Abstract
Preventive approaches against cancer have not been fully developed and applied. For example, the incidence of some types of cancer, including colon cancer, is highly dependent upon lifestyle, and therefore, amenable to prevention. Among the lifestyle factors, diet strongly affects the incidence of colon cancer; however, there are no definitive dietary recommendations that protect against this malignancy. The association between diet-derived bioactives and development of colonic neoplasms will remain ill defined if we do not take into account: (1) the identity of the metabolites present in the colonic lumen; (2) their concentrations in the colon; and (3) the effect of the colonic contents on the function of individual bioactives. We review two approaches that address these questions: the use of fecal water and in vitro models of the human colon. Compared to treatment with individual diet-derived compounds, the exposure of colon cancer cells to samples from fecal water or human colon simulators mimics closer the in vitro conditions and allows for more reliable studies on the effects of diet on colon cancer development. The rationale and the advantages of these strategies are discussed from the perspective of a specific question on how to analyze the combined effect of two types of bioactives, butyrate and polyphenol metabolites, on colon cancer cells.
Keywords: Human colon model, Fecal water, Diet, Colon cancer, Prevention, Butyrate, Polyphenols, WNT signaling
Core tip: Studies on diet and colorectal cancer are in their infancy, and the relevance of many publications on the topic is questionable due to three problems: (1) there is uncertainty about which diet-derived compounds are present in the colon; (2) most studies have focused on individual bioactives; whereas, food intake results in complex metabolite mixtures; and (3) the physiological concentrations of many colonic bioactives are unknown. Here we discuss how the use of fecal water samples and in vitro models of human colon address these problems.
INTRODUCTION
Within the past 100 years, the leading causes of death have changed dramatically[1]. Approximately a century ago, the three leading causes of death were influenza and/or pneumonia, tuberculosis, and gastrointestinal (GI) infections. However, in 1997 less than 5% of the deaths were attributed to pneumonia, influenza, and human immunodeficiency virus infection; whereas, heart disease and cancers accounted for more than 50% of all deaths[2], In 2008, the American Cancer Society projected that soon cancer will become the leading cause of death worldwide[3], and the 2010 data for United States indicate almost equal number of deaths caused by heart disease and cancer (597689 vs 574743, respectively[4]). Recent projections of mortality and causes of death by the World Health Organization also support cancer as emerging leading cause of death in both, economically developed and developing countries[5]. How are these changes explained The deaths from infectious diseases declined due to the implementation of childhood vaccinations, improvements in sanitation and hygiene, and the discovery of antibiotics. Except for the use of antibiotics, these approaches are classified as preventive measures. The more recent reduction of total cardiovascular death is also attributed to prevention; thus, massive educational efforts have raised the awareness of what constitutes a healthy lifestyle, and novel medications that control high blood pressure and cholesterol levels have been introduced into clinic. Therefore, the decreased deaths from infectious and heart diseases are mainly attributed to the development of preventive measures.
Unfortunately, the full power of prevention has not been applied in the battle against cancer. Presently, the focus is on cancer treatment, and as a result, billions of dollars are invested in drug development. The new arsenal of molecularly targeted anti-cancer drugs has raised hopes; however, it is increasingly clear that although “targeted” therapies prolong patients’ lives, their benefit is limited in time by the inevitable acquisition of drug resistance. Combination therapies that incorporate conventional chemoradiation and molecularly targeted drugs might be the next step; however, the lesson from the past is that to obtain a significant victory against any disease, we need to emphasize on primary prevention.
Similar to the trend of personalized cancer treatment, future cancer prevention measures should be stratified by phenotype, genotype, and family history. Cancer prevention strategies could include, but not be limited to, the following: (1) monitoring of the patient’s exposome (a set of biomarkers indicative of individual’s exposure to cancer promoters[6]); (2) non-invasive imaging techniques that detect the earliest stages of abnormal growth; (3) reliable dietary, physical activity, and other lifestyle recommendations; and (4) vaccines that reduce the risk for specific cancers. In addition to developing future personalized prevention approaches, it is important to expand the existing prevention strategies that address some types of cancer as a public health issue affecting large populations (e.g., educational approaches, influencing legislation, mobilizing communities). The present review focuses on the dietary approach to colorectal cancer (CRC) prevention, and addresses several problems that hinder the progress of this approach in terms of obtaining valid and unambiguous dietary recommendations.
There are over 140000 new cases of CRC and approximately 50000 CRC-related deaths a year in the United States[7]. A distinct characteristic of CRCs is that they develop slowly from benign adenomas: polyps larger than one centimeter in size have a 24% chance of progressing into carcinoma over a 20-year period[8]. The transition of benign adenomas into malignancies and the incidence of colonic neoplasms are modulated by diet-derived compounds[9]. However, studies on diet and CRC are in their infancy, and the relevance of many publications on the topic is questionable due to three problems: (1) there is uncertainty about which diet-derived compounds are present in the colon, and what their half-life; (2) most studies have focused on individual bioactives; whereas, food intake results in a complex mixture of metabolites that could modify each other’s effect on neoplastic cells; and (3) the concentrations of many bioactives in the colon are unknown; whereas compounds, for which such information is available, have been frequently analyzed at levels exceeding physiological concentrations.
Here we review two approaches that address these problems, and discuss how these strategies solve a specific question on the interaction between two dietary bioactives: butyrate and polyphenol derivatives. Both bioactives affect the risk for CRC, and although there are other dietary compounds and mechanisms proposed to be protective against the malignancy, this review is limited to one example. Our objective is to highlight the methodologies that unravel the effects of multiple dietary bioactives on colonic cells, and not to comprehensively discuss all classes of dietary bioactives and their plausible physiological effects.
WNT/catenin signaling by butyrate
In 2011, the World Cancer Research Fund and the American Institute for Cancer Research upgraded the protective effect of fiber against colon cancer from “probable” to “convincing”[10] and this effect is attributed in part to the fermentation product of fiber in the colon, butyrate. Butyrate is a short-chain fatty acid (SCFA), the production of which enables the salvage of energy from dietary fiber that would be otherwise lost. It is estimated that SCFAs contribute to about 5%-15% of the total caloric requirements in humans[11]. Various tissues in the body can utilize SCFA for energy generation; however, butyrate is the preferred fuel for the colonic epithelial cells that derive about 70% of their energy from butyrate oxidation[12,13]. Butyrate is regarded as a healthy metabolite due to its positive influence on cell growth and differentiation, as well as its anti-inflammatory properties[12,14]. Butyrate also acts as an inhibitor of histone deacetylases (HDACi). Its colonic concentration is between 2 and 10 mmol[15] and at these levels, butyrate induces apoptosis in most CRC cells in vitro. We have provided evidence that this effect is in part due to the ability of butyrate to hyperactivate the WNT/catenin signaling pathway, and several synthetic HDACis mimic the effect of butyrate on the WNT pathway and apoptosis[16,17]. The hyperactivation of WNT/catenin signaling by HDACis takes place only in colonic neoplastic cells with mutations in the pathway, and such mutations are detected in 80% of the sporadic colon cancers[18-20]. This finding is in agreement with observations that moderate levels of oncogene activities support cancer development; however, hyperactivation of oncogenic functions may result in cell death and senescence[21]. Therefore, WNT/catenin signaling is not “oncogenic” under all conditions, and sometimes its activation correlates with less aggressive cancer phenotypes[22].
Polyphenols as biological food constituents
The intake of fiber (the most important source of butyrate in the colon) is usually associated with that of other bioactive ingredients; for example, many fiber-rich foods are a source of polyphenols (e.g., cereals, fruit, and vegetables). The drinks that accompany our meals further increase the complexity of bioactives: wine, fruit juices, cocoa, tea, and coffee are all rich in polyphenols. The two main classes of dietary polyphenols are the flavonoids and the phenolic acids. In in vitro experiments, the flavonoids are powerful antioxidants; however, this activity is exhibited at concentrations exceeding the levels achievable in vivo. Thus, after consumption of 10-100 mg of a single compound, the maximum plasma levels of individual flavonoids are approximately 1-3 mol[23,24]. In addition, due to host metabolism the in vivo half-life of the precursor polyphenols is short due to their rapid conversion into metabolites, all of which exhibit diminished antioxidant activity[24-26]. More recent studies indicate that at physiological concentrations, polyphenols and their metabolites modulate cell signaling pathways[27], and exhibit anti-inflammatory activity through inhibition of COX-2 protein levels, prostanoid biogenesis, or pro-inflammatory cytokine production[28-31]. Polyphenol metabolites also exhibit anti-proliferative effect on neoplastic cells[32,33], thus, similar to butyrate, some polyphenols and their microbial metabolites exhibit a CRC protective role. For example, quercetin, a flavonol found in citrus fruit, buckwheat, and onions, suppresses the formation of aberrant crypt foci and induces apoptosis in preneoplastic human colonocytes[34,35]. Caffeic acid esters present in propolis are potent inhibitors of human colon adenocarcinoma cell growth, carcinogen-induced biochemical changes, and preneoplastic lesions in the rat colon[36,37]. A CRC-preventive role has also been reported for isoflavons, curcumin, and tea polyphenol in green tea, (-)-epigallocatechin-3-gallate (EGCG)[38].
Synergistic or antagonistic effects of butyrate and polyphenols
Since the intake of dietary fiber is frequently accompanied by that of polyphenols, it is logical to investigate whether the effect of butyrate on WNT/catenin signaling and apoptosis in CRC cells are modified by polyphenols and their metabolites. Presently, the combined effects of butyrate and polyphenol metabolites on WNT/catenin signaling are unknown; however, there have been reports on the modulation of WNT/catenin signaling by polyphenols. For example, polymeric black tea polyphenols inhibit 1,2-dimethylhydrazine-induced colorectal tumorigenesis in rats, and the researchers proposed that this effect is mediated by suppression of WNT/catenin signaling[39]. EGCG suppresses WNT/catenin transcriptional activity in HCT-116 CRC cells at concentrations of 100-200 mol, which are unachievable in vivo[40]. However, at physiologically relevant concentration of 0.5 mol[23,24,32], EGCG inhibits the enzyme glycogen synthase kinase-3 beta (GSK-3beta)[41]. This inactivation of GSK-3beta should result in accumulation of transcriptionally active Ser-37/Thr-41-dephosphorylated beta-catenin, and increased WNT transcriptional activity[42,43]. Polyphenol-rich apple juice extract, as well as the free aglycon phloretin and the flavonol quercetin, also inhibit GSK-3beta in in vitro assays[44]. In agreement with this inhibitory effect on the enzyme, quercetin at 10 mol increases WNT/catenin transcriptional activity[41]. The interpretation of these findings is difficult due to the fact that the bioavailability of the compounds has not been taken into account, or is unknown. In addition, polyphenols are biochemically transformed or completely fermented by the gut microbiota to metabolites with a modified biological activity, as discussed below. The inhibition of GSK-3beta by some polyphenols indicates that these compounds may synergize with butyrate in its effect on WNT/beta-catenin signaling. Furthermore, similar to butyrate, some polyphenols and their metabolites inhibit histone deacetylases (HDACs). Thus, fermentation of polyphenol-rich apple juice extracts with human fecal slurry revealed that polyphenol metabolites have a HDAC inhibitory function[45]. Metabolites of polyphenols in the colon, such as P Coumaric acid, 3-(4-OH-phenyl)-propionate, and caffeic acid also exhibit HDAC inhibitory function in in vitro assays with nuclear extracts from HT-29 human CC cells[46]. Therefore, similar to butyrate[16,17], polyphenol metabolites with HDAC inhibitory function may protect against CC via stabilization of beta-catenin and hyperinduction of WNT/beta-catenin signaling. Despite these data, the question of how polyphenols and their metabolites modulate the effects of butyrate on colonic neoplastic cells has remained unanswered. Several problems hinder the progress of the studies: there is little knowledge about the polyphenol derivatives present in the colon, their physiological concentrations, and how the colonic content modulates the functions of the bioactives. The main colonic species might be the polyphenol aglycones and their derivatives: phenolic and non-phenolic aromatic acids. The deglycosylation of polyphenols is catalyzed by microbial beta-glucosidases in the small intestine and primarily the colon, and this process results in aglycone forms that are more absorbable[47]. After absorption in the intestinal cells, the aglycones are metabolized to conjugates of glucuronate and sulfate, which are the major forms in plasma and urine[47]. However, these conjugates have not been detected in the colon, most likely due to the hydrolase activity of the GI microbiota[48-50].
Use of fecal water
The problems listed above are not specific to our example on the combined effect of dietary butyrate and polyphenols on colon cancer cells, as they represent a stumbling block for all studies aimed at characterization of the effects of dietary bioactives. To date, there are two approaches that address these problems: (1) performing analyses with the aqueous phase of feces (fecal water); and (2) utilizing in vitro GI models. The first approach is justified by the fact that the colonic epithelium is exposed to the fecal matter in vivo[48,51-54] and that fecal water affects the growth of colonocytes more effectively than components of the solid phase of feces[52,53]. Gas chromatography and mass spectrometry analyses of the fecal water of healthy volunteers have identified and quantified the flavonoids and their derivatives in the colon[48]. In these samples, the most prevalent flavonoids were naringenin, quercetin, formononetin, catechin, epicatechin, isorhamnetin, apigenin, and kaempferol, and they were detected at mean concentrations of 1.2, 0.63, 0.17, 0.14, 0.11, 0.10, 0.07 and 0.05 mol, respectively. All polyphenols and derivatives exhibited daily fluctuations in the same individual, and the most prevalent flavonoids naringenin, quercetin, and formononetin reached a maximum concentration of 4.04, 1.30, and 0.84 mol, respectively. Colonic derivatives of the flavonoids in the colon were detected at concentrations up to two orders of magnitude higher than these of their precursors; thus, the total monophenolic acids reached up to 740.7 mol and the total nonphenolic aromatic acids, 1.5 mmol[48]. Recent analyses of fecal water have confirmed the prevalence of the phenolic and non-phenolic aromatic acids in fecal water[28,54]. Therefore, our question of how polyphenols and their metabolites modulate the activity of butyrate may need to be re-stated to how the activity of butyrate is affected by high levels of monophenolic and nonphenolic acids.
In vitro models that mimic the human colon
The combined effect of butyrate and polyphenol metabolites on neoplastic cells, however, is even more complex. The combined effect could be modified by the presence of additional metabolites, as the intake of any diet results in a complex mixture of compounds in the colon. The physiological properties of diet-derived mixtures could be analyzed with in vitro models of the human GI tract, and one such model has been developed by TNO in the Netherlands[55]. This system closely mimics the physiological conditions in the GI tract, as established in numerous validation studies[32,56-67]. The GI system is composed of two separate models: TIM-1 that simulates the stomach and the small intestine (not further discussed here), and TIM-2 that simulates the colon[68] and contains compartments with a high density, metabolically active microbiota of human origin. The physiological conditions of the large intestine that are simulated include pH, anaerobiosis and gradual intake of pre-digested meal compounds coming from the small intestine (Figure 1). Physiological amounts of microorganisms in the TIM-2 model are maintained via dialysis mechanism. This mechanism takes up electrolytes and microbial metabolites, and ensures that the concentrations of these remain at physiological levels, preventing inhibition of the microbiota by metabolites. The in vitro GI system permits the use of an intestinal microbiota from different enterotypes and the comparison between various donors, e.g., healthy vs diseased, lean vs obese[69]. The technology also allows for controlled analyses on the colonic outputs from various diets. Thus, entire meals representative of different types of diet can be “fed” to the GI model[60] and the resulting real-time fermented samples from the TIM-2 compartments can be tested on neoplastic and normal colonic cells in vitro[32]. Although fecal water from human subjects could be used for similar studies, there are several problems associated with this approach: inter-individual differences in metabolic rates and colonic microbiota, noncompliance with diet, preferential absorption of some compounds by the colonocytes, and the impossibility of acquiring samples from different locations of the human GI tract (e.g., pre-colon, proximal colon). The last point is important, since metabolite concentrations change along the colon[11]. Using the in vitro GI system has several advantages: it is computer-controlled, allowing standardization of the experiments, it is cheaper than clinical or animal trials, and it does not have the ethical constraints associated with animal and human subject studies. Furthermore, sampling from various locations along the GI tract and at different time points enables kinetic studies of the microbial metabolism of dietary components. The application of the in vitro gut approach can facilitate the design of functional foods and dietary supplements that decrease CRC incidence. For example, utilizing the in vitro GI system, Gao et al[32] discovered that tea, citrus fruit, and soy flavonoids are metabolized in the colon to a few phenolic and aromatic acids, therefore ascertaining the exact compounds that should be screened for effects on CRC cells.
Figure 1.
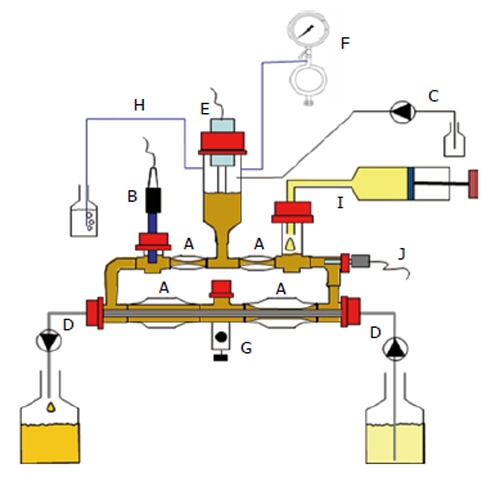
TIM-2 is a validated, computer-controlled system that simulates the human colon. The model consists of glass units with a flexible wall inside (A); Peristaltic movements, achieved by pumping warm water into the space between the glass unit and the flexible walls at regular intervals, simulate peristaltic movements and allow the lumen to be mixed and transported through the loop-shaped system. The system is kept at body temperature (37 °C). To simulate the pH in the proximal colon, the pH is set at 5.8 and controlled (B) and adjusted by secretion of 2 mol/L NaOH into the system (C). A dialysis membrane consisting of semi-permeable hollow fibres is placed in the lumen (D). Water and fermentation products are removed from the lumen through the dialysis system, thereby maintaining physiological concentrations of microbial metabolites and preventing accumulation of metabolites to toxic levels. Furthermore the model contains an inlet system for the delivery of food (I) and a level sensor to control (E) a constant volume of the luminal content. The system was kept anaerobic by flushing with nitrogen gas (F), which allowed for the growth of a dense, complex microbiota, comparable to that found in the proximal colon of humans. A: Peristaltic compartments containing fecal matter; B: pH electrode; C: Alkali pump; D: Dialysis liquid circuit with hollow fibre membrane; E: Level sensor; F: N2 gas inlet; G: Sampling port; H: Gas outlet; I: “Ileal efflux” container; J: Temperature sensor.
In addition to the studies performed with the computerized human gut TIM-2, there are numerous reports on simpler colon simulators, and the function of some of these has been validated by chemical and microbiological measurements of the intestinal contents of human sudden death victims[70]. These models are fermentation systems that closely reproduce the proximal and distal human colon in terms of physicochemical parameters by utilizing a number of different vessels and continuous or semi-continuous culturing modes[71]. For example, a two-stage compound continuous culture models consisting of a proximal vessel (with lower pH) and a distal vessel (with higher pH) inoculated with human feces have been used to evaluate how various nutrients and supplements affect genotoxicity of the colonic environment and the populations of human gut bacteria[72,73]. Continuous culture models have been applied to analyses of how certain prebiotics affect the fecal metabolite profile, the survival of probiotics, and the interactions between various colonic microbial populations[74-77]. The effect of retention time (colonic transit time) on the catabolism of organic sources of carbon and nitrogen have been analyzed by a three-stage continuous culture model, which revealed that the majority of carbohydrate breakdown and SCFA production takes place in the proximal part of the colon (in the first vessel); whereas, formation of branched-chain fatty acids and phenolic compounds, occurs primarily in the distal part (mimicked by vessels 2 and 3)[70]. Other three-stage continuous culture colonic models inoculated with human fecal material were utilized to quantitate bacteria and evaluate the fermentability of oligosaccharide sources[78,79]. Four-stage semicontinuous model systems of the human colon, in which the four compartments mimic the conditions of the ascending, transverse, descending and sigmoid colon, have been employed to investigate the effects of probiotics, prebiotics, and various synbiotic combinations[80-82].
Applied to our question of whether the apoptotic and WNT signaling-modulating functions of butyrate are affected by diet-derived polyphenol compounds and their metabolites, the strategy utilizing in vitro gut models would be a reliable approach.
Thus, digesta samples from in vitro fermentation systems or the computerized TIM-2 model, instead of individual compounds, should be used to analyze the effects of various diets on colonic cancer cells.
Screening for dietary components that increase butyrate production by the colonic microbiota
TIM-2 allows determining the potential of dietary fibers to produce butyrate by the microbiota under physiological conditions. In an extensive study comparing 17 fibers Maathuis et al[57] showed varying levels of butyrate production for each fiber, with the highest production resulting from pullulan. Interestingly, this fiber also produced high levels of lactate, an intermediate intestinal metabolite that accumulates when there is a fast fermentation of a substrate. Lactate is usually converted into propionate[83] and butyrate[84] and through cross-feeding between different members of the microbiota. Butyrate is also produced through cross-feeding from acetate; thus, using 13C-starch Maathuis et al[58] have shown that cross-feeding between Ruminococcus bromii and Eubacterium rectale results in production of butyrate from acetate[56]. Similarly, using 13C-labeled galacto-oligosaccharides it was shown that lactate, produced by Bifidobacteria and Lactobacilli, was converted into butyrate. These two cross-feeding reactions in the colon could be quantified[85,86] and an in silico model can be used to predict production of the various SCFA by the colonic microbiota.
Analyses of human fecal samples also allow for focused analyses of how dietary changes affect butyrate levels in different individuals. Thus, considerable variations in fecal butyrate concentrations have been detected among individuals consuming resistant starch or nonstarch polysaccharides in a randomized cross-over study[87]. McOrist and colleagues reported that intake of resistant starch overall increases butyrate concentrations in most, but not all, individuals[87].
Analyses with a semi-continuous colonic simulator revealed that Lactobacillus acidophilus NCFM™ in combination with lactitol increases the numbers of Bifidobacteria, and stimulates synergistically the production of butyrate[82]. Similar colonic simulation system consisting of three vessels and inoculated with fecal slurry from healthy nonmethane producing donors established the parameters of SCFA production, including this of butyrate[70].
Use of in vitro models to study the microbial metabolism of polyphenols in the colon
Approximately 90%-95% of dietary polyphenols are not absorbed in the small intestine and reach the colon intact[88]. In the case of monomeric units, studies performed with ileostomy patients have shown that almost 70% of the ingested monomeric flavanols are accumulated in the colon, with 33% corresponding to the intact parent compounds[89]. As mentioned above, the major colonic metabolites of the polyphenols are phenolic acids. Thus, (epi) catechin and the monomeric units of procyanidins are degraded into several phenolic acids, namely various substituted phenylvaleric, phenylpropionic, phenylacetic, benzoic, and hippuric acid[90-92]. Additional metabolites from catechin and epicatechin such as 5-(3,4-dihydroxyphenyl)-γ-valerolactone and 5-(3-hydroxyphenyl)-γ-valerolactone have been identified in man[90,91,93].
Under physiological conditions, the monomeric polyphenols are fermented rapidly; therefore, it is unknown whether these compounds have sufficient half-life to affect colonic (neoplastic) tissue from the luminal side, or whether the resulting microbial metabolites exert a stronger biological effect. Studies with human gut models can facilitate the answer to this question. In unpublished studies with TIM-2, we have observed that the same microbiota metabolizes different polyphenols to different low-molecular weight aromatic acids with variable hydroxylation profile and length of the aliphatic side chain (Figure 2). The number of produced microbial metabolites ranged from two (for epicatechin) to 12 (for quercetin). Even glycosylation of the polyphenols (e.g., quercetin versus rutin) affected the production of microbial metabolites, likely because different groups of colonic microorganisms ferment quercetin and rutin. Thus, compared to other polyphenols, fermentation of rutin resulted in decreased proportion of benzoic acid and other metabolites (Figure 2), as well as an about 20-fold lower absolute amount of metabolites.
Figure 2.
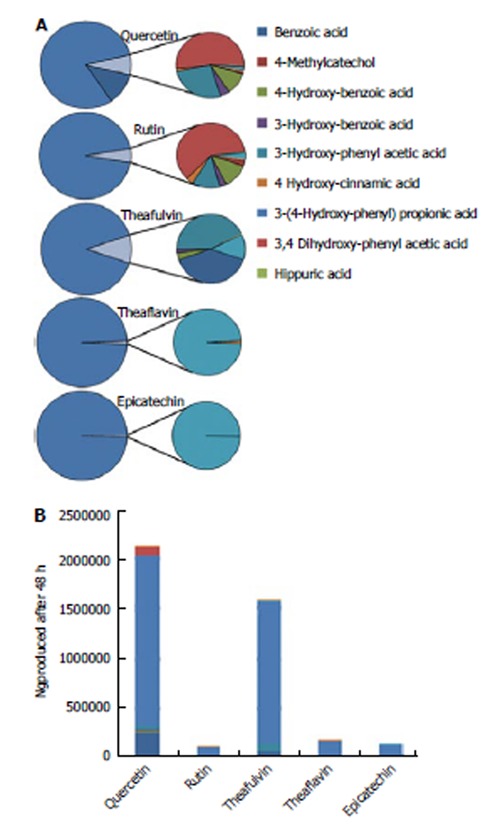
Cumulative production of ‘simple’ phenolic metabolites after 48 h fermentation of different polyphenols in TIM-2. At time zero a single shot of individuals polyphenols (1 microgram in dimethyl sulfoxide) was introduced into TIM-2 throigh the sampling port (Figure 1G). At regular intervals for the next 48 h samples were taken from the lumen and dialysate and analyzed using LC-MS for the microbial metabolites generated by the gut microbiota. The ratio (bar graph, in percentage) and absolute cumulative production (B; in ng) at t = 48 h of microbial metabolites after fermentation in TIM-2 were subsequently calculated and compared amongst the different polyphenols.
Analyses with colonic simulators allow for the detection of new colonic metabolites. In urine, the most frequent metabolite found after polyphenol ingestion is hippurate. This metabolite, a conjugate of benzoic acid and glycine, is considered to be produced by co-metabolism of the host and the microbiota. Benzoic acid is produced from the phenolic acids produced by the microbiota, and the glycine is thought to be coupled to benzoic acid in the liver. However, in the in vitro human gut TIM-2, which lacks the host metabolism component, we have shown that hippurate is also produced, indicating that the colonic microbiota by itself produces the metabolite (Figure 2).
Studies with colonic models could also address the question on the half-life of monomeric flavanols. For example, in studies on the dimeric forms of chocolate procyanidins Appeldoorn et al[90] have shown that the human microbiota produce several metabolites: 2-(3,4-dihydroxyphenyl)acetic acid, 2-(3-hydroxyphenyl)acetic acid, 2-(4-hydroxyphenyl)acetic acid and 3-(3-hydroxyphenyl)propionic acid, as well as various hydroxylated phenylvaleric acids, phenylvalerolactones,and 1-(3’,4’-dihydroxyphenyl)-3-(2’,4’,6’-trihydroxyphenyl)propan-2-ol. The researchers also indicated that the formation of smaller metabolites was due to the direct degradation of dimers instead of cleavage of the monomeric form as previously assumed[90]. It is still possible that some procyanidin dimers are converted into monomeric flavanols before being fermented into phenolic acids; however, monomeric flavanols are rapidly metabolized, and therefore their presence is difficult to analyze[94].
Finally, phenolic acids produced from flavanols by the colonic microbiota significantly inhibit pathogenic human intestinal bacteria, such as Clostridium perfringens, Staphylococcus aureus, E. coli, and Salmonella, while exhibiting a much lower inhibition of commensal bacteria and probiotics, Clostridium, Bifidobacterium and Lactobacillus[95,96]. One mechanism mediating this activity is the destabilization of the outer membrane of Salmonella species[97]. Since changes in microbiota composition influence the production of butyrate from dietary fiber, the combined effects of polyphenols and fibers need to be thoroughly investigated in colonic simulator systems that include the naturally occurring colonic microorganisms.
CONCLUSION
Analyses on individual bioactives pinpoint their molecular targets in cells; however, such studies (1) should utilize physiological concentrations of the compounds; and (2) should be accompanied by analyses with colonic digesta from different diets, since the activity of individual metabolites is likely modified by the complex colonic milieu. Such studies can be facilitated by the use of artificial GI systems and fecal water samples. This type of analyses will assist the design of functional foods and/or dietary supplements with CRC-preventive role.
Footnotes
P- Reviewers: Higgins PJ, Kanda T, Lee HC S- Editor: Qi Y L- Editor: A E- Editor: Liu SQ
References
- 1.Jones DS, Podolsky SH, Greene JA. The burden of disease and the changing task of medicine. N Engl J Med. 2012;366:2333–2338. doi: 10.1056/NEJMp1113569. [DOI] [PubMed] [Google Scholar]
- 2.Hoyert DL, Kochanek KD, Murphy SL. Deaths: final data for 1997. Natl Vital Stat Rep. 1999;47:1–104. [PubMed] [Google Scholar]
- 3.American Cancer Society (2008, December 9). Cancer Projected To Become Leading Cause Of Death Worldwide In 2010. Available from: http: //www.sciencedaily.com/releases/2008/12/081209111516.htm. Accessed on January 30, 2013.
- 4.Murphy SL, Xu J, Kochanek KD. Deaths: Final Data for 2010. Natl Vital Stat Rep. 2013;61:37–41. [PubMed] [Google Scholar]
- 5.World Health Organization. Projections of mortality and causes of death, 2015 and 2030. Geneva: World Health Organization. Available from: http: //www.who.int/healthinfo/global_burden_disease/projections/en/index.html. Accessed on August 13, 2013.
- 6.Giger JN, Davidhizar RE. Conceptual and theoretical approaches to patient care: associate versus baccalaureate degree prepared nurses. J Adv Nurs. 1990;15:1009–1015. doi: 10.1111/j.1365-2648.1990.tb01980.x. [DOI] [PubMed] [Google Scholar]
- 7. American Cancer Society, Cancer Facts and Figures 2013. Available from: http: //www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-037129.pdf. Accessed on August 3, 2013.
- 8.Stryker SJ, Wolff BG, Culp CE, Libbe SD, Ilstrup DM, MacCarty RL. Natural history of untreated colonic polyps. Gastroenterology. 1987;93:1009–1013. doi: 10.1016/0016-5085(87)90563-4. [DOI] [PubMed] [Google Scholar]
- 9.Peipins LA, Sandler RS. Epidemiology of colorectal adenomas. Epidemiol Rev. 1994;16:273–297. doi: 10.1093/oxfordjournals.epirev.a036154. [DOI] [PubMed] [Google Scholar]
- 10.World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Colorectal Cancer Report 2010 Summary. Food, Nutrition, Physical Activity, and the Prevention of Colorectal Cancer. 2011. Available from: http: //www.wcrf.org/PDFs/Colorectal cancer report summary 2011.pdf. Accessed on August 3, 2013. [Google Scholar]
- 11.Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev. 2001;81:1031–1064. doi: 10.1152/physrev.2001.81.3.1031. [DOI] [PubMed] [Google Scholar]
- 12.Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27:104–119. doi: 10.1111/j.1365-2036.2007.03562.x. [DOI] [PubMed] [Google Scholar]
- 13.Roediger WE. Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut. 1980;21:793–798. doi: 10.1136/gut.21.9.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Havenaar R. Intestinal health functions of colonic microbial metabolites: a review. Benef Microbes. 2011;2:103–114. doi: 10.3920/BM2011.0003. [DOI] [PubMed] [Google Scholar]
- 15.Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28:1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lazarova DL, Bordonaro M, Carbone R, Sartorelli AC. Linear relationship between Wnt activity levels and apoptosis in colorectal carcinoma cells exposed to butyrate. Int J Cancer. 2004;110:523–531. doi: 10.1002/ijc.20152. [DOI] [PubMed] [Google Scholar]
- 17.Bordonaro M, Lazarova DL, Sartorelli AC. The activation of beta-catenin by Wnt signaling mediates the effects of histone deacetylase inhibitors. Exp Cell Res. 2007;313:1652–1666. doi: 10.1016/j.yexcr.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/- colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 19.Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 20.Polakis P, Hart M, Rubinfeld B. Defects in the regulation of beta-catenin in colorectal cancer. Adv Exp Med Biol. 1999;470:23–32. doi: 10.1007/978-1-4615-4149-3_3. [DOI] [PubMed] [Google Scholar]
- 21.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Lucero OM, Dawson DW, Moon RT, Chien AJ. A re-evaluation of the “oncogenic” nature of Wnt/beta-catenin signaling in melanoma and other cancers. Curr Oncol Rep. 2010;12:314–318. doi: 10.1007/s11912-010-0114-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manach C, Williamson G, Morand C, Scalbert A, Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81:230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 24.Halliwell B, Rafter J, Jenner A. Health promotion by flavonoids, tocopherols, tocotrienols, and other phenols: direct or indirect effects Antioxidant or not. Am J Clin Nutr. 2005;81:268S–276S. doi: 10.1093/ajcn/81.1.268S. [DOI] [PubMed] [Google Scholar]
- 25.Manach C, Donovan JL. Pharmacokinetics and metabolism of dietary flavonoids in humans. Free Radic Res. 2004;38:771–785. doi: 10.1080/10715760410001727858. [DOI] [PubMed] [Google Scholar]
- 26.Rechner AR, Kuhnle G, Bremner P, Hubbard GP, Moore KP, Rice-Evans CA. The metabolic fate of dietary polyphenols in humans. Free Radic Biol Med. 2002;33:220–235. doi: 10.1016/s0891-5849(02)00877-8. [DOI] [PubMed] [Google Scholar]
- 27.Williams RJ, Spencer JP, Rice-Evans C. Flavonoids: antioxidants or signalling molecules. Free Radic Biol Med. 2004;36:838–849. doi: 10.1016/j.freeradbiomed.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Karlsson PC, Huss U, Jenner A, Halliwell B, Bohlin L, Rafter JJ. Human fecal water inhibits COX-2 in colonic HT-29 cells: role of phenolic compounds. J Nutr. 2005;135:2343–2349. doi: 10.1093/jn/135.10.2343. [DOI] [PubMed] [Google Scholar]
- 29.Russell WR, Drew JE, Scobbie L, Duthie GG. Inhibition of cytokine-induced prostanoid biogenesis by phytochemicals in human colonic fibroblasts. Biochim Biophys Acta. 2006;1762:124–130. doi: 10.1016/j.bbadis.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 30.Larrosa M, Luceri C, Vivoli E, Pagliuca C, Lodovici M, Moneti G, Dolara P. Polyphenol metabolites from colonic microbiota exert anti-inflammatory activity on different inflammation models. Mol Nutr Food Res. 2009;53:1044–1054. doi: 10.1002/mnfr.200800446. [DOI] [PubMed] [Google Scholar]
- 31.Monagas M, Khan N, Andrés-Lacueva C, Urpí-Sardá M, Vázquez-Agell M, Lamuela-Raventós RM, Estruch R. Dihydroxylated phenolic acids derived from microbial metabolism reduce lipopolysaccharide-stimulated cytokine secretion by human peripheral blood mononuclear cells. Br J Nutr. 2009;102:201–206. doi: 10.1017/S0007114508162110. [DOI] [PubMed] [Google Scholar]
- 32.Gao K, Xu A, Krul C, Venema K, Liu Y, Niu Y, Lu J, Bensoussan L, Seeram NP, Heber D, et al. Of the major phenolic acids formed during human microbial fermentation of tea, citrus, and soy flavonoid supplements, only 3,4-dihydroxyphenylacetic acid has antiproliferative activity. J Nutr. 2006;136:52–57. doi: 10.1093/jn/136.1.52. [DOI] [PubMed] [Google Scholar]
- 33.Lambert JD, Rice JE, Hong J, Hou Z, Yang CS. Synthesis and biological activity of the tea catechin metabolites, M4 and M6 and their methoxy-derivatives. Bioorg Med Chem Lett. 2005;15:873–876. doi: 10.1016/j.bmcl.2004.12.070. [DOI] [PubMed] [Google Scholar]
- 34.Herzog A, Kuntz S, Daniel H, Wenzel U. Identification of biomarkers for the initiation of apoptosis in human preneoplastic colonocytes by proteome analysis. Int J Cancer. 2004;109:220–229. doi: 10.1002/ijc.11692. [DOI] [PubMed] [Google Scholar]
- 35.Warren CA, Paulhill KJ, Davidson LA, Lupton JR, Taddeo SS, Hong MY, Carroll RJ, Chapkin RS, Turner ND. Quercetin may suppress rat aberrant crypt foci formation by suppressing inflammatory mediators that influence proliferation and apoptosis. J Nutr. 2009;139:101–105. doi: 10.3945/jn.108.096271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rao CV, Desai D, Rivenson A, Simi B, Amin S, Reddy BS. Chemoprevention of colon carcinogenesis by phenylethyl-3-methylcaffeate. Cancer Res. 1995;55:2310–2315. [PubMed] [Google Scholar]
- 37.Rao CV, Desai D, Simi B, Kulkarni N, Amin S, Reddy BS. Inhibitory effect of caffeic acid esters on azoxymethane-induced biochemical changes and aberrant crypt foci formation in rat colon. Cancer Res. 1993;53:4182–4188. [PubMed] [Google Scholar]
- 38.Kumar N, Shibata D, Helm J, Coppola D, Malafa M. Green tea polyphenols in the prevention of colon cancer. Front Biosci. 2007;12:2309–2315. doi: 10.2741/2233. [DOI] [PubMed] [Google Scholar]
- 39.Patel R, Ingle A, Maru GB. Polymeric black tea polyphenols inhibit 1,2-dimethylhydrazine induced colorectal carcinogenesis by inhibiting cell proliferation via Wnt/beta-catenin pathway. Toxicol Appl Pharmacol. 2008;227:136–146. doi: 10.1016/j.taap.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 40.Kim J, Zhang X, Rieger-Christ KM, Summerhayes IC, Wazer DE, Paulson KE, Yee AS. Suppression of Wnt signaling by the green tea compound (-)-epigallocatechin 3-gallate (EGCG) in invasive breast cancer cells. Requirement of the transcriptional repressor HBP1. J Biol Chem. 2006;281:10865–10875. doi: 10.1074/jbc.M513378200. [DOI] [PubMed] [Google Scholar]
- 41.Pahlke G, Ngiewih Y, Kern M, Jakobs S, Marko D, Eisenbrand G. Impact of quercetin and EGCG on key elements of the Wnt pathway in human colon carcinoma cells. J Agric Food Chem. 2006;54:7075–7082. doi: 10.1021/jf0612530. [DOI] [PubMed] [Google Scholar]
- 42.Staal FJ, Noort Mv Mv, Strous GJ, Clevers HC. Wnt signals are transmitted through N-terminally dephosphorylated beta-catenin. EMBO Rep. 2002;3:63–68. doi: 10.1093/embo-reports/kvf002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Noort M, Meeldijk J, van der Zee R, Destree O, Clevers H. Wnt signaling controls the phosphorylation status of beta-catenin. J Biol Chem. 2002;277:17901–17905. doi: 10.1074/jbc.M111635200. [DOI] [PubMed] [Google Scholar]
- 44.Kern M, Pahlke G, Ngiewih Y, Marko D. Modulation of key elements of the Wnt pathway by apple polyphenols. J Agric Food Chem. 2006;54:7041–7046. doi: 10.1021/jf0606611. [DOI] [PubMed] [Google Scholar]
- 45.Waldecker M, Kautenburger T, Daumann H, Veeriah S, Will F, Dietrich H, Pool-Zobel BL, Schrenk D. Histone-deacetylase inhibition and butyrate formation: Fecal slurry incubations with apple pectin and apple juice extracts. Nutrition. 2008;24:366–374. doi: 10.1016/j.nut.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 46.Waldecker M, Kautenburger T, Daumann H, Busch C, Schrenk D. Inhibition of histone-deacetylase activity by short-chain fatty acids and some polyphenol metabolites formed in the colon. J Nutr Biochem. 2008;19:587–593. doi: 10.1016/j.jnutbio.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 47.Kroon PA, Clifford MN, Crozier A, Day AJ, Donovan JL, Manach C, Williamson G. How should we assess the effects of exposure to dietary polyphenols in vitro. Am J Clin Nutr. 2004;80:15–21. doi: 10.1093/ajcn/80.1.15. [DOI] [PubMed] [Google Scholar]
- 48.Jenner AM, Rafter J, Halliwell B. Human fecal water content of phenolics: the extent of colonic exposure to aromatic compounds. Free Radic Biol Med. 2005;38:763–772. doi: 10.1016/j.freeradbiomed.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 49.Turner NJ, Thomson BM, Shaw IC. Bioactive isoflavones in functional foods: the importance of gut microflora on bioavailability. Nutr Rev. 2003;61:204–213. doi: 10.1301/nr.2003.jun.204-213. [DOI] [PubMed] [Google Scholar]
- 50.Hooper LV, Midtvedt T, Gordon JI. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu Rev Nutr. 2002;22:283–307. doi: 10.1146/annurev.nutr.22.011602.092259. [DOI] [PubMed] [Google Scholar]
- 51.Klinder A, Karlsson PC, Clune Y, Hughes R, Glei M, Rafter JJ, Rowland I, Collins JK, Pool-Zobel BL. Fecal water as a non-invasive biomarker in nutritional intervention: comparison of preparation methods and refinement of different endpoints. Nutr Cancer. 2007;57:158–167. doi: 10.1080/01635580701274848. [DOI] [PubMed] [Google Scholar]
- 52.Rafter JJ, Child P, Anderson AM, Alder R, Eng V, Bruce WR. Cellular toxicity of fecal water depends on diet. Am J Clin Nutr. 1987;45:559–563. doi: 10.1093/ajcn/45.3.559. [DOI] [PubMed] [Google Scholar]
- 53.Nordling MM, Glinghammar B, Karlsson PC, de Kok TM, Rafter JJ. Effects on cell proliferation, activator protein-1 and genotoxicity by fecal water from patients with colorectal adenomas. Scand J Gastroenterol. 2003;38:549–555. doi: 10.1080/00365520310002913. [DOI] [PubMed] [Google Scholar]
- 54.Pettersson J, Karlsson PC, Choi YH, Verpoorte R, Rafter JJ, Bohlin L. NMR metabolomic analysis of fecal water from subjects on a vegetarian diet. Biol Pharm Bull. 2008;31:1192–1198. doi: 10.1248/bpb.31.1192. [DOI] [PubMed] [Google Scholar]
- 55.Venema K, van den Abbeele P. Experimental models of the gut microbiome. Best Pract Res Clin Gastroenterol. 2013;27:115–126. doi: 10.1016/j.bpg.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 56.Kovatcheva-Datchary P, Egert M, Maathuis A, Rajilić-Stojanović M, de Graaf AA, Smidt H, de Vos WM, Venema K. Linking phylogenetic identities of bacteria to starch fermentation in an in vitro model of the large intestine by RNA-based stable isotope probing. Environ Microbiol. 2009;11:914–926. doi: 10.1111/j.1462-2920.2008.01815.x. [DOI] [PubMed] [Google Scholar]
- 57.Maathuis A, Hoffman A, Evans A, Sanders L, Venema K. The effect of the undigested fraction of maize products on the activity and composition of the microbiota determined in a dynamic in vitro model of the human proximal large intestine. J Am Coll Nutr. 2009;28:657–666. doi: 10.1080/07315724.2009.10719798. [DOI] [PubMed] [Google Scholar]
- 58.Maathuis AJ, van den Heuvel EG, Schoterman MH, Venema K. Galacto-oligosaccharides have prebiotic activity in a dynamic in vitro colon model using a (13)C-labeling technique. J Nutr. 2012;142:1205–1212. doi: 10.3945/jn.111.157420. [DOI] [PubMed] [Google Scholar]
- 59.Martinez RC, Cardarelli HR, Borst W, Albrecht S, Schols H, Gutiérrez OP, Maathuis AJ, de Melo Franco BD, De Martinis EC, Zoetendal EG, et al. Effect of galactooligosaccharides and Bifidobacterium animalis Bb-12 on growth of Lactobacillus amylovorus DSM 16698, microbial community structure, and metabolite production in an in vitro colonic model set up with human or pig microbiota. FEMS Microbiol Ecol. 2013;84:110–123. doi: 10.1111/1574-6941.12041. [DOI] [PubMed] [Google Scholar]
- 60.Tabernero M, Venema K, Maathuis AJ, Saura-Calixto FD. Metabolite production during in vitro colonic fermentation of dietary fiber: analysis and comparison of two European diets. J Agric Food Chem. 2011;59:8968–8975. doi: 10.1021/jf201777w. [DOI] [PubMed] [Google Scholar]
- 61.Déat E, Blanquet-Diot S, Jarrige JF, Denis S, Beyssac E, Alric M. Combining the dynamic TNO-gastrointestinal tract system with a Caco-2 cell culture model: application to the assessment of lycopene and alpha-tocopherol bioavailability from a whole food. J Agric Food Chem. 2009;57:11314–11320. doi: 10.1021/jf902392a. [DOI] [PubMed] [Google Scholar]
- 62.Mitea C, Havenaar R, Drijfhout JW, Edens L, Dekking L, Koning F. Efficient degradation of gluten by a prolyl endoprotease in a gastrointestinal model: implications for coeliac disease. Gut. 2008;57:25–32. doi: 10.1136/gut.2006.111609. [DOI] [PubMed] [Google Scholar]
- 63.Blanquet S, Zeijdner E, Beyssac E, Meunier JP, Denis S, Havenaar R, Alric M. A dynamic artificial gastrointestinal system for studying the behavior of orally administered drug dosage forms under various physiological conditions. Pharm Res. 2004;21:585–591. doi: 10.1023/b:pham.0000022404.70478.4b. [DOI] [PubMed] [Google Scholar]
- 64.Souliman S, Blanquet S, Beyssac E, Cardot JM. A level A in vitro/in vivo correlation in fasted and fed states using different methods: applied to solid immediate release oral dosage form. Eur J Pharm Sci. 2006;27:72–79. doi: 10.1016/j.ejps.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 65.Krul C, Luiten-Schuite A, Baandagger R, Verhagen H, Mohn G, Feron V, Havenaar R. Application of a dynamic in vitro gastrointestinal tract model to study the availability of food mutagens, using heterocyclic aromatic amines as model compounds. Food Chem Toxicol. 2000;38:783–792. doi: 10.1016/s0278-6915(00)00071-5. [DOI] [PubMed] [Google Scholar]
- 66.Chen L, Hébrard G, Beyssac E, Denis S, Subirade M. In vitro study of the release properties of soy-zein protein microspheres with a dynamic artificial digestive system. J Agric Food Chem. 2010;58:9861–9867. doi: 10.1021/jf101918w. [DOI] [PubMed] [Google Scholar]
- 67.Brouwers J, Anneveld B, Goudappel GJ, Duchateau G, Annaert P, Augustijns P, Zeijdner E. Food-dependent disintegration of immediate release fosamprenavir tablets: in vitro evaluation using magnetic resonance imaging and a dynamic gastrointestinal system. Eur J Pharm Biopharm. 2011;77:313–319. doi: 10.1016/j.ejpb.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 68.Minekus M, Smeets-Peeters M, Bernalier A, Marol-Bonnin S, Havenaar R, Marteau P, Alric M, Fonty G, Huis in’t Veld JH. A computer-controlled system to simulate conditions of the large intestine with peristaltic mixing, water absorption and absorption of fermentation products. Appl Microbiol Biotechnol. 1999;53:108–114. doi: 10.1007/s002530051622. [DOI] [PubMed] [Google Scholar]
- 69.Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto JM, et al. Enterotypes of the human gut microbiome. Nature. 2011;473:174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Macfarlane GT, Macfarlane S, Gibson GR. Validation of a Three-Stage Compound Continuous Culture System for Investigating the Effect of Retention Time on the Ecology and Metabolism of Bacteria in the Human Colon. Microb Ecol. 1998;35:180–187. doi: 10.1007/s002489900072. [DOI] [PubMed] [Google Scholar]
- 71.Marsh PD. The role of continuous culture in modelling the human microflora. J Chem Tech Biotechnol. 1995;64:1–9. [Google Scholar]
- 72.Christophersen CT, Petersen A, Licht TR, Conlon MA. Xylo-oligosaccharides and inulin affect genotoxicity and bacterial populations differently in a human colonic simulator challenged with soy protein. Nutrients. 2013;5:3740–3756. doi: 10.3390/nu5093740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brück WM, Graverholt G, Gibson GR. Use of batch culture and a two-stage continuous culture system to study the effect of supplemental alpha-lactalbumin and glycomacropeptide on mixed populations of human gut bacteria. FEMS Microbiol Ecol. 2002;41:231–237. doi: 10.1111/j.1574-6941.2002.tb00984.x. [DOI] [PubMed] [Google Scholar]
- 74.Sannasiddappa TH, Costabile A, Gibson GR, Clarke SR. The influence of Staphylococcus aureus on gut microbial ecology in an in vitro continuous culture human colonic model system. PLoS One. 2011;6:e23227. doi: 10.1371/journal.pone.0023227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mäkeläinen H, Forssten S, Saarinen M, Stowell J, Rautonen N, Ouwehand AC. Xylo-oligosaccharides enhance the growth of bifidobacteria and Bifidobacterium lactis in a simulated colon model. Benef Microbes. 2010;1:81–91. doi: 10.3920/BM2009.0025. [DOI] [PubMed] [Google Scholar]
- 76.De Preter V, Falony G, Windey K, Hamer HM, De Vuyst L, Verbeke K. The prebiotic, oligofructose-enriched inulin modulates the faecal metabolite profile: an in vitro analysis. Mol Nutr Food Res. 2010;54:1791–1801. doi: 10.1002/mnfr.201000136. [DOI] [PubMed] [Google Scholar]
- 77.Mäkeläinen H, Hasselwander O, Rautonen N, Ouwehand AC. Panose, a new prebiotic candidate. Lett Appl Microbiol. 2009;49:666–672. doi: 10.1111/j.1472-765X.2009.02698.x. [DOI] [PubMed] [Google Scholar]
- 78.Child MW, Kennedy A, Walker AW, Bahrami B, Macfarlane S, Macfarlane GT. Studies on the effect of system retention time on bacterial populations colonizing a three-stage continuous culture model of the human large gut using FISH techniques. FEMS Microbiol Ecol. 2006;55:299–310. doi: 10.1111/j.1574-6941.2005.00016.x. [DOI] [PubMed] [Google Scholar]
- 79.Wichienchot S, Prasertsan P, Hongpattarakere T, Gibson GR, Rastall RA. In vitro three-stage continuous fermentation of gluco-oligosaccharides produced by Gluconobacter oxydans NCIMB 4943 by the human colonic microflora. Curr Issues Intest Microbiol. 2006;7:13–18. [PubMed] [Google Scholar]
- 80.van Zanten GC, Knudsen A, Röytiö H, Forssten S, Lawther M, Blennow A, Lahtinen SJ, Jakobsen M, Svensson B, Jespersen L. The effect of selected synbiotics on microbial composition and short-chain fatty acid production in a model system of the human colon. PLoS One. 2012;7:e47212. doi: 10.1371/journal.pone.0047212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mäkeläinen HS, Mäkivuokko HA, Salminen SJ, Rautonen NE, Ouwehand AC. The effects of polydextrose and xylitol on microbial community and activity in a 4-stage colon simulator. J Food Sci. 2007;72:M153–M159. doi: 10.1111/j.1750-3841.2007.00350.x. [DOI] [PubMed] [Google Scholar]
- 82.Mäkivuokko H, Forssten S, Saarinen M, Ouwehand A, Rautonen N. Synbiotic effects of lactitol and Lactobacillus acidophilus NCFM™ in a semi-continuous colon fermentation model. Benef Microbes. 2010;1:131–137. doi: 10.3920/BM2009.0033. [DOI] [PubMed] [Google Scholar]
- 83.Venema K. Impact of Fiber on Gastrointestinal Microbiota. In: Paeschke TM, Aimutis WR, editors. Nondigestible Carbohydrates and Digestive Health. Wiley-Blackwell; 2011. pp. 125–164. [Google Scholar]
- 84.Morrison DJ, Mackay WG, Edwards CA, Preston T, Dodson B, Weaver LT. Butyrate production from oligofructose fermentation by the human faecal flora: what is the contribution of extracellular acetate and lactate. Br J Nutr. 2006;96:570–577. [PubMed] [Google Scholar]
- 85.Binsl TW, De Graaf AA, Venema K, Heringa J, Maathuis A, De Waard P, Van Beek JH. Measuring non-steady-state metabolic fluxes in starch-converting faecal microbiota in vitro. Benef Microbes. 2010;1:391–405. doi: 10.3920/BM2010.0038. [DOI] [PubMed] [Google Scholar]
- 86.de Graaf AA, Maathuis A, de Waard P, Deutz NE, Dijkema C, de Vos WM, Venema K. Profiling human gut bacterial metabolism and its kinetics using [U-13C]glucose and NMR. NMR Biomed. 2010;23:2–12. doi: 10.1002/nbm.1418. [DOI] [PubMed] [Google Scholar]
- 87.McOrist AL, Miller RB, Bird AR, Keogh JB, Noakes M, Topping DL, Conlon MA. Fecal butyrate levels vary widely among individuals but are usually increased by a diet high in resistant starch. J Nutr. 2011;141:883–889. doi: 10.3945/jn.110.128504. [DOI] [PubMed] [Google Scholar]
- 88.Clifford MN. Diet-derived phenols in plasma and tissues and their implications for health. Planta Med. 2004;70:1103–1114. doi: 10.1055/s-2004-835835. [DOI] [PubMed] [Google Scholar]
- 89.Stalmach A, Mullen W, Steiling H, Williamson G, Lean ME, Crozier A. Absorption, metabolism, and excretion of green tea flavan-3-ols in humans with an ileostomy. Mol Nutr Food Res. 2010;54:323–334. doi: 10.1002/mnfr.200900194. [DOI] [PubMed] [Google Scholar]
- 90.Appeldoorn MM, Vincken JP, Aura AM, Hollman PC, Gruppen H. Procyanidin dimers are metabolized by human microbiota with 2-(3,4-dihydroxyphenyl)acetic acid and 5-(3,4-dihydroxyphenyl)-gamma-valerolactone as the major metabolites. J Agric Food Chem. 2009;57:1084–1092. doi: 10.1021/jf803059z. [DOI] [PubMed] [Google Scholar]
- 91.Rios LY, Gonthier MP, Rémésy C, Mila I, Lapierre C, Lazarus SA, Williamson G, Scalbert A. Chocolate intake increases urinary excretion of polyphenol-derived phenolic acids in healthy human subjects. Am J Clin Nutr. 2003;77:912–918. doi: 10.1093/ajcn/77.4.912. [DOI] [PubMed] [Google Scholar]
- 92.Gonthier MP, Donovan JL, Texier O, Felgines C, Remesy C, Scalbert A. Metabolism of dietary procyanidins in rats. Free Radic Biol Med. 2003;35:837–844. doi: 10.1016/s0891-5849(03)00394-0. [DOI] [PubMed] [Google Scholar]
- 93.Das NP. Studies on flavonoid metabolism. Absorption and metabolism of (+)-catechin in man. Biochem Pharmacol. 1971;20:3435–3445. doi: 10.1016/0006-2952(71)90449-7. [DOI] [PubMed] [Google Scholar]
- 94.Aura A-M. Microbial metabolism of dietary phenolic compounds in the colon. Phytochemistry Reviews. 2008;7:407–429. [Google Scholar]
- 95.Cueva C, Moreno-Arribas MV, Martín-Alvarez PJ, Bills G, Vicente MF, Basilio A, Rivas CL, Requena T, Rodríguez JM, Bartolomé B. Antimicrobial activity of phenolic acids against commensal, probiotic and pathogenic bacteria. Res Microbiol. 2010;161:372–382. doi: 10.1016/j.resmic.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 96.Lee HC, Jenner AM, Low CS, Lee YK. Effect of tea phenolics and their aromatic fecal bacterial metabolites on intestinal microbiota. Res Microbiol. 2006;157:876–884. doi: 10.1016/j.resmic.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 97.Alakomi HL, Puupponen-Pimiä R, Aura AM, Helander IM, Nohynek L, Oksman-Caldentey KM, Saarela M. Weakening of salmonella with selected microbial metabolites of berry-derived phenolic compounds and organic acids. J Agric Food Chem. 2007;55:3905–3912. doi: 10.1021/jf070190y. [DOI] [PubMed] [Google Scholar]


