Abstract
The angiotensinogen (AGT) gene M235T polymorphism has been suggested to be linked to risk of heart failure (HF). However, association studies on the M235T polymorphism and HF risk have shown conflicting results. PubMed and China Biology Medicine (CBM) databases were systematically searched to identify relevant studies. Odds ratios (ORs) and 95% confidence intervals (CIs) were used to assess the strength of the association. A total of 1,281 HF cases and 1,376 controls were included in the analysis. The pooled data showed that there was no significant associations between the AGT M235T polymorphism and HF risk for TT vs. MM (OR = 1.17, 95%CI = 0.62–2.19, P = 0.635), MT vs. MM (OR = 0.97, 95%CI = 0.77–1.22, P = 0.776), MT/TT vs. MM (OR = 1.07, 95%CI = 0.67–1.69, P = 0.781), and TT vs. MM/MT (OR = 1.23, 95%CI = 0.86–1.76, P = 0.259). In contrast, in the HF subgroup analysis by ethnicity, the AGT M235T polymorphism had a decreased risk of HF among Asians (MT vs. MM, OR = 0.39, 95%CI = 0.17–0.92, P = 0.032). Our results suggest that the AGT M235T polymorphism is a low-penetrant risk factor for the development of HF among Asians.
The regulation of heart failure (HF) is complex and under the control of different physiological systems. Abnormalities of Ca2+ homeostasis is known to occur within cardiac myocytes in a wide variety of heart disease, including HF. It is noted that the mRNA abundance of sarcolemmal (SL) Na+/Ca2+ exchange, sarcoplasmic reticular (SR) ryanodine receptor, and SR Ca2+-ATPase are all decreased in response to stimulation by angiotensin in neonatal myocytes1. Angiotensinogen (AGT), a globular glycoprotein in the renin-angiotensin-aldosterone system (RAAS), is encoded by the AGT gene and located on chromosome 1q422. AGT is produced by the liver and changed to angiotensin I via renin. After-wards, angiotensin I is converted to angiotensin II, and the elevated cardiac angiotensin II levels alone do not directly induce cardiac hypertrophy but do increase interstitial fibrosis3. A M235T (rs699) polymorphism, encoding a threonine instead of a methionine at residue 235 of the mature protein, has been associated with higher plasma AGT levels among patients with the T allele4.
HF is an ever increasing problem worldwide and is a major health problem associated with very high morbidity and mortality5. Coronary heart disease and hypertension are major causes of HF; other underlying conditions include idiopathic dilated cardiomyopathy (IDC), valvular congenital heart disease, and diabetes mellitus. It is difficult to predict who will develop heart failure in response to myocardial injury6. Although the exact molecular mechanisms of HF are still under intensive investigation, genetic polymorphisms of major neurohormonal systems involved in the pathophysiology of HF have been discussed7.
Over the last ten years, a number of molecular epidemiological studies have been conducted to investigate the association between the AGT M235T polymorphism and HF risk, but the results remain inconsistent. In addition, a previous meta-analysis on this issue also generated conflicting results8. To assess the association of AGT M235T polymorphism with the risk of HF, we conducted a meta-analysis from all eligible case-control studies published to data.
Results
Characteristics of studies
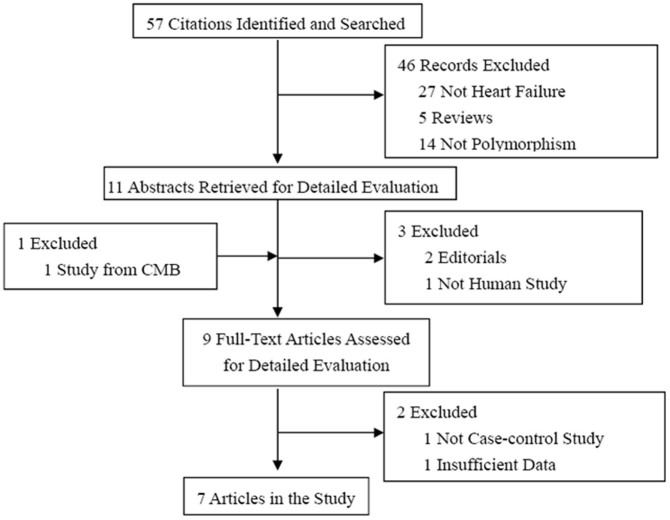
There were 57 published papers relevant to the search words. Based on the inclusion criteria, only six case-control studies were selected for this meta-analysis [9–26], and 41 studies were excluded. The flow chart of the study selection is summarized in Fig. 1. These selected studies included 1,281 cases and 1,376 control subjects. All studies were case-control studies that evaluated the association between the AGT M235T polymorphism and HF risk9,10,11,12,13,14. The distribution of genotypes in the controls of each study was in agreement with Hardy-Weinberg equilibrium except for one study9. Of these case-control studies, three reported on Europeans, while three reports were on Asians (Table 2).
Figure 1. The flow chart of the included studies.
Table 1. Quality assessment in the meta-analysis.
| Criteria | Score |
|---|---|
| A. Representativeness of cases | |
| Selected from population or hospital | 2 |
| Selected from any cardiovascular diseases service | 1 |
| Selected without clearly defined inclusion/exclusion criteria | 0 |
| B. Credibility of controls | |
| Population-based | 3 |
| Blood donors or volunteers | 2 |
| Hospital-based | 1 |
| Not described | 0 |
| C. Ascertainment of heart failure | |
| Two doctors confirmed | 2 |
| Diagnosis of heart failure by patient medical record | 1 |
| Not described | 0 |
| D. Genotyping examination | |
| Genotyping done under blinded condition | 1 |
| Not mentioned | 0 |
| E. Hardy-Weinberg equilibrium | |
| Equilibrium in controls | 2 |
| Disequilibrium in controls | 1 |
| No checked | 0 |
| F. Association assessment | |
| Assess association between genotypes and heart failure with appropriate statistics | 2 |
| Assess association between genotypes and colorectal cancer with logistic regression | 1 |
| Inappropriate statistics used | 0 |
Table 2. Study characteristics in the meta-analysis.
| First author | Publication year | Country | Ethnicity | Sample size (cases/controls) | MAFb | Quality scores | P a |
|---|---|---|---|---|---|---|---|
| Yamada | 1997 | Japan | Asian | 159/122 | 0.197 | 6 | 0.119 |
| Tiret | 2000 | France | European | 428/398 | 0.426 | 7 | 0.969 |
| Tiago | 2002 | Africa | African | 157/225 | 0.129 | 6 | 0.026 |
| Goldbergova | 2003 | Czech Republic | European | 158/200 | 0.435 | 8 | 0.808 |
| Peng | 2006 | China | Asian | 111/110 | 0.223 | 7 | 0.176 |
| Zakrzewski-Jakubiak | 2007 | Canada | European | 58/111 | 0.316 | 6 | 0.649 |
| Wu | 2009 | China | Asian | 210/210 | 0.138 | 9 | 0.998 |
aP value of Hardy-Weinberg equilibrium test.
bMAF, minor allele frequency in controls.
Main results
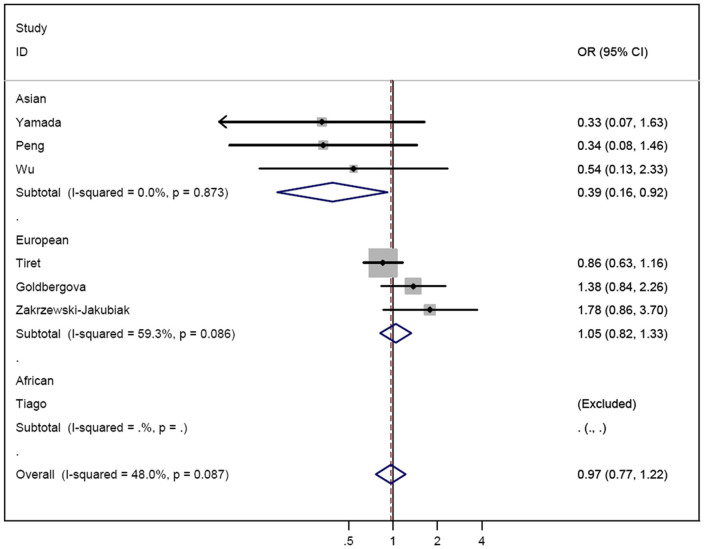
The fixed- and random-effects models were used to pool the results (Table 3). There was no evidence that the AGT M235T polymorphism was associated with HF risk in all genetic models (TT vs. MM, OR = 1.17, 95%CI = 0.62–2.19; MT vs. MM, OR = 0.97, 95%CI = 0.77–1.22; MT/TT vs. MM, OR = 1.07, 95%CI = 0.67–1.69; and TT vs. MM/MT, OR = 1.23, 95%CI = 0.86–1.76, Fig. 2). In contrast, in the subgroup analysis by ethnicity, significantly decreased risk of HF was detected among Asians for MT vs. MM (OR = 0.39, 95%CI = 0.17–0.92, Table 3).
Table 3. Meta-analysis of the AGT M235T polymorphism on HF risk.
| TT vs. MM | MT vs. MM | MT/TT vs. MM (dominant) | TT vs. MM/MT (recessive) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | na | OR (95% CI) | Pb | OR (95% CI) | Pb | OR (95% CI) | Pb | OR (95% CI) | Pb |
| Total | 7 | 1.17 (0.62–2.19)c | 0.012 | 0.97 (0.77–1.22) | 0.087 | 1.07 (0.67–1.69)c | 0.035 | 1.23 (0.86–1.76)c | 0.002 |
| Ethnicities | |||||||||
| Asian | 3 | 0.62 (0.27–1.45) | 0.467 | 0.39 (0.17–0.92) | 0.873 | 0.54 (0.24–1.22) | 0.543 | 1.39 (0.83–2.33) | 0.044 |
| European | 3 | 1.66 (0.70–3.92)c | 0.004 | 1.05 (0.82–1.33) | 0.086 | 1.31 (0.76–2.28)c | 0.014 | 1.40 (0.77–2.58) | 0.026 |
aNumber of comparisons.
bP value of Q-test for heterogeneity test.
cRandom-effects model was used when P value for heterogeneity test <0.05; otherwise, fix-effects model was used.
Figure 2. Forest plot of heart failure risk associated with the AGT M235T (MT vs. MM) among Asians and Europeans.
The squares and horizontal lines correspond to the study-specific OR and 95% CI.
Heterogeneity and sensitivity analyses
Significant heterogeneity between studies was observed in overall comparisons except for MT vs. MM model (Pheterogeneity = 0.087). The results revealed that no individual study significantly affect the pooled ORs, indicating that our results were statistically robust.
Publication bias
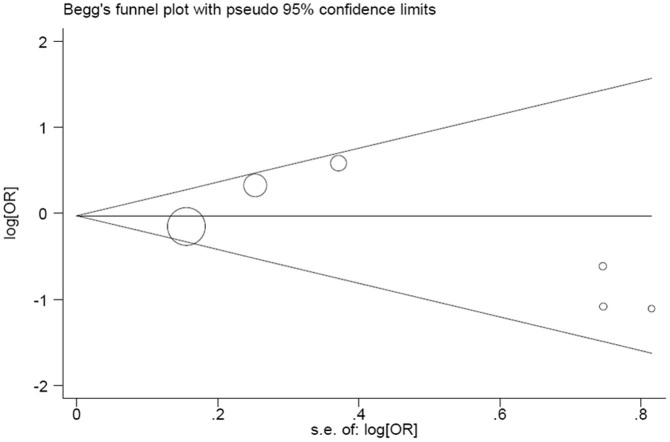
Egger's test provided no evidence for funnel plot asymmetry in the AGT M235T polymorphism in overall comparisons (t = −0.67, P = 0.541 for TT vs. MM/MT). Figure 3 showed the Begg's funnel plot on Egger's test.
Figure 3. Begg's funnel plot of AGT M235T polymorphism (MT vs. MM) and heart failure risk.
Discussion
This meta-analysis explored all available data on the association between the AGT M235T polymorphism and HF risk, including a total of 1,281 HF cases and 1,376 controls. We found that the variant MT genotype was associated with significant decreased risk of HF among Asians. Given the important roles of AGT in the regulation of RAS, it is biologically plausible that AGT polymorphism may modulate the risk of HF.
The RAAS plays an important role in the pathogenesis of HF. Following cleavage of AGT into angiotensin I by renin, production of biologically active Ang II causes vasoconstriction, stimulates sodium reabsorption15. The combinations of these abnormalities, myocardial fibrosis, and other factors modulated by RASS pathways are likely to lead to HF. The AGT gene has been widely researched as a HF candidate gene. Ishanov et al. firstly found a higher frequency of the 235T allele in hypertrophic cardiomyopathy16. The same significant association was observed in the Czech Republic women12. The results of our meta-analysis were consistent with these experimental findings. However, the effect of the AGT M235T polymorphism is still unclear. Yamada et al. indicated that there was no association between the AGT M235T polymorphism and HF risk10. Therefore, the function of the AGT M235T polymorphism needs to clarify clearly in the further.
In the subgroup analysis by ethnicity, there was a significant association found among Asians, but not Europeans, suggesting a possible role of ethnic differences in genetic backgrounds and the environment they live in. Furthermore, according to the etiology of the left ventricular dysfunction, HF can be classified with ischemic heart disease and dilated cardiomyopathy17. However, most studies did not include the analysis with these classifications. Therefore, further studies with different classifications of HF are needed to validate our findings. Although our results are not fully accord with previous meta-analysis regarding the positive association for the AGT M235T polymorphism8, we have made much more stable and detailed analysis to support our findings, which made our results much more reliable compared with previous meta-analysis, because we included more studies to improve the statistical power.
The present meta-analysis has some advantages compared to other individual studies; however, it does have some limitations. Firstly, the effect of genetic and environmental interactions was not addressed in our meta-analysis. Secondly, the results of subgroup analysis should be interpreted with caution because of limited statistical power. Thirdly, several studies have suggested that A-6G, A-20C and G-217A polymorphisms in the promoter region of AGT are implicated in the regulation of AGT gene transcription and expression, which may have an interaction with the M235T polymorphism18. However, given the limited evidence available on other AGT polymorphisms, this meta-analysis was restricted to the M235T polymorphism because it has been the most frequently studied. Therefore, we could not assess the haplotype effect on the risk of HF.
Taken together, the present meta-analysis suggests that the AGT M235T polymorphism may be involved in the risk of HF. Further large well-designed studies are still needed to determine the effects of the AGT M235T polymorphisms on HF.
Methods
Selection of relevant studies
A comprehensive search was conducted to identify for all relevant studies before August 10, 2013, using the search terms “angiotensinogen,” “polymorphism,” and “heart failure” in PubMed and China Biology Medicine (CBM) databases. Case reports, corresponding to editor, and review articles were excluded. The references cited in retrieved articles were also screened. Eligible studies included in the meta-analysis met the following criteria: (1) studies were conducted to have the evaluation of AGT M235T polymorphism and HF risk, (2) an unrelated case-control study, and (3) have available genotype frequency.
Data extraction
Two investigators independently extracted the data and reached consensus on all items. Data were collected on first author, year of publication, ethnicity of study population, quality scores, numbers of genotyped cases and controls, and Hardy-Weinberg equilibrium (HWE) of controls. Different ethnic descents were categorized as European, African, and Asian. Quality of studies was assessed according to the predefined criteria based on previous meta-analyses of observational studies19,20 (Table 1).
Statistical analysis
The strength of the associations between the AGT M235T polymorphism and HF risk was estimated by the crude odds ratios (ORs) with 95% confidence intervals (CIs). We first estimated the risk of the variant genotype TT vs. MM, and MT vs. MM, and then evaluated the dominant model (MT/TT vs. MM), and recessive model (TT vs. MM/MT). A Q-test was performed to assess the between-study heterogeneity21. Heterogeneity was considered significant if the P < 0.05 among studies, and the summary OR estimate of each study was calculated by the fixed-effects model. Otherwise, the random-effects model was used. Publication bias was invested by funnel plot, in which the standard error of log (OR) of each study was plotted against its OR. Funnel plots were assessed by the method of Egger's linear regression test. Subgroup analysis was performed according to ethnicity. Sensitivity analysis was performed by sequential omission of individual studies to ensure the stability of measuring results. All analyses were performed using the Stata software (version 10.0 StataCorp LP, College Station, TX). All P values were two-sided.
Author Contributions
Conceived and designed the experiments: J.W., Y.Z. Performed the experiments: J.W., H.H. Analyzed the data: J.W., H.H. Contributed reagents/material/analysis tools: Y.L., J.X. Wrote the manuscript: J.W., H.H., Y.Z. Reference collection and data management: J.W., H.H., Y.Z. Statistical analyses and paper writing: J.W., H.H., Y.Z. Study design: J.W., Y.Z.
References
- Ju H., Scammel-La Fleur T. & Dixon I. M. Altered mRNA abundance of calcium transport genes in cardiac myocytes induced by angiotensin II. J Mol Cell Cardiol 28, 1119–28 (1996). [DOI] [PubMed] [Google Scholar]
- Kolovou G. D. & Mavrogeni S. Genetics of Human Hypertension, the Role of Angiotensinogen. Should It be One of the Drug Target Genes? Angiology (2013). Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- van Kats J. P., Methot D., Paradis P., Silversides D. W. & Reudelhuber T. L. Use of a biological peptide pump to study chronic peptide hormone action in transgenic mice. Direct and indirect effects of angiotensin II on the heart. J Biol Chem 276, 44012–7 (2001). [DOI] [PubMed] [Google Scholar]
- Mehri S. et al. Angiotensinogen gene polymorphism in acute myocardial infarction patients. J Renin Angiotensin Aldosterone Syst 12, 42–7 (2011). [DOI] [PubMed] [Google Scholar]
- Bleumink G. S. et al. Quantifying the heart failure epidemic: prevalence, incidence rate, lifetime risk and prognosis of heart failure The Rotterdam Study. Eur Heart J 25, 1614–9 (2004). [DOI] [PubMed] [Google Scholar]
- Jackson G., Gibbs C. R., Davies M. K. & Lip G. Y. ABC of heart failure. Pathophysiology. BMJ 320, 167–70 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleumink G. S. et al. Genetic polymorphisms and heart failure. Genet Med 6, 465–74 (2004). [DOI] [PubMed] [Google Scholar]
- Chen S. et al. The M235T polymorphism in the angiotensinogen gene and heart failure: a meta-analysis. J Renin Angiotensin Aldosterone Syst (2012). Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Tiago A. D. et al. An aldosterone synthase gene variant is associated with improvement in left ventricular ejection fraction in dilated cardiomyopathy. Cardiovasc Res 54, 584–9 (2002). [DOI] [PubMed] [Google Scholar]
- Yamada Y., Ichihara S., Fujimura T. & Yokota M. Lack of association of polymorphisms of the angiotensin converting enzyme and angiotensinogen genes with nonfamilial hypertrophic or dilated cardiomyopathy. Am J Hypertens 10, 921–8 (1997). [DOI] [PubMed] [Google Scholar]
- Tiret L. et al. Lack of association between polymorphisms of eight candidate genes and idiopathic dilated cardiomyopathy: the CARDIGENE study. J Am Coll Cardiol 35, 29–35 (2000). [DOI] [PubMed] [Google Scholar]
- Goldbergova M. et al. Association of two angiotensinogen gene polymorphisms, M235T and G(-6)A, with chronic heart failure. Int J Cardiol 89, 267–72 (2003). [DOI] [PubMed] [Google Scholar]
- Zakrzewski-Jakubiak M. et al. Ten renin-angiotensin system-related gene polymorphisms in maximally treated Canadian Caucasian patients with heart failure. Br J Clin Pharmacol 65, 742–51 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. K. et al. A propensity score-based case-control study of renin-angiotensin system gene polymorphisms and diastolic heart failure. Atherosclerosis 205, 497–502 (2009). [DOI] [PubMed] [Google Scholar]
- Chapman C. M. et al. Polymorphisms in the angiotensinogen gene are associated with carotid intimal-medial thickening in females from a community-based population. Atherosclerosis 159, 209–17 (2001). [DOI] [PubMed] [Google Scholar]
- Ishanov A. et al. Angiotensinogen gene polymorphism in Japanese patients with hypertrophic cardiomyopathy. Am Heart J 133, 184–9 (1997). [DOI] [PubMed] [Google Scholar]
- Pavkova Goldbergova M. et al. Difference in angiotensinogen haplotype frequencies between chronic heart failure and advanced atherosclerosis patients - new prognostic factor? Physiol Res 60, 55–64 (2011). [DOI] [PubMed] [Google Scholar]
- Ishigami T. et al. Essential hypertension and 5′ upstream core promoter region of human angiotensinogen gene. Hypertension 30, 1325–30 (1997). [DOI] [PubMed] [Google Scholar]
- Thakkinstian A., D'Este C., Eisman J., Nguyen T. & Attia J. Meta-analysis of molecular association studies: vitamin D receptor gene polymorphisms and BMD as a case study. J Bone Miner Res 19, 419–28 (2004). [DOI] [PubMed] [Google Scholar]
- Thakkinstian A. et al. Systematic review and meta-analysis of the association between {beta}2-adrenoceptor polymorphisms and asthma: a HuGE review. Am J Epidemiol 162, 201–11 (2005). [DOI] [PubMed] [Google Scholar]
- Handoll H. H. Systematic reviews on rehabilitation interventions. Arch Phys Med Rehabil 87, 875 (2006). [DOI] [PubMed] [Google Scholar]