Abstract
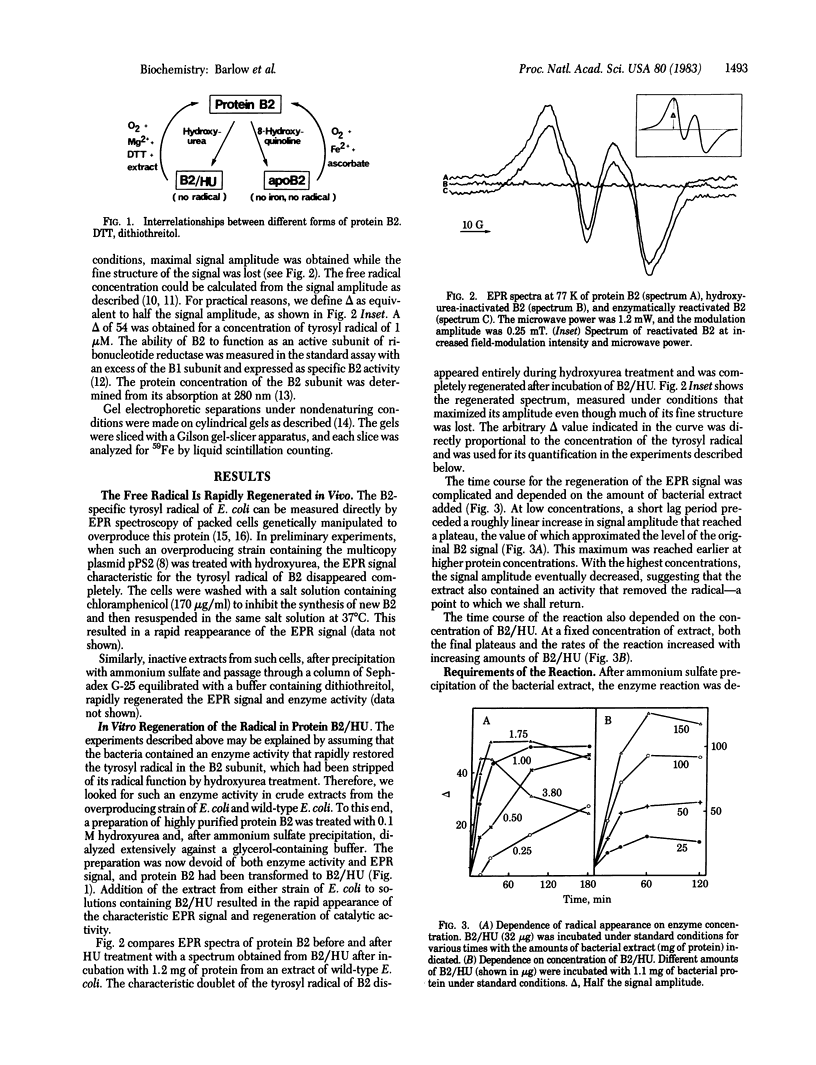
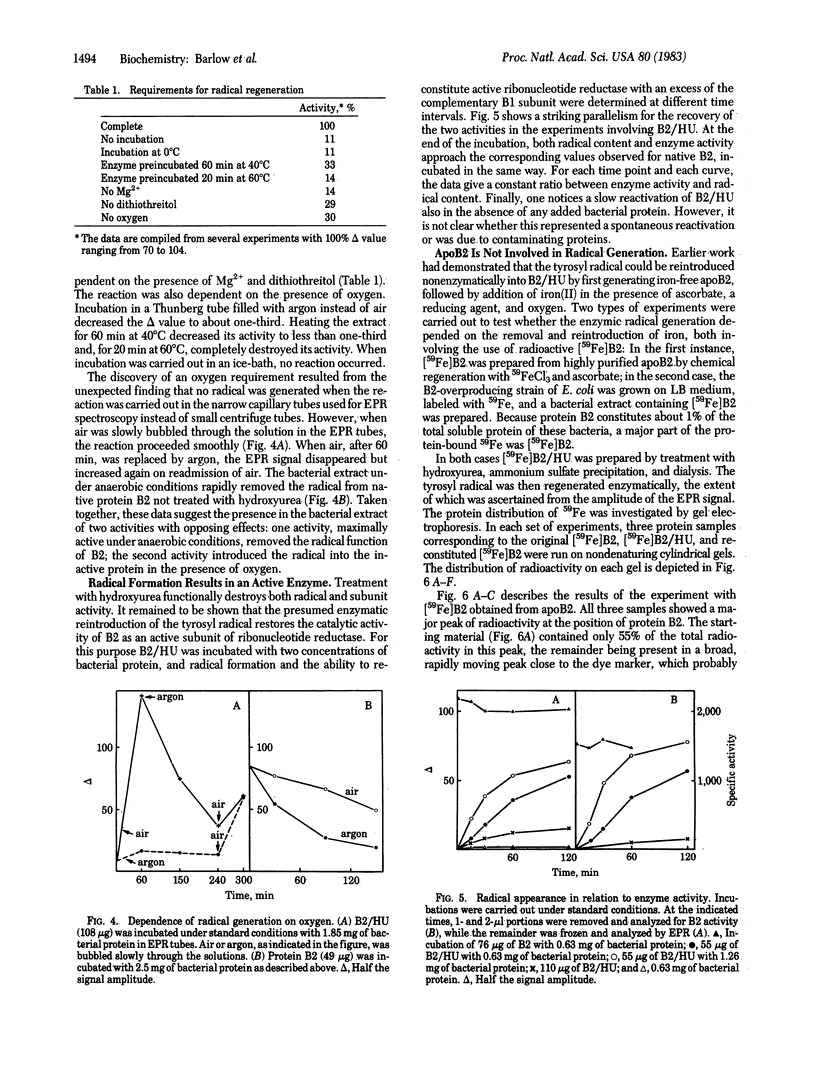
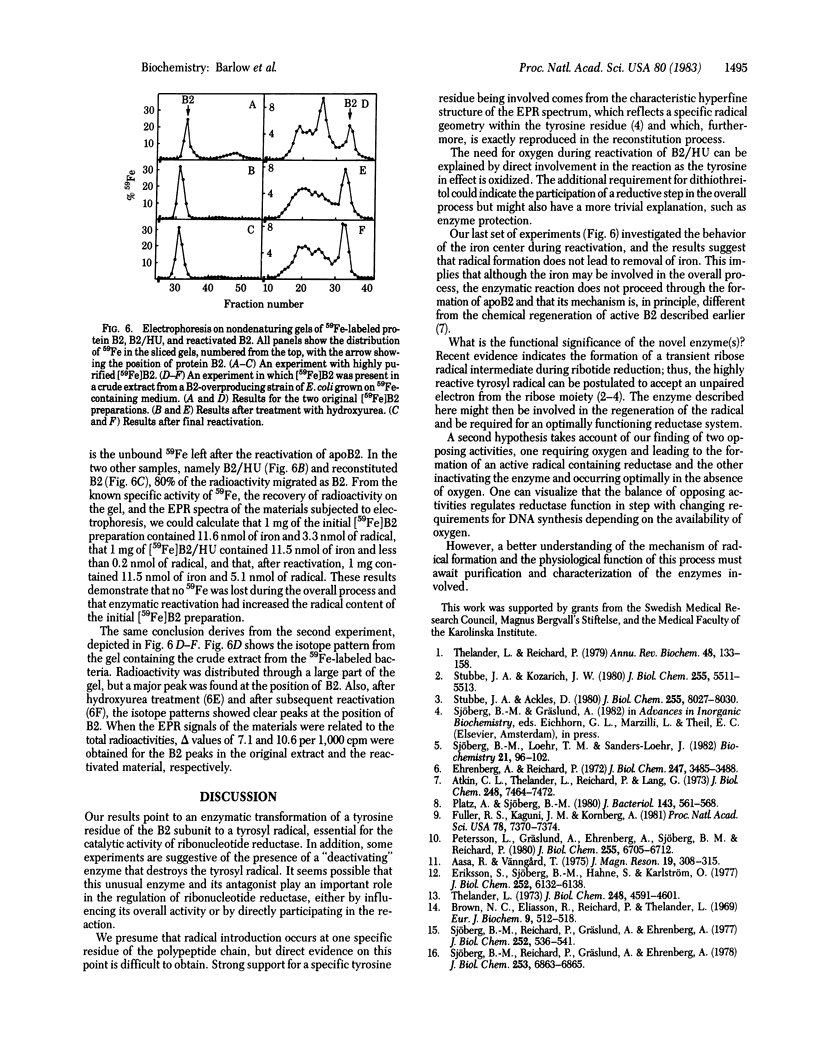
Protein B2, a subunit of ribonucleotide reductase from Escherichia coli, contains in its active form a tyrosyl free radical as part of the polypeptide chain and a dimeric iron center that stabilizes the radical. The enzyme depends on this radical for its catalytic activity. Treatment with hydroxyurea scavenges the radical without disturbing the iron center and, thereby, results in an inactive form of the subunit, B2/HU. A second inactive form, apoB2, lacking both the radical and the iron center, is obtained by treatment of B2 with 8-hydroxyquinoline. Here we describe an enzyme activity in extracts from E. coli that transforms the catalytically inactive B2/HU form into the active B2 subunit by regeneration of the tyrosyl radical. This reaction requires the presence of oxygen, dithiothreitol, and Mg2+ and does not proceed through apoB2. Under anaerobic conditions, we obtained evidence for a second activity in the bacterial extract that destroys the free radical and transforms B2 into B2/HU. We suggest that this novel type of protein modification is functionally related to the synthesis of deoxyribonucleotides and DNA.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkin C. L., Thelander L., Reichard P., Lang G. Iron and free radical in ribonucleotide reductase. Exchange of iron and Mössbauer spectroscopy of the protein B2 subunit of the Escherichia coli enzyme. J Biol Chem. 1973 Nov 10;248(21):7464–7472. [PubMed] [Google Scholar]
- Brown N. C., Eliasson R., Reichard P., Thelander L. Spectrum and iron content of protein B2 from ribonucleoside diphosphate reductase. Eur J Biochem. 1969 Jul;9(4):512–518. doi: 10.1111/j.1432-1033.1969.tb00639.x. [DOI] [PubMed] [Google Scholar]
- Ehrenberg A., Reichard P. Electron spin resonance of the iron-containing protein B2 from ribonucleotide reductase. J Biol Chem. 1972 Jun 10;247(11):3485–3488. [PubMed] [Google Scholar]
- Eriksson S., Sjöberg B. M., Hahne S. Ribonucleoside diphosphate reductase from Escherichia coli. An immunological assay and a novel purification from an overproducing strain lysogenic for phage lambdadnrd. J Biol Chem. 1977 Sep 10;252(17):6132–6138. [PubMed] [Google Scholar]
- Fuller R. S., Kaguni J. M., Kornberg A. Enzymatic replication of the origin of the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7370–7374. doi: 10.1073/pnas.78.12.7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersson L., Gräslund A., Ehrenberg A., Sjöberg B. M., Reichard P. The iron center in ribonucleotide reductase from Escherichia coli. J Biol Chem. 1980 Jul 25;255(14):6706–6712. [PubMed] [Google Scholar]
- Platz A., Sjöberg B. M. Construction and characterization of hybrid plasmids containing the Escherichia coli nrd region. J Bacteriol. 1980 Aug;143(2):561–568. doi: 10.1128/jb.143.2.561-568.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöberg B. M., Loehr T. M., Sanders-Loehr J. Raman spectral evidence for a mu-oxo bridge in the binuclear iron center of ribonucleotide reductase. Biochemistry. 1982 Jan 5;21(1):96–102. doi: 10.1021/bi00530a017. [DOI] [PubMed] [Google Scholar]
- Sjöberg B. M., Reichard P., Gräslund A., Ehrenberg A. The tyrosine free radical in ribonucleotide reductase from Escherichia coli. J Biol Chem. 1978 Oct 10;253(19):6863–6865. [PubMed] [Google Scholar]
- Sjöberg B. M., Reichard P. Nature of the free radical in ribonucleotide reductase from Escherichia coli. J Biol Chem. 1977 Jan 25;252(2):536–541. [PubMed] [Google Scholar]
- Stubbe J. A., Kozarich J. W. Fluoride, pyrophosphate, and base release from 2'-deoxy-2'-fluoronucleoside 5'-diphosphates by ribonucleoside-diphosphate reductase. J Biol Chem. 1980 Jun 25;255(12):5511–5513. [PubMed] [Google Scholar]
- Stubbe J., Ackles D. On the mechanism of ribonucleoside diphosphate reductase from Escherichia coli. Evidence for 3'-C--H bond cleavage. J Biol Chem. 1980 Sep 10;255(17):8027–8030. [PubMed] [Google Scholar]
- Thelander L. Physicochemical characterization of ribonucleoside diphosphate reductase from Escherichia coli. J Biol Chem. 1973 Jul 10;248(13):4591–4601. [PubMed] [Google Scholar]
- Thelander L., Reichard P. Reduction of ribonucleotides. Annu Rev Biochem. 1979;48:133–158. doi: 10.1146/annurev.bi.48.070179.001025. [DOI] [PubMed] [Google Scholar]


