Abstract
An intact microcirculation is vital for diffusion of oxygen and nutrients and for removal of toxins of every organ and system in the human body. The functional and/or anatomical loss of microvessels is known as rarefaction, which can compromise the normal organ function and have been suggested as a possible starting point of several diseases. The purpose of this overview is to discuss the potential underlying mechanisms leading to renal microvascular rarefaction, and the potential consequences on renal function and on the progression of renal damage. Although the kidney is a special organ that receives much more blood than its metabolic needs, experimental and clinical evidence indicates that renal microvascular rarefaction is associated to prevalent cardiovascular diseases such as diabetes, hypertension, and atherosclerosis, either as cause or consequence. On the other hand, emerging experimental evidence using progenitor cells or angiogenic cytokines supports the feasibility of therapeutic interventions capable of modifying the progressive nature of microvascular rarefaction in the kidney. This overview will also attempt to discuss the potential renoprotective mechanisms of the therapeutic targeting of the renal microcirculation.
Introduction
Changes in vascular function and structure are observed during normal development and in response to pathological insults. The microcirculation of each organ is a plastic but precisely organized functional network. In general, each nutrient vessel entering an organ branches six to eight times before becoming arterioles (10–15 µm), which in turn branch two to five times into smaller vessels, reaching diameters of 9 µm or lower where they supply blood to the capillaries. An intact microcirculation is vital for the function of every organ and system in the human body for transportation of oxygen and nutrients as well as for the removal of toxins. The plasticity of the smaller vessels embedded in the organs is a dynamic process largely induced by modifications in the environment that result in changes of their number, shape, and function, as we discuss in this overview.
The microvascular (MV) adaptation to changes in the organ or tissue surroundings has been the subject of numerous investigations. The blood flow is generally regulated according to the specific needs of the tissues as long as the arterial pressure is sufficient to sustain adequate tissue perfusion. It is generally accepted in the physiology field that “form follows function.” In the microvasculature, the “form” of the MV network in general and the single vessel in particular will follow the “function” that is imposed by the environment. For instance, if the metabolic demands in a tissue or organ increase for a prolonged period, vascularity increases by a combined augmented recruitment of quiescent vessels and, if needed, generation of new ones via a process generally called angiogenesis. On the other hand, if metabolic demands decreases, functional and structural (regression) vascularity decreases. The tissue vascularity is determined by maximum blood flow needs, and tightly regulated by a number of factors that can promote physiological or pathological vascular growth and regression.
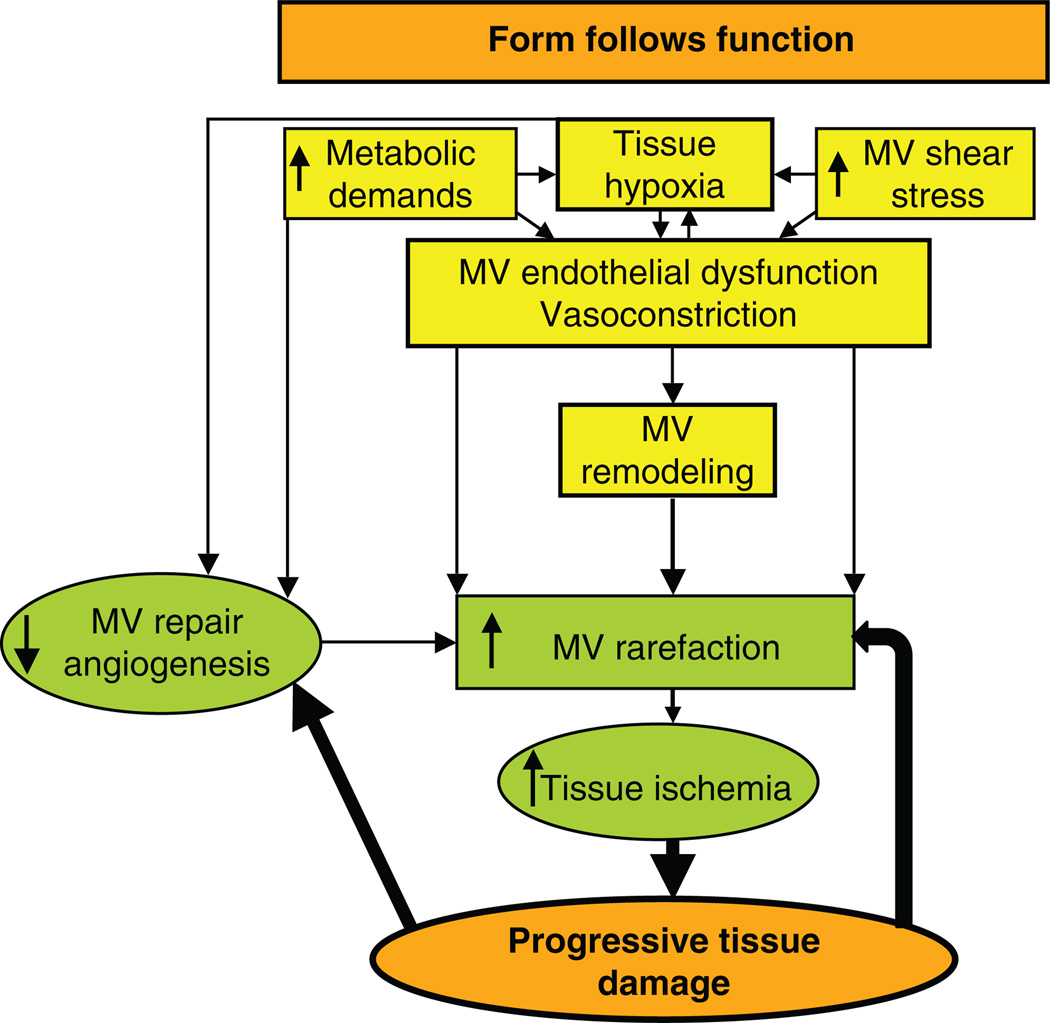
The abnormalities in the microcirculation play an important role in the progression of and are possibly the starting point of several diseases (5,14). Malfunction, structural changes, and loss are, grossly, the major deleterious changes the vessels may undergo. These not only compromise normal organ development and function, but also, more importantly, the responses to any given insult. Thus, the function determining the form includes a number of components that can induce changes in MV tone, such as increasing metabolic demands, low oxygen or glucose, increased reactive oxygen species (ROS), or augmented MV shear stress, to name a few (103,114,123). These may disrupt the balance between vasoactive substances toward MV dilatation or constriction, and/or generation of new vessels (Fig. 1). Depending on the severity and mainly, the duration of the stimulus causing long-term changes in tissue blood flow, alterations in the MV layers may consequently take place, leading to permanent and sometimes progressive modifications in the vessel, a process also known as vascular remodeling. Furthermore, the combination of functional and structural MV changes can eventuate in the loss of the contribution of these vessels to organ function, a process that is known as vascular rarefaction (Fig. 1). It is necessary to clarify that not always changes in MV tone will lead into the process of MV remodeling and eventually in MV rarefaction. Scenarios exist that vessel constriction does not necessarily lead to vessel loss. An example of this is the nonperfused capillaries that are recruited in skeletal muscle in case of a higher demand in a healthy patient.
Figure 1.
Schematic illustration describing the general process of microvascular (MV) rarefaction. Changes in the tissue environment and/or metabolic needs may lead to modification in vascular tone and progressive alterations in MV shape and morphology, ultimately resulting in MV irreversible damage and loss. This process accelerates MV damage and may subsequently lead to a feed-forward deleterious mechanism.
This overview will focus on the events leading to MV rarefaction in the kidney. I will discuss renal MV rarefaction in prevalent cardiovascular disease such as hypertension, diabetes, atherosclerosis and renovascular disease, and obesity. It should be recognized that delineating a specific causal role of MV rarefaction is quite difficult and MV rarefaction could likely be the cause as well as the consequence of the progressive nature of many kidney diseases. Finally, underlying mechanisms and the potential of targeted therapeutic interventions to protect the renal microcirculation will also be discussed.
Renal Circulation
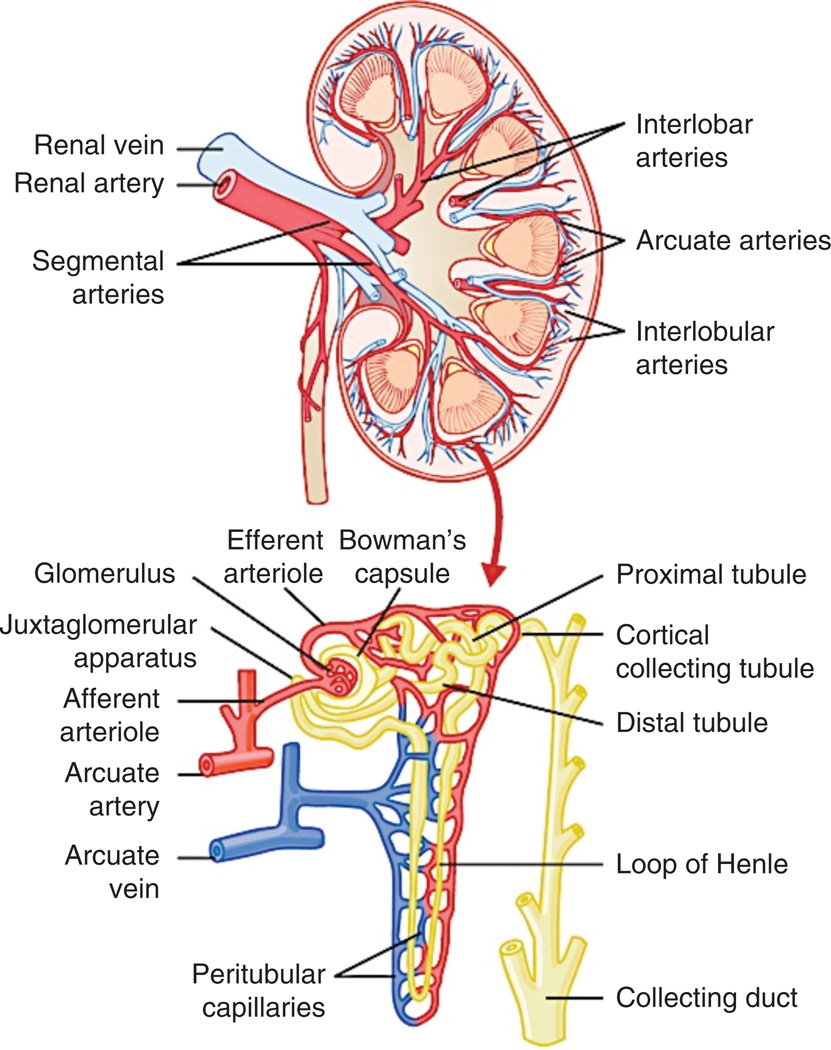
The renal circulation has unique anatomical and functional characteristics. The renal artery enters the kidney through the hilum and following the main renal artery and primary bifurcations, the renal vessels in the kidney progressively branches in interlobar, arcuate, and interlobular arteries. Then, the smaller branching order afferent arterioles lead to the glomerular capillaries and the distal ends of the capillaries of each glomerulus join together to form the efferent arterioles, followed by a second capillary network constituted by the peritubular capillaries surrounding renal tubules (Fig. 2). A distinct feature of the renal circulation is the double capillary bed in the glomerulus (where large amounts of fluid and solutes except the plasma proteins are filtered) and around the tubules, which is a key component in the filtration, secretion, and reabsorption of minerals and removal of unwanted substances from the filtrate (and therefore, from the blood) toward formation of urine. The peritubular capillaries are followed by the venous system that runs in parallel to the arteriolar vessels and progressively form the interlobular, arcuate, in-terlobar, and renal vein, which leaves the kidney beside the renal artery and ureter. Blood flow to the kidneys is normally about 22% of the cardiac output, or 1100 mL/min. The blood supply to the kidneys is one of the highest in the body since only 10% of the delivered oxygen is sufficient to satisfy the renal metabolic demands. The readers are invited to read a recent outstanding article to learn in length and depth about the characteristics and control of the renal microcirculation (119).
Figure 2.
Section of the human kidney showing the major vessels that supply the blood flow to the kidneys and schematic representation of the microcirculation of the nephron. The figure was reproduced with permission from Guyton and Hall Textbook of Medical Physiology, 12th Edition, 2011, Elsevier.
A defective renal microcirculation, also known as MV disease is a prominent pathological feature in chronic kidney disease (CKD), irrespective of the cause, and progresses as CKD evolves (59). MV disease can compromise both the renal nutrition and renal function. In general, the microcirculation is constituted by those vessels between 0 and 200 µm that are embedded within organs and are responsible for the distribution of blood within tissues (20). Partly mediated by augmented vasoconstriction and endothelial dysfunction in CKD (164), MV disease can alter renal blood flow and lead to a progressive decrease in peritubular capillary flow and consequently result in mild tubulointerstitial ischemia (142), which could constitute both a cause and/or a consequence of damage in the kidney. In the next subsections, we will discuss how defects in MV tone, shape, and number may progressively compromise renal function.
Changes in MV tone—endothelial dysfunction
The integrity of the vascular endothelium plays a pivotal role in the molecular traffic between the blood and surrounding tissue, as well as in many aspects of vascular function such as control of vascular tone, vascular permeability (58) and proliferation, and fluid balance (56). Endothelial dysfunction is the result of a combination of abnormal vasodilatory response of endothelial cells with an imbalance between substances that determines vascular tone, which are produced by or act on the endothelium. The endothelial cells are both targets and sources of vasoactive substances such as nitric oxide (NO), prostacyclin, angiotensin II, or endothelin-1, to name a few. Furthermore, endothelial dysfunction also plays a role in favoring inflammation and thrombosis via upregulation of adhesion molecules and generation of chemokines. Endothelial dysfunction has been implicated in the pathophysiology of hypertension (63), coronary artery disease (159), chronic heart failure (104), peripheral artery disease (64), diabetes (92), and chronic renal disease (144).
In addition to a reduced vasodilatory response (153), a key component causing endothelial dysfunction is the decrease in the bioavailability of NO, a potent vasodilator, anti-inflammatory, and antiaggregant gaseous molecule. Mechanisms underlying the reduced vasodilatory responses in endothelial dysfunction include reduced NO production by the endothelium, increased inactivation of NO via ROS, and reduced production of hyperpolarizing factors such as epoxyeicosatrienoic acids, hydrogen peroxide, carbon monoxide, hydrogen sulfide, or C-natriuretic peptide (63). Reduction in NO via ROS-mediated (151) quenching effects seem to be pivotal in promoting vasoconstriction and also in ROS-mediated vascular inflammation, which in turn further reduce NO bioavailability and perpetuates a vicious circle. Thus, sustained vasoconstriction due to endothelial dysfunction could lead to reductions in tissue blood flow and consequently, inadequate provision of oxygen, and nutrients as well as instability of fluid balance between intra- and extravascular spaces due to augmented vascular permeability. The increase MV permeability may facilitate the extravasation of injurious cytokines that may further extend tissue damage (25). This sequence of events is considered to play an important role as the link between hypertension, CKD, and diabetes and the augmented risk of cardiovascular events, and is observed in the kidney, as has been demonstrated in clinical (56, 58, 123) and experimental (5, 7, 30, 91) studies of both acute and chronic renal disease.
A significantly damaged endothelium may lead to a sustained renal vasoconstriction. This event could lead to inadequate perfusion of the intrarenal MV network, consequently compromise renal nutrition and ultimately renal hemodynamics and function. Thus, functional MV rarefaction (1) could serve as an important contributor for a sustained and progressive renal functional and later structural damage in renal disease, as it has been shown that interventions that can augment the bioavailability of NO may prevent such changes. Indeed, antioxidant strategies (18, 26, 32), statins (36,37), angiotensin receptor blockers or converting-enzyme inhibitors (34, 42), and endothelin-receptor blockers (27, 91) have been shown to favor renal MV function by increasing NO bioavailability through decreasing its degradation and/or stimulating sources of NO such as endothelial NO synthase (eNOS). Therefore, the severity of endothelial dysfunction plays a key role in the contribution of the vessels to renal hemodynamics and function. However, it is possible that by favoring additional deleterious mechanisms such as inflammation (61,143), thrombosis (13), and the damaging of the extravascular space (100), endothelial dysfunction may promote the transition from functional to structural MV rarefaction in the kidney.
Changes in MV structure—vascular remodeling
Vascular remodeling is a broad term that in general refers to progressive changes in vascular shape during development and disease (125). However, in the medical literature it often refers to pathological situations leading to (or caused by) changes in MV function and consequently, MV structure. Regardless of the underlying cause, a sustained vasoconstriction may lead to a deficient tissue perfusion and activation of enzymes and growth factors, which may lead to morphological modifications in capillaries, resistance, and conduit vessels. The changes could be evident from the vascular lumen to the outer layers of the vessel.
There are a number of growth factors that may directly or indirectly promote vascular remodeling. The reader is invited to consult recent outstanding contributions from other authors to review in length and depth the mechanisms and factors participating in MV remodeling (125, 150), and some of those with major involvement in this process will be discussed in this section. For instance, tissue transglutaminase is a cross-linking enzyme that has been shown to play a pivotal role in small artery inward remodeling associated with chronic low-flow states (2), as may occur following sustained vasoconstriction due to endothelial dysfunction. The persistent vasoconstriction can lead to entrenchment of reduced diameter that is sustained by tissue transglutaminase and its interaction with integrins on the organization of matrix components and vascular remodeling (36), as has been shown in clinically relevant models of renal disease. Another well-known profibrotic factor that is often involved in renal disease is transforming-growth factor (TGF)-β, a powerful inducer of epithelial-to-mesenchymal transition and renal fibrosis (37, 69). TGF-β exerts its effects via specific mediators known as smads to induce tissue proliferation and facilitate extracellular-matrix (ECM) accumulation by inhibiting matrix-metallo proteinases (MMPs) (4). Furthermore, it has direct effects on vascular proliferation (138) and remodeling by promoting the reduction of vascular lumen, adventitial fibrosis, and collagen matrix deposition around the vessel (135). Another contributing factor is connective tissue growth factor (CTGF), a cysteine-rich peptide synthesized and secreted by fibroblastic cells after activation with TGF-β. CTGF acts as a downstream mediator of TGF-β, mediates TGF-β-induced fibroblast collagen synthesis and ECM accumulation (52, 129, 130) and has direct effects on vascular remodeling (154).
At the other side of the scale, there are factors that can counterbalance the effects of TGF-β and CTGF, such as hepatocyte growth factor (HGF), an antifibrotic and proangiogenic factor that has been shown to be capable of reducing renal fibrogenesis by attenuating CTGF-mediated induction of TGF-β (82). HGF participates in tissue remodeling in the heart (19, 68), liver (85), and lungs (49), and is a powerful stimulus for vascular proliferation, repair, and angiogenesis directly and by interactions with vascular endothelial growth factor (VEGF) (19,116). HGF also seems to participate in MV protection, repair, and proliferation in the kidney exposed to chronic low flow (37, 91). Other key components for the normal development and expansion of the MV networks are the MMPs, particularly MMP-2 and 9, the most active collagenases in the kidney. Deficiencies in the renal expression and activity of MMPs has been observed in experimental models of renal disease (29) and also reported in clinical studies (115, 136). A deficiency in MMPs availability and activation may lead to abnormal development of the vasculature as well as changes in the shape of preexistent vessels due to ECM accumulation. It has been shown that MV remodeling correlates with renal scarring (89). Renal scarring may subsequently induce additional changes in vascular morphology reflecting the abnormal expansion and development of the renal vasculature against fibrotic tissue, such as increase in MV tortuousity (163). Indeed, the accumulation of ECM imposes an obstacle that may constrain and limit vascular growth and expansion, increase the interstitial pressure and consequently increase the pressure on the vessels (71). The combination of such factors may impose changes in vascular tone and diameter, as in turn ECM is a source of antiangiogenic mediators and promoters of vascular regression (45).
In summary, the process of MV remodeling in the kidney is controlled by a number of factors that seems to interact in an organized fashion. However, exogenous insults, as we discuss below, may induce an imbalance in the expression and activity of such factors leading to progressive and likely irreversible changes in MV lumen, thickness, shape, and length that can potentially deteriorate MV function.
MV Rarefaction: Definition. MV Rarefaction and the Kidney
It is generally accepted that two forms of vascular rarefaction can be distinguished: (i) functional rarefaction, which is the consequence of a pathological decrease in the number of perfused vessels without reduction of the number of vessels anatomically present, and (ii) structural rarefaction, which refers to an actual reduction in the number of anatomically present vessels in the tissue (102). Both processes are not mutually exclusive and it has been shown that functional rarefaction can progress to structural rarefaction (128).
The decrease in the availability of small vessels in the kidney can transiently or permanently deteriorate renal blood flow (RBF), glomerular filtration rate (GFR), and tubular function. The reduction of the intrarenal microvasculature could be the result of functional rarefaction (due to sustained vasoconstriction or endothelial dysfunction), structural rarefaction (due to progressive changes in vascular shape, thickness, and reductions in vascular lumen), a combined sequence of both processes, and eventually regression and loss. Many of the factors that will be discussed could also be first causing pathological changes leading to MV alterations, supporting MV rarefaction as an important pathological event that could also be considered a downstream consequence in renal disease. In the next subsections, we will focus on the mechanisms of pathological MV rarefaction and its role in potentially deteriorating the renal hemodynamics and function.
Mechanisms of microvascular rarefaction
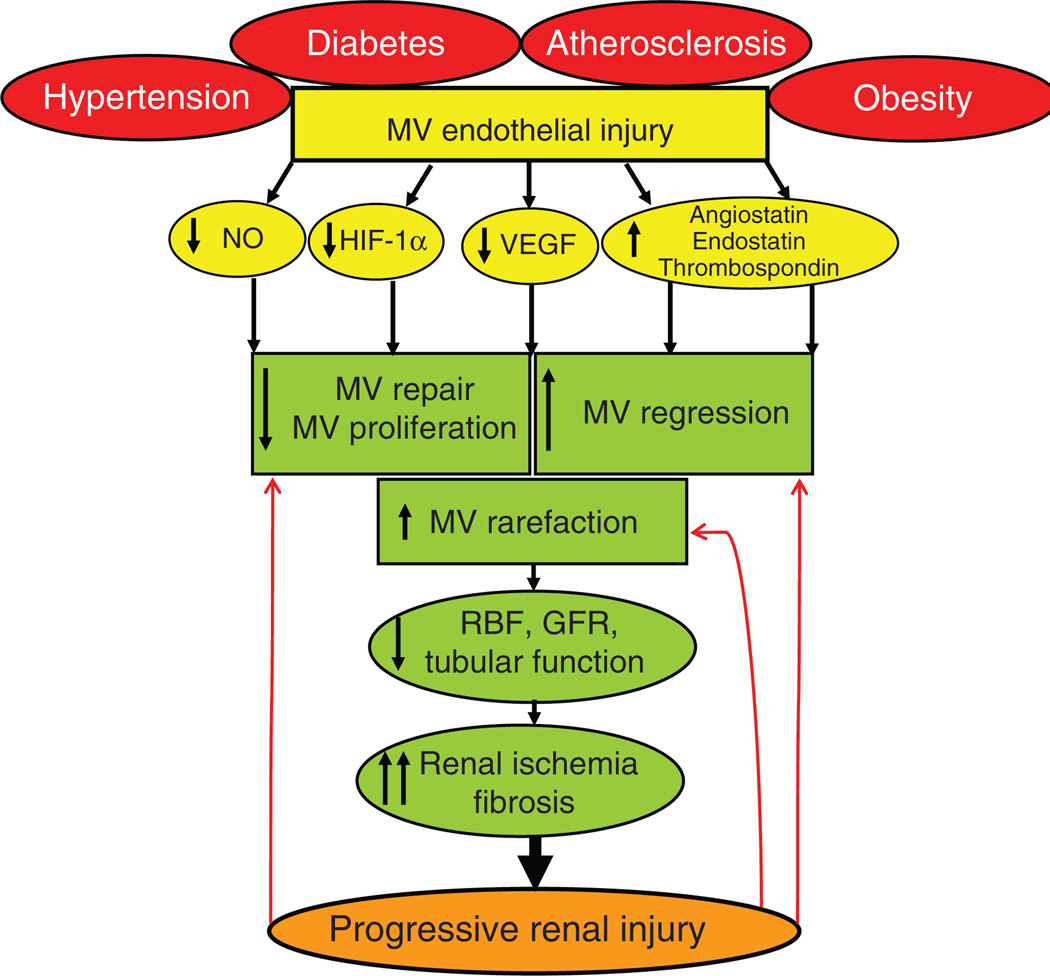
Reductions in MV number have been observed in several diseases, as we will discuss in later sections of this overview. In the kidney, there is supporting evidence showing that MV rarefaction accompanies glomerulosclerosis and tubulointerstitial fibrosis (15, 55, 87, 88, 99). Although rarefaction could theoretically occur in any vessel, it usually first compromises the capillaries and arterioles. The majority of the chronic degenerative disease processes that disrupt the function and structure of the organs are characterized for large alterations in MV number that correlate and accelerate the development and progression of tissue fibrosis. The underlying forces pushing the vessels to regress and disappear are multifactorial (Fig. 3). The initiating triggers of MV regression are largely unresolved and could be the result of a combined activation of signaling pathways, the withdrawal of survival factors, and changes in vessel perfusion (95,152). Although we may tend to think of MV rarefaction as the consequence of one or several “outside of the vessel” events, the process sometimes is initiated within the vessels by changes in vascular shape and dynamics of blood circulation, which could be promoted by substances generated and released by vascular endothelial cells. For example, recent studies have suggested that vascular dropout after acute or chronic kidney injury results from endothelial phenotypic transition and apoptosis combined with an impaired regenerative capacity (7, 91).
Figure 3.
Schematic illustration describing the potential mechanisms of renal microvascular (MV) rarefaction and consequences on renal function and structure. Sustained MV endothelial injury (irrespective of the etiology) can lead to an imbalance between factors involved in MV repair, proliferation, and regression, leading to a progressive MV rarefaction that can deteriorate renal hemodynamics, function, and tissue damage. In turn, the progression of renal damage may further stimulate MV damage and loss (red arrows) resulting in a vicious circle. NO, nitric oxide; VEGF, vascular endothelial growth factor; HIF-1α, hypoxia-induced factor-1α.
The endothelial cells are capable of producing both pro-and antiangiogenic cytokines. Indeed, an abnormal, insufficient, or absent angiogenic stimulus due to reduction in angiogenic cytokines such as VEGF has been observed in both experimental and clinical settings (24, 59). VEGF is crucial for preserving the microvasculature in general, and operates in concert with other factors to stimulate cell division, migration, endothelial cell survival, and tube formation, which are key steps for generation, repair, and maintenance of the MV networks, including those in the kidney. A major stimulus for VEGF generation is hypoxia. However, VEGF rapidly increases in acute hypoxia (118), but eventually decreases when hypoxia is prolonged (122), as we have recently demonstrated in vivo [swine model of renovascular disease (67,156)] and in vitro (renal cells) (24). This pattern suggests a biphasic regulation of VEGF that is possibly determined by the fact that cells releasing VEGF are injured or unable to secrete this cytokine as hypoxia prolongs. These in vitro findings correlate with in vivo experimental and clinical evidence suggesting that chronic reductions of renal blood flow (36, 81), progressive glomerulopathies (134), or CKD (59) are sometimes associated with significant reductions in VEGF. A recent clinical study show marked deficiencies in VEGF in CKD patients accompanied by defective vascular repair, impaired angiogenic responses, and enhanced vascular injury (59) underscoring the progressive nature of renal MV disease in CKD. Furthermore, reductions in VEGF has been also associated with (and possibly triggered by) reductions in upstream (e.g., HIF-1α and NO) and/or downstream mediators (e.g., angiopoietin-1) of VEGF-mediated angiogenic signaling in the kidney (35, 81, 162), implying that MV disease and rarefaction could be triggered by multifactorial mechanisms. In addition, in certain pathological conditions, antiangiogenic splice variants of VEGF (VEGF-b isoforms) could be released and not only inhibit MV proliferation but may also promote MV regression (9). Hence, either by reduction in its sources or altered posttranscriptional mechanisms, a poor availability of this pivotal angiogenic cytokine seems to be a key event in the process of renal MV rarefaction.
VEGF promotes vascular proliferation and repair by being one of the most powerful stimuli for mobilization of endothelial cell progenitors (73, 117). These cells stimulate angiogenesis by further promoting both the secretion of angiogenic growth factors in a paracrine fashion and by providing a source of progenitor cells that can differentiate into mature vascular endothelial cells. Thus, a deficient mobilization of cell progenitors would compromise MV proliferation and repair as well as contribute to further accelerate MV rarefaction in the ischemic tissues and augment renal damage since cell progenitors also are a source of antifibrogenic factors (35, 38). In addition, endothelial cells can release antiangiogenic mediators that can promote MV regression. Angiostatin is a MMP-induced proteolytic cleavage product of plasminogen that is capable of inhibiting angiogenesis, promoting apoptosis of endothelial cells, and disrupting capillary integrity leading to capillary dropout (6). Angiostatin is a potent inhibitor of VEGF and downstream mediators (146,161), has been shown to be persistently elevated after ischemic renal injury, and can significantly reduce VEGF-induced proliferation and repair of peritubular capillaries, consequently accelerating tubular and interstitial damage (110). Another potent extracellular antiangiogenic factors and inhibitors of cell proliferation that are highly expressed in the kidney is endostatin. Endostatin is a potent inhibitor of angiogenesis derived from type XVIII collagen that inhibits VEGF-induced endothelial cell migration and has been found to bind to both renal endothelial and epithelial cells (79, 90). Furthermore, it is highly expressed during the progression of tubule-interstitial injury and renal fibrosis (109). Endostatin has also been shown to be elevated in patients with CKD and to directly correlate with renal impairment and damage and a decrease in progenitor cells, implying a role in the progressive MV rarefaction observed in CKD (157).
Renal fibrosis is the hallmark of CKD, and the progressive accumulation of extracellular matrix during this process in the kidney may also disturb the angiogenic responses to evolving tissue ischemia. Extracellular matrix-derived signals induce cytoskeletal rearrangements that control the shape, function, and signaling events in endothelial cell-lined vessels regulating vascular proliferation and stabilization as well as remodeling and regression. The ECM constitutes an active source of potential antiangiogenic mediators and inhibitors of cell proliferation. These are highly expressed in kidneys such as such as the above-mentioned angiostatin, and also MMP-10, angiopoietin-2, and thrombospondins (78). These factors operate in concert to not only blunt angiogenesis, but they can also decrease MV development and expansion by downregulating, for example, other MMPs (27, 45). Furthermore, as a decrease in MV density induces renal fibrosis, an additional consequence of renal scarring on the existent vessels could be MV remodeling, changes in length, shape, and direction. Indeed, mechanical forces of the fibrotic tissue imposed on the vessels can lead to changes in MV morphology. These changes may also significantly slow down the normal circulation and reduce the shear stress necessary to stimulate, for example, eNOS-derived NO that maintains vascular tone in remaining vessels. These changes may lead to MV functional and eventually structural closure (95, 152), suggesting a potentially progressive deleterious mechanism in which the vessels and the extravascular tissues closely interact.
In summary, we can conclude that a decrease in the number and density of small vessels within the organs could be initiated by sustained vasoconstriction (functional rarefaction), that if it is continued, it may promote morphological changes that could ultimately lead into vascular regression and loss (structural rarefaction). In turn, an eventual loss of microvessels may further aggravate tissue damage and perpetuate a vicious circle of vasoconstriction-vascular remodeling-vascular loss-tissue injury (Fig. 3).
MV rarefaction and renal function: Effects on RBF, GFR, and tubular function
It is undoubted that renal MV rarefaction is a key pathological event in renal disease. Smaller arterioles and capillaries are the first to suffer a vascular dropout when facing acute or chronic insults and the lost of MV circulation via structural rarefaction has been shown to have an impact on renal hemodynamics (5, 81, 127) and function (103, 131). Furthermore, it has been shown that renal MV structural rarefaction plays an important role in the reduction of RBF and GFR with normal aging (149), indicating that vascular regression is also a physiological event.
Deterioration of RBF and GFR can also occur due to functional MV rarefaction. The difference in hydrostatic pressures between renal arteries and veins (pressure gradient across the renal vasculature) divided by the total renal vascular resistance is what determines RBF. The total renal vascular resistance is determined by the sum of the resistances of interlobar, arcuate, and interlobular arteries, afferent and efferent arterioles, capillaries, and veins in the kidney. An increase in the resistance of any of the vascular segments of the kidneys may reduce RBF, whereas a decrease will increase it if renal artery and vein pressures remain constant. In turn, the changes in tone in afferent and efferent arterioles and in glomerular capillary pressure are the main determinants of GFR. Therefore, the resulting modifications in RBF and GFR secondary to changes in MV tone may sometimes shift together and in a similar direction.
Although functional rarefaction of cortical microvessels could be observed in almost every CV disease with potential for kidney involvement, RBF and GFR are tightly regulated by autoregulatory mechanisms that keep them relatively constant despite marked changes in arterial pressure. However, severe endothelial dysfunction (regardless of the primary disease) due to decrease in NO bioavailability and consequent MV constriction can increase MV afferent and efferent tone, increase vascular resistance, and decrease RBF and GFR. Similarly, these vessels are richly innervated by sympathetic nerves and their activation [as occurs, for example, in hypertension or obesity (105, 106)] can induce a significant vasoconstriction, augment intrarenal MV resistance, and thus also decrease the renal hemodynamics. Other peptides that can increase MV tone and induce vasoconstriction are angiotensin II and endothelin-1. Angiotensin II plays a protective role on GFR by maintaining filtration by increasing postglomerular resistance in physiological conditions (47, 48). However, since it has been shown to be a potent renal vasoconstrictor both directly and via oxidative stress [consequently reducing NO (133)] and promoter of renal inflammation (94), fibrosis (160), and vascular injury (93), an augmented or prolonged increase in angiotensin II may contribute to the progression of renal damage. Endothelin-1 is a powerful vasoconstrictor that is released from damaged endothelial cells. Systemic and renal ET-1 have been observed to be increased in renal disease (91,165), and blockade of this pathway has been shown to improve MV tone by decreasing renal oxidative stress and augmenting bioavailability of NO, which prevented renal MV functional and structural rarefaction, preserved renal hemodynamics and function, and reduced renal inflammation, fibrosis, and apoptosis (27, 91, 139). The factors discussed above are among the major players in determining renal MV constriction that may lead to long-term deterioration of renal hemodynamics and also contribute to MV remodeling, and eventually regression and loss, as shown (22, 27, 91, 139, 165).
Following the efferent arterioles, the small vessels branch again and build the second capillary network in the kidney around the tubules. The peritubular capillaries play a crucial role in the reabsorption from and secretion to the glomerular filtrates toward formation of urine, controlling fluid homeostasis, and contributing to regulate blood pressure. Recent studies support the notion that rarefaction of peritubular capillaries represents a critical event that can irreversibly deteriorate renal function and may lead to the development of chronic renal failure and hypertension (5, 134). The loss of peritubular capillaries has been indicated as the nexus of acute kidney injury with chronic renal disease, and suggesting that capillary rarefaction is the key event that boosts the progression of renal injury (5). Indeed, the rarefaction of peritubular capillaries leading to permanent damage has been suggested in both clinical and experimental settings and accompanying hypertension, diabetes, acute and chronic renal ischemia, and aging, underscoring the importance of this MV network (5, 7, 15, 81, 87, 114). Interestingly, as peritubular capillaries are among the first renal structures that are damaged when facing either an acute or chronic insult, the capillary network surrounding the tubules seems to be feasible of repair and could expand by targeted interventions. Recent studies have shown that targeted administration of angiogenic cytokines or progenitor cells can recover the MV network, improve renal function, and significantly reduce tissue injury (24, 35,81,88), implying plasticity of this MV network and underscoring the crucial role of the renal microcirculation in preserving renal function.
CV Risk Factors as a Potential Trigger for Renal MV Rarefaction
Abnormalities of the MV system are common among the conventional CV risk factors, including hypertension, diabetes, obesity, and dyslipidemia. MV changes are hallmarks of the long-term complications of hypertension and diabetes. Furthermore, it is now clear that is also present at the early stages of hypertension and diabetes and may be important in their pathogenesis and progression (102).
In this section, I will briefly discuss the role of the most currently prevalent CV risk factors capable of inducing renal MV dysfunction, damage, remodeling, and ultimately rarefaction (Fig. 3). Changes in the renal microvasculature in these prevalent CV risk factors accompany the development and progression of renal injury. However, it is not entirely clear whether MV rarefaction is a primary initiating and causative mechanism of renal injury in these situations. This is beyond the scope of this section, and should be addressed in future studies.
Hypertension
Hypertension is a prominent cardiovascular risk factor that may cause renal MV endothelial dysfunction and remodeling, ultimately resulting in small vessel rarefaction and tissue hypoxia (58). MV rarefaction in hypertension may be either structural, associated with impaired angiogenesis or capillary apoptosis (attrition), or functional, associated with impaired recruitment of nonperfused capillaries (1). Changes in vascular structure led by hypertension can contribute to hypertensive end-organ damage as has been shown that macro and MV damage further increase blood pressure and impair tissue perfusion to target organs (127). At the MV level, hypertensive disease promotes inward (102) eutrophic or hypertrophic arteriolar remodeling and capillary rarefaction, and these abnormalities are partly determined by abnormal transmission of high blood pressure into MV networks, especially in highly perfused organs with relatively low vascular resistance, such as the kidney, heart, and brain (57). The development of renal fibrosis is one of the most common complications associated with hypertension. Renal fibrosis is thought to be a consequence of the high blood pressure and exaggerated extracellular matrix formation in mesangial and vascular smooth muscle cells as an adaptive response to the increased tension within the intrarenal circulation. An elevation of renal arterial pressure could increase vasa recta capillary pressure and renal interstitial fluid pressure (44). Consequently, the increased blood pressure could be transmitted within renal resistance vessels and possibly, glomeruli. Capillaries are relatively nondistensible and often the endothelial cell nuclei encroach on the lumen to reduce luminal cross-sectional area sometimes by more than 50%, resulting in a slowing or diversion of the blood stream to other vessels. Furthermore, capillaries contain actin filaments that may indicate some form of contractility (16). The capillary network thus can contribute to vascular resistance control by virtue of their narrow caliber, by the reduction in their number (rarefaction), or possibly through their deformations (1). These forces may extend to the renal interstitial space, further compromise the small vessels, and ultimately contribute to the development of renal fibrosis.
There are numerous factors involved in hypertension-induced renal MV damage. Circulating and/or renal generated vasoconstrictors like angiotensin II and endothelin-1 play a central role in this process and have been widely studied on their role in generation and maintenance of hypertension. Their role in MV rarefaction is underscored by studies showing that blockade of these pathways (10,91) in models of hypertension are associated with decreased MV rarefaction and augmented MV proliferation, indicating a distinct role on the microcirculation. These hormones not only induce vasoconstriction but also are powerful stimulus for activation of proinflammatory and profibrotic mechanisms as discussed earlier, leading to significant increases in vascular tone and initiating MV remodeling as well (39). Both directly and via generation of ROS, angiotensin II, and endothelin-1 exert similar actions in reducing NO bioavailability and in increasing inflammation and fibrosis in the kidney, leading to changes in MV tone, morphology, and in the surrounding renal parenchyma. Although difficult to separate since these events may occur at the same time in the kidney exposed to hypertension, endothelial dysfunction, and vasoconstriction are likely the initial steps of a deleterious sequence leading to renal MV rarefaction and subsequent tissue damage in hypertension. It has been shown that hypertension-induced MV loss could influence tissue blood flow resistance to a degree comparable with functional MV rarefaction due to vasoconstriction (70), supporting the possibility that the loss of renal microvessels can markedly alter blood flow distribution and compromise renal function.
Diabetes
A hallmark of diabetes is the generalized vascular disease due to macro- and MV complications. MV dysfunction and impaired MV recruitment has been implicated in the pathogenesis of diabetic complications. Furthermore, both by promoting pathological MV proliferation [e.g., retinopathy (84)] or rarefaction [e.g., skeletal muscle (113) and kidney (103)], the progressive changes in MV number and function during evolution of diabetes play a central role in target-organ damage.
The mechanisms behind MV dysfunction, damage, and loss in the diabetic kidney are likely the result of concurrent insults such as hyperglycemia-induced renal vascular endothelial dysfunction (66), MV insulin resistance (94), augmented advanced-glycation end products (which reduce NO bioavailability leading to sustained vasoconstriction), increased secretion of cytokines and growth factors (120,132), and diminished ECM turnover (108). In the kidney, diabetes induces significant and progressive changes in the glomerular and tubulointerstitial capillary network. Tubulointerstitial damage is a major and early feature of diabetic nephropathy that precedes glomerulosclerosis and is an important predictor of renal dysfunction. It has been reported that both the angiogenic cascade (e.g., VEGF signaling and NO) and MV density progressively decreased in the diabetic kidney, which correlates with renal dysfunction and progressive damage (103,112). In addition, the severity of glomerular capillary rarefaction correlates with the degree of glomerulosclerosis, as the endothelial cell proliferation and MV repair capability is directly proportional to the degree of glomerulosclerosis, indicating that MV rarefaction in the diabetic kidney (77) is a pivotal process for the progression of renal injury.
The importance of renal MV rarefaction in diabetes is underscored by our recent study in which we showed that MV disease in the diabetic kidney is an early and likely initiating event that triggers the progression of renal damage (112). This notion is also supported by recent clinical evidence (60). Interestingly, we observed in our study that MV rarefaction progresses as diabetes evolves, and is accompanied by blunted VEGF signaling and followed by augmented MV remodeling and early tubule-interstitial fibrosis, indicating that the damage, loss, and subsequent remodeling of the renal MV architecture in the diabetic kidney affects both developing and preexistent mature vessels (112). All these changes take place while renal function is still preserved. Therefore, the presence of renal MV and tissue damage before any changes in renal function we observed in this study support the notion that MV changes could be the initiating events in diabetic nephropathy that ultimately lead to a later deterioration of renal function in the diabetic kidney, as observed in human diabetic nephropathy.
Atherosclerosis
Atherosclerosis is a systemic and chronic inflammatory vascular disease that compromises the function and structure of small and large vessels. Atherosclerosis is associated with renal disease, both as cause and as consequence (11, 121). The buildup of vascular atherosclerotic plaques reflects the advanced stage of the disease, and has been demonstrated that earlier stages of the disease, with fatty streaks or no lipid accumulation, present significant vascular dysfunction and early structural changes (21, 27, 30).
One of the most studied consequences of atherosclerosis on the kidney is the development of obstructive lesions in the main renal artery, known as renal artery stenosis (137), one of the main causes of chronic renovascular disease in older patients (54,86). Chronic renovascular disease has a potential to develop progressive deterioration of renal function that may eventuate in CKD or end stage renal disease (ESRD) (54, 72). However, the deleterious effects of atherosclerosis on the kidney are not defined exclusively by the severity of the renal obstruction. Indeed, it has been shown that there is a lack of correlation between severity of renal dysfunction and renal stenosis suggestive of direct effects of atherosclerotic factors independent of the obstruction (101). Furthermore, this is underscored by the fact that resolution of the stenosis not always recovers renal function, as has been observed in human (148) and experimental studies (55).
Previous studies suggest that atherosclerosis has direct effects on the kidney, largely because of intrarenal MV and glomerular disease that precedes the onset and may represent the silent phase of ischemic renal disease (30, 31, 147), being capable to initiate renal injury at an early stage. Experimental studies demonstrated that diet-induced lipid abnormalities resulted in renal endothelial dysfunction, intrarenal inflammation, fibrosis, and a significant vascular dysfunction, damage, and remodeling (22, 27, 30, 31, 33) on the matured renal vasculature. Furthermore, it has been recently shown that dyslipidemia superimposed on renal artery stenosis can also accelerate not only MV dysfunction and remodeling, but also MV loss in the renal cortex, underscoring ample deleterious effects of atherogenic factors on the renal parenchyma (55).
On the other hand, dyslipidemia can also stimulate MV proliferation in the kidney, but new vessel formation under these circumstances may be an adaptive response to local tissue fibrosis and inflammation. Although this is possibly a compensatory mechanism that sustains basal renal vascular function, the augmented vasculature is likely a result of an inflammation-induced angiogenesis (21, 25) resulting in the generation of partly dysfunctional vessels. Furthermore, the newly generated vessels may likely participate on the progression of renal functional and parenchymal injury due to increased MV permeability and abnormal endothelial function (25, 27). Hence, early atherosclerosis is associated more with MV dysfunction, possibly functional rarefaction, and MV remodeling than actual loss of vessels. Nevertheless, it is possible that profibrotic factors activated during atherogenesis in the kidney can contribute at a later stage to MV loss or regression via ECM accumulation, as it was discussed earlier.
Obesity
Obesity is a major public health problem in the United States. Current data shows that over 1/3 of the US adults are obese and over 60% of adults and at least one in six children and adolescents are overweight (8,75). Obesity has been implied to have independent effects of MV function (46, 140), suggesting that it could be an important causal factor in obesity-related disorders. Nevertheless, although obesity has been shown to induce MV dysfunction, remodeling, and capillary rarefaction partly due to abnormal vasodilatation (17, 124), evidence of such changes in the kidney is scant. Obesity can injure the kidneys both directly, by physical compression, and by sustained production of numerous proinflammatory and growth-promoting factors leading to ECM proliferation, thickening of the glomerular and tubular basement membranes, MV dysfunction and remodeling, and ultimately renal fibrosis (28). We have recently shown in obese Zucker rats that obesity promotes MV proliferation and remodeling in this model, a progressive process that is likely mediated by upregulation of inflammatory factors such as TNF-α and interleukin-6 (80). This may reflect an initially compensatory mechanism that may explain, for instance, the increase in GFR observed in obesity studies. However, increased inflammation and inflammatory-induced neovascularization may further promote renal injury as obesity advances and facilitate MV remodeling and progression of renal fibrosis, initiating a vicious process. Furthermore, obesity is a risk factor for the development of hypertension, diabetes, and lipid abnormalities, all prominent renal risk factors with significant impact on the renal microcirculation, as discussed in Sections “Hypertension,” “Diabetes,” and “Atherosclerosis.”
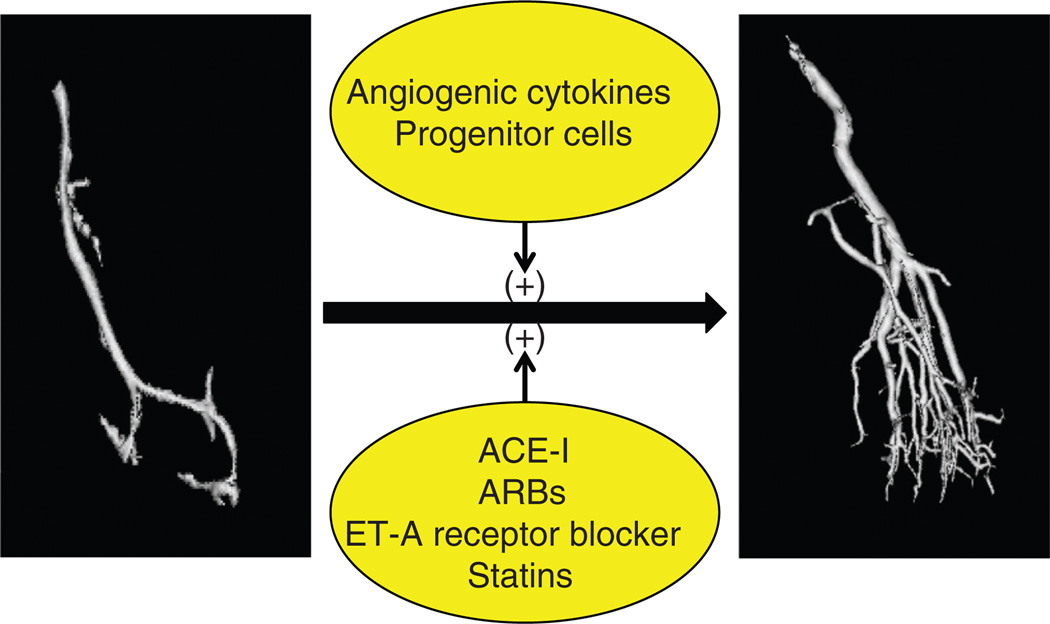
Targeting Renal MV Rarefaction: A Potential Therapeutic Intervention?
Frequently used drugs in humans such as statins (36, 41, 158), angiotensin (141) and endothelin (27,83) (91) receptor blockers, have been shown to be capable to regulate angiogenesis in different vascular beds like in the heart, brain, and kidney. For example, angiotensin-converting enzyme inhibitors and angiotensin type-1 receptor blockers, by activation of bradykinin pathways, promote the generation of VEGF, NO and, consequently, can induce angiogenesis and reduce or even reverse MV rarefaction (10). However, the effects on the intraorgan vessels are reported mainly as side observations rather than as a main effect. There is still relative low evidence supportive of targeted interventions on the renal microcirculation and in this section I will briefly discuss emerging and promising evidence.
Renal vascular rarefaction is partly the result of concurrent events that lead to controlling mechanisms of the generation of new vessels to be exhausted, negated, or defective during sustained and progressive renal injury. Therefore, the potential of targeted interventions on the renal microcirculation in decreasing the evolving injury in renal vascular disease is an exciting concept that could offer new options for renoprotection (Fig. 4). Either as a sole strategy (24, 81) or as an adjuvant renoprotective intervention to improve the success of current strategies to improve renal function such as revascularization (23), recovering the renal microcirculation could offer new options to protect the kidney in chronic renal disease from different etiologies. In addition to experimental evidence, there are a few small clinical studies that have tested the efficacy of proangiogenic therapies by direct administration of angiogenic cytokines (VEGF) (76), but the evidence supporting the administration of angiogenic factors comes mostly from studies in experimental settings (3, 74, 97).
Figure 4.
Schematic illustration of potential targeted interventions to protect the renal microcirculation, by stimulating MV proliferation, repair, and/or reducing MV damage and loss. Majority of evidence comes from experimental studies. ACE-I, angiotensin converting enzyme inhibitiors; ARBs, angiotensin receptor blockers; ET-A, endothelin-receptor A.
The VEGF pathway has been a target for investigation as a proangiogenic therapy in ischemic settings (12, 40). We have recently shown that intrarenal administration of VEGF in the stenotic kidney (in a model of chronic renovascular disease) has distinct vasculo- and renoprotective effects. Both by preventing MV damage and loss, by generation of new vessels, and possibly by stimulating vascular repair (23, 24, 81), intrarenal administration of VEGF protected the stenotic kidney (Fig. 5). Although the anatomical loss of vessels is irreversible, we showed by this approach that, partly by generation of new vessels, VEGF therapy could modify the progressive nature of MV rarefaction. These targeted effects on the renal microvessels were accompanied by substantial improvements in renal function and attenuation of renal fibrosis, supporting the pivotal role of MV rarefaction on the progression of renal injury. Although the treatment was applied at a relatively early stage of the disease but with already established and significant deterioration of renal function and MV damage and loss, the data is promising and offers potential for using it in human disease. Other promising angiogenic factors that could prevent or ameliorate MV rarefaction are angiopoietins (98, 145), cyclin kinase inhibitors (96), or HGF (51,53, 62), but studies are needed to further determine their potential use in renal disease. Therefore, challenges remain and raise the need of further studies to define the best approaches in experimental platforms since patients are diagnosed at different stages of renal disease and consequently, the severity and extent of renal MV rarefaction and subsequent damage varies.
Figure 5.
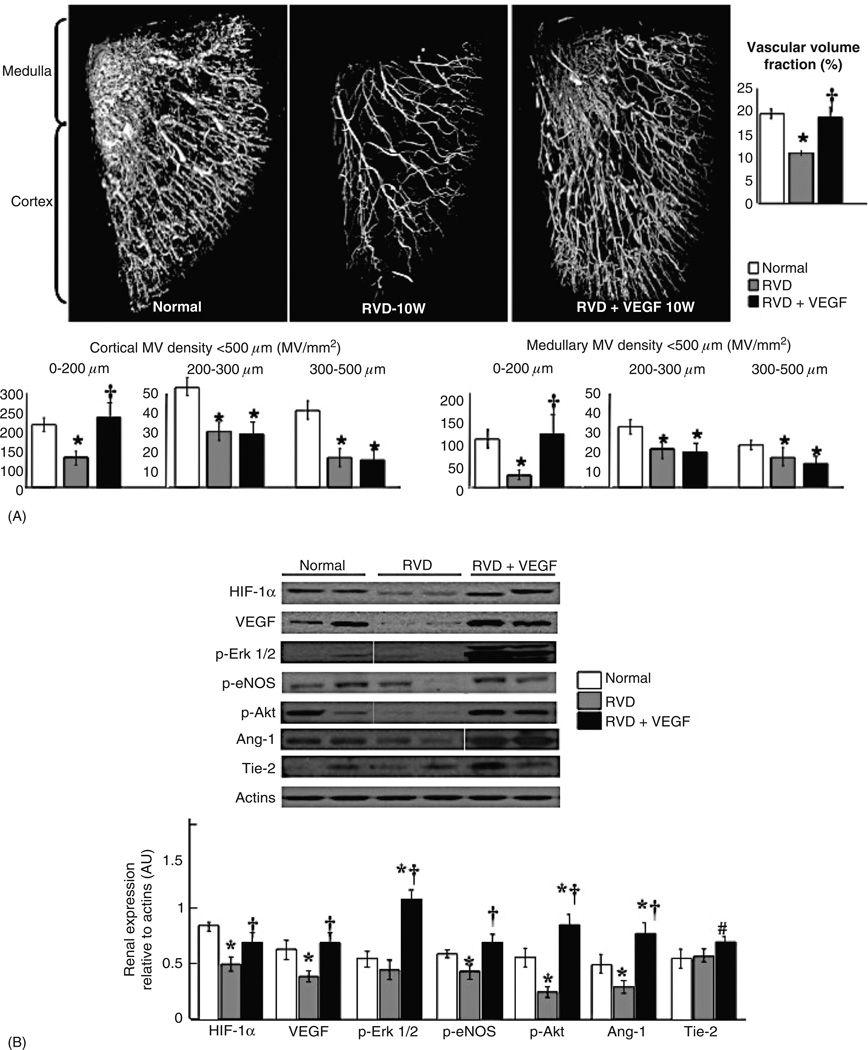
(A) Representative three-dimensional micro-CT reconstruction and quantification of the renal MV density and vascular volume fraction, and (B) representative renal protein expression and quantification of angiogenic mediators in normal, renovascular disease (RVD), and RVD + vascular endothelial growth factor (VEGF). Intrarenal VEGF therapy restored the renal expression of VEGF and angiogenic mediators and led to a significant improvement in cortical and medullary MV density compared to untreated RVD. *, P < 0.05 versus normal; †, P < 0.05 versus RVD; #, P = 0.08 versus RVD. Reproduced, with permission, from Chade et al., Am J Physiol Renal Physiol, 302: F1342–F1350, 2012.
Another emerging therapeutic field is related to the use of progenitor cells to treat ischemic tissues. Circulating and resident progenitor cells are part of the endogenous mechanisms of vascular repair healing the endothelium and generating new vessels to adequately support tissue perfusion after MV injury or loss. Defects in the number and/or function of endogenous cell progenitors have been observed in patients with atherosclerotic heart disease, diabetic nephropathy (50, 111, 126), and chronic renal disease (65, 107).
We have recently shown that renal MV dysfunction and loss (accompanied by abnormal angiogenesis) in experimental renovascular disease is partly due to a defective repair response since circulating and resident cell progenitors and the kidney showed abnormal expression of homing cell-recruitment factors (35, 38). We also showed that administration of autologous progenitor cells in the kidney recuperates renal endogenous vasculoprotective mechanisms, since this approach significantly reversed MV rarefaction in the stenotic kidney, attenuated renal dysfunction, and decreased fibrosis (35). The beneficial effects of the administration of progenitor cells are not exclusively due to the actual presence of such cells in the injured tissues. Indeed, despite a relatively low renal retention of cells, they significantly stimulate MV proliferation and protect existing vessels likely by combined autocrine and paracrine actions on the surrounding cells (35). By inducing production and release of angiogenic factors and in turn inducing mobilization of endogenous resident and circulating progenitor cells, this targeted approach protects the renal microcirculation. Therefore, reversal of MV rarefaction and generation of new vessels in the kidney seems to be a mechanism feasible to target by therapeutic interventions that could preserve renal function, since damage of the renal microcirculation seems to constitute the early steps that lead to progressive and often irreversible renal injury (23, 24, 35, 81).
Conclusion, Perspectives, and Significance
MV damage and loss as well as MV repair and generation of new vessels in the kidney seem to be processes activated due and in response to different insults that take a toll on renal function (27, 35, 36,162). An intact and healthy renal micro-circulation is vital to ensure tissue perfusion and adequate filtration and removal of toxins from the blood. Furthermore, restoration of blood flow to the injured tissues is critical to initiate a successful repair response in the kidney as in any given organ of the human body. It is possible that, as glomerulosclerosis and tubulointerstitial fibrosis are the hallmark of the advanced stages of CKD and ESRD, the presence of functional and/or structural MV rarefaction may indeed represent the initial steps of renal injury. Severity of MV damage and loss may determine the frontier between reversible and irreversible renal injury by promoting the progression of renal functional and structural damage (Fig. 6). MV rarefaction leads to tissue ischemia and can consequently further stimulate the activation of inflammatory and fibrotic cytokines leading to renal parenchymal injury, initiating a process that could eventuate in progressive and later irreversible renal injury. In turn, MV rarefaction could also be a consequence of the progression of renal injury.
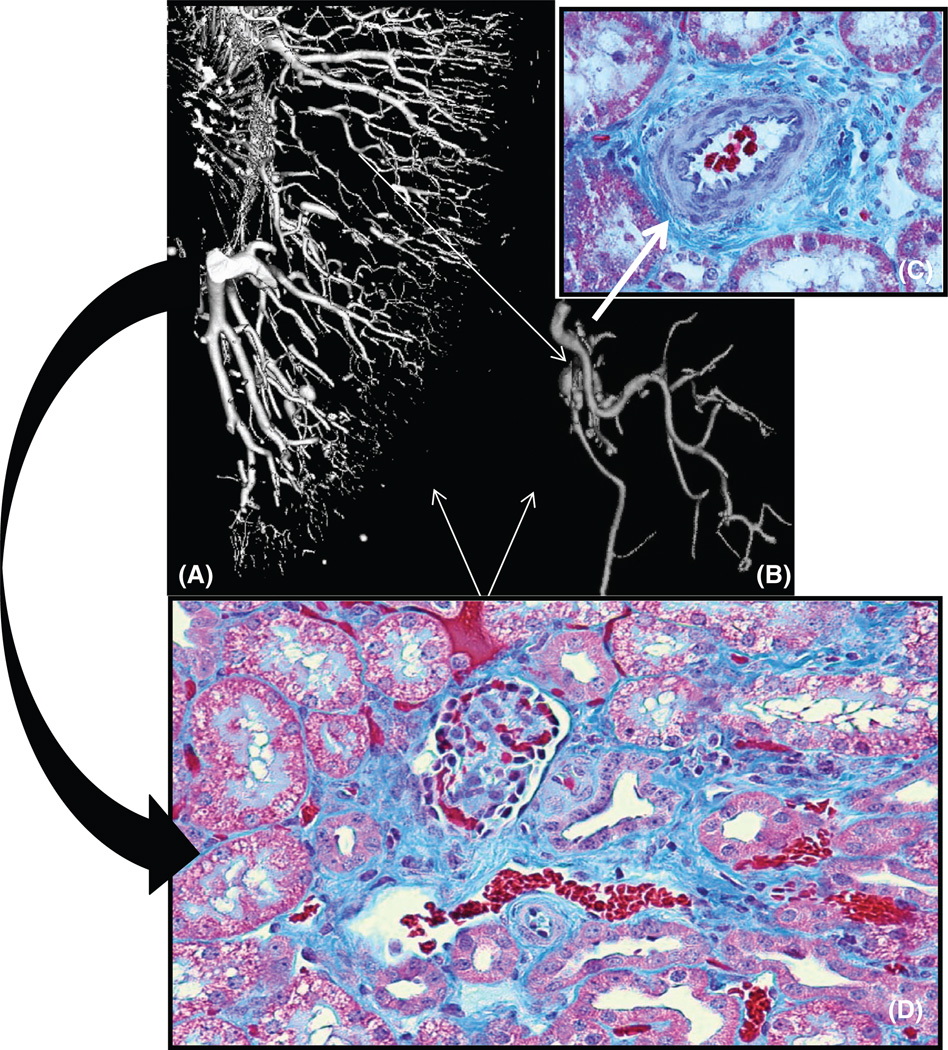
Figure 6.
Representative picture showing three-dimensional micro-CT reconstruction of the renal microvascular (MV) architecture (a), tomographically isolated microvessels (b), and renal cross sections showing MV remodeling (c, ×40) and fibrosis (d, ×20) of a kidney exposed to chronic obstruction of blood flow due to renal artery stenosis. The significant MV rarefaction (a) and remodeling (b, c) correlates with the progression of renal fibrosis (d, curved black arrow). Furthermore, the buildup of renal scarring can promotes changes in MV shape and morphology, as it could also be a source for promoters of MV regression (white arrows).
Others and we have shown that renal MV rarefaction is progressive and correlates with progressive deterioration of RBF, GFR, perfusion, and tubular function (24, 35, 55, 81). Experimental evidence from our laboratory supports the notion that such changes could partly be reversible by targeted protection of the renal microvasculature (35, 81). Reversal of MV rarefaction, by generation of new vessels that possibly shunt preexisting damage ones and/or stimulation of MV repair, likely contribute to restore filtration function in partly damaged or hibernated (43, 155) nephrons that could still be recuperated. Hence, it is possible that an underestimation of the severity of renal MV rarefaction plays a role in defining the success of established and some novel therapeutic interventions since dysfunctional or damaged vessels can progressively deteriorate renal perfusion, filtration, and tubular function. Therefore, future studies should concentrate on noninvasive assessment of the intrarenal MV architecture, distribution, and function, as additional research is needed to define optimal therapeutic strategies to protect the renal microvessels. Carefully designed prospective experimental and clinical studies are needed to determine the appropriate utilization and the timing of using such therapeutic options. Targeted interventions to enhance endogenous renoprotective mechanisms such as cell-based therapy or the use of angiogenic cytokines have shown promising results in experimental and clinical settings. However, the road ahead is still long until such options could be set as part of the therapeutic arsenal to preserve, protect, and hopefully recuperate the kidney under progressive deterioration.
Acknowledgements
This work was supported by grant HL095638 from the National Institute of Health.
References
- 1.Antonios TF, Singer DR, Markandu ND, Mortimer PS, MacGregor GA. Structural skin capillary rarefaction in essential hypertension. Hypertension. 1999;33:998–1001. doi: 10.1161/01.hyp.33.4.998. [DOI] [PubMed] [Google Scholar]
- 2.Bakker EN, Buus CL, Spaan JA, Perree J, Ganga A, Rolf TM, Sorop O, Bramsen LH, Mulvany MJ, Vanbavel E. Small artery remodeling depends on tissue-type transglutaminase. Circ Res. 2005;96:119–126. doi: 10.1161/01.RES.0000151333.56089.66. [DOI] [PubMed] [Google Scholar]
- 3.Balzer KM, Pfeiffer T, Rossbach S, Voiculescu A, Modder U, Gode-hardt E, Sandmann W. Prospective randomized trial of operative vs interventional treatment for renal artery ostial occlusive disease (RAOOD) J Vasc Surg. 2009;49:667–674. doi: 10.1016/j.jvs.2008.10.006. discussion 674–665. [DOI] [PubMed] [Google Scholar]
- 4.Baricos WH, Cortez SL, Deboisblanc M, Xin S. Transforming growth factor-beta is a potent inhibitor of extracellular matrix degradation by cultured human mesangial cells. J Am Soc Nephrol. 1999;10:790–795. doi: 10.1681/ASN.V104790. [DOI] [PubMed] [Google Scholar]
- 5.Basile DP. Rarefaction of peritubular capillaries following ischemic acute renal failure: A potential factor predisposing to progressive nephropathy. Curr Opin Nephrol Hypertens. 2004;13:1–7. doi: 10.1097/00041552-200401000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Basile DP, Fredrich K, Weihrauch D, Hattan N, Chilian WM. Angiostatin and matrix metalloprotease expression following ischemic acute renal failure. Am J Physiol Renal Physiol. 2004;286:F893–F902. doi: 10.1152/ajprenal.00328.2003. [DOI] [PubMed] [Google Scholar]
- 7.Basile DP, Friedrich JL, Spahic J, Knipe N, Mang H, Leonard EC, Changizi-Ashtiyani S, Bacallao RL, Molitoris BA, Sutton TA. Impaired endothelial proliferation and mesenchymal transition contribute to vascular rarefaction following acute kidney injury. Am J Physiol Renal Physiol. 2011;300:F721–F733. doi: 10.1152/ajprenal.00546.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baskin ML, Ard J, Franklin F, Allison DB. Prevalence of obesity in the United States. Obes Rev. 2005;6:5–7. doi: 10.1111/j.1467-789X.2005.00165.x. [DOI] [PubMed] [Google Scholar]
- 9.Bates DO, Cui TG, Doughty JM, Winkler M, Sugiono M, Shields JD, Peat D, Gillatt D, Harper SJ. VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma. Cancer Res. 2002;62:4123–4131. [PubMed] [Google Scholar]
- 10.Battegay EJ, de Miguel LS, Petrimpol M, Humar R. Effects of anti-hypertensive drugs on vessel rarefaction. Curr Opin Pharmacol. 2007;7:151–157. doi: 10.1016/j.coph.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Bax L, van der Graaf Y, Rabelink AJ, Algra A, Beutler JJ, Mali WP. Influence of atherosclerosis on age-related changes in renal size and function. Eur J Clin Invest. 2003;33:34–40. doi: 10.1046/j.1365-2362.2003.01091.x. [DOI] [PubMed] [Google Scholar]
- 12.Benest AV, Salmon AH, Wang W, Glover CP, Uney J, Harper SJ, Bates DO. VEGF and angiopoietin-1 stimulate different angiogenic phenotypes that combine to enhance functional neovascularization in adult tissue. Microcirculation. 2006;13:423–437. doi: 10.1080/10739680600775940. [DOI] [PubMed] [Google Scholar]
- 13.Biegelsen ES, Loscalzo J. Endothelial function and atherosclerosis. Coron Artery Dis. 1999;10:241–256. [PubMed] [Google Scholar]
- 14.Bobik A. The structural basis of hypertension: vascular remodelling, rarefaction and angiogenesis/arteriogenesis. J Hypertens. 2005;23:1473–1475. doi: 10.1097/01.hjh.0000174970.56965.4f. [DOI] [PubMed] [Google Scholar]
- 15.Bohle A, Mackensen-Haen S, von Gise H. Significance of tubulointerstitial changes in the renal cortex for the excretory function and concentration ability of the kidney: A morphometric contribution. Am J Nephrol. 1987;7:421–433. doi: 10.1159/000167514. [DOI] [PubMed] [Google Scholar]
- 16.Brecker CG, Shustak SR. Contractile proteins in endothelial cells: Comparison of cerebral capillaries with those in heart and skeletal muscle and with liver sinusoids. Circulation. 1972;87(45/46 Suppl II) [Google Scholar]
- 17.Caballero AE. Endothelial dysfunction in obesity and insulin resistance: A road to diabetes and heart disease. Obes Res. 2003;11:1278–1289. doi: 10.1038/oby.2003.174. [DOI] [PubMed] [Google Scholar]
- 18.Cambonie G, Comte B, Yzydorczyk C, Ntimbane T, Germain N, Le NL, Pladys P, Gauthier C, Lahaie I, Abran D, Lavoie JC, Nuyt AM. Antenatal antioxidant prevents adult hypertension, vascular dysfunction, and microvascular rarefaction associated with in utero exposure to a low-protein diet. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1236–R1245. doi: 10.1152/ajpregu.00227.2006. [DOI] [PubMed] [Google Scholar]
- 19.Caron A, Desrosiers RR, Langlois S, Beliveau R. Ischemia-reperfusion injury stimulates gelatinase expression and activity in kidney glomeruli. Can J Physiol Pharmacol. 2005;83:287–300. doi: 10.1139/y05-011. [DOI] [PubMed] [Google Scholar]
- 20.Chade AR. Renovascular disease, microcirculation, and the progression of renal injury: Role of angiogenesis. Am J Physiol Regul Integr Comp Physiol. 2011;300:R783–R790. doi: 10.1152/ajpregu.00657.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chade AR, Bentley MD, Zhu X, Rodriguez-Porcel M, Niemeyer S, Amores-Arriaga B, Napoli C, Ritman EL, Lerman A, Lerman LO. Antioxidant intervention prevents renal neovascularization in hypercholesterolemic pigs. J Am Soc Nephrol. 2004;15:1816–1825. doi: 10.1097/01.asn.0000130428.85603.6b. [DOI] [PubMed] [Google Scholar]
- 22.Chade AR, Best PJ, Rodriguez-Porcel M, Herrmann J, Zhu X, Sawamura T, Napoli C, Lerman A, Lerman LO. Endothelin-1 receptor blockade prevents renal injury in experimental hypercholesterolemia. Kidney Int. 2003;64:962–969. doi: 10.1046/j.1523-1755.2003.00170.x. [DOI] [PubMed] [Google Scholar]
- 23.Chade AR, Kelsen S. Renal microvascular disease determines the responses to revascularization in experimental renovascular disease. Circ Cardiovasc Interv. 2010;3:376–383. doi: 10.1161/CIRCINTERVENTIONS.110.951277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chade AR, Kelsen S. Reversal of renal dysfunction by targeted administration of Vegf into the stenotic kidney: A novel potential therapeutic approach. Am J Physiol Renal Physiol. 2012;302:F1342–F1350. doi: 10.1152/ajprenal.00674.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chade AR, Krier JD, Galili O, Lerman A, Lerman LO. Role of renal cortical neovascularization in experimental hypercholesterolemia. Hypertension. 2007;50:729–736. doi: 10.1161/HYPERTENSIONAHA.107.093989. [DOI] [PubMed] [Google Scholar]
- 26.Chade AR, Krier JD, Rodriguez-Porcel M, Breen JF, McKusick MA, Lerman A, Lerman LO. Comparison of acute and chronic antioxidant interventions in experimental renovascular disease. Am J Physiol Renal Physiol. 2004;286:F1079–F1086. doi: 10.1152/ajprenal.00385.2003. [DOI] [PubMed] [Google Scholar]
- 27.Chade AR, Krier JD, Textor SC, Lerman A, Lerman LO. Endothelin-a receptor blockade improves renal microvascular architecture and function in experimental hypercholesterolemia. J Am Soc Nephrol. 2006;17:3394–3403. doi: 10.1681/ASN.2006060635. [DOI] [PubMed] [Google Scholar]
- 28.Chade AR, Lerman A, Lerman LO. Kidney in early atherosclerosis. Hypertension. 2005;45:1042–1049. doi: 10.1161/01.HYP.0000167121.14254.a0. [DOI] [PubMed] [Google Scholar]
- 29.Chade AR, Mushin OP, Zhu X, Rodriguez-Porcel M, Grande JP, Textor SC, Lerman A, Lerman LO. Pathways of renal fibrosis and modulation of matrix turnover in experimental hypercholesterolemia. Hypertension. 2005;46:772–779. doi: 10.1161/01.HYP.0000184250.37607.da. [DOI] [PubMed] [Google Scholar]
- 30.Chade AR, Rodriguez-Porcel M, Grande JP, Krier JD, Lerman A, Romero JC, Napoli C, Lerman LO. Distinct renal injury in early atherosclerosis and renovascular disease. Circulation. 2002;106:1165–1171. doi: 10.1161/01.cir.0000027105.02327.48. [DOI] [PubMed] [Google Scholar]
- 31.Chade AR, Rodriguez-Porcel M, Grande JP, Zhu X, Sica V, Napoli C, Sawamura T, Textor SC, Lerman A, Lerman LO. Mechanisms of renal structural alterations in combined hypercholesterolemia and renal artery stenosis. Arterioscler Thromb Vasc Biol. 2003;23:1295–1301. doi: 10.1161/01.ATV.0000077477.40824.52. [DOI] [PubMed] [Google Scholar]
- 32.Chade AR, Rodriguez-Porcel M, Herrmann J, Krier JD, Zhu X, Lerman A, Lerman LO. Beneficial effects of antioxidant vitamins on the stenotic kidney. Hypertension. 2003;42:605–612. doi: 10.1161/01.HYP.0000089880.32275.7C. [DOI] [PubMed] [Google Scholar]
- 33.Chade AR, Rodriguez-Porcel M, Herrmann J, Zhu X, Grande JP, Napoli C, Lerman A, Lerman LO. Antioxidant intervention blunts renal injury in experimental renovascular disease. J Am Soc Nephrol. 2004;15:958–966. doi: 10.1097/01.asn.0000117774.83396.e9. [DOI] [PubMed] [Google Scholar]
- 34.Chade AR, Rodriguez-Porcel M, Rippentrop SJ, Lerman A, Lerman LO. Angiotensin II AT1 receptor blockade improves renal perfusion in hypercholesterolemia. Am J Hypertens. 2003;16:111–115. doi: 10.1016/s08957061(02)03202-8. [DOI] [PubMed] [Google Scholar]
- 35.Chade AR, Zhu X, Lavi R, Krier JD, Pislaru S, Simari RD, Napoli C, Lerman A, Lerman LO. Endothelial progenitor cells restore renal function in chronic experimental renovascular disease. Circulation. 2009;119:547–557. doi: 10.1161/CIRCULATIONAHA.108.788653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chade AR, Zhu X, Mushin OP, Napoli C, Lerman A, Lerman LO. Simvastatin promotes angiogenesis and prevents microvascular remodeling in chronic renal ischemia. Faseb J. 2006;20:1706–1708. doi: 10.1096/fj.05-5680fje. [DOI] [PubMed] [Google Scholar]
- 37.Chade AR, Zhu XY, Grande JP, Krier JD, Lerman A, Lerman LO. Simvastatin abates development of renal fibrosis in experimental renovascular disease. J Hypertens. 2008;26:1651–1660. doi: 10.1097/HJH.0b013e328302833a. [DOI] [PubMed] [Google Scholar]
- 38.Chade AR, Zhu XY, Krier JD, Jordan KL, Textor SC, Grande JP, Lerman A, Lerman LO. Endothelial progenitor cells homing and renal repair in experimental renovascular disease. Stem Cells. 2010;28:1039–1047. doi: 10.1002/stem.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chatziantoniou C, Boffa JJ, Tharaux PL, Flamant M, Ronco P, Dussaule JC. Progression and regression in renal vascular and glomerular fibrosis. Int J Exp Pathol. 2004;85:1–11. doi: 10.1111/j.0959-9673.2004.00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen F, Tan Z, Dong CY, Chen X, Guo SF. Adeno-associated virus vectors simultaneously encoding VEGF and angiopoietin-1 enhances neovascularization in ischemic rabbit hind-limbs. Acta Pharmacol Sin. 2007;28:493–502. doi: 10.1111/j.1745-7254.2007.00527.x. [DOI] [PubMed] [Google Scholar]
- 41.Chen J, Zhang ZG, Li Y, Wang Y, Wang L, Jiang H, Zhang C, Lu M, Katakowski M, Feldkamp CS, Chopp M. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol. 2003;53:743–751. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
- 42.Chen JW, Hsu NW, Wu TC, Lin SJ, Chang MS. Long-term angiotensin-converting enzyme inhibition reduces plasma asymmetric dimethylarginine and improves endothelial nitric oxide bioavailability and coronary microvascular function in patients with syndrome X. Am J Cardiol. 2002;90:974–982. doi: 10.1016/s0002-9149(02)02664-4. [DOI] [PubMed] [Google Scholar]
- 43.Cheung CM, Chrysochou C, Shurrab AE, Buckley DL, Cowie A, Kalra PA. Effects of renal volume and single-kidney glomerular filtration rate on renal functional outcome in atherosclerotic renal artery stenosis. Nephrol Dial Transplant. 2010;25:1133–1140. doi: 10.1093/ndt/gfp623. [DOI] [PubMed] [Google Scholar]
- 44.Cowley AW, Jr, Roman RJ, Krieger JE. Pathways linking renal excretion and arterial pressure with vascular structure and function. Clin Exp Pharmacol Physiol. 1991;18:21–27. doi: 10.1111/j.1440-1681.1991.tb01371.x. [DOI] [PubMed] [Google Scholar]
- 45.Davis GE, Senger DR. Extracellular matrix mediates a molecular balance between vascular morphogenesis and regression. Curr Opin Hematol. 2008;15:197–203. doi: 10.1097/MOH.0b013e3282fcc321. [DOI] [PubMed] [Google Scholar]
- 46.de Jongh RT, Serne EH, IJzerman RG, de Vries G, Stehouwer CD. Impaired microvascular function in obesity: implications for obesity-associated microangiopathy, hypertension, and insulin resistance. Circulation. 2004;109:2529–2535. doi: 10.1161/01.CIR.0000129772.26647.6F. [DOI] [PubMed] [Google Scholar]
- 47.Denton KM, Anderson WP. Glomerular ultrafiltration in rabbits with superficial glomeruli. Pflugers Arch. 1991;419:235–242. doi: 10.1007/BF00371101. [DOI] [PubMed] [Google Scholar]
- 48.Denton KM, Anderson WP, Sinniah R. Effects of angiotensin II on regional afferent and efferent arteriole dimensions and the glomerular pole. Am J Physiol Regul Integr Comp Physiol. 2000;279:R629–R638. doi: 10.1152/ajpregu.2000.279.2.R629. [DOI] [PubMed] [Google Scholar]
- 49.Desrosiers RR, Rivard ME, Grundy PE, Annabi B. Decrease in LDL receptor-related protein expression and function correlates with advanced stages of Wilms tumors. Pediatr Blood Cancer. 2006;46:40–49. doi: 10.1002/pbc.20566. [DOI] [PubMed] [Google Scholar]
- 50.Dessapt C, Karalliedde J, Hernandez-Fuentes M, Martin PP, Maltese G, Dattani N, Atkar R, Viberti G, Gnudi L. Circulating vascular progenitor cells in patients with type 1 diabetes and microalbuminuria. Diabetes Care. 2010;33:875–877. doi: 10.2337/dc09-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deuse T, Peter C, Fedak PW, Doyle T, Reichenspurner H, Zimmermann WH, Eschenhagen T, Stein W, Wu JC, Robbins RC, Schrepfer S. Hepatocyte growth factor or vascular endothelial growth factor gene transfer maximizes mesenchymal stem cell-based myocardial salvage after acute myocardial infarction. Circulation. 2009;120:S247–S254. doi: 10.1161/CIRCULATIONAHA.108.843680. [DOI] [PubMed] [Google Scholar]
- 52.Duncan MR, Frazier KS, Abramson S, Williams S, Klapper H, Huang X, Grotendorst GR. Connective tissue growth factor mediates transforming growth factor beta-induced collagen synthesis: down-regulation by cAMP. Faseb J. 1999;13:1774–1786. [PubMed] [Google Scholar]
- 53.Dworkin LD, Gong R, Tolbert E, Centracchio J, Yano N, Zanabli AR, Esparza A, Rifai A. Hepatocyte growth factor ameliorates progression of interstitial fibrosis in rats with established renal injury. Kidney Int. 2004;65:409–419. doi: 10.1111/j.1523-1755.2004.00417.x. [DOI] [PubMed] [Google Scholar]
- 54.Dworkin LD, Murphy T. Is there any reason to stent atherosclerotic renal artery stenosis? Am J Kidney Dis. 2010;56:259–263. doi: 10.1053/j.ajkd.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 55.Eirin A, Zhu XY, Urbieta-Caceres VH, Grande JP, Lerman A, Textor SC, Lerman LO. Persistent kidney dysfunction in swine renal artery stenosis correlates with outer cortical microvascular remodeling. Am J Physiol Renal Physiol. 2011;300:F1394–F1401. doi: 10.1152/ajprenal.00697.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Endemann DH, Schiffrin EL. Endothelial dysfunction. J Am Soc Nephrol. 2004;15:1983–1992. doi: 10.1097/01.ASN.0000132474.50966.DA. [DOI] [PubMed] [Google Scholar]
- 57.Feihl F, Liaudet L, Waeber B. The macrocirculation and microcirculation of hypertension. Curr Hypertens Rep. 2009;11:182–189. doi: 10.1007/s11906-009-0033-6. [DOI] [PubMed] [Google Scholar]
- 58.Fliser D. Perspectives in renal disease progression: The endothelium as a treatment target in chronic kidney disease. J Nephrol. 2011;23:369–376. [PubMed] [Google Scholar]
- 59.Futrakul N, Butthep P, Laohareungpanya N, Chaisuriya P, Ratana-banangkoon K. A defective angiogenesis in chronic kidney disease. Ren Fail. 2008;30:215–217. doi: 10.1080/08860220701813335. [DOI] [PubMed] [Google Scholar]
- 60.Futrakul N, Futrakul P. Vascular homeostasis and angiogenesis determine therapeutic effectiveness in type 2 diabetes. Int J Vasc Med. 2011;2011 doi: 10.1155/2011/971524. 971524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Galle J, Quaschning T, Seibold S, Wanner C. Endothelial dysfunction and inflammation: What is the link? Kidney Int Suppl. 2003:S45–S49. doi: 10.1046/j.1523-1755.63.s84.12.x. [DOI] [PubMed] [Google Scholar]
- 62.Gerritsen ME. HGF and VEGF: A dynamic duo. Circ Res. 2005;96:272–273. doi: 10.1161/01.RES.0000157575.66295.e0. [DOI] [PubMed] [Google Scholar]
- 63.Giles TD, Sander GE, Nossaman BD, Kadowitz PJ. Impaired vasodilation in the pathogenesis of hypertension: Focus on nitric oxide, endothelial-derived hyperpolarizing factors, and prostaglandins. J Clin Hypertens (Greenwich) 2012;14:198–205. doi: 10.1111/j.1751-7176.2012.00606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gokce N, Keaney JF, Jr, Hunter LM, Watkins MT, Nedeljkovic ZS, Menzoian JO, Vita JA. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol. 2003;41:1769–1775. doi: 10.1016/s0735-1097(03)00333-4. [DOI] [PubMed] [Google Scholar]
- 65.Goligorsky MS, Yasuda K, Ratliff B. Dysfunctional endothelial progenitor cells in chronic kidney disease. J Am Soc Nephrol. 2010;21:911–919. doi: 10.1681/ASN.2009111119. [DOI] [PubMed] [Google Scholar]
- 66.Gomes MB, Affonso FS, Cailleaux S, Almeida AL, Pinto LF, Tibirica E. Glucose levels observed in daily clinical practice induce endothelial dysfunction in the rabbit macro- and microcirculation. Fundam Clin Pharmacol. 2004;18:339–346. doi: 10.1111/j.1472-8206.2004.00248.x. [DOI] [PubMed] [Google Scholar]
- 67.Gomez SI, Warner L, Haas JA, Bolterman RJ, Textor SC, Lerman LO, Romero JC. Increased hypoxia and reduced renal tubular response to furosemide detected by BOLD magnetic resonance imaging in swine renovascular hypertension. Am J Physiol Renal Physiol. 2009;297:F981–F986. doi: 10.1152/ajprenal.90757.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Graff J, Harder S, Wahl O, Scheuermann EH, Gossmann J. Anti-inflammatory effects of clopidogrel intake in renal transplant patients: effects on platelet-leukocyte interactions, platelet CD40 ligand expression, and proinflammatory biomarkers. Clin Pharmacol Ther. 2005;78:468–476. doi: 10.1016/j.clpt.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 69.Grande JP. Role of transforming growth factor-beta in tissue injury and repair. Proc Soc Exp Biol Med. 1997;214:27–40. doi: 10.3181/00379727-214-44066. [DOI] [PubMed] [Google Scholar]
- 70.Greene AS, Tonellato PJ, Lui J, Lombard JH, Cowley AW., Jr Microvascular rarefaction and tissue vascular resistance in hypertension. Am J Physiol. 1989;256:H126–H131. doi: 10.1152/ajpheart.1989.256.1.H126. [DOI] [PubMed] [Google Scholar]
- 71.Hall JE. The kidney, hypertension, and obesity. Hypertension. 2003;41:625–633. doi: 10.1161/01.HYP.0000052314.95497.78. [DOI] [PubMed] [Google Scholar]
- 72.Hansen KJ, Edwards MS, Craven TE, Cherr GS, Jackson SA, Appel RG, Burke GL, Dean RH. Prevalence of renovascular disease in the elderly: A population-based study. J Vasc Surg. 2002;36:443–451. doi: 10.1067/mva.2002.127351. [DOI] [PubMed] [Google Scholar]
- 73.Hattori K, Dias S, Heissig B, Hackett NR, Lyden D, Tateno M, Hicklin DJ, Zhu Z, Witte L, Crystal RG, Moore MA, Rafii S. Vascular endothelial growth factor and angiopoietin-1 stimulate postnatal hematopoiesis by recruitment of vasculogenic and hematopoietic stem cells. J Exp Med. 2001;193:1005–1014. doi: 10.1084/jem.193.9.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hayashi S, Morishita R, Nakamura S, Yamamoto K, Moriguchi A, Nagano T, Taiji M, Noguchi H, Matsumoto K, Nakamura T, Higaki J, Ogihara T. Potential role of hepatocyte growth factor, a novel angiogenic growth factor, in peripheral arterial disease: Downregulation of HGF in response to hypoxia in vascular cells. Circulation. 1999;100:II301–II308. doi: 10.1161/circ.100.suppl_2.Ii-301. [DOI] [PubMed] [Google Scholar]
- 75.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291:2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 76.Henry TD, Rocha-Singh K, Isner JM, Kereiakes DJ, Giordano FJ, Simons M, Losordo DW, Hendel RC, Bonow RO, Eppler SM, Zioncheck TF, Holmgren EB, McCluskey ER. Intracoronary administration of recombinant human vascular endothelial growth factor to patients with coronary artery disease. Am Heart J. 2001;142:872–880. doi: 10.1067/mhj.2001.118471. [DOI] [PubMed] [Google Scholar]
- 77.Hohenstein B, Hausknecht B, Boehmer K, Riess R, Brekken RA, Hugo CP. Local VEGF activity but not VEGF expression is tightly regulated during diabetic nephropathy in man. Kidney Int. 2006;69:1654–1661. doi: 10.1038/sj.ki.5000294. [DOI] [PubMed] [Google Scholar]
- 78.Hugo C, Daniel C. Thrombospondin in renal disease. Nephron Exp Nephrol. 2009;111:e61–e66. doi: 10.1159/000198235. [DOI] [PubMed] [Google Scholar]
- 79.Ichinose K, Maeshima Y, Yamamoto Y, Kitayama H, Takazawa Y, Hirokoshi K, Sugiyama H, Yamasaki Y, Eguchi K, Makino H. Antian-giogenic endostatin peptide ameliorates renal alterations in the early stage of a type 1 diabetic nephropathy model. Diabetes. 2005;54:2891–2903. doi: 10.2337/diabetes.54.10.2891. [DOI] [PubMed] [Google Scholar]
- 80.Iliescu R, Chade AR. Progressive renal vascular proliferation and injury in obese Zucker rats. Microcirculation. 2010;17:250–258. doi: 10.1111/j.1549-8719.2010.00020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Iliescu R, Fernandez SR, Kelsen S, Maric C, Chade AR. Role of renal microcirculation in experimental renovascular disease. Nephrol Dial Transplant. 2010;25:1079–1087. doi: 10.1093/ndt/gfp605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Inoue T, Okada H, Kobayashi T, Watanabe Y, Kanno Y, Kopp JB, Nishida T, Takigawa M, Ueno M, Nakamura T, Suzuki H. Hepatocyte growth factor counteracts transforming growth factor-beta1, through attenuation of connective tissue growth factor induction, and prevents renal fibrogenesis in 5/6 nephrectomized mice. Fase b J. 2003;17:268–270. doi: 10.1096/fj.02-0442fje. [DOI] [PubMed] [Google Scholar]
- 83.Jesmin S, Zaedi S, Shimojo N, Iemitsu M, Masuzawa K, Yamaguchi N, Mowa CN, Maeda S, Hattori Y, Miyauchi T. Endothelin antagonism normalizes VEGF signaling and cardiac function in STZ-induced diabetic rat hearts. Am J Physiol Endocrinol Metab. 2007;292:E1030–E1040. doi: 10.1152/ajpendo.00517.2006. [DOI] [PubMed] [Google Scholar]
- 84.Josifova T, Schneider U, Henrich PB, Schrader W. Eye disorders in diabetes: Potential drug targets. Infect Disord Drug Targets. 2008;8:70–75. doi: 10.2174/187152608784746529. [DOI] [PubMed] [Google Scholar]
- 85.Kalousova M, Hodkova M, Dusilova-Sulkova S, Uhrova J, Tesar V, Zima T. Effect of hemodiafiltration on pregnancy-associated plasma protein A (PAPP-A) and related parameters. Ren Fail. 2006;28:715–721. doi: 10.1080/08860220600925412. [DOI] [PubMed] [Google Scholar]
- 86.Kalra PA, Guo H, Kausz AT, Gilbertson DT, Liu J, Chen SC, Ishani A, Collins AJ, Foley RN. Atherosclerotic renovascular disease in United States patients aged 67 years or older: risk factors, revascularization, and prognosis. Kidney Int. 2005;68:293–301. doi: 10.1111/j.1523-1755.2005.00406.x. [DOI] [PubMed] [Google Scholar]
- 87.Kang DH, Anderson S, Kim YG, Mazzalli M, Suga S, Jefferson JA, Gordon KL, Oyama TT, Hughes J, Hugo C, Kerjaschki D, Schreiner GF, Johnson RJ. Impaired angiogenesis in the aging kidney: Vascular endothelial growth factor and thrombospondin-1 in renal disease. Am J Kidney Dis. 2001;37:601–611. doi: 10.1053/ajkd.2001.22087. [DOI] [PubMed] [Google Scholar]
- 88.Kang DH, Hughes J, Mazzali M, Schreiner GF, Johnson RJ. Impaired angiogenesis in the remnant kidney model: II Vascular endothelial growth factor administration reduces renal fibrosis and stabilizes renal function. J Am Soc Nephrol. 2001;12:1448–1457. doi: 10.1681/ASN.V1271448. [DOI] [PubMed] [Google Scholar]
- 89.Kang DH, Kanellis J, Hugo C, Truong L, Anderson S, Kerjaschki D, Schreiner GF, Johnson RJ. Role of the microvascular endothelium in progressive renal disease. J Am Soc Nephrol. 2002;13:806–816. doi: 10.1681/ASN.V133806. [DOI] [PubMed] [Google Scholar]
- 90.Karihaloo A, Karumanchi SA, Barasch J, Jha V, Nickel CH, Yang J, Grisaru S, Bush KT, Nigam S, Rosenblum ND, Sukhatme VP, Cantley LG. Endostatin regulates branching morphogenesis of renal epithelial cells and ureteric bud. Proc Natl Acad Sci U S A. 2001;98:12509–12514. doi: 10.1073/pnas.221205198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kelsen S, Hall JE, Chade AR. Endothelin-A receptor blockade slows the progression of renal injury in experimental renovascular disease. Am J Physiol Renal Physiol. 2011;301:F218–F225. doi: 10.1152/ajprenal.00089.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kistorp C, Chong AY, Gustafsson F, Galatius S, Raymond I, Faber J, Lip GY, Hildebrandt P. Biomarkers of endothelial dysfunction are elevated and related to prognosis in chronic heart failure patients with diabetes but not in those without diabetes. Eur J Heart Fail. 2008;10:380–387. doi: 10.1016/j.ejheart.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 93.Kitayama H, Maeshima Y, Takazawa Y, Yamamoto Y, Wu Y, Ichinose K, Hirokoshi K, Sugiyama H, Yamasaki Y, Makino H. Regulation of angiogenic factors in angiotensin II infusion model in association with tubulointerstitial injuries. Am J Hypertens. 2006;19:718–727. doi: 10.1016/j.amjhyper.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 94.Ko SH, Cao W, Liu Z. Hypertension management and microvascular insulin resistance in diabetes. Curr Hypertens Rep. 2011;12:243–251. doi: 10.1007/s11906-010-0114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Korn C, Augustin HG. Born to die: Blood vessel regression research coming of age. Circulation. 2012;125:3063–3065. doi: 10.1161/CIRCULATIONAHA.112.112755. [DOI] [PubMed] [Google Scholar]
- 96.Lee DH, Wolstein JM, Pudasaini B, Plotkin M. INK4a deletion results in improved kidney regeneration and decreased capillary rarefaction after ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2012;302:F183–F191. doi: 10.1152/ajprenal.00407.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee JS, Kim JM, Kim KL, Jang HS, Shin IS, Jeon ES, Suh W, Byun J, Kim DK. Combined administration of naked DNA vectors encoding VEGF and bFGF enhances tissue perfusion and arteriogenesis in ischemic hindlimb. Biochem Biophys Res Commun. 2007;360:752–758. doi: 10.1016/j.bbrc.2007.06.120. [DOI] [PubMed] [Google Scholar]
- 98.Lee JS, Song SH, Kim JM, Shin IS, Kim KL, Suh YL, Kim HZ, Koh GY, Byun J, Jeon ES, Suh W, Kim DK. Angiopoietin-1 prevents hypertension and target organ damage through its interaction with endothelial Tie2 receptor. Cardiovasc Res. 2008;78:572–580. doi: 10.1093/cvr/cvn048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee LK, Meyer TW, Pollock AS, Lovett DH. Endothelial cell injury initiates glomerular sclerosis in the rat remnant kidney. J Clin Invest. 1995;96:953–964. doi: 10.1172/JCI118143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Leopold JA, Loscalzo J. Clinical importance of understanding vascular biology. Cardiol Rev. 2000;8:115–123. [PubMed] [Google Scholar]
- 101.Lerman LO, Taler SJ, Textor SC, Sheedy PF, II, Stanson AW, Romero JC. Computed tomography-derived intrarenal blood flow in renovascular and essential hypertension. Kidney Int. 1996;49:846–854. doi: 10.1038/ki.1996.117. [DOI] [PubMed] [Google Scholar]
- 102.Levy BI, Schiffrin EL, Mourad JJ, Agostini D, Vicaut E, Safar ME, Struijker-Boudier HA. Impaired tissue perfusion: a pathology common to hypertension, obesity, and diabetes mellitus. Circulation. 2008;118:968–976. doi: 10.1161/CIRCULATIONAHA.107.763730. [DOI] [PubMed] [Google Scholar]
- 103.Lindenmeyer MT, Kretzler M, Boucherot A, Berra S, Yasuda Y, Henger A, Eichinger F, Gaiser S, Schmid H, Rastaldi MP, Schrier RW, Schlondorff D, Cohen CD. Interstitial vascular rarefaction and reduced VEGF-A expression in human diabetic nephropathy. J Am Soc Nephrol. 2007;18:1765–1776. doi: 10.1681/ASN.2006121304. [DOI] [PubMed] [Google Scholar]
- 104.Linke A, Recchia F, Zhang X, Hintze TH. Acute and chronic endothelial dysfunction: Implications for the development of heart failure. Heart Fail Rev. 2003;8:87–97. doi: 10.1023/a:1022151106019. [DOI] [PubMed] [Google Scholar]
- 105.Lohmeier TE, Iliescu R, Liu B, Henegar JR, Maric-Bilkan C, Irwin ED. Systemic and renal-specific sympathoinhibition in obesity hypertension. Hypertension. 2012;59:331–338. doi: 10.1161/HYPERTENSIONAHA.111.185074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lohmeier TE, Warren S, Cunningham JT. Sustained activation of the central baroreceptor pathway in obesity hypertension. Hypertension. 2003;42:96–102. doi: 10.1161/01.HYP.0000076092.10923.FD. [DOI] [PubMed] [Google Scholar]
- 107.Lorenzen J, David S, Bahlmann FH, de Groot K, Bahlmann E, Kielstein JT, Haller H, Fliser D. Endothelial progenitor cells and cardiovascular events in patients with chronic kidney disease-a prospective follow-up study. PLoS One. 2010;5:e11477. doi: 10.1371/journal.pone.0011477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lupia E, Elliot SJ, Lenz O, Zheng F, Hattori M, Striker GE, Striker LJ. IGF-1 decreases collagen degradation in diabetic NOD mesangial cells: Implications for diabetic nephropathy. Diabetes. 1999;48:1638–1644. doi: 10.2337/diabetes.48.8.1638. [DOI] [PubMed] [Google Scholar]
- 109.Maciel TT, Coutinho EL, Soares D, Achar E, Schor N, Bellini MH. Endostatin, an antiangiogenic protein, is expressed in the unilateral ureteral obstruction mice model. J Nephrol. 2008;21:753–760. [PubMed] [Google Scholar]
- 110.Maeshima Y, Makino H. Angiogenesis and chronic kidney disease. Fibrogenesis Tissue Repair. 2010;3:13. doi: 10.1186/1755-1536-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Makino H, Okada S, Nagumo A, Sugisawa T, Miyamoto Y, Kishimoto I, Kikuchi-Taura A, Soma T, Taguchi A, Yoshimasa Y. Decreased circulating CD34+ cells are associated with progression of diabetic nephropathy. Diabet Med. 2009;26:171–173. doi: 10.1111/j.1464-5491.2008.02638.x. [DOI] [PubMed] [Google Scholar]
- 112.Maric-Bilkan C, Flynn ER, Chade AR. Microvascular disease precedes the decline in renal function in the streptozotocin-induced diabetic rat. Am J Physiol Renal Physiol. 2012;302:F308–F315. doi: 10.1152/ajprenal.00421.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Marin P, Andersson B, Krotkiewski M, Bjorntorp P. Muscle fiber composition and capillary density in women and men with NIDDM. Diabetes Care. 1994;17:382–386. doi: 10.2337/diacare.17.5.382. [DOI] [PubMed] [Google Scholar]
- 114.Mayer G. Capillary rarefaction, hypoxia, VEGF and angiogenesis in chronic renal disease. Nephrol Dial Transplant. 2011;26:1132–1137. doi: 10.1093/ndt/gfq832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.McLennan SV, Kelly DJ, Schache M, Waltham M, Dy V, Langham RG, Yue DK, Gilbert RE. Advanced glycation end products decrease mesangial cell MMP-7: A role in matrix accumulation in diabetic nephropathy? Kidney Int. 2007;72:481–488. doi: 10.1038/sj.ki.5002357. [DOI] [PubMed] [Google Scholar]
- 116.McLennan SV, Wang XY, Moreno V, Yue DK, Twigg SM. Connective tissue growth factor mediates high glucose effects on matrix degradation through tissue inhibitor of matrix metalloproteinase type1:Implications for diabetic nephropathy. Endocrinology. 2004;145:5646–5655. doi: 10.1210/en.2004-0436. [DOI] [PubMed] [Google Scholar]
- 117.Moore MA, Hattori K, Heissig B, Shieh JH, Dias S, Crystal RG, Rafii S. Mobilization of endothelial and hematopoietic stem and progenitor cells by adenovector-mediated elevation of serum levels of SDF-1, VEGF, and angiopoietin-1. Ann N Y Acad Sci. 2001;938:36–45. doi: 10.1111/j.1749-6632.2001.tb03572.x. discussion 45–37. [DOI] [PubMed] [Google Scholar]
- 118.Nakagawa T, Lan HY, Zhu HJ, Kang DH, Schreiner GF, Johnson RJ. Differential regulation of VEGF by TGF-beta and hypoxia in rat proximal tubular cells. Am J Physiol Renal Physiol. 2004;287:F658–F664. doi: 10.1152/ajprenal.00040.2004. [DOI] [PubMed] [Google Scholar]
- 119.Navar LG, Arendhorst WJ, Pallone TL, Inscho EW, Imig JD, Bell PD. The renal microcirculation. Supplement 9: Handbook of Physiology, The Cardiovascular System, Microcirculation. 2008 [Google Scholar]
- 120.O’Donovan HC, Hickey F, Brazil DP, Kavanagh DH, Oliver N, Martin F, Godson C, Crean J. Connective tissue growth factor antagonizes transforming growth factor-beta1/Smad signalling in renal mesangial cells. Biochem J. 2012;441:499–510. doi: 10.1042/BJ20110910. [DOI] [PubMed] [Google Scholar]
- 121.O’Hare AM, Glidden DV, Fox CS, Hsu CY. High prevalence of peripheral arterial disease in persons with renal insufficiency: results from the National Health and Nutrition Examination Survey 1999–2000. Circulation. 2004;109:320–323. doi: 10.1161/01.CIR.0000114519.75433.DD. [DOI] [PubMed] [Google Scholar]
- 122.Olszewska-Pazdrak B, Hein TW, Olszewska P, Carney DH. Chronic hypoxia attenuates VEGF signaling and angiogenic responses by down-regulation of KDR in human endothelial cells. Am J Physiol Cell Physiol. 2009;296:C1162–C1170. doi: 10.1152/ajpcell.00533.2008. [DOI] [PubMed] [Google Scholar]
- 123.Paravicini TM, Touyz RM. NADPH oxidases, reactive oxygen species, and hypertension: clinical implications and therapeutic possibilities. Diabetes Care. 2008;31(Suppl 2):S170–S180. doi: 10.2337/dc08-s247. [DOI] [PubMed] [Google Scholar]
- 124.Pasarica M, Sereda OR, Redman LM, Albarado DC, Hymel DT, Roan LE, Rood JC, Burk DH, Smith SR. Reduced adipose tissue oxygenation in human obesity: Evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes. 2009;58:718–725. doi: 10.2337/db08-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Peirce SM, Skalak TC. Microvascular remodeling: a complex continuum spanning angiogenesis to arteriogenesis. Microcirculation. 2003;10:99–111. doi: 10.1038/sj.mn.7800172. [DOI] [PubMed] [Google Scholar]
- 126.Pelliccia F, Pasceri V, Cianfrocca C, Vitale C, Pristipino C, Speciale G, Mercuro G, Rosano G. Endothelial progenitor cells in patients with coronary artery disease and left ventricular dysfunction. Coron Artery Dis. 2009;20:303–308. doi: 10.1097/MCA.0b013e328325765e. [DOI] [PubMed] [Google Scholar]
- 127.Pessina AC. Target organs of individuals with diabetes caught between arterial stiffness and damage to the microcirculation. J Hypertens Suppl. 2007;25:S13–S18. doi: 10.1097/01.hjh.0000271504.62325.a4. [DOI] [PubMed] [Google Scholar]
- 128.Prewitt RL, Chen II, Dowell R. Development of microvascular rarefaction in the spontaneously hypertensive rat. Am J Physiol. 1982;243:H243–H251. doi: 10.1152/ajpheart.1982.243.2.H243. [DOI] [PubMed] [Google Scholar]
- 129.Qi W, Chen X, Poronnik P, Pollock CA. Transforming growth factor-beta/connective tissue growth factor axis in the kidney. Int J Biochem Cell Biol. 2008;40:9–13. doi: 10.1016/j.biocel.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 130.Qi W, Twigg S, Chen X, Polhill TS, Poronnik P, Gilbert RE, Pollock CA. Am J Physiol Renal Physiol. 2005;288:F800–F809. doi: 10.1152/ajprenal.00179.2004. [DOI] [PubMed] [Google Scholar]
- 131.Rabelink TJ, Wijewickrama DC, de Koning EJ. Peritubular endothelium: The Achilles heel of the kidney? Kidney Int. 2007;72:926–930. doi: 10.1038/sj.ki.5002414. [DOI] [PubMed] [Google Scholar]
- 132.Raptis AE, Viberti G. Pathogenesis of diabetic nephropathy. Exp Clin Endocrinol Diabetes. 2001;109(Suppl 2):S424–S437. doi: 10.1055/s-2001-18600. [DOI] [PubMed] [Google Scholar]
- 133.Reckelhoff JF, Romero JC. Role of oxidative stress in angiotensin-induced hypertension. Am J Physiol Regul Integr Comp Physiol. 2003;284:R893–R912. doi: 10.1152/ajpregu.00491.2002. [DOI] [PubMed] [Google Scholar]
- 134.Rudnicki M, Perco P, Enrich J, Eder S, Heininger D, Bernthaler A, Wiesinger M, Sarkozi R, Noppert SJ, Schramek H, Mayer B, Oberbauer R, Mayer G. Hypoxia response and VEGF-A expression in human proximal tubular epithelial cells in stable and progressive renal disease. Lab Invest. 2009;89:337–346. doi: 10.1038/labinvest.2008.158. [DOI] [PubMed] [Google Scholar]
- 135.Ryan ST, Koteliansky VE, Gotwals PJ, Lindner V. Transforming growth factor-beta-dependent events in vascular remodeling following arterial injury. J Vasc Res. 2003;40:37–46. doi: 10.1159/000068937. [DOI] [PubMed] [Google Scholar]
- 136.Rysz J, Banach M, Stolarek RA, Pasnik J, Cialkowska-Rysz A, Koktysz R, Piechota M, Baj Z. Serum matrix metalloproteinases MMP-2 and MMP-9 and metalloproteinase tissue inhibitors TIMP-1 and TIMP-2 in diabetic nephropathy. J Nephrol. 2007;20:444–452. [PubMed] [Google Scholar]
- 137.Safian RD, Textor SC. Renal-artery stenosis. N Engl J Med. 2001;344:431–442. doi: 10.1056/NEJM200102083440607. [DOI] [PubMed] [Google Scholar]
- 138.Sakurai H, Nigam SK. Transforming growth factor-beta selectively inhibits branching morphogenesis but not tubulogenesis. Am J Physiol. 1997;272:F139–F146. doi: 10.1152/ajprenal.1997.272.1.F139. [DOI] [PubMed] [Google Scholar]
- 139.Saleh MA, Boesen EI, Pollock JS, Savin VJ, Pollock DM. Endothelin-1 increases glomerular permeability and inflammation independent of blood pressure in the rat. Hypertension. 2010;56:942–949. doi: 10.1161/HYPERTENSIONAHA.110.156570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schindler TH, Cardenas J, Prior JO, Facta AD, Kreissl MC, Zhang XL, Sayre J, Dahlbom M, Licinio J, Schelbert HR. Relationship between increasing body weight, insulin resistance, inflammation, adipocytokine leptin, and coronary circulatory function. J Am Coll Cardiol. 2006;47:1188–1195. doi: 10.1016/j.jacc.2005.10.062. [DOI] [PubMed] [Google Scholar]
- 141.Shimizu T, Okamoto H, Chiba S, Matsui Y, Sugawara T, Akino M, Nan J, Kumamoto H, Onozuka H, Mikami T, Kitabatake A. VEGF-mediated angiogenesis is impaired by angiotensin type 1 receptor blockade in cardiomyopathic hamster hearts. Cardiovasc Res. 2003;58:203–212. doi: 10.1016/s0008-6363(02)00843-x. [DOI] [PubMed] [Google Scholar]
- 142.Sila-asna M, Bunyaratvej A, Futrakul P, Futrakul N. Renal microvascular abnormality in chronic kidney disease. Ren Fail. 2006;28:609–610. doi: 10.1080/08860220600839498. [DOI] [PubMed] [Google Scholar]
- 143.Stenvinkel P. Endothelial dysfunction and inflammation-is there a link? Nephrol Dial Transplant. 2001;16:1968–1971. doi: 10.1093/ndt/16.10.1968. [DOI] [PubMed] [Google Scholar]
- 144.Stenvinkel P. Interactions between inflammation, oxidative stress, and endothelial dysfunction in end-stage renal disease. J Ren Nutr. 2003;13:144–148. doi: 10.1053/jren.2003.50018. [DOI] [PubMed] [Google Scholar]
- 145.Suh W, Lee JS, Kim KL, Song SH, Koh GY, Kim DK. Angiopoietin-1 gene therapy attenuates hypertension and target organ damage in nitric oxide synthase inhibited spontaneously hypertensive rats. Korean Circ J. 2011;41:590–595. doi: 10.4070/kcj.2011.41.10.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Takahashi S, Shinya T, Sugiyama A. Angiostatin inhibition of vascular endothelial growth factor-stimulated nitric oxide production in endothelial cells. J Pharmacol Sci. 2010;112:432–437. doi: 10.1254/jphs.10028fp. [DOI] [PubMed] [Google Scholar]
- 147.Textor SC. Ischemic nephropathy: Where are we now? J Am Soc Nephrol. 2004;15:1974–1982. doi: 10.1097/01.ASN.0000133699.97353.24. [DOI] [PubMed] [Google Scholar]
- 148.Textor SC, Wilcox CS. Renal artery stenosis: A common, treatable cause of renal failure? Annu Rev Med. 2001;52:421–442. doi: 10.1146/annurev.med.52.1.421. [DOI] [PubMed] [Google Scholar]
- 149.Urbieta Caceres VH, Syed FA, Lin J, Zhu XY, Jordan KL, Bell CC, Bentley MD, Lerman A, Khosla S, Lerman LO. Age-dependent renal cortical microvascular loss in female mice. Am J Physiol Endocrinol Metab. 2012;302:E979–E986. doi: 10.1152/ajpendo.00411.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.VanBavel E, Bakker EN, Pistea A, Sorop O, Spaan JA. Mechanics of microvascular remodeling. Clin Hemorheol Microcirc. 2006;34:35–41. [PubMed] [Google Scholar]
- 151.Vaziri ND, Ni Z, Oveisi F, Liang K, Pandian R. Enhanced nitric oxide inactivation and protein nitration by reactive oxygen species in renal insufficiency. Hypertension. 2002;39:135–141. doi: 10.1161/hy0102.100540. [DOI] [PubMed] [Google Scholar]
- 152.Wacker A, Gerhardt H. Endothelial development taking shape. Curr Opin Cell Biol. 2011;23:676–685. doi: 10.1016/j.ceb.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 153.Wang D, Iversen J, Wilcox CS, Strandgaard S. Endothelial dysfunction and reduced nitric oxide in resistance arteries in autosomal-dominant polycystic kidney disease. Kidney Int. 2003;64:1381–1388. doi: 10.1046/j.1523-1755.2003.00236.x. [DOI] [PubMed] [Google Scholar]
- 154.Wang R, Xu YJ, Liu XS, Zeng DX, Xiang M. Knockdown of connective tissue growth factor by plasmid-based short hairpin RNA prevented pulmonary vascular remodeling in cigarette smoke-exposed rats. Arch Biochem Biophys. 2011;508:93–100. doi: 10.1016/j.abb.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 155.Warncke J, David S, Kumpers P, Opherk JP, Haller H, Fliser D. A hibernating kidney - ischemic preconditioning in a renal transplant recipient with a proximal stenosis of the iliac artery. Clin Nephrol. 2008;70:168–171. doi: 10.5414/cnp70168. [DOI] [PubMed] [Google Scholar]
- 156.Warner L, Glockner JF, Woollard J, Textor SC, Romero JC, Lerman LO. Determinations of renal cortical and medullary oxygenation using blood oxygen level-dependent magnetic resonance imaging and selective diuretics. Invest Radiol. 2011;46:41–47. doi: 10.1097/RLI.0b013e3181f0213f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Watorek E, Paprocka M, Dus D, Kopec W, Klinger M. Endostatin and vascular endothelial growth factor: potential regulators of endothelial progenitor cell number in chronic kidney disease. Pol Arch Med Wewn. 2011;121:296–301. [PubMed] [Google Scholar]
- 158.Weis M, Heeschen C, Glassford AJ, Cooke JP. Statins have biphasic effects on angiogenesis. Circulation. 2002;105:739–745. doi: 10.1161/hc0602.103393. [DOI] [PubMed] [Google Scholar]
- 159.Whayne TF., Jr Coronary atherosclerosis, low-density lipoproteins and markers of thrombosis, inflammation and endothelial dysfunction. Int J Angiol. 2007;16:12–16. doi: 10.1055/s-0031-1278237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yang F, Chung AC, Huang XR, Lan HY. Angiotensin II induces connective tissue growth factor and collagen I expression via transforming growth factor-beta-dependent and -independent Smad pathways: The role of Smad3. Hypertension. 2009;54:877–884. doi: 10.1161/HYPERTENSIONAHA.109.136531. [DOI] [PubMed] [Google Scholar]
- 161.Zhu M, Bi X, Jia Q, Shangguan S. The possible mechanism for impaired angiogenesis after transient focal ischemia in type 2 diabetic GK rats: different expressions of angiostatin and vascular endothelial growth factor. Biomed Pharmacother. 2010;64:208–213. doi: 10.1016/j.biopha.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 162.Zhu XY, Chade AR, Rodriguez-Porcel M, Bentley MD, Ritman EL, Lerman A, Lerman LO. Cortical microvascular remodeling in the stenotic kidney: Role of increased oxidative stress. Arterioscler Thromb Vasc Biol. 2004;24:1854–1859. doi: 10.1161/01.ATV.0000142443.52606.81. [DOI] [PubMed] [Google Scholar]
- 163.Zhu XY, Rodriguez-Porcel M, Bentley MD, Chade AR, Sica V, Napoli C, Caplice N, Ritman EL, Lerman A, Lerman LO. Antioxidant intervention attenuates myocardial neovascularization in hypercholesterolemia. Circulation. 2004;109:2109–2115. doi: 10.1161/01.CIR.0000125742.65841.8B. [DOI] [PubMed] [Google Scholar]
- 164.Zoccali C. Endothelial dysfunction in CKD: A new player in town? Nephrol Dial Transplant. 2008;23:783–785. doi: 10.1093/ndt/gfm924. [DOI] [PubMed] [Google Scholar]
- 165.Zoja C, Cattaneo S, Fiordaliso F, Lionetti V, Zambelli V, Salio M, Corna D, Pagani C, Rottoli D, Bisighini C, Remuzzi G, Benigni A. Distinct cardiac and renal effects of ETA receptor antagonist and ACE inhibitor in experimental type 2 diabetes. Am J Physiol Renal Physiol. 2011;301:F1114–F1123. doi: 10.1152/ajprenal.00122.2011. [DOI] [PubMed] [Google Scholar]