 0 | Negative | 92 |
| 100 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | ||||
| (100) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | (1.0) | (0.0) | (1.0) | (0.0) | ||||
| 300 | 100 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | Negative | 1 | 0 | Negative | 86 |
| 100 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 2 | 1 | ||||
| (100) | (0.0) | (0.0) | (0.0) | (0.5) | (0.0) | (1.0) | (0.0) | (1.5) | (0.5) | ||||
| 600 | 100 | 0 | 0 | 2 | 0 | 0 | 2 | 0 | Negative | 4 | 2 | Negative | 44 |
| 100 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 3 | 1 | ||||
| (100) | (0.5) | (0.0) | (1.0) | (0.0) | (0.0) | (2.0) | (0.0) | (3.5) | (1.5) | ||||
| 700 | - | - | - | - | - | - | - | - | - | - | - | - | 18 |
| - | - | - | - | - | - | - | - | - | - | ||||
| (-) | (-) | (-) | (-) | (-) | (-) | (-) | (-) | (-) | (-) | ||||
| 800 EMS | 100 | 0 | 0 | 0 | 12 | 0 | 5 | 0 | Negative | 17 | 12 | Positive | 76 |
| 100 | 0 | 0 | 0 | 12 | 0 | 4 | 0 | 14 | 11 | ||||
| (100) | (0.0) | (0.0) | (0.0) | (12.0) | (0.0) | (4.5) | (0.0) | (15.5) | (11.5)** | ||||
** Significantly different from the negative control at p<0.01 (Fisher’s exact test).
a)6 hr treatment - 18 hr recovery.
b)Inclusive/exclusive gaps, means of duplicate cultures. 100 metaphases were examined per culture.
-: uncountable.
PP: Polyploid, ER: Endoreduplication, EMS: Ethylmethanesulfonate (positive control article).
RCC: Relative Cell Counts = (Cell count of treated flask/Cell count of control flask) × 100 (%).
Gaps: Chromosome type + Chromatid type gaps.
csb: Chromosome type deletions.
cse: Chromosome type exchanges.
ctb: Chromatid type deletions.
cte: Chromatid type exchanges.
Other: Metaphases with more than 10 aberrations (including gaps) or with chromosome fragmentation.
Table 13.
Chromosome aberration test in the absence of S9 mix - summary (24 hr treatment)a)
| Dose (μg/ml) | No. cells examined (mean) | Aberrations | PP+ER | No. aberrant metaphaseb) | RCC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Chromosome type | Chromatid type | Others(mean) | Gaps(mean) | No.(mean) | Decision | +Gaps | –Gaps | ||||||
|
|
|
||||||||||||
| csb(mean) | cse(mean) | ctb(mean) | cte(mean) | No.(mean) | No.(mean) | Decision | |||||||
|
| |||||||||||||
| 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Negative | 0 | 0 | Negative | 100 |
| 100 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | ||||
| (100) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | (0.5) | (0.0) | (0.5) | (0.0) | ||||
| 150 | 100 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | Negative | 3 | 1 | Negative | 101 |
| 100 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 3 | 1 | ||||
| (100) | (0.0) | (0.0) | (1.0) | (0.0) | (0.0) | (2.0) | (0.0) | (3.0) | (1.0) | ||||
| 300 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Negative | 0 | 0 | Negative | 105 |
| 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| (100) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | ||||
| 600 | 100 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | Negative | 1 | 1 | Negative | 50 |
| 100 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 2 | 1 | ||||
| (100) | (0.0) | (0.0) | (1.0) | (0.0) | (0.0) | (0.5) | (0.0) | (1.5) | (1.0) | ||||
| 700 | - | - | - | - | - | - | - | - | - | - | - | - | 19 |
| - | - | - | - | - | - | - | - | - | - | ||||
| (-) | (-) | (-) | (-) | (-) | (-) | (-) | (-) | (-) | (-) | ||||
| 600 EMS | 100 | 0 | 0 | 3 | 18 | 0 | 5 | 0 | Negative | 22 | 19 | Positive | 78 |
| 100 | 0 | 0 | 5 | 15 | 0 | 4 | 0 | 20 | 17 | ||||
| (100) | (0.0) | (0.0) | (4.0) | (16.5) | (0.0) | (4.5) | (0.0) | (21.0) | (18.0)** | ||||
** Significantly different from the negative control at p<0.01 (Fisher’s exact test).
a)24 hr treatment - 0 hr recovery.
b)Inclusive/exclusive gaps, means of duplicate cultures. 100 metaphases were examined per culture.
-: uncountable.
PP: Polyploid, ER: Endoreduplication, EMS: Ethylmethanesulfonate (positive control article).
RCC: Relative Cell Counts = (Cell count of treated flask/Cell count of control flask) × 100 (%).
Gaps: Chromosome type + Chromatid type gaps.
csb: Chromosome type deletions.
cse: Chromosome type exchanges.
ctb: Chromatid type deletions.
cte: Chromatid type exchanges.
Other: Metaphases with more than 10 aberrations (including gaps) or with chromosome fragmentation.
Mouse bone marrow micronucleus assay. No mortality and abnormality were observed. No increase in MNPCEs frequency was observed at any dose levels of SEP. The positive control, CPA, produced significant increase in the frequency of MNPCEs (p < 0.01) (Table 14). There were no significant differences in PCE:RBC ratio at any dose level of SEP. However the PCE:RBC ratio in the positive control was decreased significantly (p < 0.01).
Table 14.
Observations of micronucleus and PCE: RBC ratio
| Dose (mg/kg/day) | Animals per dose | MNPCE/2000 PCE (Mean ± S.D) | PCE:RBC Ratio (Mean ± S.D) | % Ratio |
|---|---|---|---|---|
|
| ||||
| 0 | 6 | 1.17 ± 0.75 | 0.40 ± 0.02 | 100 |
| 1250 | 6 | 0.83 ± 0.98 | 0.38 ± 0.03 | 95 |
| 2500 | 6 | 0.83 ± 0.75 | 0.37 ± 0.03 | 93 |
| 5000 | 6 | 0.83 ± 0.75 | 0.39 ± 0.02 | 98 |
| CPA 70 (mg/kg) | 6 | 61.00 ± 5.18** | 0.21 ± 0.03** | 53 |
PCE; Polychromatic erythrocyte, RBC; Red blood cells (polychromatic erythrocyte + normochromatic erythrocyte), MNPCE; Micronucleated polychromatic erythrocyte, CPA; Cyclophosphamide monohydrate.
Vehicle and Test article were orally administered to mice for two consecutive days.
CPA was intraperitoneally administered to mice once on the day of the 2nd admin.
Bone marrow smears were prepared about 24 hr after the final administration.
** Significantly different from the negative control group at p<0.01.
DISCUSSION
The present study was performed to evaluate the safety of SEP which containing DNJ in the single and 90 day repeated-dose oral toxicity. Mutagenic potential was also evaluated by in vitro bacterial reverse mutation test, in vitro chromosomal aberration assay and in vivo mouse bone marrow micronucleus assay.
The SEP did not induce acute toxicity in rats, and the approximate lethal dose was greater than 5000 mg/kg. Soft stool observed in the treatment groups was observed only on day 2 and disappeared, therefore, the sign was considered toxicologically insignificant.
In the 90 day repeated-dose oral toxicity study, no deaths and not test article related signs were observed at all dose groups. Loss of fur and reddish tear at 500 mg/kg/day were considered to be accidental changes because these signs were observed in only one animal and was lack of doseresponse.
The increases of body weight at 2000 mg/kg/day and food consumption at 1000 mg/kg/day were not considered to be test article-related effects since these changes were observed only in males without dose-response, and were within the historical control range of this strain and age (26) established in Chemon Inc. The significant increases in urinalysis (SG, KET and pH) were also considered to be of no toxicological significance, as these changes were observed during the treatment period only, and were not accompanied by meaningful morphological changes in histopatholgical examination of the kidney and other related items.
The significant changes in the hematological and serum biochemical parameters such as decreased RDW and TCho at 1000 mg/kg/day, increased WBC, ALP, CPK, K+ at 2000 mg/kg/day, and decreased TBil at 2000 mg/kg/day were not considered adverse because these changes were very slight and lack of dose-related response. Also these changes were not accompanied by any histopatholgical changes of other related items, and were within historical control ranges in rats of this strain and age (26) of Chemon Inc.
The significant increases of weights of adrenal glands and kidney in males at 500 and 2000 mg/kg/day were not accompanied by correlative findings. The significant increases in organ weights in the recovery group such as kidney, lung, brain, and liver were due to increase of fasting body weights. These changes were within the aforementioned historical control range. The necropsy findings were not related to the administration of SEP based on the results of histopathological examination. The decreased size of testis in control group was explained by the atrophy of seminiferous tubules observed in the microscopic finding. The liver-diaphragm adhesion was confirmed as hepatodiaphragmatic nodule. The protuberance of white spot in kidney at 2000 mg/kg/day was identified to be pyelonephritis. Therefore, these changes were not considered treatment-related because these changes were also found in the control group without clear dose-responsiveness, and found infrequently. The retention of clear fluid in the uterus was not attributed to the SEP but a natural change during the estrus cycle.
The protuberance of median lobe in liver, decreased size of adrenal gland, and discoloration of yellowish in caudate lobe in the liver found at necropsy of the recovery group were identified to be hepatodiaphragmatic nodule, accessory nodule in adrenal cortex, and focal necrosis of hepatocyte and fibrosis with brown pigmentation, respectively. They were not considered to be test article-related because they were observed in control group or found infrequently. Besides, other lesions which have been well known to occur spontaneously in Sprague-Dawley rats of same age were observed.
In summary, the present study evaluated the toxicity and genotoxicity of the Silkworm extract powders containing the DNJ. The Silkworm extract powder was not genotoxic as tested in a bacterial reverse mutation test, a chromosomal aberration assay and mouse bone marrow micronucleus assay and 90 day repeated oral administration of test article no significant toxic effects in rats.
Accordingly, under the present experimental conditions, SEP is not mutagenic both in vitro and in vivo assays, and the approximate lethal dose in single-dose oral toxicity study was higher than 5000 mg/kg in rats. The 90 day repeated-dose study demonstrated that at doses up to 2000 mg/kg/day is safe and does not cause adverse effects. The No-Observed-Adverse-Effect-Level (NOAEL) was 2000 mg/ kg/day for both male and female rats, and no target organ was identified.
Acknowledgments
This work was carried out with the support of “Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ009125)” Rural Development Administration, Republic of Korea.
References
- 1.Bulletin of the World Health Organization. WHO News. Bull. W. H. O. (2004);82:635–636. [PMC free article] [PubMed] [Google Scholar]
- 2.Hughes A.B., Rudge A.J. Deoxynojirimycin: synthesis and biological activity. Nat. Prod. Rep. (1994);11:135–162. doi: 10.1039/np9941100135. [DOI] [PubMed] [Google Scholar]
- 3.Yoshikuni Y. Inhibition of intestinal alpha-glucosidase activity and postprandial hyperglycemia by moranoline and its N-alkyl derivatives. Agric. Biol. Chem. (1988);52:121–128. doi: 10.1271/bbb1961.52.121. [DOI] [PubMed] [Google Scholar]
- 4.Yoshikuni Y., Ezure Y., Aoyagi Y., Enomoto H. Inhibition of intestinal alpha-glucosidase and postprandial hyperglycemia by N-substituted moranoline derivatives. J. Pharmacobiodyn. (1988);11:356–362. doi: 10.1248/bpb1978.11.356. [DOI] [PubMed] [Google Scholar]
- 5.Junge B., Matzke M., Stltefuss J. Chemistry and structure activity relationships of glucosidase inhibitors. Handb. Exp. Pharmacol. (1996);119:411–482. [Google Scholar]
- 6.Kimura Y., Tabata Y. Controlled release of stromal cell-derived factor-1 from gelatin hydrogels enhances angiogenesis. J. Biomater. Sci. Poym. Ed. (2010);21:37–51. doi: 10.1163/156856209X410193. [DOI] [PubMed] [Google Scholar]
- 7.Kong W.H., Oh S.H., Ahn Y.R., Kim K.W., Kim J.H., Seo S.W. Antiobesity effects and improvement of insulin sensitivity by 1-deoxynojirimycin in animal models. J. Agric. Food Chem. (2008);56:2613–2619. doi: 10.1021/jf073223i. [DOI] [PubMed] [Google Scholar]
- 8.Asai A., Nakagawa K., Higuchi O., Kimura T., Kojima Y., Kariya J., Miyazawa T., Oikawa S. Effect of mulberry leaf extract with enriched 1-deoxynojirimycin content on postprandial glycemic control in subjects with impaired glucose metabolism. J. Diabetes Invest. (2011);2:318–323. doi: 10.1111/j.2040-1124.2011.00101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vichasilp C., Nakangwa K., Sookwong P., Higuchi O., Kimura F., Miyazawa T. A novel gelatin crossslinking method retards release of mulberry 1-deoxynojirimycin providing a prolonged hypoglycaemic effect. Food chem. (2012);134:1823–1830. doi: 10.1016/j.foodchem.2012.03.086. [DOI] [PubMed] [Google Scholar]
- 10.Yoshikaki A., Hivonu M. The structure of moranoline, a piperidine alkaloid form Morus species. Nippon Nogei Kagaku Kaishi. (1976);50:571–572. doi: 10.1271/nogeikagaku1924.50.11_571. [DOI] [Google Scholar]
- 11.Kimura T., Nakagaw K., Kubota H., Kojima Y., Goto Y., Yamaqishi K., Oita S., Oikawa S., Miyazawa T. Food-grade mulberry powder enriched with 1-deoxynojirimycin suppresses the elevation of postprandial blood glucose in humans. J. Agric. Food Chem. (2007);55:5869–5874. doi: 10.1021/jf062680g. [DOI] [PubMed] [Google Scholar]
- 12.Asano N., Tomioka E., Kizu H., Matsui K. Sugars with nitrogen in the ring isolated from the leaves of Morus bombycis. Carbohydr. Res. (1994);253:235–245. doi: 10.1016/0008-6215(94)80068-5. [DOI] [PubMed] [Google Scholar]
- 13.Asano N., Oseki K., Tomioka E., Kizu H., Matsui K. N-containing sugars from Morus alba and their glycosidase inhibitory activities. Carbohydr. Res. (1994);259:243–255. doi: 10.1016/0008-6215(94)84060-1. [DOI] [PubMed] [Google Scholar]
- 14.Asano N., Yamashita T., Yasuda K., Ikeda K., Kizu H., Kameda Y., Kata A., Nash R.J., Lee H.S., Ryu K.S. Polyhydroxylated alkaloids isolated from mulberrytrees (Morus alba L.) and silkworms (Bombyx mori L.). J Agric. Food Chem. (2001);49:4208–4213. doi: 10.1021/jf010567e. [DOI] [PubMed] [Google Scholar]
- 15.Kim J.W., Kim S.U., Lee H.S., Kim I., Ahn M.Y., Ryu K.S. Determination of 1-deoxynojirimycin in Morus alba L. leaves by derivatization with 9-fluorenylmethyl chloroformate followed by reversedphase high-performance liquid chromatography. J Chromatogr. A. (2003);1002:93–99. doi: 10.1016/S0021-9673(03)00728-3. [DOI] [PubMed] [Google Scholar]
- 16.Yin H., Shi X.Q., Sun B., Ye J.J., Duan Z.A., Zhou X.L., Cui W.Z., Wu X.F. Accumulation of 1-deoxynojirimycin in silkworm, Bombyx mori L. J. Zhejiang Univ. Sci. B. (2010);11:286–291. doi: 10.1631/jzus.B0900344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akai H. New physiological functions of silk material. Shokuhin Kaihatsu. (1999);34:45–47. [Google Scholar]
- 18.Gotoh K., Izumi H., Kanamoto T., Tamada Y., Nakashima H. Sulfate fibroin, a novel sulfated peptide derived from silk, inhibits human immunodeficiency virus replication in vitro. Biosci. Biotechnol. Biochem. (2000);64:1664–1670. doi: 10.1271/bbb.64.1664. [DOI] [PubMed] [Google Scholar]
- 19.Hong S.E., Park K.J., Suh B.S., Do M.S., Hyun C.K. Effect of silk fibroin hydrolysate on adipocyte metabolism in db/db mice. Korean J. Pharmacogn. (2002);33:312–318. [Google Scholar]
- 20.Suzuki N., Fuzimura A., Nagai T., Mizumoto I., Itami T., Hatate H., Nozawa T., Kato N., Nomoto T., Yoda B. Antioxidative activity of animal and vegetable dietary fibers. BioFactors. (2004);21:329–333. doi: 10.1002/biof.552210164. [DOI] [PubMed] [Google Scholar]
- 21.Koyama H., Utakoji T., Ono T. A new cell line derived from newborn Chinese hamster lung tissue. Gann. (1970);61:161–167. [PubMed] [Google Scholar]
- 22.Maron D.M., Ames B.N. Revised methods for the Salmonella mutagenicity test. Mutat. Res. (1983);113:173–215. doi: 10.1016/0165-1161(83)90010-9. [DOI] [PubMed] [Google Scholar]
- 23.Green M.H., Muriel W.J. Mutagen testing using TRP+ reversion in Escherichia coli . Mutat. Res. (1976);38:3–32. doi: 10.1016/0165-1161(76)90076-5. [DOI] [PubMed] [Google Scholar]
- 24.Japanese Environmental Mutagen Society-Mammalian Mutagenicity Study Group. Atlas of chromosome aberration by chemicals. JEMS-MMS; Tokyo, Japan: (1988). [Google Scholar]
- 25.Schmid W. The micronucleus test. Mutat. Res. (1975);31:9–15. doi: 10.1016/0165-1161(75)90058-8. [DOI] [PubMed] [Google Scholar]
- 26.Han Z.Z., Xu H.D., Kim K.H., Ahn T.H., Bae J.S., Lee J.Y., Gil K.H., Lee J.Y., Woo S.J., Yoo H.J., Lee H.K., Kim K.H., Park C.K., Zhang H.S., Song S.W. Reference data of the main physiological parameters in control Spague-Dawley rats from pre-clinical toxicity studies. Lab. Anim. Res. (2010);26:153–164. doi: 10.5625/lar.2010.26.2.153. [DOI] [Google Scholar]