Abstract
Background
Sarcomere adaptation has been proposed as a mechanism for the adjustment of rectus muscle length in regulating binocular alignment. The purpose of this study was to investigate whether horizontal rectus muscle paths have abnormal lengths in subjects with intermittent or alternating strabismus.
Methods
High-resolution, surface coil magnetic resonance imaging was obtained in 2 mm thick axial planes in strabismic patients who had not undergone prior surgery and normal control subjects. The lengths of horizontal rectus muscle paths were measured digitally in central gaze for the fixating eye only and compared.
Results
A total of 12 strabismic subjects and 13 controls were included: 8 subjects had esotropia averaging 30Δ, and 4 had exotropia averaging 47Δ. The sample had 80% power to detect muscle path length changes of at least the typical surgical doses appropriate to strabismus surgery for correction of the mean deviations in each group, had such changes existed. Mean (± standard deviation) medial rectus path length was 35.0 ± 4.1 mm in controls, not significantly different from 36.3 ± 1.7 mm in exotropia (P = 0.56) or 35.8 ± 2.9 mm in esotropia (P = 0.62). Mean lateral rectus path length in controls was 35.7 ± 4.0 mm, not significantly different from the values of 39.6 ± 3.8 mm in exotropia (P = 0.09) and 37.8 ± 3.3 (P = 0.19) mm in esotropia.
Conclusions
Horizontal rectus muscle path lengths are not significantly abnormal in commonly encountered intermittent or alternating esotropia and exotropia.
Why does an esotropic eye turn inward? In incomitant strabismus, that is, paralytic or mechanical strabismus, end organ extraocular muscle function is clearly affected. However, in the more common types of comitant strabismus, the possible role of abnormal muscle structure and function remains enigmatic.1 Are there abnormalities of the horizontal rectus muscles, or is strabismus related to maldevelopment of normal cerebral visuomotor circuits?
Animal studies have shown that if a skeletal muscle is passively stretched or shortened, sarcomere length initially increases or decreases, respectively, resulting in suboptimal actin–myosin overlap and decreased contractile efficiency.2-4 However, if skeletal muscle length is maintained for several weeks, sarcomere adaption occurs. If the skeletal muscle is stretched, new sarcomeres are laid down in series (end to end), thus allowing each sarcomere to return to its initial optimum length. Conversely, if the muscle is shortened, sarcomeres drop out serially so that sarcomere length returns to normal. The same process also occurs in response to muscle length changes induce by chronic nerve stimulation.2 Biological mechanisms mediating these sarcomere adaptations are unclear,5 but the physiologic benefit is evidently to maintain optimum actin and myosin filament overlap to maximize efficiency of muscle contraction.
In 1994 Scott6 demonstrated that extraocular muscles in monkeys, like skeletal muscles, could adapt their sarcomere lengths. He sutured one eye of a monkey to the lateral orbital wall in a position of 30°-45° of exotropia. After maintaining exotropia for 2 months, sarcomere length in the treated eye was similar to those of the control eye; since the muscle lengths themselves were presumably altered, this implied that the numbers of sarcomeres in each muscle had changed. This modification of extraocular muscle length by sarcomere adaptation has been hypothesized to explain persistence of strabismus in Duane syndrome, and following transient injury, botulinum paralysis, or surgical recession.6-8
Guyton and colleagues9 elaborated the sarcomere adaptation concept to include a three-level feedback system as the basis for several types of “sensory” strabismus, suggesting that changes in vergence tonus in strabismus drive extraocular muscle length adaptation bilaterally, in turn decreasing the need for chronic changes in vergence tonus. Guyton9-10 has hypothesized that this mechanism may explain increasing “basic” deviation in accommodative esotropia, torsional deviation associated with A and V patterns,11 recurrent esotropia with presbyopia,12 divergence insufficiency in presbyopic patients,9 and cyclovertical deviations mimicking superior oblique paresis.10
Alternatively, work in primate models13-16 and clinical observations argues against primary extraocular muscle abnormality as the cause of comitant strabismus. Tychsen and colleagues15 found no differences in horizontal rectus cross-sectional areas, extraocular muscle paths, innervation densities, or cytoarchitecture in monkeys with spontaneous early-onset esotropia ranging from 7Δ to 24Δ compared with normal animals on high-resolution MRI and whole-orbit histopathology.15
The present study sought to determine whether abnormalities in path lengths of the horizontal rectus muscles are associated with commonly encountered intermittent or alternating esotropia and exotropia, testing the common presumption that muscles shorten in the direction of the habitual deviation. An experimentally accessible parameter might at first be thought to be overall muscle rest length, the length of a completely relaxed muscle not subjected to external mechanical loading. Presumably rest length would change because of sarcomere adaptation. The observed lengths of extraocular muscles change from their rest lengths as a function of elastic tensile loading, among other factors. Rest length, however, is not an observable parameter in vivo, since its measurement under zero loading would require extraocular muscle extirpation. Furthermore, since living muscles contract and relax to change their lengths during duction, any interpretable study must be performed with the eye in the same, reference, position. Central gaze seems a reasonable choice of reference position. However, if rectus muscles always followed the shortest paths from origin to insertion, then their lengths could only vary with globe and orbit size but would never otherwise vary as long as the eye remained in the reference position. Until recognition of the role of the orbital connective tissues in regulation of extraocular muscle paths, the foregoing consideration made it seem likely that rectus muscles would always have the same lengths in the reference eye position, regardless of the presence of strabismus. Were this true, there could be no empirical way of interpreting muscle lengths in the pathophysiology of strabismus; however, it is empirically possible to measure extraocular muscle path length.
Anatomical17,18 and functional imaging studies19-21 have established that the orbital pulley system inflects rectus muscle paths in both orthogonal directions transverse to their long axes, especially for the lateral rectus .22 Inflected or curved paths are necessarily longer than straight paths. Indeed, markedly bowed, elongated muscle paths have been demonstrated in older adult patients with acquired strabismus due to sagging eye syndrome.23 The authors postulated a similar phenomenon in other forms of strabismus. In esotropia, for example, a shortened medial rectus might hypothetically have increased tensile force in central gaze and resist path inflection by the pulley system, resulting in a straighter path and shorter length than normal. A lengthened lateral rectus, on the other hand, might hypothetically have less tensile force and thus a larger inflection in central gaze, resulting in an longer path than normal.24 Because rectus muscle path length has not heretofore been studied in other forms of strabismus, the present study examined path lengths of the medial rectus and lateral rectus muscles in concomitant esotropia and exotropia, including intermittent or alternating forms. While the approach of measuring path length in standard gaze position still cannot determine resting (ie, externally unloaded) length, it represents the only possible systematic method of evaluating the role of muscle length in strabismus.
Subjects and Methods
Written informed consent was obtained according to a protocol approved by the Institutional Review Board of the University of California–Los Angeles and in conformity with the Health Insurance Portability and Accountability Act of 1996. Paid control subjects underwent complete examinations to verify normal corrected vision, normal ocular versions, orthotropia in all gazes positions, and normal Titmus stereopsis of 40 arcsec. Subjects with esotropia or exotropia were recruited from an academic strabismus practice into a long-term, prospective study on strabismus and underwent sensorimotor evaluation and MRI. The ongoing study includes an overabundance of potentially eligible subjects. We selected from the study database the first alphabetically consecutive 13 controls and 12 strabismic cases with adequate imaging quality for analysis. Exclusion criteria included cases of paralytic or restrictive strabismus. No patient had a history of strabismus surgery. Heterotropia was measured at distance and near by prism and cover testing.
A 1.5-T MRI scanner (Signa; General Electric, Milwaukee, WI) was used for imaging using T1-25-26 or T2-weighted fast spin-echo sequences.27 These scanning protocols provide equivalent measurements. Crucial technical aspects, detailed elsewhere,20, 28-29 include use of a dual-phase surface coil array (Medical Advances, Milwaukee, WI) and fixation targets. High-resolution (312 μm) axial images of 2 mm thickness and matrix of 256 × 256 perpendicular to the long axis of the orbit were obtained in target-controlled central gaze.
To avoid confounding measurements by contractile changes in muscle path lengths induced by varying eye position, measurements were obtained in central gaze only, on both eyes in normal patients and on the fixating eye in strabismic patients. This constraint imposed a uniform reference eye position on all measurements. In order to verify gaze position, we measured the angle of the fixating eye. A line was drawn on the MRI image from the corneal apex through the lens center to the retina, and a second line was drawn from this point to the cranial vertical. The resulting angle was taken as gaze direction. Image analysis was similar to published methods.23,30-32 Although investigators were unmasked to diagnosis, they did not have a strong prior hypothesis regarding the expected effect of strabismus on muscle path lengths. Moreover, because the muscle path lengths were measured before review of clinical data, interpretive bias is unlikely. Digital MRIs were quantified using ImageJ64 (provided in the public domain by W. Rasband, National Institutes of Health, Bethesda, MD, http://rsb.info.nih.gov/ij/, 1997-2009). Lengths were obtained from manually drawn curved paths, consisting of adjacent points, bisecting muscle width from orbital apex to the visible scleral insertion. The X and Y coordinates of each point were recorded. The length of the path defined by these points was then computed by using the Euclidean distance formula: length = √(X2 + Y2) (Figures 1-3). A line drawn from corneal apex through lens center to retina was used to measure globe axial length . Comparison of means was by the t test, with 0.05 considered significant.
FIG 1.
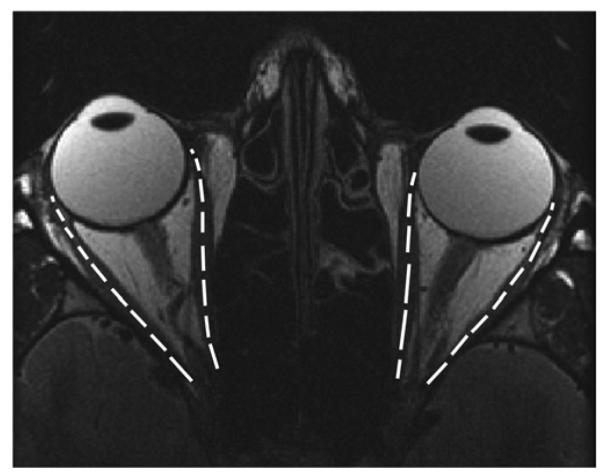
T2-weighted fast spin-echo axial magnetic resonance imaging (MRI) of a control subject overlaid with lengths of the horizontal extraocular muscles (right medial rectus muscle [MR], 35.9 mm; right lateral rectus muscle [LR], 37.2 mm; left MR, 33.8 mm, left LR, 36.2 mm).
FIG 3.
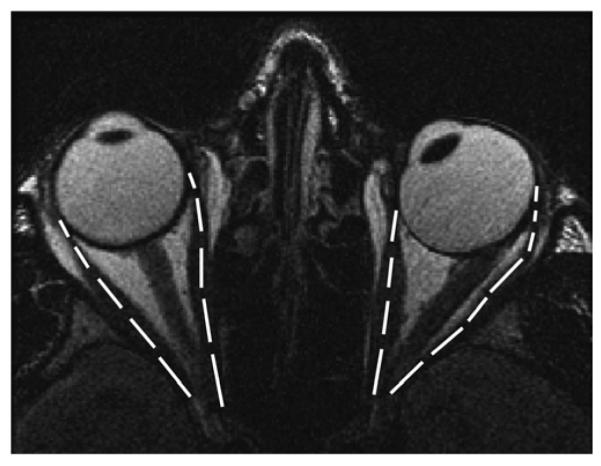
Axial T2-weighted fast spin-echo MRI of patient 4 with 50Δ alternating esotropia (right eye fixating) overlaid with horizontal muscle lengths (right MR, 39 mm; right LR, 37.5 mm; left MR, 31.1 mm; left LR, 42.8 mm). Note that MR length in the left nonfixating, esotropic eye was 31.1 mm, whereas with the left eye centrally fixating, length of the same MR was 36.9 mm.
Results
Control data were obtained from 13 orthotropic adults (mean age, 32.5 ± 18.5; range, 21-68; 6 females) and 12 adult strabismic subjects (mean age, 46.6 ± 24.7, range, 14-84; 6 females). Eight subjects had esotropia (mean age, 53.1 ± 24.3 years; range, 16-84 years; 4 females) and 4 had exotropia (mean age, 33.7 ± 23.0 years; range 14-67 years; 2 females). Strabismic and control subjects did not differ significantly in age (P = 0.12). Subjects with divergence paralysis esotropia were significantly older (76.3 ± 9.3 years) than the other groups (P = 0.03). The esotropic group comprised 3 patients with divergence paralysis esotropia, 3 patients with decompensated intermittent esotropia, and 2 patients with pattern strabismus. The exotropic group included 2 patients whose deviations were intermittent, and 2 patients with pattern strabismus (See Table 1). Esotropic subjects had concomitant deviations averaging 30.8Δ ± 26Δ at distance and 30.3Δ ± 31.4Δ at near; exotropic subjects had concomitant deviations averaging 47.5Δ ± 31.7Δ at distance and 47.2Δ ± 23Δ at near. Strabismus duration ranged from 1 to 20 years for esotropic subjects and 3 to 20 years for exotropic subjects. Best-corrected visual acuity ranged from 20/12 to 20/25 in the esotropic group and from 20/15 to 20/20 in the exotropic group. Controls subjects had 20/16 to 20/20 acuities.
Table 1.
Strabismic subject characteristics and extraocular muscle path lengths
| Sex | Age, years |
Muscle length, mm |
Strabismus, PD |
Pattern | Stereopsis, arcsec |
Strabismus duration, |
Fixing eye in MRI |
MRI findings | ||
|---|---|---|---|---|---|---|---|---|---|---|
| LR | MR | years | ||||||||
| 1 | F | 16 | 35.9 | 37.2 | ET 55 | V | Nil | 5 | Right | Normal |
| 2 | F | 53 | 33.2 | 36.0 | ET 25 | None | 40 | 20 | Right | Normal |
| 3 | M | 28 | 39.9 | 39.8 | ET 75 | None | Nil | 3.5 | Right | Normal |
| 4a | M | 37 | 39.0 | 37.5 | ET 50 | None | Nil | 1.5 | Right | Normal |
| 4b | M | 37 | 37.8 | 37.0 | ET 50 | None | Nil | 1.5 | Left | Normal |
| 5 | F | 62 | 33.4 | 31.6 | ET 18 | A | 80 | 1.25 | Left | LR-SR band rupture |
| 6 | M | 84 | 36.8 | 32.5 | Orthoc | None | 140 | 1 | Right | LR-SR band rupture |
| 7 | F | 66 | 43.1 | 32.4 | ET 12 | None | 80 | 10 | Right | LR-SR band rupture |
| 8 | M | 79 | 41.0 | 38.0 | ET 12 | None | 3000 | 3 | Right | LR sagging OU |
| 9 | F | 14 | 37.7 | 38.0 | 0 | A | 200 | 3 | Left | LR superior to MR, |
| Down XT 45 | SR nasal to IR; | |||||||||
| Both globes intort in | ||||||||||
| downgaze | ||||||||||
| 10 | M | 28 | 37.3 | 34.2 | XT 65 | None | 400 | 20 | Left | Normal |
| 11 | M | 26 | 38.2 | 35.6 | X (T)60 | V | Not done | N/A | Left | Extorted orbits |
| 12 | F | 67 | 45.2 | 37.3 | XT 60 | None | Nil | N/A | Right | Extorted orbits, |
| nonspherical globes | ||||||||||
ET, esotropia; IR, inferior rectus muscle; LR, lateral rectus muscle; MR, medial rectus muscle; NA, not available; Ortho, orthotropia; OU, both eyes/each eye; PD, prism diopters; SR, superior rectus muscle; X(T), (intermittent) exotropia.
Patient 4 measured with right eye fixing.
Patient 4 measured with left eye fixing to center right eye.
Average esotropia of patient 6 in lateral gaze was 7 PD.
Six exotropic subjects were myopic (range, −5.00 D to −0.25 D), 1 hyperopic (+1.00 D), and 1 emmetropic (pseudophakia bilaterally). All 3 subjects with divergence paralysis esotropia were bilaterally pseudophakic. Three exotropic subjects were myopic (range, −9.00 to −0.25D), and one emmetropic. Axial length of the esotropic group was 23.59 ± 1.07 mm; of the exotropic group, 24.44 ± 1.43 mm; and of controls, 23.49 ± 2.21 mm, without statistically significant differences (P > 0.11). Stereo threshold ranged from nil to 40 arcsec in the esotropic group and was 200 arcsec in the 2 exotropic patients who were measured; the controls had 40 arcsec stereopsis (P = 0.29).
The mean fixating eye angle of strabismic subjects was +7.86° ± 4.84°, not significantly different from +3.65° ± 5.46° in controls (P = 0.26). This angle is slightly temporal to straight ahead and corresponds to an expected positive angle kappa. Mean (±SD) medial rectus path length was 35.0±4.1 mm in controls (19 muscles), not significantly different from 36.3 ± 1.7 mm in exotropia (4 muscles, P = 0.56) or 35.8 ± 2.9 mm in esotropia (9 muscles, P = 0.62). Mean lateral rectus path length in controls was 35.7 ± 4.0 mm (17 muscles), not significantly different from the values of 39.6 ± 3.8 mm in exotropia (4 muscles, P = 0.09) and 37.8 ± 3.3 mm (9 muscles, P = 0.19) in esotropia. In divergence paralysis esotropia, mean medial rectus path length was 34.3 ± 3.2 mm (P = 0.77). Mean lateral rectus path length was moderately but not significantly longer than in controls at 39.6 ± 3.7 mm (P = 0.09, Figure 4).
FIG 4.
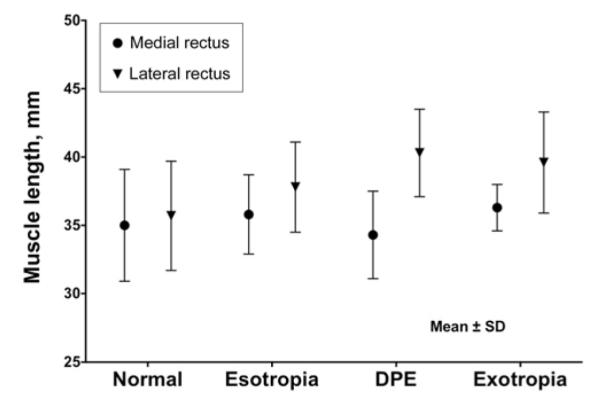
Horizontal rectus extraocular muscle length ± standard error of the mean.
Since the study was exploratory, statistical power computations were performed post hoc using measured variances. Thresholds for detectable muscle path length changes were estimated from surgical dosage tables of Parks and Mitchell33 for the mean values of 30Δ esotropia and 47Δ exotropia observed in this study. Thus we considered adequacy of the sample size for detection of at least 4.5 mm medial rectus shortening or 6 mm lateral rectus lengthening in esotropia and at least 6 mm medial rectus lengthening or 8 mm lateral rectus shortening in exotropia. We assumed alpha (P value) of 5%, and beta of 80%, representing a 20% chance of incorrectly failing to reject the null hypothesis of no difference between control and strabismic subjects, and thus erroneously missing a real difference. Given foregoing assumptions, a sample size of only 8 subjects in the control and strabismic groups is required for the medial rectus muscle in esotropia, 5 subjects in each group for the lateral rectus in esotropia, and 3 subjects in each group for both the medial rectus muscle and lateral rectus muscle in exotropia. Since the actual data set included 13 controls, 8 subjects with esotropia, and 4 subjects with exotropia, the conclusion appears statistically reasonable that there were no significant differences in medial rectus and lateral rectus path lengths in patients who had concomitant esotropia and exotropia. Indeed, for the lateral rectus muscle, the current sample appears sufficient to have had 95% power to detect at least 6 mm path elongation in esotropia.
Discussion
The present study compared medial and lateral rectus path lengths in central gaze in patients with common esotropia and exotropia to those of normal controls. In patients with long-standing, comitant strabismus, muscle path length changes might be expected to have become fully developed, if this phenomenon exists. Except for a trend for lateral rectus path elongation in divergence paralysis esotropia, the present study found no change in path lengths of the medial and lateral rectus muscles in central fixation by axial MRI. There was even a nonsignificant trend toward abnormal lengthening of the lateral rectus path in exotropic subjects. Although it is possible that small changes in path lengths might have been detected had the study included many more strabismic subjects, no trends suggested this.
Our findings agree with those of Narasimhan and colleagues14 in orthotropic, naturally, and artificially strabismic monkeys. These authors found no significant differences among the three groups in horizontal rectus muscle sizes or paths as measured by MRI nor histological differences in connective tissues, in the pulley system, or in muscle size, structure, or innervation.14 Similarly, Tychsen and colleagues15 found no evidence of a structural or innervational extraocular muscle abnormality in monkeys with naturally occurring early-onset esotropia. Recently, Joshi and Das16 demonstrated that in monkeys with sensory-induced exotropia, central innervation to extraocular muscles sets the state of strabismus. Mechanical factors such as muscle length adaptation (for horizontal misalignment) and pulley heterotopy or static torsion (for A patterns) likely did not play major roles in sensory-induced strabismus.
Potentially the extraocular muscles of strabismic patients harbored abnormalities undetectable by MRI , but the present study can reasonably exclude extraocular muscle path length abnormalities as a significant association. For example, it might be the case that despite normal extraocular muscle path length in strabismic patients, muscle contractile force could be abnormal due to changes in the fiber type and distribution,34-35 changes in innervation, atrophy or hypertrophy. Using MRI, Schoeff and colleagues36 demonstrated supernormal medial rectus cross section and contractility in 12 adults with concomitant esotropia. It is also possible that actual extraocular muscle rest length, a conceptually unmeasurable parameter in vivo, is altered in strabismus, perhaps by changes in the numbers of sarcomeres or rearrangement of the configuration of muscle fibers. However, rest length can only be measured with a muscle under zero innervation and zero external mechanical tension—conditions unattainable in vivo.
Recently, Chaudhuri and Demer23 reported elongation lateral and medial rectus muscle elongation in 11 patients with divergence paralysis esotropia. In the current study, although lateral rectus path tended to be longer in esotropic patients than in controls, the path elongation did not reach statistical significance (P = 0.09), likely because only 3 subjects in the current study had divergence paralysis esotropia. Subjects with divergence paralysis in the present study cohort, unlike that of Chaudhuri and Demer,23 included 1 case with esotropia only in lateral gazes.
Results of this study should be understood within the context of limitations imposed by small sample size and the measuring technique. Because of low MRI contrast of extraocular muscles against adjacent tissues, we consistently considered muscle contact point with the sclera as the anterior end point for measurement of path length. Actual path lengths of the horizontal rectus muscles, including their tendons, obviously exceed our stated measurements. However, since rectus muscle insertions are highly uniform among normal and strabismic people,37 no bias should result from using the scleral contact point in both groups. As emphasized, the study could not measure resting lengths of rectus muscle or sarcomere size or number.
FIG 2.
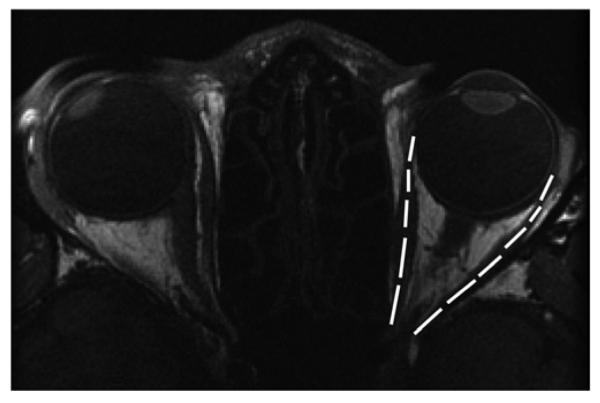
T1-weighted MRI of patient 10 with 65Δ alternating exotropia (left eye fixating) overlaid with lengths of the left MR and LR (left MR, 34.2 mm; left LR, 37.2 mm).
Acknowledgments
The authors thank Nicolasa De Salles for technical assistance.
Supported by US Public Health Service, National Eye Institute grants EY08313 and EY00331, and Research to Prevent Blindness. Joseph Demer is Leonard Apt Professor of Ophthalmology.
Presented at the 39th Annual Meeting of the American Association for Pediatric Ophthalmology and Strabismus, Boston, Massachusetts, April 3-7, 2013.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kushner BJ. Incomitant strabismus: Does extraocular muscle form denote function? Arch Ophthalmol. 2010;128:164–9. doi: 10.1001/archophthalmol.2010.301. [DOI] [PubMed] [Google Scholar]
- 2.Tabary J-C, Tardieu C, Tardieu G, Tabary C. Experimental rapid sarcomere loss with concomitant hypoextensibility. Muscle Nerve. 1981;4:198–203. doi: 10.1002/mus.880040305. [DOI] [PubMed] [Google Scholar]
- 3.Williams PE, Catanese T, Lucey EG, Goldspink G. The importance of stretch and contractile activity in the prevention of connective tissue accumulation in muscle. J Anat. 1988;158:109–14. [PMC free article] [PubMed] [Google Scholar]
- 4.Goldspink G, Williams P. Cellular mechanisms involved in the determination of muscle length and mass during growth; problems arising from imbalance between antagonists muscle groups. In: Scott AB, editor. Proceedings of the Mechanics of Strabismus Symposium. The Smith-Kettlewell Eye Research Institute; San Francisco, CA: 1992. pp. 195–206. [Google Scholar]
- 5.Goldspink G, Williams P, Simpson H. Gene expression in response to muscle stretch (review) Clin Orthop Relat Res. 2002;(suppl):S146–52. doi: 10.1097/00003086-200210001-00017. [DOI] [PubMed] [Google Scholar]
- 6.Scott AB. Change of eye muscle sarcomeres according to eye position. J Pediatr Ophthalmol Strabismus. 1994;31:85–8. doi: 10.3928/0191-3913-19940301-05. [DOI] [PubMed] [Google Scholar]
- 7.Collins CC, Jampolsky A, Howe PS. Proc Mechanics of Strabismus Symp. Smith Kettlewell Eye Research Institute; San Francisco: 1992. Mechanical limitations in rotation; pp. 19–40. [Google Scholar]
- 8.Castanera de Molina A, Giner-Munoz ML. Short stiff extraocular muscles: mechanics involved in EOM adaptations to squint. In: Parito-Diaz J, Hauviller V, editors. XII Congreso del Consejo Latinoamericano de Estrabismo (CLADE) Garfica Lifra; La Plata: 1997. pp. 503–8. [Google Scholar]
- 9.Guyton DL. The 10th Bielschowsky Lecture. Changes in strabismus over time: the roles of vergence tonus and muscle length adaptation. Binocular Vision and Strabismus Quarterly. 2006;21:81–92. [PubMed] [Google Scholar]
- 10.Guyton DL. Ocular torsion reveals the mechanisms of cyclovertical strabismus: the Weisenfeld Lecture. Invest Ophthalmol Vis Sci. 2008;49:847–57. doi: 10.1167/iovs.07-0739. [DOI] [PubMed] [Google Scholar]
- 11.Guyton DL, Weingarten PE. Sensory torsion as the cause of primary oblique muscle overaction/underaction and A-and V-pattern strabismus. Binocular Vision and Eye Muscle Surg Quarterly. 1994;9:209–36. [Google Scholar]
- 12.Wright WW, Goltzer KC, Guyton DL. Esotropia associated with early presbyopia caused by inappropriate muscle length adaptation. J AAPOS. 2005;9:563–6. doi: 10.1016/j.jaapos.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Das VE, Mustari MJ. Correlation of cross-axis eye movements and motoneuron activity in non-human primates with “A” pattern strabismus. Invest Ophthalmol Vis Sci. 2007;48:665–74. doi: 10.1167/iovs.06-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Narasimhan A, Tychsen L, Poukens V, Demer JL. Horizontal rectus muscle anatomy in naturally and artificial strabismic monkeys. Invest Ophthalmol Vis Sci. 2007;48:2576–88. doi: 10.1167/iovs.06-0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tychsen L, Richards M, Wong A, et al. Spectrum of infantile esotropia in primates: Behavior, Brains, and orbits. J AAPOS. 2008;12:375–80. doi: 10.1016/j.jaapos.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joshi AC, Das VE. Responses of medial rectus motoneurons in monkeys with strabismus. Invest Ophthalmol Vis Sci. 2011;52:6697–705. doi: 10.1167/iovs.11-7402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demer JL, Miller JM, Poukens V, Vinters HV, Glasgow B. Evidence for fibromuscular pulleys of the recti extraocular muscles. Invest Ophthalmol Vis Sci. 1995;36:1125–36. [PubMed] [Google Scholar]
- 18.Demer JL. Pivotal role of orbital connective tissues in binocular alignment and strabismus: the Friedenwald lecture. Invest Ophthalmol Vis Sci. 2004;45:729–38. doi: 10.1167/iovs.03-0464. [DOI] [PubMed] [Google Scholar]
- 19.Clark RA, Miller JM, Demer JL. Location and stability of rectus muscle pulleys: muscle paths as a function of gaze. Invest Ophthalmol Vis Sci. 1997;38:227–40. [PubMed] [Google Scholar]
- 20.Demer JL, Kono R, Wright W. Magnetic resonance imaging of human extraocular muscles in convergence. J Neurophysiol. 2003;89:2072–85. doi: 10.1152/jn.00636.2002. [DOI] [PubMed] [Google Scholar]
- 21.Demer JL. Current concepts of mechanical and neural factors in ocular motility. Curr Opin Neurol. 2006;19:4–13. doi: 10.1097/01.wco.0000198100.87670.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demer JL. Inflection in inactive lateral rectus muscle: Evidence suggesting focal mechanical effects of connective tissues. Invest Ophthalmol Vis Sci. 2008;49:4858–64. doi: 10.1167/iovs.08-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chaudhuri Z, Demer JL. Sagging eye syndrome: connective tissue involution as a cause of horizontal and vertical strabismus in older patients. JAMA Ophthalmol. 2013;131:619–25. doi: 10.1001/jamaophthalmol.2013.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clark RA, Demer JL. Posterior inflection of weakened lateral rectus path: connective tissue factors reduce response to lateral rectus recession. Am J Ophthalmol. 2009;147:127–33. doi: 10.1016/j.ajo.2008.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Demer JL. A 12-year, prospective study of extraocular muscle imaging in complex strabismus. J AAPOS. 2003;6:337–47. doi: 10.1067/mpa.2002.129040. [DOI] [PubMed] [Google Scholar]
- 26.Demer JL, Miller JM. Orbital imaging in strabismus surgery. In: Rosenbaum AL, Santiago AP, editors. Clinical Strabismus Management: Principles and Techniques. Saunders; Philadelphia: WB: 1999. pp. 84–98. [Google Scholar]
- 27.Demer JL, Dusyanth A. T2-weighted fast spin-echo magnetic resonance imaging of extraocular muscles. J AAPOS. 2011;15:17–23. doi: 10.1016/j.jaapos.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clark RA, Miller JM, Rosenbaum AL, Demer JL. Heterotopic muscle pulleys or oblique muscle dysfunction? J AAPOS. 1998;2:17–25. doi: 10.1016/s1091-8531(98)90105-7. [DOI] [PubMed] [Google Scholar]
- 29.Demer JL, Miller JM, Koo EY, Rosenbaum AL. Quantitative magnetic resonance morphometry of extraocular muscles: A new diagnostic tool in paralytic strabismus. J Pediatr Ophthalmol Strabismus. 1994;31:177–88. doi: 10.3928/0191-3913-19940501-10. [DOI] [PubMed] [Google Scholar]
- 30.Clark RA, Demer JL. Functional morphometry of horizontal rectus extraocular muscles during ocular duction. Inv Ophthalmol Vis Sci. 2012;53:7375–9. doi: 10.1167/iovs.12-9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clark RA, Demer JL. Enhanced vertical rectus contractility by magnetic resonance imaging in superior oblique palsy. Arch Ophthalmol. 2011;129:904–8. doi: 10.1001/archophthalmol.2011.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Demer JL, Clark RA, Lim KH, Engle EC. Magnetic resonance imaging evidence for widespread orbital dysinnervation in dominant Duane’s retraction syndrome linked to the DURS2 locus. Inv Ophthalmol Vis Sci. 2007;48:194–202. doi: 10.1167/iovs.06-0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parks MM, Mitchell PR, Wheeler MB. Concomitant Esodeviations. In: Tasman W, Jaeger EA, editors. Duane’s Foundations of Clinical Ophthalmology. Vol 1. Lippincott Williams and Wilkins; Philadelphia: 2002. p. 12. [Google Scholar]
- 34.Spencer RF, Porter J. Biological organization of the extraocular muscles. In: Buttner-Ennerver JA, editor. Progress in Brain Research: Neuroanatomy of the Oculomotor System. Elsevier; Amsterdam: 2006. pp. 43–80. [DOI] [PubMed] [Google Scholar]
- 35.Lennerstrand G. Strabismus and eye muscle function. Acta Ophthalmol Scand. 2007;85:711–23. doi: 10.1111/j.1600-0420.2007.00853.x. [DOI] [PubMed] [Google Scholar]
- 36.Schoeff K, Chaudhuri Z, Demer JL. Functional magnetic resonance imaging of horizontal rectus muscles in esotropia. J AAPOS. 2013;17:16–21. doi: 10.1016/j.jaapos.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Apt L, Call NB. An anatomical reevaluation of rectus muscle insertions. Ophthalmic Surgery. 1982;13:108–12. [PubMed] [Google Scholar]


