Abstract
Gastroenteropancreatic neuroendocrine neoplasms are heterogeneous in their clinical behavior and require therapies specially tailored according to staging, grading, origin and expression of peptide receptors. Despite extensive scientific efforts, the therapy options are still not satisfactory. The main reasons are due to the lack of a broad mechanistic knowledge, an insufficient classification of specific diagnostic sub-groups, and predictive markers. GEP-NEN tumors evade early diagnosis because of slow asymptomatic growth behavior and are frequently not detected until metastasized. How signaling networks contribute to tumor progression and how these networks interact remains unclear in large parts. In this review we summarize the knowledge on the growth factor responsive non-angiogenetic pathways in sporadic GEP-NENs, highlight promising mechanistic research approaches, and describe important therapy targets.
Keywords: Gastroenteropancreatic neuroendocrine neoplasms, signal transduction, growth factors, kinases, biotherapy, molecular biology, inhibitor.
Introduction
GEP-NENs (Gastroenteropancreatic neuroendocrine neoplasms) emerge from various neuroendocrine cells of the gastroenteropancreatic system and represent the largest subgroup of neuroendocrine neoplasms. This heterogenic entity of solid tumors, formerly termed GEP-NETs or GEP-NECs (gastroenteropancreatic neuroendocrine tumors and carcinomas) or “carcinoids”, displays a broad spectrum of characteristics concerning behavior during growth and differentiation, functional aspects, localization and prognosis.
Although they are ranked among rare neoplastic diseases in general, their incidence has increased exponentially throughout the last decade. Currently, GEP-NENs state the second most common gastrointestinal malignancy after colorectal cancer 1.
The majority of GEP-NENs is characterized by slow proliferating, well differentiated G1 phenotypes (WHO/ENETS classification 2010, refer to table 1), which are often diagnosed late in the developmental course by the occurrence of metastases (NEN G1, previously termed WDNET: well differentiated neuroendocrine tumor). In contrast, a small G3 subgroup of rapidly growing and poorly differentiated GEP-NENs display a behavior that is comparable to those of prevalent solid carcinoma entities (NEN G3 or NEC, previously called PDNEC: poorly differentiated neuroendocrine carcinoma). The third group, characterized by an intermediate malignancy or unclear behavior, NEN G2, approximates the former description of a well differentiated neuroendocrine carcinoma. The tumor grade is dependent on the proliferative behavior marked by mitoses and by the Ki‑67 antigen, which is a proliferation-related antigen and is immunohistochemically analyzed during diagnosis by default (refer to table 1). In contrast to previous classifications or the TNM staging, the differentiation of the tumor cells is not involved in grading decisions, which still hampers the evaluation of slowly growing poorly differentiated or highly proliferating well differentiated tumors and does not provide insights regarding invasive behavior. However, the main advantage of the current grading is the possibility to predict proliferation behavior and thus facilitate decisions regarding surgical resection, chemo-, radio- and biotherapy or monitoring.
Table 1.
WHO 2010 grading for (neuro-)endocrine tumors.
| Grade | Mitotic count (10 HPF) | Ki-67 index (%) |
|---|---|---|
| G1 | <2 | ≤2 |
| G2 | 2-20 | 3-20 |
| G3 | >20 | >20 |
Several signaling cascades influence malignant transformation, progression and metastasis in neuroendocrine cancers including RTKs (receptor tyrosine kinases) and GPCRs (G-protein coupled receptors) downstream signaling, which regulate Ras/Raf, MAPK, PI3K-Akt-mTOR and JNK and lead to DNA synthesis and cell proliferation. Pancreatic NENs are highly vascularized and nourished. Accordingly, they exhibit a vast expression of growth factors such as VEGF (vascular endothelial growth factor), PDGF (platelet-derived growth factor), IGF-1 (insulin-like growth factor 1), bFGF (basic fibroblast growth factor), TGF-α and -β (transforming growth factor) and PIGF (placental growth factor). Not surprisingly, aberrant receptor activity, including those of the IGF-1R (IGF-1 receptor) and FGFR3 (FGF receptor 3), and highly activated downstream signaling is frequent in pNENs 2-5.
Analogous to the pancreatic subgroup, gastrointestinal NENs overexpress VEGF, bFGF, TGF α and -β, PDGF, IGF-1 and its corresponding receptors PDGFR (PDGF receptor), IGF-IR, EGFR (epidermal growth factor receptor), VEGFR (VEGF receptor), and c-kit (stem cell factor receptor). They thus exhibit increased growth factor, pro-angiogenic and typically pro-secretory signaling 6-16. This work will focus on the major growth-factor related signaling networks, namely the PI3 kinase and the MAP kinase cascades and their importance for GEP-NEN therapy and diagnosis today and for future therapy approaches.
Aberrant receptor activity induces sustained growth factor signaling in GEP-NENs and activates the PI3K and MAPK signaling network
IGF receptors
The IGF-1R is one of the crucial RTKs in gastroenteropancreatic neuroendocrine tumor growth factor biology. The intrinsic RTK activity of IGF-1R is activated upon binding of its respective ligand and leading to auto-phosphorylation of intracellular tyrosine residues in the juxtamembrane and C-terminal domains. Those phosphorylated tyrosines serve as docking stations for insulin receptor substrates, such as IRS-1 and Src resulting in PI3K signaling via Grb2/SOS and Ras and MAPK pathways 17.
NEN cells have been shown to secrete high amounts of IGF‑1. In gastrinomas, increased levels of both IGF-1 and the corresponding IGF-1R were associated with tumor growth, aggressiveness, and progression 18. Furthermore, other studies have displayed that the expression of IGF-1R is decreased in functionally inactive neuroendocrine tumors of different offspring in relation to their functional analogues. These findings and further in vitro experiments suggest that IGF-1 is not only a major autocrine regulator of neuroendocrine tumor growth but also of neuroendocrine secretion itself. Inhibition of IGF-1R activity, e.g. by direct inhibition or by blocking its regulators, such as HSP90 (heat shock protein 90), resulted in decreased PI3K and ERK1/2 (extracellular signal-regulated kinase) signaling and induction of cell cycle arrest and apoptosis 14, 18-26. Additionally, an alternatively spliced IGF-1R mRNA transcript could be detected with a higher abundance in neuroendocrine tumors of different offspring, suggesting that post-transcriptional mechanisms may cause regulatory aberrations 19.
In addition to aberrant receptor and ligand abundance, an important regulator of IGF signaling was found to be significantly up-regulated in metastatic NENs in two gene expression studies: IGFBP3 (IGF binding protein 3), which is considered to maintain the serum level of IGF-1 in a tissue specific pro- or antiproliferative manner. IGFBP3 was overexpressed in >80% of lymph node or distant metastases versus <60% in primary pNEN lesions 27-29. Those data might indicate a stoma or tumor cell-controlled regulation of a distinct IGF-1 homeostasis and allocation even in target tissues with a completely different composition. Adaptive and cooperative behavior of metastasizing NEN cells in the context of circulation and homing should be further explored in the future.
Therefore, IGF-1 and its receptor IGF-R1 are highly expressed in GEP-NENs with an altered abundance which depends on IGF binding factors and the relative ratio of specific receptor isoforms. IGF-1 has been shown to be a major autocrine regulator of neuroendocrine tumor growth and of neuroendocrine secretion.
EGF receptors and FGF
The EGFR belongs to the HER receptor family that consists of EGFR (HER1 or erbB1), erbB2 (HER2), erbB3 (HER3) and erb4 (HER4). Gastrointestinal and pancreatic NENs express and activate EGFRs. In immunohistochemical analyses of NENs located in different primary locations, 96% of the specimens were positive for EGFR expression and 63% were positive for phosphorylated EGFR 6. Another study demonstrated a significantly higher expression (> 91%) in metastatic and non-metastatic gastrointestinal NENs in contrast to <25% in primary and metastatic pNEN 30. A third study retrospectively evaluated the expression of EGFR and one of its ligands, TGF-α (transforming growth factor alpha), in pNENs, demonstrating that 63% of the tumors were positive for TGF-alpha and 65% were positive for the intracellular and/or extracellular domain of EGFR, but failed to prove a correlation with size, functional status, secretory profile, or biologic behavior 31. These data were confirmed by Nilsson and colleagues, who showed that several human neuroendocrine tumors express both TGF-alpha and EGF receptors in vivo and in vitro, suggesting an autocrine mechanism 9.
Di Florio and colleagues recently demonstrated that gastrointestinal hormones and neurotransmitters stimulate the growth of the human BON, QGP-1 and the rat Rin-14B-cell lines in an EGFR dependent manner through activation of PKC and Src kinases, matrix metalloproteinase activation and the generation of reactive oxygen species 32.
Although a recent analysis of pancreatic neuroendocrine tumors uncovered frequent single nucleotide polymorphisms (SNPs) in PDGFRA and EGFR, no druggable EGFR, KIT or PDGFRA mutation could be found in these receptors to date. Nevertheless, elevated copy number of the EGFR and HER-2/neu loci could be detected in 38% and 33% of the cases and high expression of PDGFRA in 65%, respectively 21.
The non-secretory protein FGF13 has recently been described as new progression marker in pNENs. Although FGF13 is insufficient to stimulate FGF receptors, it was demonstrated to be an independent predictor of a shorter progression-free survival associated with positive Ki-67 staining. Furthermore FGF overexpression correlates with the occurrence of liver metastases and shortened disease-free survival in patients that underwent complete tumor resection 33. FGF13 has recently been identified as microtubule-stabilizing protein in neuronal cells that promotes neuronal migration in the cerebral cortex. These processes might also facilitate dissemination of neuroendocrine tumor cells that share at least some characteristics with neuronal cells, but the underlying mechanisms remain unknown 34.
Taken together, these studies have accounted for high growth factor abundance in GEP-NENs. Although SNPs in several growth factor receptors have been demonstrated, no druggable mutation could be found to date. Therefore growth factor receptors might serve as targets for anti-growth receptor therapy in patients with GEP‑NENs, as several IGF-1R and EGFR inhibitors are currently under clinical assessment (refer to Supplementary Material: suppl. 1). Nevertheless further subgroup-specific genetic and post-transcriptional analyses are necessary to clarify the role of the distinct receptor aberrations and improve the therapeutic potential of growth factor receptors as therapeutic targets in GEP-NENs.
Somatostatin receptors mediate anti-proliferative signals, inhibit PI3K and MAPK signaling and are important therapeutic targets in GEP-NENs
Somatostatin signaling
The cyclopeptide family of SSTs (somatostatins), which function as somatotropin-release inhibiting factors, is distributed throughout the central nervous system and peripheral organs and can be found in endocrine, immune and neuronal cells, as well as in certain tumors. Its preserved peptide structure indicates a fundamental regulatory function in vertebrate hormone homeostasis.
The human genome includes five non-allelic genes that encode for five or six distinct transmembrane domain G-protein-coupled SSTRs (somatostatin receptors). The gene encoding SSTR2 produces two splice variants (SSTR2A and SSTR2B) in mouse and presumably in humans as well 35-38, whereas the other genes are intronless and generate one receptor in each case 39-42. The natural ligands of SSTR1-5 (SST-14, SST-28 and cortistatin) are bound with a high affinity. Nevertheless the majority of (longer acting) synthetic peptide analogues, namely MS201-995 (octreotide), RC-160 (vapreotide), BIM 23014 (lanreotide) and MK 678 (Seglitide), only interact with the subtypes SSTR 2, 3 and 5 to a satisfactory extent. Moreover, Pasireotide (SOM 230) shows higher binding capacity towards SSTR1 43, 44.
Somatostatin receptor signaling is complex. Its activation mediates cell cycle arrest, apoptosis and is involved in the regulation of hormone secretion in endocrine target cells. Appropriately, binding of somatostatin and its analogues to SSTRs triggers a number of intracellular signaling events, initiated by specific G-Protein activation. These G-proteins function as transducers and in turn modulate the activity of several key enzymes including adenylyl cyclase, phospho-tyrosine phosphatases (PTPs) and MAPKs (mitogen activated kinases) 45-47. Inhibitory effects of SST on adenylyl cyclase, cAMP production and several distinct ion channels regulate both endocrine and exocrine secretion 39, 48, 49. Intracellular PTPs exert anti-proliferative activity by deactivation of intracellular PI3K and MAPK signaling. Phosphatases, such as SHP-1 and SHP-2, mediate SST-induced cell cycle arrest in many cell lines in vitro. SHP-2 is also considered to inactivate insulin and epidermal growth factor binding RTKs and to repress C-Raf and MAPK signaling, whereas SHP-1 is involved in dephosphorylation of the p85 PI3K regulatory subunit and IRS-1 and thus, decreased PDK-1 (phosphoinositide-dependent kinase 1) and Akt activity. It furthermore inhibits S phase entry through the overexpression of p27kip1 and increase of hypo-phosphorylated retinoblastoma gene product (Rb) 47, 50-61. A third PTP is involved in the SST-induced proliferation arrest: DEP-1 (density enhanced phosphatase-1, also termed PTPη in rats) is a negative regulator of Src-mediated growth factor receptor activity and participates in cellular differentiation 62-66.
The mechanism of SST signaling has been confirmed in different cellular models indicating that this modular multi-effector pathway, which is induced by several SSTRs, transmitted by similar kinases and PTPs and resulting in the activation of final effector PTPs, is a common mechanism in various cells 67, 68. Furthermore the downstream signaling of the distinct SSTRs differs in certain subtype specific functions: e.g. whereas both SSTR2 and 5 regulate GH secretion, insulin secretion is predominantly controlled by SSTR5. Glucagon secretion and immune response are SSTR2 dependent (reviewed in 39).
Somatostatin receptors in GEP-NEN therapy
Gastroenteropancreatic neuroendocrine neoplasms express all five subtypes of SSTRs, although at a variable extent and in various combinations. The most prevalent receptor is SSTR2A, which has been immunohistochemically detected in 84% of the GEP-NEN specimens in a recent study. SSTR3, 4, 5 and 1 have been found expressed in 84, 44, 32 and 32%, respectively 69. This data agree with previous studies in insulinomas where the SSTR2, 1 and 3 have been found to be predominantly expressed 70. In midgut NENs SSTR2 is the most prominent as well, but followed by SSTR1 and SSTR5, and, with a less frequency, by SSTR3 and SSTR4. 70-73. Comparable findings have been recently published in a cohort of 67 NENs, with an expression of SSTR1, 2a, 3, 4 and 5 in 42, 63, 6, 32 and 65% of the cases, respectively. Interestingly, the SSTR5 immunoreactivity was correlated with the presence of metastases and angioinvasion 74. Other studies have previously demonstrated a positive correlation of SSTR2 and SSTR5 expression with a better prognosis and higher differentiation 75-80.
Although corresponding data is rare, some studies have demonstrated a frequent co-expression of SSTRs and the D2R (dopamine D2 receptor) in GEP-NENs 38, 77, 81. Co-expression of SSTR5 and D2R is assumed to contribute to a hetero-oligomerization and creation of a novel receptor with enhanced functional activity 82. A Co-expression of SSTR2 and SSTR5 with D2R and its correlation with low tumor grade has been described in mixed NENs 77. The high frequency of SSTR1, SSTR2, and SSTR5 expression and inverse correlation with COX2, a cytochrome C oxidase and component of the respiratory chain, was recently demonstrated. The study also accounted for a better prognosis concomitant with a generally high SSTR expression 76.
Additionally, one group was also able to demonstrate a high expression of the human SSTR2B which raises further questions, whether this splice variant exists in humans 38.
Data concerning the SSTR receptor distribution pattern in different subgroups was published for SSTR2A, which was present in a high frequency in carcinoids and gastrinomas, but detectable in only half of the analyzed insulinomas, suggesting one reason for the high number of octreotide insensitive insulinomas 83, 84. Another study demonstrated a higher SSTR expression in non-pancreatic NENs versus pancreatic NENs for all receptor isoforms 78.
This high heterogeneity of data, beyond the fact that SSTR2 exerts to be the most prevalent of the generally highly expressed SSTRs in GEP-NENs, is summarized in table 2 which highlights the need for better defined criteria. For example, a discussion about scoring systems as criteria in the use of mono- versus polyclonal antibodies is still ongoing 69, 85-88.
Table 2.
SSTR expression in GEP-NENs. Several studies have been conducted to date, but with very heterogeneous results. SSTR2 is presumed to be the most prevalently expressed isoform.
| Reference | Tissue | Method | SSTR1 | SSTR2 | SSTR3 | SSTR4 | SSTR5 |
|---|---|---|---|---|---|---|---|
| Papotti 2002 73 | GEP-NENs | RT-PCR | 90,1% | 84,8% | 78,8% | 24,2% | 42,4% |
| IHC pAb | 68,2% | 36,4% | 63,6% | ||||
| Reubi 2003 70 | Midgut NENs | receptor auto-radiography | 50-60% | 90-100% | 10-20% | < 10% | ~ 50% |
| Srirajaskanthan 2009 77 | Low grade | IHC pAb | 100% | 100% | |||
| Interm. gr. | 94,4% | 94,4% | |||||
| High grade | 66,7% | 66,7% | |||||
| Corleto 2009 75 | functioning NEN | RT-PCR | 73% | 100% | 64% | 9% | 64% |
| Zamora 2010 78 | GEP-NENs | IHC | 46% | 86% | 26% | 24% | 62% |
| Kim 2011 76 | GEP-NENs | IHC m/pAb | 84% | 72% | 55% | ||
| Diakatou 2011 38 | GEP-NENS | IHC pAb | 39% | 62% (2A) 49% (2B) |
38% | 15% | 38% |
| Kaemmerer 2012 69 | GEP-NENs | IHC mAb | 32% | 84% | 84% | 44% | 32% |
| Schmid 2012 74 | GEP-NENs | IHC mAb | 42% | 63% | 6% | 32% | 65% |
Furthermore the trafficking of SSTRs has become more and more interesting in somatostatin receptor-positive cancer entities and GEP-NENs. Concerning the internalization of SSTRs and its regulation in NENs, mainly controversially discussed immunohistochemical data is available. The involved agonists, phosphorylation sites and phosphatases in NENs are almost unknown, but basic neuroscience research has increasingly focused on this fundamental aspect of SSTR regulation. 89-95.
Throughout two decades, efforts have already been made to translate the knowledge of neuroendocrine SSTR expression into effective GEP-NEN therapies. During this process a multitude of somatostatin peptide agonists and antagonists and non-peptide agonists and antagonists have been developed, of which the agonists have found their way into clinical applications (refer to Supplementary Material: suppl. 2).
The clinical use of SST analogues is limited in clinical application due to a poor oral bioavailability, short half-life and immunogenicity. Non-peptide analogues are considered to be more advantageous as synthesis can be directed upon a higher specificity, bioavailability and less immunogenicity. Furthermore, somatostatin analogue-conjugated radioligands are applied in PRRT (peptide receptor radionuclide therapy) and scintigraphy-based diagnosis (Reviewed in 96-106).
The two octapeptide analogues octreotide and lanreotide, which are also available as long-acting repeatable (LAR) depot formulation, bind to SSTR2 with a high and to SSTR5 with a moderate affinity. They inhibit the release of neuroendocrine hormones and have been shown to prolong disease stability in >50% of patients with progressive GEP-NEN disease. Although no objective tumor responses were observed in several studies, including the randomized, prospective Phase III PROMID trial in patients with metastatic neuroendocrine midgut tumors, patients profit from prolonged time to progression 107-117. The results of the Phase III CLARINET-study using lanreotide in progressive neuroendocrine tumors of various sites are awaited later on this year. Further SST analogues, binding to the other SSTRs, such as Pasireotide, which binds to SSTR1, 2, 3 and 5, have been clinically assessed so far and chimeric molecules directed towards SSTRs and DRs (dopastatins) are under development and preclinical assessment in GEP‑NENs 45, 118-123.
In summary, the SSTRs are highly expressed in GEP-NENs. They have evolved into establishing therapy targets, although to date the individual expression patterns of the patients have not been related to therapeutic outcome.
The PI3K-pathway is frequently deregulated in many human cancers entities including neuroendocrine neoplasms of the gastroenteropancreatic system
PI3 Kinases
The lipid kinase family of PI3Ks (Phosphatidylinositol 3-kinases) consists of three classes (I-III) that promote the phosphorylation of 3-hydroxyl-phosphoinositides. Whereas heterodimeric class I PI3Ks influence cellular proliferation, insulin signaling and inflammation, the monomeric class II PI3Ks determine the regulation of membrane trafficking. The sole class III member is involved in autophagy. The most important subclass in human cancers is formed by class IA PI3Ks 124, 125.
Extracellular growth factor signaling such as by VEGF, PDGF, IGF-1, FGF and TGF- α and -β is transmitted by RTKs to PI3Ks and results in their activation 2, 3. Activated class I PI3Ks convert their substrate PI(4,5)P2 (phosphatidylinositol 4,5-biphosphate) into its triple-phosphorylated form PI(3,4,5)P3 (phosphatidylinositol (3,4,5)-triphosphate). Subsequently, PI(3,4,5)P3 recruits proteins that contain a PH-domain (Pleckstrin homology domain) into proximity and thus functions as a docking site for Akt and PDK-1, allowing the latter to phosphorylate Akt at T308 (Threonin-308) 125, 126. Phosphorylation on both, T308 by PDK-1 and on S473 (Serin-473) by mTORC2 is required for the full activation of Akt kinase activity 127.
The PIK3CA gene, which encodes for p110α (the catalytic subunit of class I PI3K) and is considered as the only relevant catalytic subunit in the context of cancer associated mutations, was found mutated in only 1.4% and 8% of pNENs, respectively 128, 129. Data about PI3K-p85α subunit mutation nor PI3K amplification in NENs have not been published to date.
The regulatory impact of PI3K could be validated by preclinical studies with PI3K inhibitors. LY294002, a quercetin analogue and PI3K inhibitor, decreased cell proliferation in non-gastrointestinal neuroendocrine cell lines when applied as single agent or combined with rapamycin 130, 131. Studies with LY294002 treatment of rat-derived GEP-NEN cell lines propose an inhibitory effect of LY294002 on the VEGF secretion by neoplastic endocrine cells 132. The mTORC2-PI3K-mediated activation of the ERK cascade during mTOR inhibition of NENs was demonstrated through stimulation of human neuroendocrine BON (pNEN), GOT-1 (ileal NEN), KRJ-I (ileal NEN), H-STS (hepatic metastasis of ileal NEN) and NCI-H727 (bronchial carcinoid) cell lines with single and dual inhibitors 133-135. Previous studies on BON cells have demonstrated that LY294002 blocks the constitutive activation of PI3K and ERKs, respectively. PI3K, but not the ERK cascade, regulates expression of cyclin D1 and p27kip1, induced by an autocrine IGF-I loop, in BON cells 136. Not least, PI3K signaling is negatively involved in NE secretion, as demonstrated by PI3K subunit p110α-inhibition in vitro. Li et al. demonstrated that inhibition of p110α increases neurotensin granule trafficking by up-regulating α-tubulin acetylation and regulating Ras-related protein Rab27A in BON and QGP-1 cells 137.
A vast number of agents and inhibitors interfering with PI3 kinases and upstream receptors have been developed so far and are currently in different stages of clinical testing (refer to Supplementary Material: suppl. 3). The majority have not been assessed in GEP-NENs thus far.
The physiological inhibition and termination of PI3K signaling by degradation of PI(3,4,5)P3 is mediated by two major types of phosphatases. The SH2 domain-containing inositol phosphatases SHIP1 and SHIP2 dephosphorylate position 5 of the inositol ring and produce PI (3,4)P2. The loss of SHIP2 results in a significant increase of insulin sensitivity, indicating that this phosphatase is a crucial regulator of PI3K signaling downstream of insulin 138.
In summary, PI3 Kinases show up to be rarely mutated in GEP-NENs but contribute to several feedback loops as signal transduction mediators. They are therefore prominent targets for dual and combined targeting therapy approaches but their importance for GEP-NEN disease remains to be further explored in clinical and in vivo contexts.
The tumor suppressor PTEN
Another crucial phosphatase and tumor suppressor protein involved in growth factor signaling is PTEN (phosphatase and tensin homologue). After recruitment from cytosol to plasma membrane, PTEN dephosphorylates position 3 of PI(3,4,5)P3 and produces PI(4,5)P2. Loss of PTEN is frequently observed in a wide variety of human cancers, with the highest incidence found in endometrium, central nervous system, skin, and prostate cancers 139, 140. Beyond its function as upstream regulator of PI3K signaling, loss of PTEN was linked to occurrence of metastases and is regarded as critical marker for therapy resistance and sensitivity towards mTOR inhibition 139, 141-144.
The activity of PTEN is lost through diverse mechanisms in many different entities of cancer, but the majority of PTEN mutations induce truncations of the protein. PTEN is often mutated in tumor-prone germ line diseases and in cancer-associated somatic mutations 145-147.
The impact of PTEN towards cellular integrity is not limited to its cytoplasm-located lipid phosphatase activity. PTEN is localized in the nucleus under various conditions, such as cell differentiation and cell cycle arrest under stress and apoptotic stimuli, e.g. by regulating the APC/C (anaphase-promoting complex/cyclosome) 148-152.
Nuclear localization has been linked to maintenance of chromosome stability. The absence of nuclear PTEN is thus linked to tumor aggression, which suggests that the nuclear localization of PTEN is tumor-suppressive 148, 153-155. PTEN also influences cytoskeletal remodeling processes and thereby controls cell size, cell invasion and migration 156, 157.
Interesting work with regard to the sequestration of PTEN was published by Putz and colleagues in 2012. They demonstrated that mono-ubiquitinated PTEN can be exosomally trafficked between cells. In target cells, internalized PTEN had functional activity, which led to a reduction in the abundance of pAkt and a decrease in the extent of target cell proliferation 158. These findings disclose new insight into tumor-stroma interactions and highlight the unlimited influence of tumor-associated cells on tumor cell signaling.
In analogy to many other cancers, PTEN is frequently affected in pancreatic neuroendocrine neoplasms (pNENs). PTEN gene mutations are rare events in 7 and 9% of the cases respectively, but reduced PTEN expression is a frequently observed phenomenon in sporadic pNENs. Allelic loss of heterozygosity of PTEN, which is located at 10q23.3, was identified in one-third of sporadic pNENs. In 25% of pNENs a somatic deletion of 10q occurs 33, 128, 154, 159-162.
In nonfunctioning endocrine tumors of the pancreas alterations in microRNA expression have been related to endocrine and acinar neoplastic transformation and progression of malignancy. Assuming that MicroRNA-21 regulates the expression of PTEN in hepatocellular cancer, this scenario is as well discussed as a potential inhibitory mechanism of PTEN down-regulation in pNENs 163-165.
In primary pNENs, low expression of PTEN correlates with advanced WHO phenotype and higher proliferation index. Moreover, hyper-phosphorylation of mTOR is associated with significantly lower 5-year overall survival 166.
Several studies have demonstrated that the subcellular localization of PTEN is altered in both sporadic and VHL-associated pNENs, although there are contradictory conclusions whether cytoplasmic or nuclear localization is sufficient to predict therapy outcome or whether an increased or decreased nuclear to cytoplasmatic ratio is associated with worse prognosis 33, 154, 167, 168.
In vitro data concerning the response of neuroendocrine pancreatic (BON) and insulinoma (CM) cell lines towards triciribine inhibition suggest that PTEN might also influence the sensitivity of pNENs cells to Akt inhibition 169-171.
In general, in GEP-NENs the expression of PTEN was found to correlate with response to streptozotocin-based cytostatic therapy and to systemic therapy with streptozotocin and doxorubicin 168. As observed in the pNENs subclass study, PTEN expression in overall GEP-NENs was prevalently effected in tumors with lower differentiation in contrast to those with well differentiated phenotype 172.
PTEN might therefore serve as therapy response and malignancy marker, especially in combination with TSC2 expression analyses (see below). The mechanisms that lead to the down regulation of PTEN remain almost unexplored.
The role of Akt
The Akt family of serine/threonine kinases (Akt1/2/3, also known as protein kinase B, PKBα/β/γ) is the key mediator of PI3K signaling and connector to several interrelated pathways. It is involved in the majority of cellular processes, such as protein synthesis and cell growth, survival, proliferation and metabolism. Presuming that these processes constitute the backbone of cancer development and progression 173, Akt isoforms represent a highly prominent target for GEP-NET therapy research and for drug development in general.
The most prevalent member of the Akt family of protein kinases is Akt1, which is implicated in cell growth and survival. Akt2 is predominantly expressed in muscle and adipocytes and is in involved in the insulin-mediated regulation of glucose homeostasis. The distribution of Akt3 is almost limited to testes and brain 174-178.
Full Akt activation depends on the concomitant phosphorylation of two distinct sites that can be activated independently: the PDK-1-catalyzed T308 phosphorylation inside of the activation loop serves as readout of PI3K activation. In contrast, the phosphorylation of S473 in the hydrophobic motif of the C-terminal tail indicates a mTORC2 to Akt feedback signaling activity or is induced by PIKK (PI3 kinase-related kinase) superfamily or DNA-PK. Activity of Akt is detected by a fivefold increase upon S473 phosphorylation 179-184. Furthermore the role of the Akt phosphorylation site is not limited to activation enhancement, but also required for target specification. One of the most prevalent downstream targets of Akt T308 is TSC2 (tuberous sclerosis complex 2). Akt mediated phosphorylation of the TSC2 subunit hinders TSC1/2 complex formation and activates the GAP function of TSC2 toward the small GTPase Rheb. Hereupon Rheb activates mTORC1 at S2448, leading to the phosphorylation of the downstream effectors 4EBP1 (Eukaryotic translation initiation factor 4E-binding protein 1) and p70S6K (70 kDa ribosomal protein S6 kinase 1, also termed S6K) 185. Phospho-(T308)-Akt mediated PI3K signaling also determines cellular metabolism, cell cycle progression and insulin signaling by affecting the subcellular localization of GSK3 (glycogen synthase kinase 3) 125, 186-189. Interestingly, in vitro and in vivo studies on deletion of Rictor, mSIN1 (stress-activated map kinase interacting protein 1) or mLST8 (mammalian lethal with SEC13 protein 8) suggest, that the inhibition of mTORC2 mediated S473 phosphorylation selectively affects Akt substrates FOXO1 and FOXO3a (O-Family Forkheadbox proteins), with little effect on Akt substrates GSK3 and TSC2 183, 190. The discrepancy between S473 phosphorylation and resulting Akt activity indicates that S473 phosphorylation might not be the major regulator of Akt activity 191.
Nevertheless, activated Akt promotes survival signaling by inhibition of pro-apoptotic proteins, such as BIM (Bcl-2 interacting mediator of cell death) and BAD (BCL2-associated agonist of cell death) by phosphorylation, leading to their sequestration and degradation. Akt also inhibits the expression of pro-apoptotic genes and cell cycle inhibitors such as p21cip1 and p27kip1 by targeting their transcription factors and stimulates cyclin D1- and c-Myc-controlled cell cycle progression by inhibiting their negative regulators, e.g. FOXOs and GSK3.
Relatively few studies are available on Akt phosphorylation and expression in GEP-NENs. Weak expression of Akt or presence of the PI3-signaling antagonist PTEN was associated with response to systemic chemotherapy and Akt overexpression has been correlated to shorter median survival rates for patients with well-differentiated and poorly differentiated tumors 168.
Shah and colleagues demonstrated a phospho-S473 activation of Akt in 76% of 98 differently graded NEN-tissue samples, indicating that activation of Akt may contribute to GEP-NEN tumorigenesis. Unfortunately the data failed to correlate Akt S473 phosphorylation to tumor grade by multi-variant statistical analysis 6. These findings were supported by further immunohistochemical analysis of enteropancreatic NENs respecting Akt S473 phosphorylation. In a subsequent study S473-activated Akt was found in 61 % of the tissues, but also failed to correlate with tumor grade, tumor size or the presence of metastases 192.
Nevertheless, NEN patients treated with everolimus and octreotide in an open-label phase II trial (NCT00113360) showed a significant longer PFS in the case of T308-phosphorylated Akt in pretreatment and on-treatment tumor biopsies. Even partial responders were more likely to have an increased Akt T308 phosphorylation in comparison to non-responders. Therefore, although baseline Akt activation is associated with a more aggressive clinical course, increase of Akt activation has been considered a predictor of rapamycin response. These data have been supported by several preclinical studies on rapamycin treated cell lines of various offspring in vitro and xenografted 193-195. In vitro experiments on neuroendocrine cell lines have furthermore evaluated an isoform specific impact of Akt on NEN cancerogenesis that might be important for therapeutic approaches. Only the directed down-regulation of Akt1 and Akt3 decreased the phosphorylation of classical Akt downstream targets such as GSK3α/β, MDM2 and p70S6K and suppressed cell viability. Inhibition and knockdown of Akt1 and Akt3 resulted in decreased ERK1/2 phosphorylation and Akt3 ablation induced apoptosis in BON cells, indicating that this isoform is particularly relevant to neuroendocrine cell survival. Akt1 and Akt3 seem to be important for NEN cell viability while Akt2 may have antitumor activity as demonstrated by pan and isoform-selective knockdown of Akt. The data suggest a particular role for these Akt isoforms in NEN activity 196.
Several specific inhibitors of pan-Akt, Akt isoforms and PDK-1, an Akt regulator, are commercially available. Only a few of them are already under clinical and preclinical investigation in GEP-NENs (refer to Supplementary Material: suppl. 4).
In conclusion, the Akt proteins serve as crucial feedback mediators within the growth factor response network. Nevertheless, the sole focus on pan-Akt S473 phosphorylation might not suffice to understand the complex interplay between various isoforms, phosphorylation sites and scaffold regulators. Akt activation and subtype distribution has a high potential to predict therapy response, but the importance of the specific patterns in vitro and in clinical settings remains to be elucidated.
mTOR complexes and feedback activation
The most important downstream mediator of PI3K-Akt signaling is the serine/threonine kinase mTOR mammalian target of rapamycin. It is encoded by the FRAP 1 gene and contributes to a multitude of cancer associated cellular processes. It forms two distinct protein complexes: mTORC1 and mTORC2. Whereas mTORC1 is sensitive to rapalogues such as rapamycin, everolimus and temsirolimus, mTORC2 is considered resistant to rapamycin and insensitive to nutrient signals 197.
The mTOR complex 1 is formed by the mTOR protein itself, associated with RAPTOR (Regulatory associated protein of mTOR), and two negative regulators, PRAS40 (proline rich Akt substrate 40 kDa) and DEPTOR (DEP domain containing mTOR interacting protein) 198-201. RAPTOR functions as a scaffold and positively modulates the mTOR kinase reaction towards the mTORC1 key targets 4EBP1 and p70S6K in vivo 198. The second subunit of the mTORC1 complex, mLST8, is considered to bind to the kinase domain of mTOR and to regulate its kinase activity positively. It is also considered to maintain the interaction between mTOR and either RAPTOR or RICTOR (Rapamycin-insensitive companion of mTOR), which is part of the mTORC2 complex, and thus, to be important for shuttling mTOR between the two complexes and for sustaining the intracellular equilibrium of mTORC1 and mTORC2 183, 202, 203. Whereas PRAS40 inhibits the mTORC1 activity via raptor, DEPTOR was identified to interact directly with mTOR in both mTORC1 and mTORC2 complexes. However, DEPTOR overexpression leads to decreased p70S6K phosphorylation and to mTORC2-mediated signaling back to Akt, monitored by S473 phosphorylation 201, 204.
PI3K-mTORC1 signaling controls the transcription of many genes, some of which are involved in metabolic pathways and regulate nutrient-responsive transcription programs 205-208. Its major downstream effectors, including p70S6K and 4EBP1 are regulated by phosphorylation 209. Subsequently, activated p70S6K phosphorylates S6 (40S ribosomal protein S6) and other ribosomal proteins and elongation factors and therefore enhances the translation of mRNAs and promotes proteins synthesis 210. Although p70S6K can also be activated by mTOR independent pathways such as PDK-1, MAPK and SAPK (stress-activated protein kinase), the mTORC1-mediated phosphorylation at T389 is required for its complete activation 211.
Hypo-phosphorylated 4EBP1 inhibits the initiation of protein translation by binding and inactivating eIF4E (eukaryotic translation initiation factor 4E). Phosphorylation of 4EBP1 by mTORC1 promotes dissociation from eIF4E and thereby facilitates eIF4E-dependent translation initiation 212.
Furthermore, mTORC1 controls the activity of many other proteins, such as ODC (ornithine decarboxylase), glycogen synthase, HIF-1α (hypoxia-inducible factor 1α), eEF2 kinase (eukaryotic elongation factor 2 kinase), PKCδ and PKCɛ (protein kinases C delta and epsilon), PP2A (protein phosphatase 2A), p21cip1 and p27Kip1 cyclin-dependent kinase inhibitors and STAT3 (signal transducer and activator of transcription 3) 213-223. Accordingly, the impact of the mTORC1 complex spans a multitude of cellular processes that modulate cellular behavior in response to local circumstances and links availability of growth factors, nutrients and energy to cell growth, survival, proliferation, angiogenesis and motility.
The rapamycin-insensitive mTOR Complex 2 consists of mTOR mLST8, mSin1, PROTOR1/PRR5 and 5 PROTOR2/PRR5L (proline-rich protein), HSP70 (heat shock 70 kDa protein) and DEPTOR.
The mSin1 protein contains a Ras binding domain and a PH-domain, which allows localizing the protein near the plasma membrane. It is therefore considered to be important for the assembly and dynamic localization of mTORC2 and to the phosphorylation of Akt at S473. HSP70 assures proper assembly of the protein complex under physiological conditions and following heat shock 190, 224-227. HSP70, which has various cellular functions beyond maintaining proper protein structures, has been found to exist in a neuroendocrine tumor specific truncated isoform in NENs. The authors conclude, that the altered HSP70 isoform equilibrium might contribute to apoptosis inhibition or might be based on similarities with neuronal cells, as protein folding and protection against aggregate formation is of particular importance in the nervous system 228.
The mTOR complex 2 is activated by growth factors, G protein-coupled receptor ligands and cytokines. It phosphorylates PKC-α and paxillin (a focal adhesion-associated adaptor protein) and regulates the activity of the small GTPases Rac and Rho which control motility, invasion and cytoskeletal assembly. Beside motility aspects that play distinct roles in metastasis, the Akt S473 feedback activation, that is mediated by mTORC2 following mTORC1 inhibition, is one of the crucial mechanisms for PI3K cancer signaling 181, 229-231.
In summary, two major feedback loops control PI3K-signaling, which could potentially impact therapy approaches, especially with regard to monotherapy (refer to figure 1): Activation of p70S6K by mTORC1 causes feedback inhibition of IGF-1/insulin signaling by phosphorylating IRS-1 (insulin receptor substrate 1), causing IRS-1 degradation, and leads to decreased PI3K signaling and reduced Akt T308 phosphorylation. Reciprocally, rapalogue-induced inhibition of mTORC1 consequently inhibits p70S6K phosphorylation, but relieves this feedback and induces Akt T308 re-phosphorylation and thus increased mTORC2 activation. Subsequent Akt S473 phosphorylation follows in an mTORC2-dependent manner, which attenuates the therapeutic effects of rapalogues in tumors, in model systems and in patients as well 204, 232, 233. This feedback activation could be obviated in serum free in vitro conditions, due to the known requirement for growth factors for mTORC1 to PI3K feedback loops 204. For some cancer entities it is evident that even inhibition of both, mTORC1 and mTORC2, e.g. by ATP-competitive mTOR kinase inhibitor AZD8055, did not result in a persistent inhibition of PI3K signaling: although sustained inhibition of mTORC2 activity and AKT S473 phosphorylation was detectable, a distinct activation of RTK signaling occurred, which induced PI3K signaling and reinduction of T308 phosphorylation 234.
Figure 1.
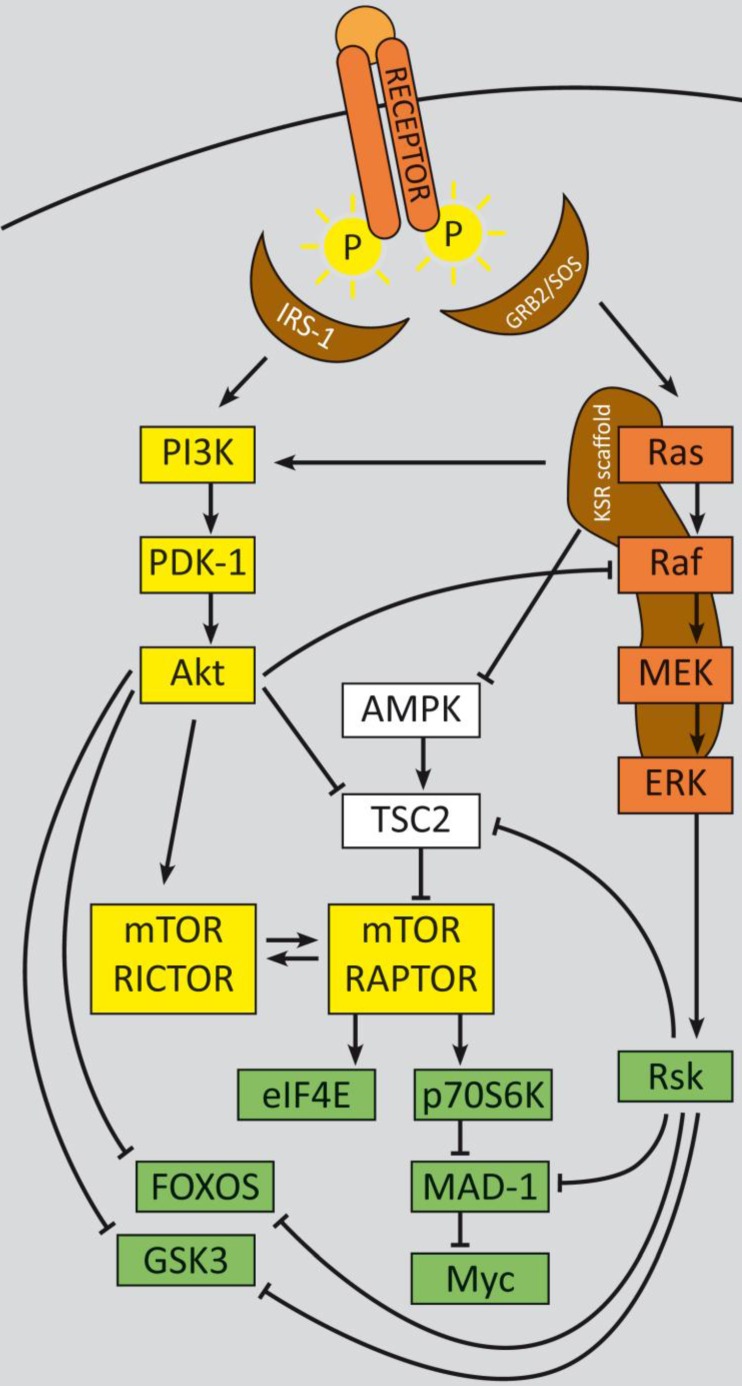
PI3K signaling feedbacks: PI3K signaling is highly activated and de-regulated in GEP‑NENs. Several feedback loops contribute to this effect 330, 331. RTK activation by ligand binding triggers a phosphorylation cascade onto IRS-1 and PI3K. The latter phosphorylates PIP2 and thereby activates PDK-1 and Akt at T308. Akt in turn inhibits TSC2 by phosphorylation, which leads to mTOR activation. The mTOR complex 1 activates translation via eIF4A and p70S6K and cell cycle progression, whereas mTORC2 relieves a feedback loop by fully-activating Akt at S273. S6K promotes a negative feedback onto IRS‑1 and thereby counteracts mTORC1 inhibition. The two major negative regulators, PTEN and TSC2 are frequently down regulated in GEP-NENs which lead to a highly deregulated Akt activation 33, 128.
Expression and activation level of mTOR in GEP-NENs has been analyzed in a vast number of studies and experiments but mainly failed to correlate to clinical outcome in a clear statistically significant matter. Nevertheless, a distinct tendency of high malignant NEN to show highly activated mTOR expression was obvious. Catena and colleagues demonstrated that mTOR was expressed in the majority (80%) of poorly differentiated neuroendocrine carcinoma patients (WHO 2000 classification) however with no relationship to tumor origin or proliferation rate determined by MIB-1 (Ki-67 antibody for paraffin-embedded tissue specimens) 235. Another immunhistochemical analysis detected high levels of S2448 phosphorylated mTOR in 67% of poorly differentiated neuroendocrine carcinomas (including positive staining of all large cell neuroendocrine carcinomas) in contrast to 27% mTOR activation in well-differentiated tumors and carcinomas (WHO 2000 classification). Further statistical validation failed due to the low number of analyzed patients 236.
Significant correlation of mTOR expression with primary tumor location and metastatic status in GEP-NENs could be demonstrated by Kasajima and colleagues. Expression of mTOR, 4EBP4 and phosphorylated p70S6K was found to be higher in foregut than in midgut tumors. Furthermore, higher proliferation indices (indicated by Ki-67) were associated with significantly higher mTOR and 4EBP1 expression, as well as with higher activation of the mTOR targets 4EBP1, p70S6K and eIF4E (indicated by phosphorylation) 237.
One of the major regulatory proteins of mTOR activation, TSC2, is frequently down-regulated in pNENs and correlates with worse prognosis concerning overall survival, time to progression and disease-free survival. Low levels of TSC2 significantly correlate with functional tumor status and aggressiveness 33. Although neither TSC2 nor PTEN were significant independent prognostic indicators regarding occurrence of metastases in a multivariate analysis by Missiaglia and colleagues, conjoint low levels of PTEN and TSC2 are assumed to be related to liver metastasis and might predict response to PI3K-pathway inhibitors. While somatic mutation of TSC2 and PTEN was found in only less than 10% of pNENs, 85% of primary pNENs showed altered protein levels of TSC2, PTEN or both. 33, 128.
Germline mutations in TSC1 and TSC2 tumor suppressor genes, resulting in the activation of the mTORC1 pathway, have been described in patients with tuberous sclerosis complex. TSC is an autosomal dominant genetic disorder and most of the occurring neoplasms are benign with an early onset 238. A study on 219 TSC patients revealed that the incidence of pNENs was 1.8% as compared to the rate of 0,002% in the general population. Therefore it seems likely that pNENs are a dominant pancreatic pathology in the setting of TSC and aberrant TSC1 and TSC2 proteins may play a crucial role in the development of pancreatic neuroendocrine lesions 238, 239, 114, 240-242.
In summary, several studies have highlighted the importance of high mTOR expression and activation, activation of its targets as well as the functional impact of the low expression of its direct negative regulator, TSC2. Relevant somatic mutations that influence the expression of either TSC2 or mTOR have not been detected thus far. Additional regulatory mechanisms such as transcriptional or post-transcriptional gene silencing might be involved in their regulation and require further investigation.
Inhibition of mTOR as therapeutic strategy
Analyses of mTOR aberrations have furnished the rationale for use of inhibitors of mTOR and its downstream targets (refer to Supplementary Material: suppl. 5) 33, 165: The mTORC1 inhibitor and rapalogue everolimus (RAD001, Afinitor®) is the most clinically advanced and the furthest developed target directed therapy in GEP-NENs. It was approved by the FDA for advanced well-differentiated pNENs in 2011.
Studies on genetic determinants of rapalogue response have demonstrated that drug-resistant cells (such as HT-29, HCT116, and DLD-1) carried mutations in both PIK3CA and KRAS/BRAF. Everolimus-sensitive cells displayed PI3K pathway alterations but no mutations in the KRAS/BRAF genes, rendering neuroendocrine tumors a favorable target for everolimus treatment 239. This effect is assumed to be dependent on mTOR-Ras/Raf crosstalk and its outcome depends on Raf mutational status (paradoxical activation, see below).
Nevertheless in preclinical studies of everolimus, inhibition of mTORC1 in neuroendocrine cell lines was demonstrated to induce growth inhibition and induction of apoptosis 240. However it also led to a global upregulation of upstream PI3K signaling and to cross-activation of Ras/Raf/Erk signaling via mTORC1-p70S6K-IRS-1 mediated negative feedback loops. This cross-activation resulted in upregulation of VEGF secretion, through a raise of NF-κB (nuclear factor-κB)-mediated VEGF expression and HIF-1α induction 134, 135, 195. In vivo, Rapamycin monotherapy was notably efficacious in pNEN bearing transgenic mice and prolonged survival concomitant with stable disease. Nevertheless, the tumors developed resistance 241. Preclinical xenograft studies on mouse cells that mimic PDNEC revealed a significant deduction of tumor mass and Ki-67 in response to everolimus treatment, suggesting further exploration in poorly differentiated neuroendocrine cancers, that particularly lack therapy options to date 242.
Three human phase II or III studies have been conducted, including 600 patients with advanced pNENs 114, 243, 244. The response rate as assessed by conventional Response Evaluation Criteria in Solid Tumors (RECIST) criteria has been shown to be very low; however, everolimus significantly affected progression-free survival. The RADIANT-3 clinical study, which led to FDA approval, resulted in a stable disease of 73% and 51% in the everolimus and placebo arms respectively, rather than a partial response with 5% and 2%.
Concluding, everolimus delayed tumor progression without changing the pattern of progression among patients with advanced pNENs 244, 245. Recent therapeutic approaches have therefore focused on inhibitors of alternative components of the PI3K pathway, dual target inhibitors and effective combinatory bio/chemotherapies.
Outlook: PI3 signaling in diagnosis and therapy
The multiple results of PI3K signaling analyzes in GEP-NENs are summarized in table 3. Noticeably, with few exceptions, no study detected serious activating mutations of PI3K-pathway mediators. In contrast to other cancer entities, where activating mutations in receptors or the PI3 kinase itself are frequent, GEP-NENs thus lack appropriate druggable targets. Nevertheless, a high level of deregulation (as indicated by extensive activation of kinases) triggers proliferation, neoangiogenesis and a secretory phenotype. The complex interactions within the autoregulatory network bypass the current therapeutic approaches. It is therefore all the more important to identify bottle neck factors that might not have been recognized as key players to date. Evaluating the role of regulator non-coding RNAs might also answer some of the questions, why the neuroendocrine growth factors pathways are deregulated to that high extent although almost no appreciable mutations could be detected responsible to date. Furthermore, the tumor stroma and tumor-associated cells also contribute to a growth factor-saturated microenvironment in general. Studying their importance for GEP-NEN cancerogenesis and metastasis might also reveal a high potential of prognostic factors and therapeutic interventions.
Table 3.
Summary of studies that analyzed the role of PI3K signaling in GEP-NENs.
| Study results | Reference |
|---|---|
| Expression of multiple growth factors is very high in GEP-NENs | 2-16. |
| Expression of IGF-1 and IGF-1R is elevated in GEP-NENs | 14, 18-26 |
| Elevated copy number of the EGFR and HER-2/neu loci might contribute to high receptor expression | 21, 246 |
| EGFR expression is high in GEP-NENs and correlates with metastasis in pNENs | 6, 9, 30, 31 |
| PIK3CA mutations are rare events | 128, 129. |
| PI3K signaling stimulates the ERK pathway of NE cells in vitro | 133-135 |
| PI3K signaling triggers secretion in vitro | 132, 136, 137 |
| PTEN mutations are rare events, but LOH may contribute to malignant transformation in (pancreatic) NENs |
33, 128, 154, 161, 162 |
| Low PTEN expression correlates with prognosis and response to therapy | 166, 168, 169, 172 |
| Subcellular localization of PTEN might serve as prognosis marker | 33, 154, 167, 168 |
| Akt overexpression associated with shorter median survival in with well- and poorly differentiated tumors | 168 |
| Phospho-T308 Akt might serve as marker of rapamycin response | 193-195 |
| Phospho-S473 Akt might not serve as a valuable marker | 6, 192 |
| Subtype specific activity: Akt1 and Akt3 pro-survival; Akt2 pro-apoptotic | 196 |
| Expression and activation of mTOR, 4EBP4 and p70S6K is associated with higher proliferation index and might correlate with low differentiation | 235-237 |
| Low level of TSC2 is correlated with tumor status and aggressiveness | 33, 128 |
The MAPK pathways promotes growth and cancer progression as well as feedback bypasses to PI3K signaling
The MAPK cascades
Alteration of the Ras-MAPK cascades has frequently been described in human cancer. These pathways comprise several kinases that transmit extracellular signals from growth factors, chemokines and ECM signals and regulate cell growth, differentiation, proliferation, apoptosis and migration. They are classified in several interconnected branches constructed of functional analogues of MAPKs (mitogen-activated kinases), their MAPK kinases and the latter's MAPKK kinases. The four most important branches are (1) the ERK/MAPK, (2) the JNK (c-Jun amino-terminal kinase)/SAPK pathway, (3) the p38 pathway and (4) the BMK (big mitogen-activated protein kinase)/ERK 5 pathway 247. We will therefore focus on the classical ERK/MAPK cascade and glance to other important members.
The classical cascade is induced by ligand binding to RTKs, such as VEGFR or EGFR, leading to its dimerization and auto-phosphorylation of the intracellular c-terminal region. Thereby binding sites for adaptor proteins are generated that in turn recruit GEFs (guanine nucleotide exchange factors) to the plasma membrane. GEFs facilitate the binding of GTP to Ras proteins. GTP-bound Ras recruits Raf kinases and activates the latter's serine/threonine kinase function to phosphorylate MEK and induce the phosphorylation of ERK effector kinases 248.
Abnormal activation of receptor tyrosine kinases or gain-of-function mutations in the RAS and BRAF genes have been frequently identified in a vast number of cancers. Multiple cross links and feedback loops to other mitogen pathways induce therapy resistance and bypass inhibitory approaches. The MAPK pathway contributes to neuroendocrine cancerogenesis, although many aspects in GEP-NENs remain to be explored.
The role of Ras
The Ras superfamily is divided into five main families which in total comprise more than 150 members in humans: Ras, Rho, Rab, Arf, and Ran. The Ras family members are the most intensively studied. Three human RAS genes encode four Ras protein isoforms, designated as H-Ras, N‑Ras, K-Ras4A and K-Ras4B. Ras proteins exhibit GTPase function and a structure that is related to the Gα subunit of heterotrimeric G proteins. G proteins are molecular switches that oscillate from inactive GDP-bound to active GTP-bound states 249. The cyclic process of GDP/GTP is facilitated by GEF and GAP (GTPase activating proteins) classes of regulatory proteins that build a three-protein-complex with Ras.
The Ras proteins are anchored in close proximity to adaptor proteins such as Grb2 (growth factor receptor bound protein 2) and GEFs in the plasma membrane by farnesylation. The SOS family (son of sevenless) of RasGEFs, facilitates the exchange of Ras bound GDP with GTP and thereby activates Ras by conformational change 250.
Activating point mutations of the three Ras family genes are common in human cancers (30%) with a very high incidence in pancreatic adenocarcinoma, colorectal and lung cancers. Mutations in other Ras superfamily GTPases are rare and thus, their hyper-activation requires alternative induction mechanisms 251-255. Deregulation of their regulators is a common event.
Aberrant signaling from growth factor receptors, in particular, RTKs and GPCRs (G protein-coupled receptors), or up-regulated gene expression can lead to aberrant GEF regulation and thus to enhanced activity of small GTPase proteins of the Ras superfamily.
GAPs, which return the GTPase to its GDP-bound inactive state, have been shown to exert crucial roles in curtailing GTPase activity in cancer. Since activation of Ras superfamily GEFs has been frequently described in human cancers, loss of GAP activity permits uncontrolled GTPase activity and can promote cancer 256-262.
In contrast to other entities such as pulmonary neuroendocrine carcinomas, mutations in the Ras genes are uncommon and rarely documented in GEP-NENs. Early studies could demonstrate that Ras mutations are virtually absent in gastroenteropancreatic neuroendocrine neoplasms 21, 263-265. H‑Ras and K‑Ras expression could be detected in 65% and 10%, respectively. However, further information regarding its activity has not been generated to date 266, but much more comprehensive data is available for the Ras downstream targets, especially for B-Raf.
Wild type Raf and the importance of paradoxical activation
Activation of Raf family members (A-Raf, B-Raf and C-Raf or Raf-1) is initiated by binding of their Ras binding domain to Ras‑GTP and release from a 14-3-3 dimer bound to the N-terminal phosphorylation site. Concomitant conformational changes stimulate their serine/threonine kinase activity, dimerization and trigger sequential phosphorylation and activation of their targets MEK and ERK 267, 268.
B-Raf is the family member most easily activated by Ras, since both A-Raf and C-Raf need additional steps, such as phosphorylation of activating residues and dephosphorylation of negative regulatory residues, to reach maximal activation 269, 270. Furthermore the kinase activity of B-Raf is higher than those of the other family members 271.
Recent studies discovered that besides its involvement in oncogenic Ras signaling, BRAF itself is also mutated at a high frequency in human cancers, especially in melanoma (30-60%), thyroid cancer (30-50%) and ovarian cancer (~30%) 272. A very common BRAF mutation, B-RAFV600E, is involved in the expression of hypoxia-inducible factor-1α and VEGF and thus contributes to neoangiogenesis in those entities 273-275.
Overexpression or gain of function mutation of full-length Raf (or the truncated catalytic domain) leads to the activation of the ERK pathway and increases proliferation and tumor growth. Although there is no evidence that Raf activation participates in senescence evasion to date, it has been demonstrated to retrain apoptosis by regulating the expression and/or the activity of Bcl-2 family members 276.
Activated Raf is also involved in EMT (epithelial to mesenchymal transition), invasion and metastasis by promoting the production of TGFβ. Additionally, both B-Raf and C-Raf antagonistically control cell contractility and migration: B-Raf increases Rho-dependent contractility and opposes migration in an ERK-dependent manner, whereas C-Raf reduces contractility and increases migration by interfering with the activity of the cytoskeleton-based Rho effector ROCK2 (Rok-α) 277-284.
The C-Raf protein appears as part of a multiprotein complex composed of HSP90, p50, and several scaffold proteins, such as 14-3-3. This complex is required for controlling its stability and activation status as well as its activity 285-287. The phosphorylation state of C-Raf is influenced by multiple further protein kinases, including Src, PKC (protein kinase C) family members, the p21cip1-activated protein kinase PAK, and Akt 288-291.
In the case of the ubiquitous C-Raf, other targets potentially contributing to tumor progression have been identified such as the NF-κB, Rb and BAD 292-294. C-Raf also contributes to genomic instability. Loss of RKIP (Raf kinase inhibitor protein) or C-Raf overexpression lowers the activity of the Aurora-B kinase, allowing cells to bypass the spindle assembly checkpoint 295.
The serine/threonine phosphorylation of MEK proteins as an intermediate step of the MAPK cascade features two special objectives: to enhance the cooperativity of activation of the MAPK and to allow modulation by other signaling events. In the case of the ERK1/2 pathway, amplification occurs at the Raf-MEK step, because MEK1 is much more abundant than Raf. Another controlling feature of this step in the MAPK cascade depends on the dual phosphorylation of the MAPK by MEK. The tyrosine residues of ERK1/2 are phosphorylated with a higher affinity than threonine, leading to a nonprocessive phosphorylation and to the establishment of a threshold. The tyrosine phosphorylated proteins remain in an inactive state and accumulate until the threshold is reached. Subsequently the kinases are rapidly converted to the active state by threonine phosphorylation 296-303.
MEK mutations are rare events in human cancers with an incidence of 3% in melanomas and 2% in colon carcinomas 304.
The data concerning Raf and MEK downstream signaling and the discussion whether raf inhibition is reasonable or not is very contradictory and is still in the focus of preclinical investigation. Several further inhibitors of Raf and MEK are under preclinical assessment in GEP-NENs however with not very promising results to date (refer to Supplementary Material: suppl. 6).
Genetic analyses demonstrated that B-Raf mutations are rare in GEP-NENs 263, 305-307, with the exception of colorectal NENs, which have been demonstrated to harbor ~ 60% KRAS and BRAF mutations, but presumably due to its high content of adenoma and/or an adenocarcinoma cells 308.
Nevertheless the small GTPase Rap1 and B-Raf are highly expressed in GEP-NEN specimens, and both contribute to ERK1/2 and E26-like kinase (Elk-1) activation in NE cell lines 309. Disrupting Raf-MEK-Erk signaling by the B-Raf inhibitor Raf265 significantly decreased Bcl-2 level and sensitized to TRAIL signaling in neuroendocrine cell lines. Raf265 inhibited Erk1/2 phosphorylation but in turn induced Akt phosphorylation and VEGF secretion, suggesting the existence of a compensatory feedback loop onto PI3K-Akt signaling 135, 310, 311.
In vitro experiments demonstrated the crucial functional role of another Raf isoform: C-Raf activating retroviral transduction of BON cells resulted in high levels of phosphorylated ERK1/2 as well and caused remarkable morphologic and functional changes, such as reductions in 5-HT (5-hydroxytryptamine, the neurotransmitter serotonin), chromogranin A (marker of large dense core vesicles), and synaptophysin (marker of small synaptic vesicles) levels, which are strong neuroendocrine-related secretion markers. Accordingly, treatment of C-Raf-transducted BON cells with MEK inhibitors blocked morphological changes and hormone suppression but not ERK1/2 phosphorylation, indicating a dependency of NE dedifferentiation on MEK-mediated Raf to ERK signaling. Furthermore, activation of the C-Raf signaling cascade in BON cells resulted in significant decrease in cellular adhesion and migration in a FAK (focal adhesion kinase) dependent manner 312-314. Although a reduction of NE marker production, especially those of chromogranin A and synaptophysin, might be generally referred to worse differentiation in GEP-NENs in vitro and in vivo 315-317, cellular adhesion and migration are a prerequisite for invasion and metastatic dissemination and thus, markers of malignant phenotypes. Paradoxically, studies with several ATP-competitive C‑Raf activators could demonstrate, that wild type Raf activation can not only suppress the expression of chromogranin A, but also the proliferation via p21cip1 upregulation in p21 wild type NEN cells 318-321. These data conform with several publications that have proved an insensitivity of wild type B‑Raf cell lines from several cancer entities to ATP-competitive Raf inhibitors. On the other hand, the exposure to Raf inhibitors resulted in a dose-dependent and sustained paradox activation of mitogen-activated protein kinase signaling in cells and tumors with wild type B-Raf. These paradoxical effects of Raf inhibition were seen in various malignant and normal cells in vitro, xenografted and in vivo, leading to entry into the cell cycle, enhanced proliferation, and significantly stimulated tumor growth in vivo (refer to figure 2). Moreover, even in the clinical setting this paradoxical ERK-activation by the B-raf-Inhibitor Vemurafenib could be observed and led to a restriction on use of the drug 322. The mechanism for this paradox activation is assumed to depend on an inhibitor-induced C-Raf/C-Raf or B-Raf/C-Raf homo- and heterodimerization, respectively, following sustained upstream signaling via Ras or enhanced RTK activation such as via IGF-1R or HER2 20, 323-329.
Figure 2.
Paradoxical activation of Raf downstream signaling following treatment with ATP-competitive Raf inhibitors lead to an enhanced proliferation of B-Raf wild type cells in vitro and in vivo (adapted from Cichowski et al. 327): a) mutant B-Raf constitutively activates MAPK signaling in cancer cells; b) ATP-competitive inhibition of mutant B-Raf counteracts ERK activation and leads to tumor growth reduction; c) ATP-competitive wild type Raf inhibition in normal cells results in increased ERK activation and proliferation in vitro, and in hyperplasia in normal tissues of mice in vivo 323; d) and e) sustained RTK or Ras dependent upstream signaling activates MAPK signaling under ATP-competitive wild type Raf inhibition 323, 324, 330.
Assuming that ATP-competitive wild type Raf inhibition indeed enhances paradoxical mitogen ERK downstream signaling and C-Raf activation in GEP-NENs (under the prerequisite of wild type Ras) and thus inhibits the neuroendocrine phenotype 318, the p70S6K/PI3K/RAS crosstalk under rapamycin treatment 330, 331 might emerge as an desirable side effect. This might e.g. comprise the reduced secretion of the bioactive hormones and thus alleviate the symptoms of functional active neuroendocrine tumors, but better understanding requires further mechanistic exploration.
Cross-regulation of Raf by inhibition of the PI3K-Akt axis might lead to unexpected and (under certain circumstances) harmful therapeutic outcome. Although Raf mutations are rare events in GEP-NENs tumors, subgroup-specific assessment of Raf and Akt inhibitors might improve therapeutic precision.
The MAP Kinases ERK1/2 and their downstream effectors
ERK1/2 and other MAP kinases target a vast number of effector proteins, including membrane proteins, such as phospholipase A2, cytoplasmic proteins, such as downstream kinases and cytoskeletal proteins, and nuclear proteins, such as transcription factors. In response to cytokines, stress and chemotactic factors, the MAPK p38 and ERK2 regulate transcription by phosphorylation of MAPKAP kinase-2, which induces phosphorylation of the transcriptions factors CREB (c-AMP response element binding Protein) and ATF-1 (activating transcription factor) in cells and thus regulate the transcription of a large variety of genes.
Furthermore a recent publication demonstrated that p38-ATF/CREB signal transduction pathway can coordinately induce (promote transcription and RNA stability) and repress (promote RNA decay) transcript levels for distinct sets of genes, without shutting off transcription itself. This Stress-Activated RNA decay provides a mechanism to reduce the expression of target genes as it is required for cellular decisions in response to stress and other stimuli 332.
Ras responsive genes can be transcriptionally activated by ETS/AP-1 transcription factors. Hereby AP-1 comprises c-Jun, c-Fos and ATF-2 and controls the early transcriptional response to extracellular signals 333-340. The TCFs (ternary complex factors) are substrates of the MAPKs ERK1/2, JNK/SAPK and p38. These targets, such as Elk-1, mediate transcription of genes containing SREs (serum response elements) in their promoters 341-343.
ERK2 has been shown to phosphorylate SRC-1 (steroid receptor coactivator-1), which shows histone acetyltransferase activity and is a coactivator of steroid nuclear receptors. SRC-1 also interacts with CREB to enhance estrogen and progesterone receptor-mediated gene activation 344-346.
Not least, MAP kinase pathways are considered to phosphorylate STAT3 (signal transducers and activators of transcription) and thus stimulate cytokine production, induction of pro-angiogenetic factors and invasion in tumor cells 347-353.
MAPKs also contribute to chromatin remodeling, for instance by phosphorylating Rsk2 (ribosomal protein S6 kinase), which can subsequently phosphorylate histone H3, or by interacting with topoisomerase II. Further targets, Msk1 and 2 (mitogen and stress-activated protein kinase), are able to phosphorylate CREB and its co-factors, but also are very potent histone H3 and HMG-14 kinases 334, 354-360.
These mitogen effector functions of MAPK has put forward the development of several MAPK inhibitors in recent years (refer to Supplementary Material: suppl. 7). Few of those are under preclinical assessment for GEP-NEN therapy.
The activation of MAP Kinases in GEP-NENs is complex and due to multifunctional effects (such as differentiation and proliferation) not yet fully understood. Phosphorylated and thus activated ERK could be detected very frequently in an early immunohistochemically analysis of the MAPK pathway in GEP-NENs 263. These data were supported by a second study where 96% of the analyzed specimens were positive for phospho-ERK1/2 and ERK activation could be related to EGFR and Akt phosphorylation, suggesting a simultaneous induction of Akt and ERK mediated pathways in NENs under EGFR kinase activity 6. Consistently, in vitro treatment of neuroendocrine cell lines with EGFR or Raf inhibitors resulted in a time and dose-dependent dephosphorylation of ERK1/2 309, 361.
In summary, analogous to PI3K signaling, the MAPK pathway is highly activated but the triggering mechanisms remain unclear. It is highly involved in the generation of the NE phenotype as inhibition of MAPK signaling results in impaired secretion and migration (summarized in table 4).
Table 4.
Summary of study results concerning MAPK signaling in GEP-NENs.
| Study results | Reference |
|---|---|
| Ras is expressed in GEP-NENs but mutations are rare | 21, 263-266 |
| Raf mutations are rare events | 263, 305-307 |
| Rap1 and B-Raf are highly expressed in GEP-NEN | 309 |
| Raf inhibition triggers feedback activation of Akt | 135, 310, 311 |
| MAPK signaling triggers NE secretion and migration in vitro |
312-314 |
| Raf activation induces a paradox inhibition of proliferation and NE secretion | 318-321 |
| ERK activation is frequent in GEP-NENs | 6, 263, 309, 361 |
PI3K and MAPK signaling are highly interwoven pathways and cooperate in therapy resistance in GEP-NENs
Although both, the PI3K and the MAPK pathway can be activated by the same RTKs, the agonists only partially overlap, since e.g. insulin, and IGF-1 are weak Ras-ERK activators, but strong PI3K-mTORC1 activators. Nevertheless the response of a certain pathway to specific growth factors depends on the factor's abundance, the receptor status in relation to expression and localization and the expression level of pathway mediators as well as of required docking and scaffold proteins 362-364.
The PI3K pathway can be cross-activated upstream by direct Ras-GTP interaction with PI3 Kinases or downstream by ERK and Rsk mediated phosphorylation of TSC2 and RAPTOR. In the latter case, phosphorylation inhibits the GAP function of TSC and promotes mTORC1 activity 365-369.
Furthermore, studies have demonstrated that also the KSR (kinase suppressor of Ras) scaffold, which maintains the co-localization of RAF, MEK, and ERK during ERK activation, interacts with mTOR, RAPTOR, RICTOR, and the TSC2-activating kinases AMPK and GSK3 366, 370-372. Consequently both, the PI3K and the MAPK pathways frequently converge on the same downstream targets and promote cell survival, proliferation, metabolism, and motility (refer to figure 3). Prominent proteins that are regulated by both pathways are the FOXOs and the c-Myc early transcription factors, as well as BAD and GSK3. FOXOs suppress cell survival and proliferation and once phosphorylated they undergo nuclear export and ubiquitin-proteasome-mediated degradation, whereas c-Myc induces pro-survival genes 373-378.
Figure 3.
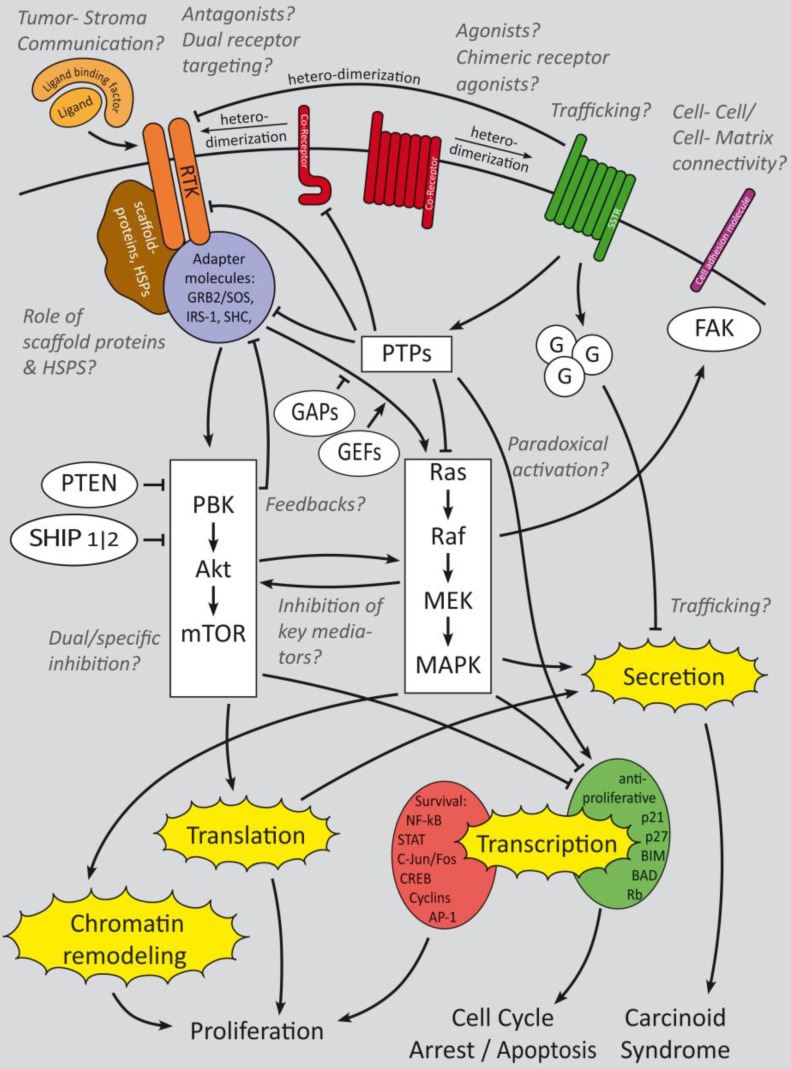
Summary of growth factor-induced cellular mechanisms and inhibitory effects of somatostatin signaling in GEP-NENs. The major receptors of interest are RTKs, SSTRs and their Co-receptors. Cell adhesion molecules are of minor research interest but might have an interesting potential. Whereas RTK activate downstream PI3K and mitogen activated signaling that triggers gene regulation and secretion, SSTRs activate G-protein coupled signaling which has a predominant regulatory effect onto growth factor signaling cascades in GEP-NENs. Beside the main questions, which crosstalks are relevant for GEP-NEN cancerogenesis and how to inhibit their key mediators, the effect of paradoxical activation has not been clinically evaluated. Trafficking, intercellular and cell-matrix interactions have been analyzed only in a small number of publications 90, 92, 94 but might have a high potential for therapeutic and prognostic issues. Ligand binding factors and receptor agonists and antagonists are of high research interest and the focus is on dual receptor targeting to develop a more subgroup specific therapy approach 27-29. Italic: major fields of interest in current research and remaining open questions.
Furthermore, Rsk, Akt and p70S6K share the ability to induce protein translation by regulating initiation and elongation factors, such as eIF4B (eukaryotic initiation factor 4B), eEF2K (eukaryotic elongation factor 2 kinase), RPS6 (ribosomal protein S6) and eEF2 365, 379-381.
A very important cross-inhibitory mechanism in the context of therapy resistance is the crosstalk of C‑Raf and Akt. Highly activated Akt suppresses Raf kinase activity by phosphorylation of S259. Phospho-S259 results in binding of the 14-3-3 protein and in Raf inactivation 289, 331, 382-386. Consequently, inhibition of PI3K signaling can activate Raf as demonstrated for everolimus in GEP-NETs 204. This mechanism is discussed to be one reason why mTOR inhibition has limited impact in GEP-NET therapy. Nevertheless the effect of paradoxical MAPK pathway activation has not been integrated in this debate to date and requires further investigation.
In summary, the PI3K and the MAPK pathways share overlapping activation patterns and converge on a number of common targets (refer to figure 4), although with varying affinities. The role of distinct posttranscriptional modification sites and multi-protein-complex assembly is analyzed in a large number of more detailed publications. Inhibiting one of those non-specific pathway mediators thus harbors the danger of activating other cascades and states the rationale for many dual inhibition approaches, unless with little success in GEP-NENs so far.
Figure 4.
Major crosstalks between the PI3K and the MAPK cascades. The PI3K and the MAPK pathway are highly interconnected and can be activated by the same RTKs, dependent on the triggering ligand. Direct Ras-GTP interaction with PI3 Kinases can trigger Ras-activated PI3K signaling 365-369. The KSR scaffold is also a potent regulator of key PI3K signaling mediators. Several cross-activations and -inhibitions have been assessed to date. For instance, Akt can inhibit Ras function 289, 331, 382-386 and Rsk can interfere with TSC2 365-369. Furthermore, both pathways converge on the same downstream targets, such as the FOXO proteins, GSK3, Myc or p70S6K 373-378, and thus promote cancer associated-phenotypes.
Conclusion
In sporadic GEP-NENs, growth factor receptor downstream signaling is ensured by large networks of triggering kinases, stabilizing scaffold proteins and regulating phosphatases, as well as by various transcription factors and co-factors. This network is highly upregulated and triggers cell growth, proliferation and secretion. Although basic cell-biological research has elucidated a vast number of mechanisms and interrelations that might explain observations that have been made in GEP-NENs, the majority of molecular information still remains to be elucidated. In contrast to cancer entities, where the oncogenic input can be “translated” in a manageable number of mutated proteins which facilitates the development of “target-directed” therapies, main sources of growth factor deregulation in sporadic GEP-NENs have not been identified to date. Genomic studies have revealed a number of chromosomal alterations but their relation to specific genes remains unclear. Subgroup specific data is rare due to the already limited number of cases, but might be important to understand differences between distinct groups of patients. Moreover, the “crosstalk” of different signaling pathways in a NEN cell may be much more complex and interactive as it is already known and the inhibition of one pathway can easily activate others by feedback loops, as described for mTOR inhibition.
In this review we have compiled several studies on the molecular basis for growth factor induced mitogen downstream-signaling in GEP-NENs. The two most promising and approved therapies in GEP-NENs are target-directed approaches, namely targeting the mTOR protein and somatostatin receptors. Recommendations for the diagnostics and therapy of gastroenteropancreatic neuroendocrine neoplasms are given by experts of the European Neuroendocrine Tumor Society in consensus conferences and are updated regularly 387. Certainly, somatostatin analogues have been introduced as anti-proliferative agents after the positive results of the PROMID study 116 and are used in midgut tumors, whereas Everolimus and Sunitinib have their indication in metastatic pNENs 244, 388. Everolimus might possibly also evolve as therapeutic option in midgut NENs 389 as results of RADIANT-4 study are awaited.
Nevertheless the therapy outcome depends on the abundance and activation pattern of these targets. Subgrouping data by therapy predictive biological markers, such as the phosphorylation status of Akt or the SSTR expression pattern, might improve the therapeutic precision for choosing the right therapy. Here we provide some evidence that even the most advanced therapeutic agent, everolimus, which is widely used in GEP-NENs therapy and was approved by the FDA two years ago, may still hold some drawbacks which may diminish its success.
In preclinical models Everolimus leads to a global upregulation of upstream PI3K signaling and cross-activation of Ras/Raf/Erk signaling via negative feedback loops. Therefore, recent therapeutic approaches focus on inhibitors of alternative components of the PI3K pathway, dual target inhibitors and effective combinatory bio/chemotherapies. Another example is the crosstalk between B-Raf/C-Raf and Ras/MEK/ERK-pathway. In the clinical setting, the use of a B-Raf-inhibitor in a melanoma patient led to the (paradoxical) activation and progression of a second malignancy (n-Ras mutated AML) in the same patient 322. This effect was reversed by withdrawing the inhibitor - but it impressively demonstrated the need for further investigations on the crosstalks and the complexity of different pathways.
There is also a need for entity-specific research data concerning processes that trigger GEP-NEN-specific cellular behavior, such as intra- and intercellular trafficking, tumor-stroma interactions or the regulatory role of scaffold proteins. Although a large variety of inhibitors and molecular pathway regulator are available to date, their application is almost limited to their assessment for clinical purposes rather than to identify their mechanisms of actions. For instance, the observations that mutations in key mediators of growth factor activated pathways are rare in GEP-NENs, is an important fact for novel therapeutic strategies and, especially in the case of Raf, fundamental to define a certain outcome. Without understanding the underlying mechanism, interpretation of unexpected study results will remain unsatisfying. The importance of molecular markers and genetic data to predict therapy outcome before starting cost-intensive clinical trials has been increased dramatically due to the enormous rise of available substances in the context of growth factor signaling. Significant mechanistic knowledge, deducted from generally valid cancerogenetic contexts, needs to be analyzed for its validity in GEP-NENs and thus to furnish stronger rationales for distinct target directed and subtype specific therapeutic trials. Therefore the identification and assessment of new markers and their evolution throughout neuroendocrine cancerogenesis is a promising field of research. Detailed pathway analyses are essential to understand the limitations of the current therapies and to elucidate new possibilities of molecular imaging and targeting in this heterogeneous cancer entity.
Supplementary Material
Suppl. 1-7.
Acknowledgments
The support of the German Wilhelm und Ingeburg Dinse Gedächtnis-Stiftung and of the Sonnenfeld Stiftung Berlin is gratefully acknowledged.
The authors would like to thank Liliana H. Mochmann for critically reading the manuscript, the colleagues of the Theranostics Research Network, especially Prof. Dieter Hörsch and Dr. Daniel Kämmerer, for providing specialist advice and support, and Florentine Lewens and Helma Freitag for technical assistance and support.
References
- 1.Schimmack S, Svejda B, Lawrence B, Kidd M, Modlin IM. The diversity and commonalities of gastroenteropancreatic neuroendocrine tumors. Langenbecks Arch Surg. 2011;396:273–98. doi: 10.1007/s00423-011-0739-1. doi:10.1007/s00423-011-0739-1. [DOI] [PubMed] [Google Scholar]
- 2.Capdevila J, Salazar R, Halperin I, Abad A, Yao JC. Innovations therapy: mammalian target of rapamycin (mTOR) inhibitors for the treatment of neuroendocrine tumors. Cancer Metastasis Rev. 2011;30(Suppl 1):27–34. doi: 10.1007/s10555-011-9290-3. doi:10.1007/s10555-011-9290-3. [DOI] [PubMed] [Google Scholar]
- 3.Capdevila J, Tabernero J. A shining light in the darkness for the treatment of pancreatic neuroendocrine tumors. Cancer Discov. 2011;1:213–21. doi: 10.1158/2159-8290.CD-11-0151. doi:10.1158/2159-8290.cd-11-0151. [DOI] [PubMed] [Google Scholar]
- 4.Hilfenhaus G, Gohrig A, Pape UF, Neumann T, Jann H, Zdunek D. et al. Placental growth factor supports neuroendocrine tumor growth and predicts disease prognosis in patients. Endocr Relat Cancer. 2013;20:305–19. doi: 10.1530/ERC-12-0223. doi:10.1530/erc-12-0223. [DOI] [PubMed] [Google Scholar]
- 5.Oberstein PE, Saif MW. Update on prognostic and predictive biomarkers for pancreatic neuroendocrine tumors. JOP. 2012;13:368–71. doi: 10.6092/1590-8577/965. doi:10.6092/1590-8577/965. [DOI] [PubMed] [Google Scholar]
- 6.Shah T, Hochhauser D, Frow R, Quaglia A, Dhillon AP, Caplin ME. Epidermal growth factor receptor expression and activation in neuroendocrine tumours. J Neuroendocrinol. 2006;18:355–60. doi: 10.1111/j.1365-2826.2006.01425.x. doi:10.1111/j.1365-2826.2006.01425.x. [DOI] [PubMed] [Google Scholar]
- 7.Christofori G, Naik P, Hanahan D. Vascular endothelial growth factor and its receptors, flt-1 and flk-1, are expressed in normal pancreatic islets and throughout islet cell tumorigenesis. Mol Endocrinol. 1995;9:1760–70. doi: 10.1210/mend.9.12.8614412. [DOI] [PubMed] [Google Scholar]
- 8.La Rosa S, Chiaravalli AM, Capella C, Uccella S, Sessa F. Immunohistochemical localization of acidic fibroblast growth factor in normal human enterochromaffin cells and related gastrointestinal tumours. Virchows Arch. 1997;430:117–24. doi: 10.1007/BF01008032. [DOI] [PubMed] [Google Scholar]
- 9.Nilsson O, Wangberg B, Kolby L, Schultz GS, Ahlman H. Expression of transforming growth factor alpha and its receptor in human neuroendocrine tumours. Int J Cancer. 1995;60:645–51. doi: 10.1002/ijc.2910600514. [DOI] [PubMed] [Google Scholar]
- 10.Chaudhry A, Oberg K, Gobl A, Heldin CH, Funa K. Expression of transforming growth factors beta 1, beta 2, beta 3 in neuroendocrine tumors of the digestive system. Anticancer Res. 1994;14:2085–91. [PubMed] [Google Scholar]
- 11.Krishnamurthy S, Dayal Y. Immunohistochemical expression of transforming growth factor alpha and epidermal growth factor receptor in gastrointestinal carcinoids. Am J Surg Pathol. 1997;21:327–33. doi: 10.1097/00000478-199703000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Chaudhry A, Papanicolaou V, Oberg K, Heldin CH, Funa K. Expression of platelet-derived growth factor and its receptors in neuroendocrine tumors of the digestive system. Cancer Res. 1992;52:1006–12. [PubMed] [Google Scholar]
- 13.Van Gompel JJ, Chen H. Insulin-like growth factor 1 signaling in human gastrointestinal carcinoid tumor cells. Surgery. 2004;136:1297–302. doi: 10.1016/j.surg.2004.06.061. doi:10.1016/j.surg.2004.06.061. [DOI] [PubMed] [Google Scholar]
- 14.Hopfner M, Baradari V, Huether A, Schofl C, Scherubl H. The insulin-like growth factor receptor 1 is a promising target for novel treatment approaches in neuroendocrine gastrointestinal tumours. Endocr Relat Cancer. 2006;13:135–49. doi: 10.1677/erc.1.01090. doi:10.1677/erc.1.01090. [DOI] [PubMed] [Google Scholar]
- 15.La Rosa S, Uccella S, Finzi G, Albarello L, Sessa F, Capella C. Localization of vascular endothelial growth factor and its receptors in digestive endocrine tumors: correlation with microvessel density and clinicopathologic features. Hum Pathol. 2003;34:18–27. doi: 10.1053/hupa.2003.56. doi:10.1053/hupa.2003.56. [DOI] [PubMed] [Google Scholar]
- 16.Gross DJ, Munter G, Bitan M, Siegal T, Gabizon A, Weitzen R. et al. The role of imatinib mesylate (Glivec) for treatment of patients with malignant endocrine tumors positive for c-kit or PDGF-R. Endocr Relat Cancer. 2006;13:535–40. doi: 10.1677/erc.1.01124. doi:10.1677/erc.1.01124. [DOI] [PubMed] [Google Scholar]
- 17.Samani AA, Yakar S, LeRoith D, Brodt P. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev. 2007;28:20–47. doi: 10.1210/er.2006-0001. doi:10.1210/er.2006-0001. [DOI] [PubMed] [Google Scholar]
- 18.Furukawa M, Raffeld M, Mateo C, Sakamoto A, Moody TW, Ito T. et al. Increased expression of insulin-like growth factor I and/or its receptor in gastrinomas is associated with low curability, increased growth, and development of metastases. Clin Cancer Res. 2005;11:3233–42. doi: 10.1158/1078-0432.CCR-04-1915. doi:10.1158/1078-0432.ccr-04-1915. [DOI] [PubMed] [Google Scholar]
- 19.Vitale L, Lenzi L, Huntsman SA, Canaider S, Frabetti F, Casadei R. et al. Differential expression of alternatively spliced mRNA forms of the insulin-like growth factor 1 receptor in human neuroendocrine tumors. Oncol Rep. 2006;15:1249–56. [PubMed] [Google Scholar]
- 20.von Wichert G, Jehle PM, Hoeflich A, Koschnick S, Dralle H, Wolf E. et al. Insulin-like growth factor-I is an autocrine regulator of chromogranin A secretion and growth in human neuroendocrine tumor cells. Cancer Res. 2000;60:4573–81. [PubMed] [Google Scholar]
- 21.Gilbert JA, Adhikari LJ, Lloyd RV, Halfdanarson TR, Muders MH, Ames MM. Molecular markers for novel therapeutic strategies in pancreatic endocrine tumors. Pancreas. 2013;42:411–21. doi: 10.1097/MPA.0b013e31826cb243. doi:10.1097/MPA.0b013e31826cb243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wulbrand U, Wied M, Zofel P, Goke B, Arnold R, Fehmann H. Growth factor receptor expression in human gastroenteropancreatic neuroendocrine tumours. Eur J Clin Invest. 1998;28:1038–49. doi: 10.1046/j.1365-2362.1998.00397.x. [DOI] [PubMed] [Google Scholar]
- 23.Nilsson O, Wangberg B, McRae A, Dahlstrom A, Ahlman H. Growth factors and carcinoid tumours. Acta Oncol. 1993;32:115–24. doi: 10.3109/02841869309083899. [DOI] [PubMed] [Google Scholar]
- 24.Nilsson O, Wangberg B, Theodorsson E, Skottner A, Ahlman H. Presence of IGF-I in human midgut carcinoid tumours--an autocrine regulator of carcinoid tumour growth? Int J Cancer. 1992;51:195–203. doi: 10.1002/ijc.2910510206. [DOI] [PubMed] [Google Scholar]
- 25.Gloesenkamp C, Nitzsche B, Lim AR, Normant E, Vosburgh E, Schrader M. et al. Heat shock protein 90 is a promising target for effective growth inhibition of gastrointestinal neuroendocrine tumors. Int J Oncol. 2012;40:1659–67. doi: 10.3892/ijo.2012.1328. doi:10.3892/ijo.2012.1328. [DOI] [PubMed] [Google Scholar]
- 26.Richardson PG, Mitsiades CS, Laubach JP, Lonial S, Chanan-Khan AA, Anderson KC. Inhibition of heat shock protein 90 (HSP90) as a therapeutic strategy for the treatment of myeloma and other cancers. Br J Haematol. 2011;152:367–79. doi: 10.1111/j.1365-2141.2010.08360.x. doi:10.1111/j.1365-2141.2010.08360.x. [DOI] [PubMed] [Google Scholar]
- 27.Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 28.Hansel DE, Rahman A, House M, Ashfaq R, Berg K, Yeo CJ. et al. Met proto-oncogene and insulin-like growth factor binding protein 3 overexpression correlates with metastatic ability in well-differentiated pancreatic endocrine neoplasms. Clin Cancer Res. 2004;10:6152–8. doi: 10.1158/1078-0432.CCR-04-0285. doi:10.1158/1078-0432.ccr-04-0285. [DOI] [PubMed] [Google Scholar]
- 29.Maitra A, Hansel DE, Argani P, Ashfaq R, Rahman A, Naji A. et al. Global expression analysis of well-differentiated pancreatic endocrine neoplasms using oligonucleotide microarrays. Clin Cancer Res. 2003;9:5988–95. [PubMed] [Google Scholar]
- 30.Papouchado B, Erickson LA, Rohlinger AL, Hobday TJ, Erlichman C, Ames MM. et al. Epidermal growth factor receptor and activated epidermal growth factor receptor expression in gastrointestinal carcinoids and pancreatic endocrine carcinomas. Mod Pathol. 2005;18:1329–35. doi: 10.1038/modpathol.3800427. doi:10.1038/modpathol.3800427. [DOI] [PubMed] [Google Scholar]
- 31.Srivastava A, Alexander J, Lomakin I, Dayal Y. Immunohistochemical expression of transforming growth factor alpha and epidermal growth factor receptor in pancreatic endocrine tumors. Hum Pathol. 2001;32:1184–9. doi: 10.1053/hupa.2001.28959. doi:10.1053/hupa.2001.28959. [DOI] [PubMed] [Google Scholar]
- 32.Di Florio A, Sancho V, Moreno P, Delle Fave G, Jensen RT. Gastrointestinal hormones stimulate growth of Foregut Neuroendocrine Tumors by transactivating the EGF receptor. Biochim Biophys Acta. 2013;1833:573–82. doi: 10.1016/j.bbamcr.2012.11.021. doi:10.1016/j.bbamcr.2012.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Missiaglia E, Dalai I, Barbi S, Beghelli S, Falconi M, della Peruta M. et al. Pancreatic endocrine tumors: expression profiling evidences a role for AKT-mTOR pathway. J Clin Oncol. 2010;28:245–55. doi: 10.1200/JCO.2008.21.5988. doi:10.1200/jco.2008.21.5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu QF, Yang L, Li S, Wang Q, Yuan XB, Gao X. et al. Fibroblast growth factor 13 is a microtubule-stabilizing protein regulating neuronal polarization and migration. Cell. 2012;149:1549–64. doi: 10.1016/j.cell.2012.04.046. doi:10.1016/j.cell.2012.04.046. [DOI] [PubMed] [Google Scholar]
- 35.Ono K, Suzuki T, Miki Y, Taniyama Y, Nakamura Y, Noda Y. et al. Somatostatin receptor subtypes in human non-functioning neuroendocrine tumors and effects of somatostatin analogue SOM230 on cell proliferation in cell line NCI-H727. Anticancer Res. 2007;27:2231–9. [PubMed] [Google Scholar]
- 36.Taniyama Y, Suzuki T, Mikami Y, Moriya T, Satomi S, Sasano H. Systemic distribution of somatostatin receptor subtypes in human: an immunohistochemical study. Endocr J. 2005;52:605–11. doi: 10.1507/endocrj.52.605. [DOI] [PubMed] [Google Scholar]
- 37.Pawlikowski M, Pisarek H, Winczyk K. Immunohistochemical detection of dopamine D2 receptors in neuroendocrine tumours. Endokrynol Pol. 2011;62:388–91. [PubMed] [Google Scholar]
- 38.Diakatou E, Kaltsas G, Tzivras M, Kanakis G, Papaliodi E, Kontogeorgos G. Somatostatin and dopamine receptor profile of gastroenteropancreatic neuroendocrine tumors: an immunohistochemical study. Endocr Pathol. 2011;22:24–30. doi: 10.1007/s12022-011-9149-8. doi:10.1007/s12022-011-9149-8. [DOI] [PubMed] [Google Scholar]
- 39.Patel YC. Somatostatin and its receptor family. Front Neuroendocrinol. 1999;20:157–98. doi: 10.1006/frne.1999.0183. doi:10.1006/frne.1999.0183. [DOI] [PubMed] [Google Scholar]
- 40.Sellers LA, Alderton F, Carruthers AM, Schindler M, Humphrey PP. Receptor isoforms mediate opposing proliferative effects through gbetagamma-activated p38 or Akt pathways. Mol Cell Biol. 2000;20:5974–85. doi: 10.1128/mcb.20.16.5974-5985.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosskopf D, Schurks M, Manthey I, Joisten M, Busch S, Siffert W. Signal transduction of somatostatin in human B lymphoblasts. Am J Physiol Cell Physiol. 2003;284:C179–90. doi: 10.1152/ajpcell.00160.2001. doi:10.1152/ajpcell.00160.2001. [DOI] [PubMed] [Google Scholar]
- 42.Meyerhof W. The elucidation of somatostatin receptor functions: a current view. Rev Physiol Biochem Pharmacol. 1998;133:55–108. doi: 10.1007/BFb0000613. [DOI] [PubMed] [Google Scholar]
- 43.Bruns C, Lewis I, Briner U, Meno-Tetang G, Weckbecker G. SOM230: a novel somatostatin peptidomimetic with broad somatotropin release inhibiting factor (SRIF) receptor binding and a unique antisecretory profile. Eur J Endocrinol. 2002;146:707–16. doi: 10.1530/eje.0.1460707. [DOI] [PubMed] [Google Scholar]
- 44.Appetecchia M, Baldelli R. Somatostatin analogues in the treatment of gastroenteropancreatic neuroendocrine tumours, current aspects and new perspectives. J Exp Clin Cancer Res. 2010;29:19.. doi: 10.1186/1756-9966-29-19. doi:10.1186/1756-9966-29-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weckbecker G, Lewis I, Albert R, Schmid HA, Hoyer D, Bruns C. Opportunities in somatostatin research: biological, chemical and therapeutic aspects. Nat Rev Drug Discov. 2003;2:999–1017. doi: 10.1038/nrd1255. doi:10.1038/nrd1255. [DOI] [PubMed] [Google Scholar]
- 46.Moller LN, Stidsen CE, Hartmann B, Holst JJ. Somatostatin receptors. Biochim Biophys Acta. 2003;1616:1–84. doi: 10.1016/s0005-2736(03)00235-9. [DOI] [PubMed] [Google Scholar]
- 47.Florio T. Somatostatin/somatostatin receptor signalling: phosphotyrosine phosphatases. Mol Cell Endocrinol. 2008;286:40–8. doi: 10.1016/j.mce.2007.08.012. doi:10.1016/j.mce.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 48.Reichlin S. Somatostatin. N Engl J Med. 1983;309:1495–501. doi: 10.1056/NEJM198312153092406. doi:10.1056/nejm198312153092406. [DOI] [PubMed] [Google Scholar]
- 49.Scherubl H, Hescheler J, Riecken EO. Molecular mechanisms of somatostatin's inhibition of hormone release: participation of voltage-gated calcium channels and G-proteins. Horm Metab Res Suppl. 1993;27:1–4. [PubMed] [Google Scholar]
- 50.Zapata PD, Ropero RM, Valencia AM, Buscail L, Lopez JI, Martin-Orozco RM. et al. Autocrine regulation of human prostate carcinoma cell proliferation by somatostatin through the modulation of the SH2 domain containing protein tyrosine phosphatase (SHP)-1. J Clin Endocrinol Metab. 2002;87:915–26. doi: 10.1210/jcem.87.2.8194. [DOI] [PubMed] [Google Scholar]
- 51.Thangaraju M, Sharma K, Liu D, Shen SH, Srikant CB. Interdependent regulation of intracellular acidification and SHP-1 in apoptosis. Cancer Res. 1999;59:1649–54. [PubMed] [Google Scholar]
- 52.Florio T, Morini M, Villa V, Arena S, Corsaro A, Thellung S. et al. Somatostatin inhibits tumor angiogenesis and growth via somatostatin receptor-3-mediated regulation of endothelial nitric oxide synthase and mitogen-activated protein kinase activities. Endocrinology. 2003;144:1574–84. doi: 10.1210/en.2002-220949. [DOI] [PubMed] [Google Scholar]
- 53.Florio T, Thellung S, Arena S, Corsaro A, Bajetto A, Schettini G. et al. Somatostatin receptor 1 (SSTR1)-mediated inhibition of cell proliferation correlates with the activation of the MAP kinase cascade: role of the phosphotyrosine phosphatase SHP-2. J Physiol Paris. 2000;94:239–50. doi: 10.1016/s0928-4257(00)00214-x. [DOI] [PubMed] [Google Scholar]
- 54.Held-Feindt J, Forstreuter F, Pufe T, Mentlein R. Influence of the somatostatin receptor sst2 on growth factor signal cascades in human glioma cells. Brain Res Mol Brain Res. 2001;87:12–21. doi: 10.1016/s0169-328x(00)00225-4. [DOI] [PubMed] [Google Scholar]
- 55.Dent P, Wang Y, Gu YZ, Wood SL, Reardon DB, Mangues R. et al. S49 cells endogenously express subtype 2 somatostatin receptors which couple to increase protein tyrosine phosphatase activity in membranes and down-regulate Raf-1 activity in situ. Cell Signal. 1997;9:539–49. doi: 10.1016/s0898-6568(97)00048-x. [DOI] [PubMed] [Google Scholar]
- 56.Reardon DB, Dent P, Wood SL, Kong T, Sturgill TW. Activation in vitro of somatostatin receptor subtypes 2, 3, or 4 stimulates protein tyrosine phosphatase activity in membranes from transfected Ras-transformed NIH 3T3 cells: coexpression with catalytically inactive SHP-2 blocks responsiveness. Mol Endocrinol. 1997;11:1062–9. doi: 10.1210/mend.11.8.9960. [DOI] [PubMed] [Google Scholar]
- 57.Theodoropoulou M, Zhang J, Laupheimer S, Paez-Pereda M, Erneux C, Florio T. et al. Octreotide, a somatostatin analogue, mediates its antiproliferative action in pituitary tumor cells by altering phosphatidylinositol 3-kinase signaling and inducing Zac1 expression. Cancer Res. 2006;66:1576–82. doi: 10.1158/0008-5472.CAN-05-1189. doi:10.1158/0008-5472.can-05-1189. [DOI] [PubMed] [Google Scholar]
- 58.Bousquet C, Delesque N, Lopez F, Saint-Laurent N, Esteve JP, Bedecs K. et al. sst2 somatostatin receptor mediates negative regulation of insulin receptor signaling through the tyrosine phosphatase SHP-1. J Biol Chem. 1998;273:7099–106. doi: 10.1074/jbc.273.12.7099. [DOI] [PubMed] [Google Scholar]
- 59.Bousquet C, Guillermet-Guibert J, Saint-Laurent N, Archer-Lahlou E, Lopez F, Fanjul M. et al. Direct binding of p85 to sst2 somatostatin receptor reveals a novel mechanism for inhibiting PI3K pathway. EMBO J. 2006;25:3943–54. doi: 10.1038/sj.emboj.7601279. doi:10.1038/sj.emboj.7601279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pages P, Benali N, Saint-Laurent N, Esteve JP, Schally AV, Tkaczuk J. et al. sst2 somatostatin receptor mediates cell cycle arrest and induction of p27(Kip1). Evidence for the role of SHP-1. J Biol Chem. 1999;274:15186–93. doi: 10.1074/jbc.274.21.15186. [DOI] [PubMed] [Google Scholar]
- 61.Sharma K, Srikant CB. Induction of wild-type p53, Bax, and acidic endonuclease during somatostatin-signaled apoptosis in MCF-7 human breast cancer cells. Int J Cancer. 1998;76:259–66. doi: 10.1002/(sici)1097-0215(19980413)76:2<259::aid-ijc14>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 62.Ostman A, Hellberg C, Bohmer FD. Protein-tyrosine phosphatases and cancer. Nat Rev Cancer. 2006;6:307–20. doi: 10.1038/nrc1837. doi:10.1038/nrc1837. [DOI] [PubMed] [Google Scholar]
- 63.Iuliano R, Trapasso F, Le Pera I, Schepis F, Sama I, Clodomiro A. et al. An adenovirus carrying the rat protein tyrosine phosphatase eta suppresses the growth of human thyroid carcinoma cell lines in vitro and in vivo. Cancer Res. 2003;63:882–6. [PubMed] [Google Scholar]
- 64.Keane MM, Lowrey GA, Ettenberg SA, Dayton MA, Lipkowitz S. The protein tyrosine phosphatase DEP-1 is induced during differentiation and inhibits growth of breast cancer cells. Cancer Res. 1996;56:4236–43. [PubMed] [Google Scholar]
- 65.Pera IL, Iuliano R, Florio T, Susini C, Trapasso F, Santoro M. et al. The rat tyrosine phosphatase eta increases cell adhesion by activating c-Src through dephosphorylation of its inhibitory phosphotyrosine residue. Oncogene. 2005;24:3187–95. doi: 10.1038/sj.onc.1208510. doi:10.1038/sj.onc.1208510. [DOI] [PubMed] [Google Scholar]
- 66.Martelli ML, Trapasso F, Bruni P, Berlingieri MT, Battaglia C, Vento MT. et al. Protein tyrosine phosphatase-eta expression is upregulated by the PKA-dependent and is downregulated by the PKC-dependent pathways in thyroid cells. Exp Cell Res. 1998;245:195–202. doi: 10.1006/excr.1998.4257. doi:10.1006/excr.1998.4257. [DOI] [PubMed] [Google Scholar]
- 67.Arena S, Pattarozzi A, Massa A, Esteve JP, Iuliano R, Fusco A. et al. An intracellular multi-effector complex mediates somatostatin receptor 1 activation of phospho-tyrosine phosphatase eta. Mol Endocrinol. 2007;21:229–46. doi: 10.1210/me.2006-0081. doi:10.1210/me.2006-0081. [DOI] [PubMed] [Google Scholar]
- 68.Ferjoux G, Lopez F, Esteve JP, Ferrand A, Vivier E, Vely F. et al. Critical role of Src and SHP-2 in sst2 somatostatin receptor-mediated activation of SHP-1 and inhibition of cell proliferation. Mol Biol Cell. 2003;14:3911–28. doi: 10.1091/mbc.E03-02-0069. doi:10.1091/mbc.E03-02-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaemmerer D, Peter L, Lupp A, Schulz S, Sanger J, Baum RP. et al. Comparing of IRS and Her2 as immunohistochemical scoring schemes in gastroenteropancreatic neuroendocrine tumors. Int J Clin Exp Pathol. 2012;5:187–94. [PMC free article] [PubMed] [Google Scholar]
- 70.Reubi JC, Waser B. Concomitant expression of several peptide receptors in neuroendocrine tumours: molecular basis for in vivo multireceptor tumour targeting. Eur J Nucl Med Mol Imaging. 2003;30:781–93. doi: 10.1007/s00259-003-1184-3. doi:10.1007/s00259-003-1184-3. [DOI] [PubMed] [Google Scholar]
- 71.Reubi JC. Peptide receptors as molecular targets for cancer diagnosis and therapy. Endocr Rev. 2003;24:389–427. doi: 10.1210/er.2002-0007. [DOI] [PubMed] [Google Scholar]
- 72.Reubi JC. Somatostatin and other Peptide receptors as tools for tumor diagnosis and treatment. Neuroendocrinology. 2004;80(Suppl 1):51–6. doi: 10.1159/000080742. doi:10.1159/000080742. [DOI] [PubMed] [Google Scholar]
- 73.Papotti M, Bongiovanni M, Volante M, Allia E, Landolfi S, Helboe L. et al. Expression of somatostatin receptor types 1-5 in 81 cases of gastrointestinal and pancreatic endocrine tumors. A correlative immunohistochemical and reverse-transcriptase polymerase chain reaction analysis. Virchows Arch. 2002;440:461–75. doi: 10.1007/s00428-002-0609-x. doi:10.1007/s00428-002-0609-x. [DOI] [PubMed] [Google Scholar]
- 74.Schmid HA, Lambertini C, van Vugt HH, Barzaghi-Rinaudo P, Schafer J, Hillenbrand R. et al. Monoclonal antibodies against the human somatostatin receptor subtypes 1-5: development and immunohistochemical application in neuroendocrine tumors. Neuroendocrinology. 2012;95:232–47. doi: 10.1159/000330616. doi:10.1159/000330616. [DOI] [PubMed] [Google Scholar]
- 75.Corleto VD, Falconi M, Panzuto F, Milione M, De Luca O, Perri P. et al. Somatostatin receptor subtypes 2 and 5 are associated with better survival in well-differentiated endocrine carcinomas. Neuroendocrinology. 2009;89:223–30. doi: 10.1159/000167796. doi:10.1159/000167796. [DOI] [PubMed] [Google Scholar]
- 76.Kim HS, Lee HS, Kim WH. Clinical significance of protein expression of cyclooxygenase-2 and somatostatin receptors in gastroenteropancreatic neuroendocrine tumors. Cancer Res Treat. 2011;43:181–8. doi: 10.4143/crt.2011.43.3.181. doi:10.4143/crt.2011.43.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Srirajaskanthan R, Watkins J, Marelli L, Khan K, Caplin ME. Expression of somatostatin and dopamine 2 receptors in neuroendocrine tumours and the potential role for new biotherapies. Neuroendocrinology. 2009;89:308–14. doi: 10.1159/000179899. doi:10.1159/000179899. [DOI] [PubMed] [Google Scholar]
- 78.Zamora V, Cabanne A, Salanova R, Bestani C, Domenichini E, Marmissolle F. et al. Immunohistochemical expression of somatostatin receptors in digestive endocrine tumours. Dig Liver Dis. 2010;42:220–5. doi: 10.1016/j.dld.2009.07.018. doi:10.1016/j.dld.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 79.Reubi JC, Kvols LK, Waser B, Nagorney DM, Heitz PU, Charboneau JW. et al. Detection of somatostatin receptors in surgical and percutaneous needle biopsy samples of carcinoids and islet cell carcinomas. Cancer Res. 1990;50:5969–77. [PubMed] [Google Scholar]
- 80.Oconnor JM, Belli S, Pesce V, Bestani C, Dominichini E, Mendez G, Somatostatin receptor (sstr) expression and proliferative index (ki 67) in 100 patients (pts) with gastroenteropancreatic neuroendocrine tumors (gep-nets) Clinical-pathological correlation. 37th ESMO Conference. Vienna. 2012.
- 81.O'Toole D, Saveanu A, Couvelard A, Gunz G, Enjalbert A, Jaquet P. et al. The analysis of quantitative expression of somatostatin and dopamine receptors in gastro-entero-pancreatic tumours opens new therapeutic strategies. Eur J Endocrinol. 2006;155:849–57. doi: 10.1530/eje.1.02307. doi:10.1530/eje.1.02307. [DOI] [PubMed] [Google Scholar]
- 82.Rocheville M, Lange DC, Kumar U, Patel SC, Patel RC, Patel YC. Receptors for dopamine and somatostatin: formation of hetero-oligomers with enhanced functional activity. Science. 2000;288:154–7. doi: 10.1126/science.288.5463.154. [DOI] [PubMed] [Google Scholar]
- 83.Kulaksiz H, Eissele R, Rossler D, Schulz S, Hollt V, Cetin Y. et al. Identification of somatostatin receptor subtypes 1, 2A, 3, and 5 in neuroendocrine tumours with subtype specific antibodies. Gut. 2002;50:52–60. doi: 10.1136/gut.50.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Imam H, Eriksson B, Lukinius A, Janson ET, Lindgren PG, Wilander E. et al. Induction of apoptosis in neuroendocrine tumors of the digestive system during treatment with somatostatin analogs. Acta Oncol. 1997;36:607–14. doi: 10.3109/02841869709001323. [DOI] [PubMed] [Google Scholar]
- 85.Kaemmerer D, Lupp A, Peter L, Fischer E, Schulz S, Kloppel G. et al. Correlation of monoclonal and polyclonal somatostatin receptor 5 antibodies in pancreatic neuroendocrine tumors. Int J Clin Exp Pathol. 2013;6:49–54. [PMC free article] [PubMed] [Google Scholar]
- 86.Lupp A, Hunder A, Petrich A, Nagel F, Doll C, Schulz S. Reassessment of sst(5) somatostatin receptor expression in normal and neoplastic human tissues using the novel rabbit monoclonal antibody UMB-4. Neuroendocrinology. 2011;94:255–64. doi: 10.1159/000329876. doi:10.1159/000329876. [DOI] [PubMed] [Google Scholar]
- 87.Lupp A, Nagel F, Doll C, Rocken C, Evert M, Mawrin C. et al. Reassessment of sst3 somatostatin receptor expression in human normal and neoplastic tissues using the novel rabbit monoclonal antibody UMB-5. Neuroendocrinology. 2012;96:301–10. doi: 10.1159/000337659. doi:10.1159/000337659. [DOI] [PubMed] [Google Scholar]
- 88.Fischer T, Doll C, Jacobs S, Kolodziej A, Stumm R, Schulz S. Reassessment of sst2 somatostatin receptor expression in human normal and neoplastic tissues using the novel rabbit monoclonal antibody UMB-1. J Clin Endocrinol Metab. 2008;93:4519–24. doi: 10.1210/jc.2008-1063. doi:10.1210/jc.2008-1063. [DOI] [PubMed] [Google Scholar]
- 89.Kao YJ, Ghosh M, Schonbrunn A. Ligand-dependent mechanisms of sst2A receptor trafficking: role of site-specific phosphorylation and receptor activation in the actions of biased somatostatin agonists. Mol Endocrinol. 2011;25:1040–54. doi: 10.1210/me.2010-0398. doi:10.1210/me.2010-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Petrich A, Mann A, Kliewer A, Nagel F, Strigli A, Martens JC. et al. Phosphorylation of threonine 333 regulates trafficking of the human sst5 somatostatin receptor. Mol Endocrinol. 2013;27:671–82. doi: 10.1210/me.2012-1329. doi:10.1210/me.2012-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Janson ET, Westlin JE, Ohrvall U, Oberg K, Lukinius A. Nuclear localization of 111In after intravenous injection of [111In-DTPA-D-Phe1]-octreotide in patients with neuroendocrine tumors. J Nucl Med. 2000;41:1514–8. [PubMed] [Google Scholar]
- 92.Jacobs S, Schulz S. Intracellular trafficking of somatostatin receptors. Mol Cell Endocrinol. 2008;286:58–62. doi: 10.1016/j.mce.2007.10.005. doi:10.1016/j.mce.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 93.Poll F, Lehmann D, Illing S, Ginj M, Jacobs S, Lupp A. et al. Pasireotide and octreotide stimulate distinct patterns of sst2A somatostatin receptor phosphorylation. Mol Endocrinol. 2010;24:436–46. doi: 10.1210/me.2009-0315. doi:10.1210/me.2009-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Csaba Z, Peineau S, Dournaud P. Molecular mechanisms of somatostatin receptor trafficking. J Mol Endocrinol. 2012;48:R1–12. doi: 10.1530/JME-11-0121. doi:10.1530/jme-11-0121. [DOI] [PubMed] [Google Scholar]
- 95.Barbieri F, Bajetto A, Pattarozzi A, Gatti M, Wurth R, Thellung S. et al. Peptide receptor targeting in cancer: the somatostatin paradigm. Int J Pept. 2013;2013:926295.. doi: 10.1155/2013/926295. doi:10.1155/2013/926295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bodei L, Cremonesi M, Grana CM, Chinol M, Baio SM, Severi S. et al. Yttrium-labelled peptides for therapy of NET. Eur J Nucl Med Mol Imaging. 2012;39(Suppl 1):S93–102. doi: 10.1007/s00259-011-2002-y. doi:10.1007/s00259-011-2002-y. [DOI] [PubMed] [Google Scholar]
- 97.Bodei L, Ferone D, Grana CM, Cremonesi M, Signore A, Dierckx RA. et al. Peptide receptor therapies in neuroendocrine tumors. J Endocrinol Invest. 2009;32:360–9. doi: 10.1007/BF03345728. [DOI] [PubMed] [Google Scholar]
- 98.Giovacchini G, Nicolas G, Forrer F. Peptide receptor radionuclide therapy with somatostatin analogues in neuroendocrine tumors. Anticancer Agents Med Chem. 2012;12:526–42. doi: 10.2174/187152012800617803. [DOI] [PubMed] [Google Scholar]
- 99.Kam BL, Teunissen JJ, Krenning EP, de Herder WW, Khan S, van Vliet EI. et al. Lutetium-labelled peptides for therapy of neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2012;39(Suppl 1):S103–12. doi: 10.1007/s00259-011-2039-y. doi:10.1007/s00259-011-2039-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kwekkeboom DJ, de Herder WW, Krenning EP. Somatostatin receptor-targeted radionuclide therapy in patients with gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011;40:173–85. doi: 10.1016/j.ecl.2010.12.003. ix. doi:10.1016/j.ecl.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 101.Horsch D, Bert T, Schrader J, Hommann M, Kaemmerer D, Petrovitch A. et al. Pancreatic neuroendocrine neoplasms. Minerva Gastroenterol Dietol. 2012;58:401–26. [PubMed] [Google Scholar]
- 102.Horsch D, Ezziddin S, Haug A, Gratz KF, Dunkelmann S, Krause BJ. et al. Peptide receptor radionuclide therapy for neuroendocrine tumors in Germany: first results of a multi-institutional cancer registry. Recent Results Cancer Res. 2013;194:457–65. doi: 10.1007/978-3-642-27994-2_25. doi:10.1007/978-3-642-27994-2_25. [DOI] [PubMed] [Google Scholar]
- 103.Horsch D, Grabowski P, Schneider CP, Petrovitch A, Kaemmerer D, Hommann M. et al. Current treatment options for neuroendocrine tumors. Drugs Today (Barc) 2011;47:773–86. doi: 10.1358/dot.2011.47.10.1673555. doi:10.1358/dot.2011.47.10.1673555. [DOI] [PubMed] [Google Scholar]
- 104.Sundin A. Radiological and nuclear medicine imaging of gastroenteropancreatic neuroendocrine tumours. Best Pract Res Clin Gastroenterol. 2012;26:803–18. doi: 10.1016/j.bpg.2012.12.004. doi:10.1016/j.bpg.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 105.Modlin IM, Pavel M, Kidd M, Gustafsson BI. Review article: somatostatin analogues in the treatment of gastroenteropancreatic neuroendocrine (carcinoid) tumours. Aliment Pharmacol Ther. 2010;31:169–88. doi: 10.1111/j.1365-2036.2009.04174.x. doi:10.1111/j.1365-2036.2009.04174.x. [DOI] [PubMed] [Google Scholar]
- 106.Culler MD, Oberg K, Arnold R, Krenning EP, Sevilla I, Diaz JA. Somatostatin analogs for the treatment of neuroendocrine tumors. Cancer Metastasis Rev. 2011;30(Suppl 1):9–17. doi: 10.1007/s10555-011-9293-0. doi:10.1007/s10555-011-9293-0. [DOI] [PubMed] [Google Scholar]
- 107.Arnold R, Benning R, Neuhaus C, Rolwage M, Trautmann ME. Gastroenteropancreatic endocrine tumors: effect of Sandostatin on tumor growth. The German Sandostatin Study Group. Metabolism. 1992;41:116–8. doi: 10.1016/0026-0495(92)90044-b. [DOI] [PubMed] [Google Scholar]
- 108.Arnold R, Trautmann ME, Creutzfeldt W, Benning R, Benning M, Neuhaus C. et al. Somatostatin analogue octreotide and inhibition of tumour growth in metastatic endocrine gastroenteropancreatic tumours. Gut. 1996;38:430–8. doi: 10.1136/gut.38.3.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Boden G, Ryan IG, Eisenschmid BL, Shelmet JJ, Owen OE. Treatment of inoperable glucagonoma with the long-acting somatostatin analogue SMS 201-995. N Engl J Med. 1986;314:1686–9. doi: 10.1056/NEJM198606263142606. doi:10.1056/nejm198606263142606. [DOI] [PubMed] [Google Scholar]
- 110.di Bartolomeo M, Bajetta E, Buzzoni R, Mariani L, Carnaghi C, Somma L. et al. Clinical efficacy of octreotide in the treatment of metastatic neuroendocrine tumors. A study by the Italian Trials in Medical Oncology Group. Cancer. 1996;77:402–8. doi: 10.1002/(SICI)1097-0142(19960115)77:2<402::AID-CNCR25>3.0.CO;2-4. doi:10.1002/(sici)1097-0142(19960115)77:2<402::aid-cncr25>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 111.Kvols LK, Moertel CG, O'Connell MJ, Schutt AJ, Rubin J, Hahn RG. Treatment of the malignant carcinoid syndrome. Evaluation of a long-acting somatostatin analogue. N Engl J Med. 1986;315:663–6. doi: 10.1056/NEJM198609113151102. doi:10.1056/nejm198609113151102. [DOI] [PubMed] [Google Scholar]
- 112.Saltz L, Trochanowski B, Buckley M, Heffernan B, Niedzwiecki D, Tao Y. et al. Octreotide as an antineoplastic agent in the treatment of functional and nonfunctional neuroendocrine tumors. Cancer. 1993;72:244–8. doi: 10.1002/1097-0142(19930701)72:1<244::aid-cncr2820720143>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 113.Strosberg J, Gardner N, Kvols L. Survival and prognostic factor analysis of 146 metastatic neuroendocrine tumors of the mid-gut. Neuroendocrinology. 2009;89:471–6. doi: 10.1159/000197899. doi:10.1159/000197899. [DOI] [PubMed] [Google Scholar]
- 114.Yao JC, Phan AT, Chang DZ, Wolff RA, Hess K, Gupta S. et al. Efficacy of RAD001 (everolimus) and octreotide LAR in advanced low- to intermediate-grade neuroendocrine tumors: results of a phase II study. J Clin Oncol. 2008;26:4311–8. doi: 10.1200/JCO.2008.16.7858. doi:10.1200/jco.2008.16.7858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jann H, Denecke T, Koch M, Pape UF, Wiedenmann B, Pavel M. Impact of Octreotide LAR on Tumour Growth Control as First-Line Treatment in Neuroendocrine Tumours of Pancreatic Origin. Neuroendocrinology. 2013 doi: 10.1159/000353785. doi:10.1159/000353785. [DOI] [PubMed] [Google Scholar]
- 116.Rinke A, Muller HH, Schade-Brittinger C, Klose KJ, Barth P, Wied M. et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27:4656–63. doi: 10.1200/JCO.2009.22.8510. doi:10.1200/jco.2009.22.8510. [DOI] [PubMed] [Google Scholar]
- 117.Plockinger U, Wiedenmann B. Treatment of gastroenteropancreatic neuroendocrine tumors. Virchows Arch. 2007;451(Suppl 1):S71–80. doi: 10.1007/s00428-007-0446-z. doi:10.1007/s00428-007-0446-z. [DOI] [PubMed] [Google Scholar]
- 118.Wolin EM, Hu K, Hughes G, Bouillaud E, Giannone V, Resendiz KH. Safety, tolerability, pharmacokinetics, and pharmacodynamics of a long-acting release (LAR) formulation of pasireotide (SOM230) in patients with gastroenteropancreatic neuroendocrine tumors: results from a randomized, multicenter, open-label, phase I study. Cancer Chemother Pharmacol. 2013. doi:10.1007/s00280-013-2202-1. [DOI] [PMC free article] [PubMed]
- 119.Kvols LK, Oberg KE, O'Dorisio TM, Mohideen P, de Herder WW, Arnold R. et al. Pasireotide (SOM230) shows efficacy and tolerability in the treatment of patients with advanced neuroendocrine tumors refractory or resistant to octreotide LAR: results from a phase II study. Endocr Relat Cancer. 2012;19:657–66. doi: 10.1530/ERC-11-0367. doi:10.1530/erc-11-0367. [DOI] [PubMed] [Google Scholar]
- 120.Jaquet P, Gunz G, Saveanu A, Barlier A, Dufour H, Taylor J. et al. BIM-23A760, a chimeric molecule directed towards somatostatin and dopamine receptors, vs universal somatostatin receptors ligands in GH-secreting pituitary adenomas partial responders to octreotide. J Endocrinol Invest. 2005;28:21–7. [PubMed] [Google Scholar]
- 121.Jaquet P, Gunz G, Saveanu A, Dufour H, Taylor J, Dong J. et al. Efficacy of chimeric molecules directed towards multiple somatostatin and dopamine receptors on inhibition of GH and prolactin secretion from GH-secreting pituitary adenomas classified as partially responsive to somatostatin analog therapy. Eur J Endocrinol. 2005;153:135–41. doi: 10.1530/eje.1.01950. doi:10.1530/eje.1.01950. [DOI] [PubMed] [Google Scholar]
- 122.Zitzmann K, Andersen S, Vlotides G, Spottl G, Zhang S, Datta R. et al. The Novel SSTR2/D2R Chimeric Compound BIM-23A758 Decreases the Viability of Human GOT1 Midgut Carcinoid Cells. Neuroendocrinology. 2013 doi: 10.1159/000353784. doi:10.1159/000353784. [DOI] [PubMed] [Google Scholar]
- 123.Toumpanakis C, Caplin ME. Update on the role of somatostatin analogs for the treatment of patients with gastroenteropancreatic neuroendocrine tumors. Semin Oncol. 2013;40:56–68. doi: 10.1053/j.seminoncol.2012.11.006. doi:10.1053/j.seminoncol.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 124.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–19. doi: 10.1038/nrg1879. doi:10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 125.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–7. doi: 10.1126/science.296.5573.1655. doi:10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 126.Lawlor MA, Alessi DR. PKB/Akt: a key mediator of cell proliferation, survival and insulin responses? J Cell Sci. 2001;114:2903–10. doi: 10.1242/jcs.114.16.2903. [DOI] [PubMed] [Google Scholar]
- 127.Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB. et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7:261–9. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 128.Jiao Y, Shi C, Edil BH, de Wilde RF, Klimstra DS, Maitra A. et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science. 2011;331:1199–203. doi: 10.1126/science.1200609. doi:10.1126/science.1200609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Reidy-Lagunes DL, Vakiani E, Segal MF, Hollywood EM, Tang LH, Solit DB. et al. A phase 2 study of the insulin-like growth factor-1 receptor inhibitor MK-0646 in patients with metastatic, well-differentiated neuroendocrine tumors. Cancer. 2012;118:4795–800. doi: 10.1002/cncr.27459. doi:10.1002/cncr.27459. [DOI] [PubMed] [Google Scholar]
- 130.Couderc C, Poncet G, Villaume K, Blanc M, Gadot N, Walter T. et al. Targeting the PI3K/mTOR pathway in murine endocrine cell lines: in vitro and in vivo effects on tumor cell growth. Am J Pathol. 2011;178:336–44. doi: 10.1016/j.ajpath.2010.11.023. doi:10.1016/j.ajpath.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pitt SC, Chen H, Kunnimalaiyaan M. Inhibition of phosphatidylinositol 3-kinase/Akt signaling suppresses tumor cell proliferation and neuroendocrine marker expression in GI carcinoid tumors. Ann Surg Oncol. 2009;16:2936–42. doi: 10.1245/s10434-009-0591-5. doi:10.1245/s10434-009-0591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Villaume K, Blanc M, Gouysse G, Walter T, Couderc C, Nejjari M. et al. VEGF secretion by neuroendocrine tumor cells is inhibited by octreotide and by inhibitors of the PI3K/AKT/mTOR pathway. Neuroendocrinology. 2010;91:268–78. doi: 10.1159/000289569. doi:10.1159/000289569. [DOI] [PubMed] [Google Scholar]
- 133.Pfragner R, Behmel A, Hoger H, Beham A, Ingolic E, Stelzer I. et al. Establishment and characterization of three novel cell lines - P-STS, L-STS, H-STS - derived from a human metastatic midgut carcinoid. Anticancer Res. 2009;29:1951–61. [PubMed] [Google Scholar]
- 134.Svejda B, Kidd M, Kazberouk A, Lawrence B, Pfragner R, Modlin IM. Limitations in small intestinal neuroendocrine tumor therapy by mTor kinase inhibition reflect growth factor-mediated PI3K feedback loop activation via ERK1/2 and AKT. Cancer. 2011;117:4141–54. doi: 10.1002/cncr.26011. doi:10.1002/cncr.26011. [DOI] [PubMed] [Google Scholar]
- 135.Zitzmann K, Ruden J, Brand S, Goke B, Lichtl J, Spottl G. et al. Compensatory activation of Akt in response to mTOR and Raf inhibitors - a rationale for dual-targeted therapy approaches in neuroendocrine tumor disease. Cancer Lett. 2010;295:100–9. doi: 10.1016/j.canlet.2010.02.018. doi:10.1016/j.canlet.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 136.von Wichert G, Haeussler U, Greten FR, Kliche S, Dralle H, Bohm BO. et al. Regulation of cyclin D1 expression by autocrine IGF-I in human BON neuroendocrine tumour cells. Oncogene. 2005;24:1284–9. doi: 10.1038/sj.onc.1208264. doi:10.1038/sj.onc.1208264. [DOI] [PubMed] [Google Scholar]
- 137.Li J, Song J, Cassidy MG, Rychahou P, Starr ME, Liu J. et al. PI3K p110alpha/Akt signaling negatively regulates secretion of the intestinal peptide neurotensin through interference of granule transport. Mol Endocrinol. 2012;26:1380–93. doi: 10.1210/me.2012-1024. doi:10.1210/me.2012-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Clement S, Krause U, Desmedt F, Tanti JF, Behrends J, Pesesse X. et al. The lipid phosphatase SHIP2 controls insulin sensitivity. Nature. 2001;409:92–7. doi: 10.1038/35051094. doi:10.1038/35051094. [DOI] [PubMed] [Google Scholar]
- 139.Hafsi S, Pezzino FM, Candido S, Ligresti G, Spandidos DA, Soua Z. et al. Gene alterations in the PI3K/PTEN/AKT pathway as a mechanism of drug-resistance (review) Int J Oncol. 2012;40:639–44. doi: 10.3892/ijo.2011.1312. doi:10.3892/ijo.2011.1312. [DOI] [PubMed] [Google Scholar]
- 140.Maehama T, Dixon JE. PTEN: a tumour suppressor that functions as a phospholipid phosphatase. Trends Cell Biol. 1999;9:125–8. doi: 10.1016/s0962-8924(99)01519-6. [DOI] [PubMed] [Google Scholar]
- 141.Zhang S, Yu D. PI(3)king apart PTEN's role in cancer. Clin Cancer Res. 2010;16:4325–30. doi: 10.1158/1078-0432.CCR-09-2990. doi:10.1158/1078-0432.ccr-09-2990. [DOI] [PubMed] [Google Scholar]
- 142.Ramaswamy S, Nakamura N, Vazquez F, Batt DB, Perera S, Roberts TM. et al. Regulation of G1 progression by the PTEN tumor suppressor protein is linked to inhibition of the phosphatidylinositol 3-kinase/Akt pathway. Proc Natl Acad Sci U S A. 1999;96:2110–5. doi: 10.1073/pnas.96.5.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Myers MP, Pass I, Batty IH, Van der Kaay J, Stolarov JP, Hemmings BA. et al. The lipid phosphatase activity of PTEN is critical for its tumor supressor function. Proc Natl Acad Sci U S A. 1998;95:13513–8. doi: 10.1073/pnas.95.23.13513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Furnari FB, Huang HJ, Cavenee WK. The phosphoinositol phosphatase activity of PTEN mediates a serum-sensitive G1 growth arrest in glioma cells. Cancer Res. 1998;58:5002–8. [PubMed] [Google Scholar]
- 145.Leslie NR, Foti M. Non-genomic loss of PTEN function in cancer: not in my genes. Trends Pharmacol Sci. 2011;32:131–40. doi: 10.1016/j.tips.2010.12.005. doi:10.1016/j.tips.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 146.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8:627–44. doi: 10.1038/nrd2926. doi:10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Salmena L, Carracedo A, Pandolfi PP. Tenets of PTEN tumor suppression. Cell. 2008;133:403–14. doi: 10.1016/j.cell.2008.04.013. doi:10.1016/j.cell.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 148.Song MS, Carracedo A, Salmena L, Song SJ, Egia A, Malumbres M. et al. Nuclear PTEN regulates the APC-CDH1 tumor-suppressive complex in a phosphatase-independent manner. Cell. 2011;144:187–99. doi: 10.1016/j.cell.2010.12.020. doi:10.1016/j.cell.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lachyankar MB, Sultana N, Schonhoff CM, Mitra P, Poluha W, Lambert S. et al. A role for nuclear PTEN in neuronal differentiation. J Neurosci. 2000;20:1404–13. doi: 10.1523/JNEUROSCI.20-04-01404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ginn-Pease ME, Eng C. Increased nuclear phosphatase and tensin homologue deleted on chromosome 10 is associated with G0-G1 in MCF-7 cells. Cancer Res. 2003;63:282–6. [PubMed] [Google Scholar]
- 151.Gil A, Andres-Pons A, Fernandez E, Valiente M, Torres J, Cervera J. et al. Nuclear localization of PTEN by a Ran-dependent mechanism enhances apoptosis: Involvement of an N-terminal nuclear localization domain and multiple nuclear exclusion motifs. Mol Biol Cell. 2006;17:4002–13. doi: 10.1091/mbc.E06-05-0380. doi:10.1091/mbc.E06-05-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chang CJ, Mulholland DJ, Valamehr B, Mosessian S, Sellers WR, Wu H. PTEN nuclear localization is regulated by oxidative stress and mediates p53-dependent tumor suppression. Mol Cell Biol. 2008;28:3281–9. doi: 10.1128/MCB.00310-08. doi:10.1128/mcb.00310-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gimm O, Attie-Bitach T, Lees JA, Vekemans M, Eng C. Expression of the PTEN tumour suppressor protein during human development. Hum Mol Genet. 2000;9:1633–9. doi: 10.1093/hmg/9.11.1633. [DOI] [PubMed] [Google Scholar]
- 154.Perren A, Komminoth P, Saremaslani P, Matter C, Feurer S, Lees JA. et al. Mutation and expression analyses reveal differential subcellular compartmentalization of PTEN in endocrine pancreatic tumors compared to normal islet cells. Am J Pathol. 2000;157:1097–103. doi: 10.1016/S0002-9440(10)64624-X. doi:10.1016/s0002-9440(10)64624-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Trotman LC, Wang X, Alimonti A, Chen Z, Teruya-Feldstein J, Yang H. et al. Ubiquitination regulates PTEN nuclear import and tumor suppression. Cell. 2007;128:141–56. doi: 10.1016/j.cell.2006.11.040. doi:10.1016/j.cell.2006.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tamura M, Gu J, Takino T, Yamada KM. Tumor suppressor PTEN inhibition of cell invasion, migration, and growth: differential involvement of focal adhesion kinase and p130Cas. Cancer Res. 1999;59:442–9. [PubMed] [Google Scholar]
- 157.Kim JS, Xu X, Li H, Solomon D, Lane WS, Jin T. et al. Mechanistic analysis of a DNA damage-induced, PTEN-dependent size checkpoint in human cells. Mol Cell Biol. 2011;31:2756–71. doi: 10.1128/MCB.01323-10. doi:10.1128/mcb.01323-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Putz U, Howitt J, Doan A, Goh CP, Low LH, Silke J. et al. The tumor suppressor PTEN is exported in exosomes and has phosphatase activity in recipient cells. Sci Signal. 2012;5:ra70.. doi: 10.1126/scisignal.2003084. doi:10.1126/scisignal.2003084. [DOI] [PubMed] [Google Scholar]
- 159.Speel EJ, Richter J, Moch H, Egenter C, Saremaslani P, Rutimann K. et al. Genetic differences in endocrine pancreatic tumor subtypes detected by comparative genomic hybridization. Am J Pathol. 1999;155:1787–94. doi: 10.1016/S0002-9440(10)65495-8. doi:10.1016/s0002-9440(10)65495-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Floridia G, Grilli G, Salvatore M, Pescucci C, Moore PS, Scarpa A. et al. Chromosomal alterations detected by comparative genomic hybridization in nonfunctioning endocrine pancreatic tumors. Cancer Genet Cytogenet. 2005;156:23–30. doi: 10.1016/j.cancergencyto.2004.04.015. doi:10.1016/j.cancergencyto.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 161.Dacic S, Finkelstein SD, Baksh FK, Swalsky PA, Barnes LE, Yousem SA. Small-cell neuroendocrine carcinoma displays unique profiles of tumor-suppressor gene loss in relationship to the primary site of formation. Hum Pathol. 2002;33:927–32. doi: 10.1053/hupa.2002.126875. [DOI] [PubMed] [Google Scholar]
- 162.Krausch M, Raffel A, Anlauf M, Schott M, Willenberg H, Lehwald N. et al. Loss of PTEN expression in neuroendocrine pancreatic tumors. Horm Metab Res. 2011;43:865–71. doi: 10.1055/s-0031-1291333. doi:10.1055/s-0031-1291333. [DOI] [PubMed] [Google Scholar]
- 163.Roldo C, Missiaglia E, Hagan JP, Falconi M, Capelli P, Bersani S. et al. MicroRNA expression abnormalities in pancreatic endocrine and acinar tumors are associated with distinctive pathologic features and clinical behavior. J Clin Oncol. 2006;24:4677–84. doi: 10.1200/JCO.2005.05.5194. doi:10.1200/jco.2005.05.5194. [DOI] [PubMed] [Google Scholar]
- 164.Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133:647–58. doi: 10.1053/j.gastro.2007.05.022. doi:10.1053/j.gastro.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cingarlini S, Bonomi M, Corbo V, Scarpa A, Tortora G. Profiling mTOR pathway in neuroendocrine tumors. Target Oncol. 2012;7:183–8. doi: 10.1007/s11523-012-0226-9. doi:10.1007/s11523-012-0226-9. [DOI] [PubMed] [Google Scholar]
- 166.Han X, Ji Y, Zhao J, Xu X, Lou W. Expression of PTEN and mTOR in pancreatic neuroendocrine tumors. Tumour Biol. 2013. doi:10.1007/s13277-013-0849-1. [DOI] [PubMed]
- 167.Weisbrod AB, Zhang L, Jain M, Barak S, Quezado MM, Kebebew E. Altered PTEN, ATRX, CHGA, CHGB, and TP53 Expression Are Associated with Aggressive VHL-Associated Pancreatic Neuroendocrine Tumors. Horm Cancer. 2013;4:165–75. doi: 10.1007/s12672-013-0134-1. doi:10.1007/s12672-013-0134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.O'Toole D, Couvelard A, Rebours V, Zappa M, Hentic O, Hammel P. et al. Molecular markers associated with response to chemotherapy in gastro-entero-pancreatic neuroendocrine tumors. Endocr Relat Cancer. 2010;17:847–56. doi: 10.1677/ERC-09-0204. doi:10.1677/erc-09-0204. [DOI] [PubMed] [Google Scholar]
- 169.Gloesenkamp CR, Nitzsche B, Ocker M, Di Fazio P, Quint K, Hoffmann B. et al. AKT inhibition by triciribine alone or as combination therapy for growth control of gastroenteropancreatic neuroendocrine tumors. Int J Oncol. 2012;40:876–88. doi: 10.3892/ijo.2011.1256. doi:10.3892/ijo.2011.1256. [DOI] [PubMed] [Google Scholar]
- 170.Evers BM, Ishizuka J, Townsend CM Jr, Thompson JC. The human carcinoid cell line, BON. A model system for the study of carcinoid tumors. Ann N Y Acad Sci. 1994;733:393–406. doi: 10.1111/j.1749-6632.1994.tb17289.x. [DOI] [PubMed] [Google Scholar]
- 171.Gueli N, Toto A, Palmieri G, Carmenini G, Delpino A, Ferrini U. In vitro growth of a cell line originated from a human insulinoma. J Exp Clin Cancer Res. 1987. pp. 281–5.
- 172.Wang L, Ignat A, Axiotis CA. Differential expression of the PTEN tumor suppressor protein in fetal and adult neuroendocrine tissues and tumors: progressive loss of PTEN expression in poorly differentiated neuroendocrine neoplasms. Appl Immunohistochem Mol Morphol. 2002;10:139–46. doi: 10.1097/00129039-200206000-00008. [DOI] [PubMed] [Google Scholar]
- 173.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. doi:10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 174.Chen WS, Xu PZ, Gottlob K, Chen ML, Sokol K, Shiyanova T. et al. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 2001;15:2203–8. doi: 10.1101/gad.913901. doi:10.1101/gad.913901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB 3rd. et al. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta) Science. 2001;292:1728–31. doi: 10.1126/science.292.5522.1728. doi:10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 176.Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem. 2001;276:38349–52. doi: 10.1074/jbc.C100462200. doi:10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- 177.Garofalo RS, Orena SJ, Rafidi K, Torchia AJ, Stock JL, Hildebrandt AL. et al. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKB beta. J Clin Invest. 2003;112:197–208. doi: 10.1172/JCI16885. doi:10.1172/jci16885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yang ZZ, Tschopp O, Hemmings-Mieszczak M, Feng J, Brodbeck D, Perentes E. et al. Protein kinase B alpha/Akt1 regulates placental development and fetal growth. J Biol Chem. 2003;278:32124–31. doi: 10.1074/jbc.M302847200. doi:10.1074/jbc.M302847200. [DOI] [PubMed] [Google Scholar]
- 179.Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P. et al. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–51. [PMC free article] [PubMed] [Google Scholar]
- 180.Alessi DR. Discovery of PDK1, one of the missing links in insulin signal transduction. Colworth Medal Lecture. Biochem Soc Trans. 2001;29:1–14. doi: 10.1042/0300-5127:0290001. [DOI] [PubMed] [Google Scholar]
- 181.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–101. doi: 10.1126/science.1106148. doi:10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 182.Bozulic L, Hemmings BA. PIKKing on PKB: regulation of PKB activity by phosphorylation. Curr Opin Cell Biol. 2009;21:256–61. doi: 10.1016/j.ceb.2009.02.002. doi:10.1016/j.ceb.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 183.Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J. et al. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell. 2006;11:859–71. doi: 10.1016/j.devcel.2006.10.007. doi:10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 184.Garcia-Martinez JM, Moran J, Clarke RG, Gray A, Cosulich SC, Chresta CM. et al. Ku-0063794 is a specific inhibitor of the mammalian target of rapamycin (mTOR) Biochem J. 2009;421:29–42. doi: 10.1042/BJ20090489. doi:10.1042/bj20090489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Inoki K, Li Y, Xu T, Guan KL. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003;17:1829–34. doi: 10.1101/gad.1110003. doi:10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS. et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–68. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 187.Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499–511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Vadlakonda L, Pasupuleti M, Pallu R. Role of PI3K-AKT-mTOR and Wnt Signaling Pathways in Transition of G1-S Phase of Cell Cycle in Cancer Cells. Front Oncol. 2013;3:85.. doi: 10.3389/fonc.2013.00085. doi:10.3389/fonc.2013.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Markman B, Dienstmann R, Tabernero J. Targeting the PI3K/Akt/mTOR pathway--beyond rapalogs. Oncotarget. 2010;1:530–43. doi: 10.18632/oncotarget.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY. et al. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–37. doi: 10.1016/j.cell.2006.08.033. doi:10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 191.Vincent EE, Elder DJ, Thomas EC, Phillips L, Morgan C, Pawade J. et al. Akt phosphorylation on Thr308 but not on Ser473 correlates with Akt protein kinase activity in human non-small cell lung cancer. Br J Cancer. 2011;104:1755–61. doi: 10.1038/bjc.2011.132. doi:10.1038/bjc.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ghayouri M, Boulware D, Nasir A, Strosberg J, Kvols L, Coppola D. Activation of the serine/theronine protein kinase Akt in enteropancreatic neuroendocrine tumors. Anticancer Res. 2010;30:5063–7. [PubMed] [Google Scholar]
- 193.Noh WC, Mondesire WH, Peng J, Jian W, Zhang H, Dong J. et al. Determinants of rapamycin sensitivity in breast cancer cells. Clin Cancer Res. 2004;10:1013–23. doi: 10.1158/1078-0432.ccr-03-0043. [DOI] [PubMed] [Google Scholar]
- 194.Breuleux M, Klopfenstein M, Stephan C, Doughty CA, Barys L, Maira SM. et al. Increased AKT S473 phosphorylation after mTORC1 inhibition is rictor dependent and does not predict tumor cell response to PI3K/mTOR inhibition. Mol Cancer Ther. 2009;8:742–53. doi: 10.1158/1535-7163.MCT-08-0668. doi:10.1158/1535-7163.mct-08-0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Meric-Bernstam F, Akcakanat A, Chen H, Do KA, Sangai T, Adkins F. et al. PIK3CA/PTEN mutations and Akt activation as markers of sensitivity to allosteric mTOR inhibitors. Clin Cancer Res. 2012;18:1777–89. doi: 10.1158/1078-0432.CCR-11-2123. doi:10.1158/1078-0432.ccr-11-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zitzmann K, Vlotides G, Brand S, Lahm H, Spottl G, Goke B. et al. Perifosine-mediated Akt inhibition in neuroendocrine tumor cells: role of specific Akt isoforms. Endocr Relat Cancer. 2012;19:423–34. doi: 10.1530/ERC-12-0074. doi:10.1530/erc-12-0074. [DOI] [PubMed] [Google Scholar]
- 197.Yang Q, Guan KL. Expanding mTOR signaling. Cell Res. 2007;17:666–81. doi: 10.1038/cr.2007.64. doi:10.1038/cr.2007.64. [DOI] [PubMed] [Google Scholar]
- 198.Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, Hidayat S. et al. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002;110:177–89. doi: 10.1016/s0092-8674(02)00833-4. [DOI] [PubMed] [Google Scholar]
- 199.Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H. et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–75. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 200.Sancak Y, Thoreen CC, Peterson TR, Lindquist RA, Kang SA, Spooner E. et al. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell. 2007;25:903–15. doi: 10.1016/j.molcel.2007.03.003. doi:10.1016/j.molcel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 201.Peterson TR, Laplante M, Thoreen CC, Sancak Y, Kang SA, Kuehl WM. et al. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell. 2009;137:873–86. doi: 10.1016/j.cell.2009.03.046. doi:10.1016/j.cell.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kim DH, Sarbassov DD, Ali SM, Latek RR, Guntur KV, Erdjument-Bromage H. et al. GbetaL, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between raptor and mTOR. Mol Cell. 2003;11:895–904. doi: 10.1016/s1097-2765(03)00114-x. [DOI] [PubMed] [Google Scholar]
- 203.Zeng Z, Sarbassov dos D, Samudio IJ, Yee KW, Munsell MF, Ellen Jackson C. et al. Rapamycin derivatives reduce mTORC2 signaling and inhibit AKT activation in AML. Blood. 2007;109:3509–12. doi: 10.1182/blood-2006-06-030833. doi:10.1182/blood-2006-06-030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.O'Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D. et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–8. doi: 10.1158/0008-5472.CAN-05-2925. doi:10.1158/0008-5472.can-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Shepherd PR, Nave BT, O'Rahilly S. The role of phosphoinositide 3-kinase in insulin signalling. J Mol Endocrinol. 1996;17:175–84. doi: 10.1677/jme.0.0170175. [DOI] [PubMed] [Google Scholar]
- 206.Peng T, Golub TR, Sabatini DM. The immunosuppressant rapamycin mimics a starvation-like signal distinct from amino acid and glucose deprivation. Mol Cell Biol. 2002;22:5575–84. doi: 10.1128/MCB.22.15.5575-5584.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hannan KM, Brandenburger Y, Jenkins A, Sharkey K, Cavanaugh A, Rothblum L. et al. mTOR-dependent regulation of ribosomal gene transcription requires S6K1 and is mediated by phosphorylation of the carboxy-terminal activation domain of the nucleolar transcription factor UBF. Mol Cell Biol. 2003;23:8862–77. doi: 10.1128/MCB.23.23.8862-8877.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Mayer C, Zhao J, Yuan X, Grummt I. mTOR-dependent activation of the transcription factor TIF-IA links rRNA synthesis to nutrient availability. Genes Dev. 2004;18:423–34. doi: 10.1101/gad.285504. doi:10.1101/gad.285504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Nojima H, Tokunaga C, Eguchi S, Oshiro N, Hidayat S, Yoshino K. et al. The mammalian target of rapamycin (mTOR) partner, raptor, binds the mTOR substrates p70 S6 kinase and 4E-BP1 through their TOR signaling (TOS) motif. J Biol Chem. 2003;278:15461–4. doi: 10.1074/jbc.C200665200. doi:10.1074/jbc.C200665200. [DOI] [PubMed] [Google Scholar]
- 210.Faivre S, Kroemer G, Raymond E. Current development of mTOR inhibitors as anticancer agents. Nat Rev Drug Discov. 2006;5:671–88. doi: 10.1038/nrd2062. doi:10.1038/nrd2062. [DOI] [PubMed] [Google Scholar]
- 211.Dennis PB, Pullen N, Kozma SC, Thomas G. The principal rapamycin-sensitive p70(s6k) phosphorylation sites, T-229 and T-389, are differentially regulated by rapamycin-insensitive kinase kinases. Mol Cell Biol. 1996;16:6242–51. doi: 10.1128/mcb.16.11.6242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Pause A, Belsham GJ, Gingras AC, Donze O, Lin TA, Lawrence JC Jr. et al. Insulin-dependent stimulation of protein synthesis by phosphorylation of a regulator of 5'-cap function. Nature. 1994;371:762–7. doi: 10.1038/371762a0. doi:10.1038/371762a0. [DOI] [PubMed] [Google Scholar]
- 213.Redpath NT, Foulstone EJ, Proud CG. Regulation of translation elongation factor-2 by insulin via a rapamycin-sensitive signalling pathway. EMBO J. 1996;15:2291–7. [PMC free article] [PubMed] [Google Scholar]
- 214.Seidel ER, Ragan VL. Inhibition by rapamycin of ornithine decarboxylase and epithelial cell proliferation in intestinal IEC-6 cells in culture. Br J Pharmacol. 1997;120:571–4. doi: 10.1038/sj.bjp.0700936. doi:10.1038/sj.bjp.0700936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Azpiazu I, Saltiel AR, DePaoli-Roach AA, Lawrence JC. Regulation of both glycogen synthase and PHAS-I by insulin in rat skeletal muscle involves mitogen-activated protein kinase-independent and rapamycin-sensitive pathways. J Biol Chem. 1996;271:5033–9. doi: 10.1074/jbc.271.9.5033. [DOI] [PubMed] [Google Scholar]
- 216.Hudson CC, Liu M, Chiang GG, Otterness DM, Loomis DC, Kaper F. et al. Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin. Mol Cell Biol. 2002;22:7004–14. doi: 10.1128/MCB.22.20.7004-7014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Huffman TA, Mothe-Satney I, Lawrence JC Jr. Insulin-stimulated phosphorylation of lipin mediated by the mammalian target of rapamycin. Proc Natl Acad Sci U S A. 2002;99:1047–52. doi: 10.1073/pnas.022634399. doi:10.1073/pnas.022634399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Parekh D, Ziegler W, Yonezawa K, Hara K, Parker PJ. Mammalian TOR controls one of two kinase pathways acting upon nPKCdelta and nPKCepsilon. J Biol Chem. 1999;274:34758–64. doi: 10.1074/jbc.274.49.34758. [DOI] [PubMed] [Google Scholar]
- 219.Peterson RT, Desai BN, Hardwick JS, Schreiber SL. Protein phosphatase 2A interacts with the 70-kDa S6 kinase and is activated by inhibition of FKBP12-rapamycinassociated protein. Proc Natl Acad Sci U S A. 1999;96:4438–42. doi: 10.1073/pnas.96.8.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Huang S, Liu LN, Hosoi H, Dilling MB, Shikata T, Houghton PJ. p53/p21(CIP1) cooperate in enforcing rapamycin-induced G(1) arrest and determine the cellular response to rapamycin. Cancer Res. 2001;61:3373–81. [PubMed] [Google Scholar]
- 221.Nourse J, Firpo E, Flanagan WM, Coats S, Polyak K, Lee MH. et al. Interleukin-2-mediated elimination of the p27Kip1 cyclin-dependent kinase inhibitor prevented by rapamycin. Nature. 1994;372:570–3. doi: 10.1038/372570a0. doi:10.1038/372570a0. [DOI] [PubMed] [Google Scholar]
- 222.Usui I, Haruta T, Iwata M, Takano A, Uno T, Kawahara J. et al. Retinoblastoma protein phosphorylation via PI 3-kinase and mTOR pathway regulates adipocyte differentiation. Biochem Biophys Res Commun. 2000;275:115–20. doi: 10.1006/bbrc.2000.3201. doi:10.1006/bbrc.2000.3201. [DOI] [PubMed] [Google Scholar]
- 223.Yokogami K, Wakisaka S, Avruch J, Reeves SA. Serine phosphorylation and maximal activation of STAT3 during CNTF signaling is mediated by the rapamycin target mTOR. Curr Biol. 2000;10:47–50. doi: 10.1016/s0960-9822(99)00268-7. [DOI] [PubMed] [Google Scholar]
- 224.Frias MA, Thoreen CC, Jaffe JD, Schroder W, Sculley T, Carr SA. et al. mSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2s. Curr Biol. 2006;16:1865–70. doi: 10.1016/j.cub.2006.08.001. doi:10.1016/j.cub.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 225.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H. et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–302. doi: 10.1016/j.cub.2004.06.054. doi:10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 226.Pearce LR, Huang X, Boudeau J, Pawlowski R, Wullschleger S, Deak M. et al. Identification of Protor as a novel Rictor-binding component of mTOR complex-2. Biochem J. 2007;405:513–22. doi: 10.1042/BJ20070540. doi:10.1042/bj20070540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Martin J, Masri J, Bernath A, Nishimura RN, Gera J. Hsp70 associates with Rictor and is required for mTORC2 formation and activity. Biochem Biophys Res Commun. 2008;372:578–83. doi: 10.1016/j.bbrc.2008.05.086. doi:10.1016/j.bbrc.2008.05.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Zierhut B, Mechtler K, Gartner W, Daneva T, Base W, Weissel M. et al. Heat shock protein 70 (Hsp70) subtype expression in neuroendocrine tissue and identification of a neuroendocrine tumour-specific Hsp70 truncation. Endocr Relat Cancer. 2004;11:377–89. doi: 10.1677/erc.0.0110377. [DOI] [PubMed] [Google Scholar]
- 229.Hresko RC, Mueckler M. mTOR.RICTOR is the Ser473 kinase for Akt/protein kinase B in 3T3-L1 adipocytes. J Biol Chem. 2005;280:40406–16. doi: 10.1074/jbc.M508361200. doi:10.1074/jbc.M508361200. [DOI] [PubMed] [Google Scholar]
- 230.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF. et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–68. doi: 10.1016/j.molcel.2006.03.029. doi:10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 231.Gulhati P, Bowen KA, Liu J, Stevens PD, Rychahou PG, Chen M. et al. mTORC1 and mTORC2 regulate EMT, motility, and metastasis of colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Res. 2011;71:3246–56. doi: 10.1158/0008-5472.CAN-10-4058. doi:10.1158/0008-5472.can-10-4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue P, Fu H. et al. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005;65:7052–8. doi: 10.1158/0008-5472.CAN-05-0917. doi:10.1158/0008-5472.can-05-0917. [DOI] [PubMed] [Google Scholar]
- 233.Shi Y, Yan H, Frost P, Gera J, Lichtenstein A. Mammalian target of rapamycin inhibitors activate the AKT kinase in multiple myeloma cells by up-regulating the insulin-like growth factor receptor/insulin receptor substrate-1/phosphatidylinositol 3-kinase cascade. Mol Cancer Ther. 2005;4:1533–40. doi: 10.1158/1535-7163.MCT-05-0068. doi:10.1158/1535-7163.mct-05-0068. [DOI] [PubMed] [Google Scholar]
- 234.Rodrik-Outmezguine VS, Chandarlapaty S, Pagano NC, Poulikakos PI, Scaltriti M, Moskatel E. et al. mTOR kinase inhibition causes feedback-dependent biphasic regulation of AKT signaling. Cancer Discov. 2011;1:248–59. doi: 10.1158/2159-8290.CD-11-0085. doi:10.1158/2159-8290.cd-11-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Catena L, Bajetta E, Milione M, Ducceschi M, Valente M, Dominoni F. et al. Mammalian target of rapamycin expression in poorly differentiated endocrine carcinoma: clinical and therapeutic future challenges. Target Oncol. 2011;6:65–8. doi: 10.1007/s11523-011-0171-z. doi:10.1007/s11523-011-0171-z. [DOI] [PubMed] [Google Scholar]
- 236.Shida T, Kishimoto T, Furuya M, Nikaido T, Koda K, Takano S. et al. Expression of an activated mammalian target of rapamycin (mTOR) in gastroenteropancreatic neuroendocrine tumors. Cancer Chemother Pharmacol. 2010;65:889–93. doi: 10.1007/s00280-009-1094-6. doi:10.1007/s00280-009-1094-6. [DOI] [PubMed] [Google Scholar]
- 237.Kasajima A, Pavel M, Darb-Esfahani S, Noske A, Stenzinger A, Sasano H. et al. mTOR expression and activity patterns in gastroenteropancreatic neuroendocrine tumours. Endocr Relat Cancer. 2011;18:181–92. doi: 10.1677/ERC-10-0126. doi:10.1677/erc-10-0126. [DOI] [PubMed] [Google Scholar]
- 238.Larson AM, Hedgire SS, Deshpande V, Stemmer-Rachamimov AO, Harisinghani MG, Ferrone CR. et al. Pancreatic neuroendocrine tumors in patients with tuberous sclerosis complex. Clin Genet. 2012;82:558–63. doi: 10.1111/j.1399-0004.2011.01805.x. doi:10.1111/j.1399-0004.2011.01805.x. [DOI] [PubMed] [Google Scholar]
- 239.Mendoza MC, Er EE, Blenis J. The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem Sci. 2011;36:320–8. doi: 10.1016/j.tibs.2011.03.006. doi:10.1016/j.tibs.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Zitzmann K, De Toni EN, Brand S, Goke B, Meinecke J, Spottl G. et al. The novel mTOR inhibitor RAD001 (everolimus) induces antiproliferative effects in human pancreatic neuroendocrine tumor cells. Neuroendocrinology. 2007;85:54–60. doi: 10.1159/000100057. doi:10.1159/000100057. [DOI] [PubMed] [Google Scholar]
- 241.Chiu CW, Nozawa H, Hanahan D. Survival benefit with proapoptotic molecular and pathologic responses from dual targeting of mammalian target of rapamycin and epidermal growth factor receptor in a preclinical model of pancreatic neuroendocrine carcinogenesis. J Clin Oncol. 2010;28:4425–33. doi: 10.1200/JCO.2010.28.0198. doi:10.1200/jco.2010.28.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Bollard J, Couderc C, Blanc M, Poncet G, Lepinasse F, Hervieu V. et al. Antitumor effect of everolimus in preclinical models of high-grade gastroenteropancreatic neuroendocrine carcinomas. Neuroendocrinology. 2013;97:331–40. doi: 10.1159/000347063. doi:10.1159/000347063. [DOI] [PubMed] [Google Scholar]
- 243.Yao JC, Lombard-Bohas C, Baudin E, Kvols LK, Rougier P, Ruszniewski P. et al. Daily oral everolimus activity in patients with metastatic pancreatic neuroendocrine tumors after failure of cytotoxic chemotherapy: a phase II trial. J Clin Oncol. 2010;28:69–76. doi: 10.1200/JCO.2009.24.2669. doi:10.1200/jco.2009.24.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E. et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–23. doi: 10.1056/NEJMoa1009290. doi:10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Yao JC, Phan AT, Jehl V, Shah G, Meric-Bernstam F. Everolimus in advanced pancreatic neuroendocrine tumors: the clinical experience. Cancer Res. 2013;73:1449–53. doi: 10.1158/0008-5472.CAN-12-3923. doi:10.1158/0008-5472.can-12-3923. [DOI] [PubMed] [Google Scholar]
- 246.Azzoni C, Bottarelli L, Cecchini S, Lagrasta C, Pizzi S, D'Adda T. et al. Involvement of HER-2/neu and metastasis-related proteins in the development of ileal neuroendocrine tumors. Virchows Arch. 2011;458:525–36. doi: 10.1007/s00428-011-1069-y. doi:10.1007/s00428-011-1069-y. [DOI] [PubMed] [Google Scholar]
- 247.Cuevas BD, Abell AN, Johnson GL. Role of mitogen-activated protein kinase kinase kinases in signal integration. Oncogene. 2007;26:3159–71. doi: 10.1038/sj.onc.1210409. doi:10.1038/sj.onc.1210409. [DOI] [PubMed] [Google Scholar]
- 248.Chong H, Vikis HG, Guan KL. Mechanisms of regulating the Raf kinase family. Cell Signal. 2003;15:463–9. doi: 10.1016/s0898-6568(02)00139-0. [DOI] [PubMed] [Google Scholar]
- 249.Vetter IR, Wittinghofer A. The guanine nucleotide-binding switch in three dimensions. Science. 2001;294:1299–304. doi: 10.1126/science.1062023. doi:10.1126/science.1062023. [DOI] [PubMed] [Google Scholar]
- 250.Shields JM, Pruitt K, McFall A, Shaub A, Der CJ. Understanding Ras: 'it ain't over 'til it's over'. Trends Cell Biol. 2000;10:147–54. doi: 10.1016/s0962-8924(00)01740-2. [DOI] [PubMed] [Google Scholar]
- 251.Kawesha A, Ghaneh P, Andren-Sandberg A, Ograed D, Skar R, Dawiskiba S. et al. K-ras oncogene subtype mutations are associated with survival but not expression of p53, p16(INK4A), p21(WAF-1), cyclin D1, erbB-2 and erbB-3 in resected pancreatic ductal adenocarcinoma. Int J Cancer. 2000;89:469–74. doi: 10.1002/1097-0215(20001120)89:6<469::aid-ijc1>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 252.Moskaluk CA, Hruban RH, Kern SE. p16 and K-ras gene mutations in the intraductal precursors of human pancreatic adenocarcinoma. Cancer Res. 1997;57:2140–3. [PubMed] [Google Scholar]
- 253.Kimura W, Zhao B, Futakawa N, Muto T, Makuuchi M. Significance of K-ras codon 12 point mutation in pancreatic juice in the diagnosis of carcinoma of the pancreas. Hepatogastroenterology. 1999;46:532–9. [PubMed] [Google Scholar]
- 254.Jancik S, Drabek J, Radzioch D, Hajduch M. Clinical relevance of KRAS in human cancers. J Biomed Biotechnol. 2010;2010:150960.. doi: 10.1155/2010/150960. doi:10.1155/2010/150960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Ellenbroek SI, Collard JG. Rho GTPases: functions and association with cancer. Clin Exp Metastasis. 2007;24:657–72. doi: 10.1007/s10585-007-9119-1. doi:10.1007/s10585-007-9119-1. [DOI] [PubMed] [Google Scholar]
- 256.Vigil D, Cherfils J, Rossman KL, Der CJ. Ras superfamily GEFs and GAPs: validated and tractable targets for cancer therapy? Nat Rev Cancer. 2010;10:842–57. doi: 10.1038/nrc2960. doi:10.1038/nrc2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Vigil D, Martin TD, Williams F, Yeh JJ, Campbell SL, Der CJ. Aberrant overexpression of the Rgl2 Ral small GTPase-specific guanine nucleotide exchange factor promotes pancreatic cancer growth through Ral-dependent and Ral-independent mechanisms. J Biol Chem. 2010;285:34729–40. doi: 10.1074/jbc.M110.116756. doi:10.1074/jbc.M110.116756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Prenen H, Tejpar S, Van Cutsem E. New strategies for treatment of KRAS mutant metastatic colorectal cancer. Clin Cancer Res. 2010;16:2921–6. doi: 10.1158/1078-0432.CCR-09-2029. doi:10.1158/1078-0432.ccr-09-2029. [DOI] [PubMed] [Google Scholar]
- 259.Salhia B, Tran NL, Chan A, Wolf A, Nakada M, Rutka F. et al. The guanine nucleotide exchange factors trio, Ect2, and Vav3 mediate the invasive behavior of glioblastoma. Am J Pathol. 2008;173:1828–38. doi: 10.2353/ajpath.2008.080043. doi:10.2353/ajpath.2008.080043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Patel V, Rosenfeldt HM, Lyons R, Servitja JM, Bustelo XR, Siroff M. et al. Persistent activation of Rac1 in squamous carcinomas of the head and neck: evidence for an EGFR/Vav2 signaling axis involved in cell invasion. Carcinogenesis. 2007;28:1145–52. doi: 10.1093/carcin/bgm008. doi:10.1093/carcin/bgm008. [DOI] [PubMed] [Google Scholar]
- 261.Qin J, Xie Y, Wang B, Hoshino M, Wolff DW, Zhao J. et al. Upregulation of PIP3-dependent Rac exchanger 1 (P-Rex1) promotes prostate cancer metastasis. Oncogene. 2009;28:1853–63. doi: 10.1038/onc.2009.30. doi:10.1038/onc.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Lambert JM, Lambert QT, Reuther GW, Malliri A, Siderovski DP, Sondek J. et al. Tiam1 mediates Ras activation of Rac by a PI(3)K-independent mechanism. Nat Cell Biol. 2002;4:621–5. doi: 10.1038/ncb833. doi:10.1038/ncb833. [DOI] [PubMed] [Google Scholar]
- 263.Tannapfel A, Vomschloss S, Karhoff D, Markwarth A, Hengge UR, Wittekind C. et al. BRAF gene mutations are rare events in gastroenteropancreatic neuroendocrine tumors. Am J Clin Pathol. 2005;123:256–60. [PubMed] [Google Scholar]
- 264.Paraskevakou H, Saetta A, Skandalis K, Tseleni S, Athanassiadis A, Davaris PS. Morphological-histochemical study of intestinal carcinoids and K-ras mutation analysis in appendiceal carcinoids. Pathol Oncol Res. 1999;5:205–10. doi: 10.1053/paor.1999.0193. [DOI] [PubMed] [Google Scholar]
- 265.Dammann R, Schagdarsurengin U, Liu L, Otto N, Gimm O, Dralle H. et al. Frequent RASSF1A promoter hypermethylation and K-ras mutations in pancreatic carcinoma. Oncogene. 2003;22:3806–12. doi: 10.1038/sj.onc.1206582. doi:10.1038/sj.onc.1206582. [DOI] [PubMed] [Google Scholar]
- 266.Liedke M, Karnbach C, Kalinin V, Herbst B, Frilling A, Broelsch CE. [Detection of H-ras and K-ras in tumors of gastrointestinal-pancreatic system] Langenbecks Arch Chir Suppl Kongressbd. 1998;115:255–9. [PubMed] [Google Scholar]
- 267.Morrison DK, Cutler RE. The complexity of Raf-1 regulation. Curr Opin Cell Biol. 1997;9:174–9. doi: 10.1016/s0955-0674(97)80060-9. [DOI] [PubMed] [Google Scholar]
- 268.Wittinghofer A, Nassar N. How Ras-related proteins talk to their effectors. Trends Biochem Sci. 1996;21:488–91. doi: 10.1016/s0968-0004(96)10064-5. [DOI] [PubMed] [Google Scholar]
- 269.Mason CS, Springer CJ, Cooper RG, Superti-Furga G, Marshall CJ, Marais R. Serine and tyrosine phosphorylations cooperate in Raf-1, but not B-Raf activation. EMBO J. 1999;18:2137–48. doi: 10.1093/emboj/18.8.2137. doi:10.1093/emboj/18.8.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Zhang BH, Guan KL. Activation of B-Raf kinase requires phosphorylation of the conserved residues Thr598 and Ser601. EMBO J. 2000;19:5429–39. doi: 10.1093/emboj/19.20.5429. doi:10.1093/emboj/19.20.5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Emuss V, Garnett M, Mason C, Marais R. Mutations of C-RAF are rare in human cancer because C-RAF has a low basal kinase activity compared with B-RAF. Cancer Res. 2005;65:9719–26. doi: 10.1158/0008-5472.CAN-05-1683. doi:10.1158/0008-5472.can-05-1683. [DOI] [PubMed] [Google Scholar]
- 272.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S. et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. doi:10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 273.Kumar SM, Yu H, Edwards R, Chen L, Kazianis S, Brafford P. et al. Mutant V600E BRAF increases hypoxia inducible factor-1alpha expression in melanoma. Cancer Res. 2007;67:3177–84. doi: 10.1158/0008-5472.CAN-06-3312. doi:10.1158/0008-5472.can-06-3312. [DOI] [PubMed] [Google Scholar]
- 274.Sharma A, Tran MA, Liang S, Sharma AK, Amin S, Smith CD. et al. Targeting mitogen-activated protein kinase/extracellular signal-regulated kinase kinase in the mutant (V600E) B-Raf signaling cascade effectively inhibits melanoma lung metastases. Cancer Res. 2006;66:8200–9. doi: 10.1158/0008-5472.CAN-06-0809. doi:10.1158/0008-5472.can-06-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Sharma A, Trivedi NR, Zimmerman MA, Tuveson DA, Smith CD, Robertson GP. Mutant V599EB-Raf regulates growth and vascular development of malignant melanoma tumors. Cancer Res. 2005;65:2412–21. doi: 10.1158/0008-5472.CAN-04-2423. doi:10.1158/0008-5472.can-04-2423. [DOI] [PubMed] [Google Scholar]
- 276.Balmanno K, Cook SJ. Tumour cell survival signalling by the ERK1/2 pathway. Cell Death Differ. 2009;16:368–77. doi: 10.1038/cdd.2008.148. doi:10.1038/cdd.2008.148. [DOI] [PubMed] [Google Scholar]
- 277.Lehmann K, Janda E, Pierreux CE, Rytomaa M, Schulze A, McMahon M. et al. Raf induces TGFbeta production while blocking its apoptotic but not invasive responses: a mechanism leading to increased malignancy in epithelial cells. Genes Dev. 2000;14:2610–22. doi: 10.1101/gad.181700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Sobczak I, Galabova-Kovacs G, Sadzak I, Kren A, Christofori G, Baccarini M. B-Raf is required for ERK activation and tumor progression in a mouse model of pancreatic beta-cell carcinogenesis. Oncogene. 2008;27:4779–87. doi: 10.1038/onc.2008.128. doi:10.1038/onc.2008.128. [DOI] [PubMed] [Google Scholar]
- 279.Riesco-Eizaguirre G, Rodriguez I, De la Vieja A, Costamagna E, Carrasco N, Nistal M. et al. The BRAFV600E oncogene induces transforming growth factor beta secretion leading to sodium iodide symporter repression and increased malignancy in thyroid cancer. Cancer Res. 2009;69:8317–25. doi: 10.1158/0008-5472.CAN-09-1248. doi:10.1158/0008-5472.can-09-1248. [DOI] [PubMed] [Google Scholar]
- 280.Janda E, Lehmann K, Killisch I, Jechlinger M, Herzig M, Downward J. et al. Ras and TGF[beta] cooperatively regulate epithelial cell plasticity and metastasis: dissection of Ras signaling pathways. J Cell Biol. 2002;156:299–313. doi: 10.1083/jcb.200109037. doi:10.1083/jcb.200109037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Pritchard CA, Hayes L, Wojnowski L, Zimmer A, Marais RM, Norman JC. B-Raf acts via the ROCKII/LIMK/cofilin pathway to maintain actin stress fibers in fibroblasts. Mol Cell Biol. 2004;24:5937–52. doi: 10.1128/MCB.24.13.5937-5952.2004. doi:10.1128/mcb.24.13.5937-5952.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Ehrenreiter K, Kern F, Velamoor V, Meissl K, Galabova-Kovacs G, Sibilia M. et al. Raf-1 addiction in Ras-induced skin carcinogenesis. Cancer Cell. 2009;16:149–60. doi: 10.1016/j.ccr.2009.06.008. doi:10.1016/j.ccr.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 283.Ehrenreiter K, Piazzolla D, Velamoor V, Sobczak I, Small JV, Takeda J. et al. Raf-1 regulates Rho signaling and cell migration. J Cell Biol. 2005;168:955–64. doi: 10.1083/jcb.200409162. doi:10.1083/jcb.200409162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Piazzolla D, Meissl K, Kucerova L, Rubiolo C, Baccarini M. Raf-1 sets the threshold of Fas sensitivity by modulating Rok-alpha signaling. J Cell Biol. 2005;171:1013–22. doi: 10.1083/jcb.200504137. doi:10.1083/jcb.200504137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Schulte TW, Blagosklonny MV, Ingui C, Neckers L. Disruption of the Raf-1-Hsp90 molecular complex results in destabilization of Raf-1 and loss of Raf-1-Ras association. J Biol Chem. 1995;270:24585–8. doi: 10.1074/jbc.270.41.24585. [DOI] [PubMed] [Google Scholar]
- 286.Schulte TW, Blagosklonny MV, Romanova L, Mushinski JF, Monia BP, Johnston JF. et al. Destabilization of Raf-1 by geldanamycin leads to disruption of the Raf-1-MEK-mitogen-activated protein kinase signalling pathway. Mol Cell Biol. 1996;16:5839–45. doi: 10.1128/mcb.16.10.5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Stancato LF, Chow YH, Hutchison KA, Perdew GH, Jove R, Pratt WB. Raf exists in a native heterocomplex with hsp90 and p50 that can be reconstituted in a cell-free system. J Biol Chem. 1993;268:21711–6. [PubMed] [Google Scholar]
- 288.Kolch W, Heidecker G, Kochs G, Hummel R, Vahidi H, Mischak H. et al. Protein kinase C alpha activates RAF-1 by direct phosphorylation. Nature. 1993;364:249–52. doi: 10.1038/364249a0. doi:10.1038/364249a0. [DOI] [PubMed] [Google Scholar]
- 289.Zimmermann S, Moelling K. Phosphorylation and regulation of Raf by Akt (protein kinase B) Science. 1999;286:1741–4. doi: 10.1126/science.286.5445.1741. [DOI] [PubMed] [Google Scholar]
- 290.Jin S, Zhuo Y, Guo W, Field J. p21-activated Kinase 1 (Pak1)-dependent phosphorylation of Raf-1 regulates its mitochondrial localization, phosphorylation of BAD, and Bcl-2 association. J Biol Chem. 2005;280:24698–705. doi: 10.1074/jbc.M413374200. doi:10.1074/jbc.M413374200. [DOI] [PubMed] [Google Scholar]
- 291.Diaz B, Barnard D, Filson A, MacDonald S, King A, Marshall M. Phosphorylation of Raf-1 serine 338-serine 339 is an essential regulatory event for Ras-dependent activation and biological signaling. Mol Cell Biol. 1997;17:4509–16. doi: 10.1128/mcb.17.8.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Baumann B, Weber CK, Troppmair J, Whiteside S, Israel A, Rapp UR. et al. Raf induces NF-kappaB by membrane shuttle kinase MEKK1, a signaling pathway critical for transformation. Proc Natl Acad Sci U S A. 2000;97:4615–20. doi: 10.1073/pnas.080583397. doi:10.1073/pnas.080583397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Kinkade R, Dasgupta P, Carie A, Pernazza D, Carless M, Pillai S. et al. A small molecule disruptor of Rb/Raf-1 interaction inhibits cell proliferation, angiogenesis, and growth of human tumor xenografts in nude mice. Cancer Res. 2008;68:3810–8. doi: 10.1158/0008-5472.CAN-07-6672. doi:10.1158/0008-5472.can-07-6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Polzien L, Baljuls A, Rennefahrt UE, Fischer A, Schmitz W, Zahedi RP. et al. Identification of novel in vivo phosphorylation sites of the human proapoptotic protein BAD: pore-forming activity of BAD is regulated by phosphorylation. J Biol Chem. 2009;284:28004–20. doi: 10.1074/jbc.M109.010702. doi:10.1074/jbc.M109.010702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Eves EM, Shapiro P, Naik K, Klein UR, Trakul N, Rosner MR. Raf kinase inhibitory protein regulates aurora B kinase and the spindle checkpoint. Mol Cell. 2006;23:561–74. doi: 10.1016/j.molcel.2006.07.015. doi:10.1016/j.molcel.2006.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Alessi DR, Saito Y, Campbell DG, Cohen P, Sithanandam G, Rapp U. et al. Identification of the sites in MAP kinase kinase-1 phosphorylated by p74raf-1. EMBO J. 1994;13:1610–9. doi: 10.1002/j.1460-2075.1994.tb06424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Zheng CF, Guan KL. Activation of MEK family kinases requires phosphorylation of two conserved Ser/Thr residues. EMBO J. 1994;13:1123–31. doi: 10.1002/j.1460-2075.1994.tb06361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Ferrell JE Jr. Tripping the switch fantastic: how a protein kinase cascade can convert graded inputs into switch-like outputs. Trends Biochem Sci. 1996;21:460–6. doi: 10.1016/s0968-0004(96)20026-x. [DOI] [PubMed] [Google Scholar]
- 299.Ferrell JE Jr. How responses get more switch-like as you move down a protein kinase cascade. Trends Biochem Sci. 1997;22:288–9. doi: 10.1016/s0968-0004(97)82217-7. [DOI] [PubMed] [Google Scholar]
- 300.Ferrell JE Jr. Building a cellular switch: more lessons from a good egg. Bioessays. 1999;21:866–70. doi: 10.1002/(SICI)1521-1878(199910)21:10<866::AID-BIES9>3.0.CO;2-1. doi:10.1002/(sici)1521-1878(199910)21:10<866::aid-bies9>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 301.Ferrell JE Jr, Bhatt RR. Mechanistic studies of the dual phosphorylation of mitogen-activated protein kinase. J Biol Chem. 1997;272:19008–16. doi: 10.1074/jbc.272.30.19008. [DOI] [PubMed] [Google Scholar]
- 302.Huang CY, Ferrell JE Jr. Ultrasensitivity in the mitogen-activated protein kinase cascade. Proc Natl Acad Sci U S A. 1996;93:10078–83. doi: 10.1073/pnas.93.19.10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Robbins DJ, Cobb MH. Extracellular signal-regulated kinases 2 autophosphorylates on a subset of peptides phosphorylated in intact cells in response to insulin and nerve growth factor: analysis by peptide mapping. Mol Biol Cell. 1992;3:299–308. doi: 10.1091/mbc.3.3.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Murugan AK, Dong J, Xie J, Xing M. MEK1 mutations, but not ERK2 mutations, occur in melanomas and colon carcinomas, but none in thyroid carcinomas. Cell Cycle. 2009;8:2122–4. doi: 10.4161/cc.8.13.8710. [DOI] [PubMed] [Google Scholar]
- 305.Wang GG, Yao JC, Worah S, White JA, Luna R, Wu TT. et al. Comparison of genetic alterations in neuroendocrine tumors: frequent loss of chromosome 18 in ileal carcinoid tumors. Mod Pathol. 2005;18:1079–87. doi: 10.1038/modpathol.3800389. doi:10.1038/modpathol.3800389. [DOI] [PubMed] [Google Scholar]
- 306.Rindi G, Candusso ME, Solcia E. Molecular aspects of the endocrine tumours of the pancreas and the gastrointestinal tract. Ital J Gastroenterol Hepatol. 1999;31(Suppl 2):S135–8. [PubMed] [Google Scholar]
- 307.Perren A, Schmid S, Locher T, Saremaslani P, Bonvin C, Heitz PU. et al. BRAF and endocrine tumors: mutations are frequent in papillary thyroid carcinomas, rare in endocrine tumors of the gastrointestinal tract and not detected in other endocrine tumors. Endocr Relat Cancer. 2004;11:855–60. doi: 10.1677/erc.1.00841. doi:10.1677/erc.1.00841. [DOI] [PubMed] [Google Scholar]
- 308.Karkouche R, Bachet JB, Sandrini J, Mitry E, Penna C, Cote JF. et al. Colorectal neuroendocrine carcinomas and adenocarcinomas share oncogenic pathways. A clinico-pathologic study of 12 cases. Eur J Gastroenterol Hepatol. 2012;24:1430–7. doi: 10.1097/MEG.0b013e3283583c87. doi:10.1097/MEG.0b013e3283583c87. [DOI] [PubMed] [Google Scholar]
- 309.Karhoff D, Sauer S, Schrader J, Arnold R, Fendrich V, Bartsch DK. et al. Rap1/B-Raf signaling is activated in neuroendocrine tumors of the digestive tract and Raf kinase inhibition constitutes a putative therapeutic target. Neuroendocrinology. 2007;85:45–53. doi: 10.1159/000100508. doi:10.1159/000100508. [DOI] [PubMed] [Google Scholar]
- 310.Mordant P, Loriot Y, Leteur C, Calderaro J, Bourhis J, Wislez M. et al. Dependence on phosphoinositide 3-kinase and RAS-RAF pathways drive the activity of RAF265, a novel RAF/VEGFR2 inhibitor, and RAD001 (Everolimus) in combination. Mol Cancer Ther. 2010;9:358–68. doi: 10.1158/1535-7163.MCT-09-1014. doi:10.1158/1535-7163.mct-09-1014. [DOI] [PubMed] [Google Scholar]
- 311.Zitzmann K, de Toni E, von Ruden J, Brand S, Goke B, Laubender RP. et al. The novel Raf inhibitor Raf265 decreases Bcl-2 levels and confers TRAIL-sensitivity to neuroendocrine tumour cells. Endocr Relat Cancer. 2011;18:277–85. doi: 10.1530/ERC-10-0108. doi:10.1530/erc-10-0108. [DOI] [PubMed] [Google Scholar]
- 312.Sippel RS, Carpenter JE, Kunnimalaiyaan M, Lagerholm S, Chen H. Raf-1 activation suppresses neuroendocrine marker and hormone levels in human gastrointestinal carcinoid cells. Am J Physiol Gastrointest Liver Physiol. 2003;285:G245–54. doi: 10.1152/ajpgi.00420.2002. doi:10.1152/ajpgi.00420.2002. [DOI] [PubMed] [Google Scholar]
- 313.Sippel RS, Chen H. Activation of the ras/raf-1 signal transduction pathway in carcinoid tumor cells results in morphologic transdifferentiation. Surgery. 2002;132:1035–9. doi: 10.1067/msy.2002.128877. discussion 9. doi:10.1067/msy.2002.128877. [DOI] [PubMed] [Google Scholar]
- 314.Ning L, Chen H, Kunnimalaiyaan M. Focal adhesion kinase, a downstream mediator of Raf-1 signaling, suppresses cellular adhesion, migration, and neuroendocrine markers in BON carcinoid cells. Mol Cancer Res. 2010;8:775–82. doi: 10.1158/1541-7786.MCR-09-0525. doi:10.1158/1541-7786.mcr-09-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Georgieva I, Koychev D, Wang Y, Holstein J, Hopfenmuller W, Zeitz M. et al. ZM447439, a novel promising aurora kinase inhibitor, provokes antiproliferative and proapoptotic effects alone and in combination with bio- and chemotherapeutic agents in gastroenteropancreatic neuroendocrine tumor cell lines. Neuroendocrinology. 2010;91:121–30. doi: 10.1159/000258705. doi:10.1159/000258705. [DOI] [PubMed] [Google Scholar]
- 316.Kanakis G, Kaltsas G. Biochemical markers for gastroenteropancreatic neuroendocrine tumours (GEP-NETs) Best Pract Res Clin Gastroenterol. 2012;26:791–802. doi: 10.1016/j.bpg.2012.12.006. doi:10.1016/j.bpg.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 317.O'Toole D, Grossman A, Gross D, Delle Fave G, Barkmanova J, O'Connor J. et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: biochemical markers. Neuroendocrinology. 2009;90:194–202. doi: 10.1159/000225948. doi:10.1159/000225948. [DOI] [PubMed] [Google Scholar]
- 318.Cook MR, Pinchot SN, Jaskula-Sztul R, Luo J, Kunnimalaiyaan M, Chen H. Identification of a novel Raf-1 pathway activator that inhibits gastrointestinal carcinoid cell growth. Mol Cancer Ther. 2010;9:429–37. doi: 10.1158/1535-7163.MCT-09-0718. doi:10.1158/1535-7163.mct-09-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Pinchot SN, Adler JT, Luo Y, Ju J, Li W, Shen B. et al. Tautomycin suppresses growth and neuroendocrine hormone markers in carcinoid cells through activation of the Raf-1 pathway. Am J Surg. 2009;197:313–9. doi: 10.1016/j.amjsurg.2008.10.007. doi:10.1016/j.amjsurg.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Lee JH, Lee JS, Kim SE, Moon BS, Kim YC, Lee SK. et al. Tautomycetin inhibits growth of colorectal cancer cells through p21cip/WAF1 induction via the extracellular signal-regulated kinase pathway. Mol Cancer Ther. 2006;5:3222–31. doi: 10.1158/1535-7163.MCT-06-0455. doi:10.1158/1535-7163.mct-06-0455. [DOI] [PubMed] [Google Scholar]
- 321.Van Gompel JJ, Kunnimalaiyaan M, Holen K, Chen H. ZM336372, a Raf-1 activator, suppresses growth and neuroendocrine hormone levels in carcinoid tumor cells. Mol Cancer Ther. 2005;4:910–7. doi: 10.1158/1535-7163.MCT-04-0334. doi:10.1158/1535-7163.mct-04-0334. [DOI] [PubMed] [Google Scholar]
- 322.Callahan MK, Rampal R, Harding JJ, Klimek VM, Chung YR, Merghoub T. et al. Progression of RAS-mutant leukemia during RAF inhibitor treatment. N Engl J Med. 2012;367:2316–21. doi: 10.1056/NEJMoa1208958. doi:10.1056/NEJMoa1208958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Carnahan J, Beltran PJ, Babij C, Le Q, Rose MJ, Vonderfecht S. et al. Selective and potent Raf inhibitors paradoxically stimulate normal cell proliferation and tumor growth. Mol Cancer Ther. 2010;9:2399–410. doi: 10.1158/1535-7163.MCT-10-0181. doi:10.1158/1535-7163.mct-10-0181. [DOI] [PubMed] [Google Scholar]
- 324.Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464:427–30. doi: 10.1038/nature08902. doi:10.1038/nature08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Kaplan FM, Shao Y, Mayberry MM, Aplin AE. Hyperactivation of MEK-ERK1/2 signaling and resistance to apoptosis induced by the oncogenic B-RAF inhibitor, PLX4720, in mutant N-RAS melanoma cells. Oncogene. 2011;30:366–71. doi: 10.1038/onc.2010.408. doi:10.1038/onc.2010.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Rushworth LK, Hindley AD, O'Neill E, Kolch W. Regulation and role of Raf-1/B-Raf heterodimerization. Mol Cell Biol. 2006;26:2262–72. doi: 10.1128/MCB.26.6.2262-2272.2006. doi:10.1128/mcb.26.6.2262-2272.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Cichowski K, Janne PA. Drug discovery: inhibitors that activate. Nature. 2010;464:358–9. doi: 10.1038/464358a. doi:10.1038/464358a. [DOI] [PubMed] [Google Scholar]
- 328.Ritt DA, Monson DM, Specht SI, Morrison DK. Impact of feedback phosphorylation and Raf heterodimerization on normal and mutant B-Raf signaling. Mol Cell Biol. 2010;30:806–19. doi: 10.1128/MCB.00569-09. doi:10.1128/mcb.00569-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Hatzivassiliou G, Song K, Yen I, Brandhuber BJ, Anderson DJ, Alvarado R. et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431–5. doi: 10.1038/nature08833. doi:10.1038/nature08833. [DOI] [PubMed] [Google Scholar]
- 330.Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A. et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008;118:3065–74. doi: 10.1172/JCI34739. doi:10.1172/jci34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Carracedo A, Pandolfi PP. The PTEN-PI3K pathway: of feedbacks and cross-talks. Oncogene. 2008;27:5527–41. doi: 10.1038/onc.2008.247. doi:10.1038/onc.2008.247. [DOI] [PubMed] [Google Scholar]
- 332.Gao J, Wagnon JL, Protacio RM, Glazko GV, Beggs M, Raj V, A stress-activated, p38 MAPK-ATF/CREB pathway regulates post-transcriptional, sequence-dependent decay of target RNAs. Mol Cell Biol. 2013. doi:10.1128/mcb.00349-13. [DOI] [PMC free article] [PubMed]
- 333.Hollenhorst PC. RAS/ERK pathway transcriptional regulation through ETS/AP-1 binding sites. Small GTPases. 2012;3:154–8. doi: 10.4161/sgtp.19630. doi:10.4161/sgtp.19630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Rolli M, Kotlyarov A, Sakamoto KM, Gaestel M, Neininger A. Stress-induced stimulation of early growth response gene-1 by p38/stress-activated protein kinase 2 is mediated by a cAMP-responsive promoter element in a MAPKAP kinase 2-independent manner. J Biol Chem. 1999;274:19559–64. doi: 10.1074/jbc.274.28.19559. [DOI] [PubMed] [Google Scholar]
- 335.Xing J, Kornhauser JM, Xia Z, Thiele EA, Greenberg ME. Nerve growth factor activates extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways to stimulate CREB serine 133 phosphorylation. Mol Cell Biol. 1998;18:1946–55. doi: 10.1128/mcb.18.4.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Tan Y, Rouse J, Zhang A, Cariati S, Cohen P, Comb MJ. FGF and stress regulate CREB and ATF-1 via a pathway involving p38 MAP kinase and MAPKAP kinase-2. EMBO J. 1996;15:4629–42. [PMC free article] [PubMed] [Google Scholar]
- 337.Derijard B, Hibi M, Wu IH, Barrett T, Su B, Deng T. et al. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–37. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 338.Hibi M, Lin A, Smeal T, Minden A, Karin M. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 1993;7:2135–48. doi: 10.1101/gad.7.11.2135. [DOI] [PubMed] [Google Scholar]
- 339.Chen RH, Abate C, Blenis J. Phosphorylation of the c-Fos transrepression domain by mitogen-activated protein kinase and 90-kDa ribosomal S6 kinase. Proc Natl Acad Sci U S A. 1993;90:10952–6. doi: 10.1073/pnas.90.23.10952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Chen RH, Juo PC, Curran T, Blenis J. Phosphorylation of c-Fos at the C-terminus enhances its transforming activity. Oncogene. 1996;12:1493–502. [PubMed] [Google Scholar]
- 341.O'Donnell A, Odrowaz Z, Sharrocks AD. Immediate-early gene activation by the MAPK pathways: what do and don't we know? Biochem Soc Trans. 2012;40:58–66. doi: 10.1042/BST20110636. doi:10.1042/bst20110636. [DOI] [PubMed] [Google Scholar]
- 342.Janknecht R, Ernst WH, Pingoud V, Nordheim A. Activation of ternary complex factor Elk-1 by MAP kinases. EMBO J. 1993;12:5097–104. doi: 10.1002/j.1460-2075.1993.tb06204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Gille H, Kortenjann M, Thomae O, Moomaw C, Slaughter C, Cobb MH. et al. ERK phosphorylation potentiates Elk-1-mediated ternary complex formation and transactivation. EMBO J. 1995;14:951–62. doi: 10.1002/j.1460-2075.1995.tb07076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Rowan BG, Weigel NL, O'Malley BW. Phosphorylation of steroid receptor coactivator-1. Identification of the phosphorylation sites and phosphorylation through the mitogen-activated protein kinase pathway. J Biol Chem. 2000;275:4475–83. doi: 10.1074/jbc.275.6.4475. [DOI] [PubMed] [Google Scholar]
- 345.Kamei Y, Xu L, Heinzel T, Torchia J, Kurokawa R, Gloss B. et al. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell. 1996;85:403–14. doi: 10.1016/s0092-8674(00)81118-6. [DOI] [PubMed] [Google Scholar]
- 346.Smith CL, Onate SA, Tsai MJ, O'Malley BW. CREB binding protein acts synergistically with steroid receptor coactivator-1 to enhance steroid receptor-dependent transcription. Proc Natl Acad Sci U S A. 1996;93:8884–8. doi: 10.1073/pnas.93.17.8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Schuringa JJ, Dekker LV, Vellenga E, Kruijer W. Sequential activation of Rac-1, SEK-1/MKK-4, and protein kinase Cdelta is required for interleukin-6-induced STAT3 Ser-727 phosphorylation and transactivation. J Biol Chem. 2001;276:27709–15. doi: 10.1074/jbc.M009821200. doi:10.1074/jbc.M009821200. [DOI] [PubMed] [Google Scholar]
- 348.Schuringa JJ, Jonk LJ, Dokter WH, Vellenga E, Kruijer W. Interleukin-6-induced STAT3 transactivation and Ser727 phosphorylation involves Vav, Rac-1 and the kinase SEK-1/MKK-4 as signal transduction components. Biochem J. 2000. 347 Pt 1: 89-96. [PMC free article] [PubMed]
- 349.Briest F, Berndt A, Clement J, Junker K, Eggeling F, Grimm S. et al. Tumor-stroma interactions in tumorigenesis: lessons from stem cell biology. Front Biosci (Elite Ed) 2012;4:1871–87. doi: 10.2741/509. [DOI] [PubMed] [Google Scholar]
- 350.Zugowski C, Lieder F, Muller A, Gasch J, Corvinus FM, Moriggl R. et al. STAT3 controls matrix metalloproteinase-1 expression in colon carcinoma cells by both direct and AP-1-mediated interaction with the MMP-1 promoter. Biol Chem. 2011;392:449–59. doi: 10.1515/BC.2011.038. doi:10.1515/bc.2011.038. [DOI] [PubMed] [Google Scholar]
- 351.Tsareva SA, Moriggl R, Corvinus FM, Wiederanders B, Schutz A, Kovacic B. et al. Signal transducer and activator of transcription 3 activation promotes invasive growth of colon carcinomas through matrix metalloproteinase induction. Neoplasia. 2007;9:279–91. doi: 10.1593/neo.06820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Bode JG, Ehlting C, Haussinger D. The macrophage response towards LPS and its control through the p38(MAPK)-STAT3 axis. Cell Signal. 2012;24:1185–94. doi: 10.1016/j.cellsig.2012.01.018. doi:10.1016/j.cellsig.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 353.Dijkgraaf EM, Welters MJ, Nortier JW, van der Burg SH, Kroep JR. Interleukin-6/interleukin-6 receptor pathway as a new therapy target in epithelial ovarian cancer. Curr Pharm Des. 2012;18:3816–27. doi: 10.2174/138161212802002797. [DOI] [PubMed] [Google Scholar]
- 354.Brami-Cherrier K, Roze E, Girault JA, Betuing S, Caboche J. Role of the ERK/MSK1 signalling pathway in chromatin remodelling and brain responses to drugs of abuse. J Neurochem. 2009;108:1323–35. doi: 10.1111/j.1471-4159.2009.05879.x. doi:10.1111/j.1471-4159.2009.05879.x. [DOI] [PubMed] [Google Scholar]
- 355.Soloaga A, Thomson S, Wiggin GR, Rampersaud N, Dyson MH, Hazzalin CA. et al. MSK2 and MSK1 mediate the mitogen- and stress-induced phosphorylation of histone H3 and HMG-14. EMBO J. 2003;22:2788–97. doi: 10.1093/emboj/cdg273. doi:10.1093/emboj/cdg273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Deak M, Clifton AD, Lucocq LM, Alessi DR. Mitogen- and stress-activated protein kinase-1 (MSK1) is directly activated by MAPK and SAPK2/p38, and may mediate activation of CREB. EMBO J. 1998;17:4426–41. doi: 10.1093/emboj/17.15.4426. doi:10.1093/emboj/17.15.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Shapiro PS, Whalen AM, Tolwinski NS, Wilsbacher J, Froelich-Ammon SJ, Garcia M. et al. Extracellular signal-regulated kinase activates topoisomerase IIalpha through a mechanism independent of phosphorylation. Mol Cell Biol. 1999;19:3551–60. doi: 10.1128/mcb.19.5.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Sassone-Corsi P, Mizzen CA, Cheung P, Crosio C, Monaco L, Jacquot S. et al. Requirement of Rsk-2 for epidermal growth factor-activated phosphorylation of histone H3. Science. 1999;285:886–91. doi: 10.1126/science.285.5429.886. [DOI] [PubMed] [Google Scholar]
- 359.Clayton AL, Mahadevan LC. MAP kinase-mediated phosphoacetylation of histone H3 and inducible gene regulation. FEBS Lett. 2003;546:51–8. doi: 10.1016/s0014-5793(03)00451-4. [DOI] [PubMed] [Google Scholar]
- 360.Dunn KL, Espino PS, Drobic B, He S, Davie JR. The Ras-MAPK signal transduction pathway, cancer and chromatin remodeling. Biochem Cell Biol. 2005;83:1–14. doi: 10.1139/o04-121. doi:10.1139/o04-121. [DOI] [PubMed] [Google Scholar]
- 361.Hopfner M, Sutter AP, Gerst B, Zeitz M, Scherubl H. A novel approach in the treatment of neuroendocrine gastrointestinal tumours. Targeting the epidermal growth factor receptor by gefitinib (ZD1839) Br J Cancer. 2003;89:1766–75. doi: 10.1038/sj.bjc.6601346. doi:10.1038/sj.bjc.6601346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Clerk A, Aggeli IK, Stathopoulou K, Sugden PH. Peptide growth factors signal differentially through protein kinase C to extracellular signal-regulated kinases in neonatal cardiomyocytes. Cell Signal. 2006;18:225–35. doi: 10.1016/j.cellsig.2005.04.005. doi:10.1016/j.cellsig.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 363.Weng LP, Smith WM, Brown JL, Eng C. PTEN inhibits insulin-stimulated MEK/MAPK activation and cell growth by blocking IRS-1 phosphorylation and IRS-1/Grb-2/Sos complex formation in a breast cancer model. Hum Mol Genet. 2001;10:605–16. doi: 10.1093/hmg/10.6.605. [DOI] [PubMed] [Google Scholar]
- 364.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117–34. doi: 10.1016/j.cell.2010.06.011. doi:10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell. 2010;40:310–22. doi: 10.1016/j.molcel.2010.09.026. doi:10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. doi:10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Kodaki T, Woscholski R, Hallberg B, Rodriguez-Viciana P, Downward J, Parker PJ. The activation of phosphatidylinositol 3-kinase by Ras. Curr Biol. 1994;4:798–806. doi: 10.1016/s0960-9822(00)00177-9. [DOI] [PubMed] [Google Scholar]
- 368.Suire S, Hawkins P, Stephens L. Activation of phosphoinositide 3-kinase gamma by Ras. Curr Biol. 2002;12:1068–75. doi: 10.1016/s0960-9822(02)00933-8. [DOI] [PubMed] [Google Scholar]
- 369.Roux PP, Ballif BA, Anjum R, Gygi SP, Blenis J. Tumor-promoting phorbol esters and activated Ras inactivate the tuberous sclerosis tumor suppressor complex via p90 ribosomal S6 kinase. Proc Natl Acad Sci U S A. 2004;101:13489–94. doi: 10.1073/pnas.0405659101. doi:10.1073/pnas.0405659101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.McKay MM, Morrison DK. Integrating signals from RTKs to ERK/MAPK. Oncogene. 2007;26:3113–21. doi: 10.1038/sj.onc.1210394. doi:10.1038/sj.onc.1210394. [DOI] [PubMed] [Google Scholar]
- 371.Dougherty MK, Ritt DA, Zhou M, Specht SI, Monson DM, Veenstra TD. et al. KSR2 is a calcineurin substrate that promotes ERK cascade activation in response to calcium signals. Mol Cell. 2009;34:652–62. doi: 10.1016/j.molcel.2009.06.00. doi:10.1016/j.molcel.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Costanzo-Garvey DL, Pfluger PT, Dougherty MK, Stock JL, Boehm M, Chaika O. et al. KSR2 is an essential regulator of AMP kinase, energy expenditure, and insulin sensitivity. Cell Metab. 2009;10:366–78. doi: 10.1016/j.cmet.2009.09.010. doi:10.1016/j.cmet.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Yang JY, Zong CS, Xia W, Yamaguchi H, Ding Q, Xie X. et al. ERK promotes tumorigenesis by inhibiting FOXO3a via MDM2-mediated degradation. Nat Cell Biol. 2008;10:138–48. doi: 10.1038/ncb1676. doi:10.1038/ncb1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–74. doi: 10.1016/j.cell.2007.06.009. doi:10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Tang ED, Nunez G, Barr FG, Guan KL. Negative regulation of the forkhead transcription factor FKHR by Akt. J Biol Chem. 1999;274:16741–6. doi: 10.1074/jbc.274.24.16741. [DOI] [PubMed] [Google Scholar]
- 376.Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24:7410–25. doi: 10.1038/sj.onc.1209086. doi:10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- 377.Sears R, Nuckolls F, Haura E, Taya Y, Tamai K, Nevins JR. Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability. Genes Dev. 2000;14:2501–14. doi: 10.1101/gad.836800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Zhu J, Blenis J, Yuan J. Activation of PI3K/Akt and MAPK pathways regulates Myc-mediated transcription by phosphorylating and promoting the degradation of Mad1. Proc Natl Acad Sci U S A. 2008;105:6584–9. doi: 10.1073/pnas.0802785105. doi:10.1073/pnas.0802785105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Pearce LR, Komander D, Alessi DR. The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol. 2010;11:9–22. doi: 10.1038/nrm2822. doi:10.1038/nrm2822. [DOI] [PubMed] [Google Scholar]
- 380.Holz MK, Ballif BA, Gygi SP, Blenis J. mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell. 2005;123:569–80. doi: 10.1016/j.cell.2005.10.024. doi:10.1016/j.cell.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 381.van Gorp AG, van der Vos KE, Brenkman AB, Bremer A, van den Broek N, Zwartkruis F. et al. AGC kinases regulate phosphorylation and activation of eukaryotic translation initiation factor 4B. Oncogene. 2009;28:95–106. doi: 10.1038/onc.2008.367. doi:10.1038/onc.2008.367. [DOI] [PubMed] [Google Scholar]
- 382.Moelling K, Schad K, Bosse M, Zimmermann S, Schweneker M. Regulation of Raf-Akt Cross-talk. J Biol Chem. 2002;277:31099–106. doi: 10.1074/jbc.M111974200. doi:10.1074/jbc.M111974200. [DOI] [PubMed] [Google Scholar]
- 383.Reusch HP, Zimmermann S, Schaefer M, Paul M, Moelling K. Regulation of Raf by Akt controls growth and differentiation in vascular smooth muscle cells. J Biol Chem. 2001;276:33630–7. doi: 10.1074/jbc.M105322200. doi:10.1074/jbc.M105322200. [DOI] [PubMed] [Google Scholar]
- 384.Rommel C, Clarke BA, Zimmermann S, Nunez L, Rossman R, Reid K. et al. Differentiation stage-specific inhibition of the Raf-MEK-ERK pathway by Akt. Science. 1999;286:1738–41. doi: 10.1126/science.286.5445.1738. [DOI] [PubMed] [Google Scholar]
- 385.Wang X, Hawk N, Yue P, Kauh J, Ramalingam SS, Fu H. et al. Overcoming mTOR inhibition-induced paradoxical activation of survival signaling pathways enhances mTOR inhibitors' anticancer efficacy. Cancer Biol Ther. 2008;7:1952–8. doi: 10.4161/cbt.7.12.6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Mi R, Ma J, Zhang D, Li L, Zhang H. Efficacy of combined inhibition of mTOR and ERK/MAPK pathways in treating a tuberous sclerosis complex cell model. J Genet Genomics. 2009;36:355–61. doi: 10.1016/S1673-8527(08)60124-1. doi:10.1016/s1673-8527(08)60124-1. [DOI] [PubMed] [Google Scholar]
- 387.Pavel M, Baudin E, Couvelard A, Krenning E, Oberg K, Steinmuller T. et al. ENETS Consensus Guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology. 2012;95:157–76. doi: 10.1159/000335597. doi:10.1159/000335597. [DOI] [PubMed] [Google Scholar]
- 388.Raymond E, Hammel P, Dreyer C, Maatescu C, Hentic O, Ruszniewski P. et al. Sunitinib in pancreatic neuroendocrine tumors. Target Oncol. 2012;7:117–25. doi: 10.1007/s11523-012-0220-2. doi:10.1007/s11523-012-0220-2. [DOI] [PubMed] [Google Scholar]
- 389.Pavel ME, Hainsworth JD, Baudin E, Peeters M, Horsch D, Winkler RE. et al. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet. 2011;378:2005–12. doi: 10.1016/S0140-6736(11)61742-X. doi:10.1016/s0140-6736(11)61742-x. [DOI] [PubMed] [Google Scholar]
- 390.Strosberg JR, Chan JA, Ryan DP, Meyerhardt JA, Fuchs CS, Abrams T. et al. A multi-institutional, phase II open-label study of ganitumab (AMG 479) in advanced carcinoid and pancreatic neuroendocrine tumors. Endocr Relat Cancer. 2013;20:383–90. doi: 10.1530/ERC-12-0390. doi:10.1530/erc-12-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Bao XH, Takaoka M, Hao HF, Wang ZG, Fukazawa T, Yamatsuji T. et al. Esophageal cancer exhibits resistance to a novel IGF-1R inhibitor NVP-AEW541 with maintained RAS-MAPK activity. Anticancer Res. 2012;32:2827–34. [PubMed] [Google Scholar]
- 392.Baumann P, Hagemeier H, Mandl-Weber S, Franke D, Schmidmaier R. Myeloma cell growth inhibition is augmented by synchronous inhibition of the insulin-like growth factor-1 receptor by NVP-AEW541 and inhibition of mammalian target of rapamycin by Rad001. Anticancer Drugs. 2009;20:259–66. doi: 10.1097/CAD.0b013e328328d18b. doi:10.1097/CAD.0b013e328328d18b. [DOI] [PubMed] [Google Scholar]
- 393.Cunningham MP, Thomas H, Marks C, Green M, Fan Z, Modjtahedi H. Co-targeting the EGFR and IGF-IR with anti-EGFR monoclonal antibody ICR62 and the IGF-IR tyrosine kinase inhibitor NVP-AEW541 in colorectal cancer cells. Int J Oncol. 2008;33:1107–13. [PubMed] [Google Scholar]
- 394.Garcia-Echeverria C, Pearson MA, Marti A, Meyer T, Mestan J, Zimmermann J. et al. In vivo antitumor activity of NVP-AEW541-A novel, potent, and selective inhibitor of the IGF-IR kinase. Cancer Cell. 2004;5:231–9. doi: 10.1016/s1535-6108(04)00051-0. [DOI] [PubMed] [Google Scholar]
- 395.Gariboldi MB, Ravizza R, Monti E. The IGFR1 inhibitor NVP-AEW541 disrupts a pro-survival and pro-angiogenic IGF-STAT3-HIF1 pathway in human glioblastoma cells. Biochem Pharmacol. 2010;80:455–62. doi: 10.1016/j.bcp.2010.05.011. doi:10.1016/j.bcp.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 396.Hagerstrand D, Lindh MB, Pena C, Garcia-Echeverria C, Nister M, Hofmann F. et al. PI3K/PTEN/Akt pathway status affects the sensitivity of high-grade glioma cell cultures to the insulin-like growth factor-1 receptor inhibitor NVP-AEW541. Neuro Oncol. 2010;12:967–75. doi: 10.1093/neuonc/noq029. doi:10.1093/neuonc/noq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Ioannou N, Seddon AM, Dalgleish A, Mackintosh D, Modjtahedi H. Treatment with a combination of the ErbB (HER) family blocker afatinib and the IGF-IR inhibitor, NVP-AEW541 induces synergistic growth inhibition of human pancreatic cancer cells. BMC Cancer. 2013;13:41.. doi: 10.1186/1471-2407-13-41. doi:10.1186/1471-2407-13-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Isebaert SF, Swinnen JV, McBride WH, Haustermans KM. Insulin-like growth factor-type 1 receptor inhibitor NVP-AEW541 enhances radiosensitivity of PTEN wild-type but not PTEN-deficient human prostate cancer cells. Int J Radiat Oncol Biol Phys. 2011;81:239–47. doi: 10.1016/j.ijrobp.2011.03.030. doi:10.1016/j.ijrobp.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 399.Maiso P, Ocio EM, Garayoa M, Montero JC, Hofmann F, Garcia-Echeverria C. et al. The insulin-like growth factor-I receptor inhibitor NVP-AEW541 provokes cell cycle arrest and apoptosis in multiple myeloma cells. Br J Haematol. 2008;141:470–82. doi: 10.1111/j.1365-2141.2008.07049.x. doi:10.1111/j.1365-2141.2008.07049.x. [DOI] [PubMed] [Google Scholar]
- 400.Moser C, Schachtschneider P, Lang SA, Gaumann A, Mori A, Zimmermann J. et al. Inhibition of insulin-like growth factor-I receptor (IGF-IR) using NVP-AEW541, a small molecule kinase inhibitor, reduces orthotopic pancreatic cancer growth and angiogenesis. Eur J Cancer. 2008;44:1577–86. doi: 10.1016/j.ejca.2008.04.003. doi:10.1016/j.ejca.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 401.Mukohara T, Shimada H, Ogasawara N, Wanikawa R, Shimomura M, Nakatsura T. et al. Sensitivity of breast cancer cell lines to the novel insulin-like growth factor-1 receptor (IGF-1R) inhibitor NVP-AEW541 is dependent on the level of IRS-1 expression. Cancer Lett. 2009;282:14–24. doi: 10.1016/j.canlet.2009.02.056. doi:10.1016/j.canlet.2009.02.056. [DOI] [PubMed] [Google Scholar]
- 402.Premkumar DR, Jane EP, Pollack IF. Co-administration of NVP-AEW541 and dasatinib induces mitochondrial-mediated apoptosis through Bax activation in malignant human glioma cell lines. Int J Oncol. 2010;37:633–43. doi: 10.3892/ijo_00000712. [DOI] [PubMed] [Google Scholar]
- 403.Tanno B, Mancini C, Vitali R, Mancuso M, McDowell HP, Dominici C. et al. Down-regulation of insulin-like growth factor I receptor activity by NVP-AEW541 has an antitumor effect on neuroblastoma cells in vitro and in vivo. Clin Cancer Res. 2006;12:6772–80. doi: 10.1158/1078-0432.CCR-06-1479. doi:10.1158/1078-0432.ccr-06-1479. [DOI] [PubMed] [Google Scholar]
- 404.Tazzari PL, Tabellini G, Bortul R, Papa V, Evangelisti C, Grafone T. et al. The insulin-like growth factor-I receptor kinase inhibitor NVP-AEW541 induces apoptosis in acute myeloid leukemia cells exhibiting autocrine insulin-like growth factor-I secretion. Leukemia. 2007;21:886–96. doi: 10.1038/sj.leu.2404643. doi:10.1038/sj.leu.2404643. [DOI] [PubMed] [Google Scholar]
- 405.Zumsteg A, Caviezel C, Pisarsky L, Strittmatter K, Garcia-Echeverria C, Hofmann F. et al. Repression of malignant tumor progression upon pharmacologic IGF1R blockade in a mouse model of insulinoma. Mol Cancer Res. 2012;10:800–9. doi: 10.1158/1541-7786.MCR-11-0522. doi:10.1158/1541-7786.mcr-11-0522. [DOI] [PubMed] [Google Scholar]
- 406.He Y, Zhang J, Zheng J, Du W, Xiao H, Liu W. et al. The insulin-like growth factor-1 receptor kinase inhibitor, NVP-ADW742, suppresses survival and resistance to chemotherapy in acute myeloid leukemia cells. Oncol Res. 2010;19:35–43. doi: 10.3727/096504010x12828372551821. [DOI] [PubMed] [Google Scholar]
- 407.Warshamana-Greene GS, Litz J, Buchdunger E, Garcia-Echeverria C, Hofmann F, Krystal GW. The insulin-like growth factor-I receptor kinase inhibitor, NVP-ADW742, sensitizes small cell lung cancer cell lines to the effects of chemotherapy. Clin Cancer Res. 2005;11:1563–71. doi: 10.1158/1078-0432.CCR-04-1544. doi:10.1158/1078-0432.ccr-04-1544. [DOI] [PubMed] [Google Scholar]
- 408.Warshamana-Greene GS, Litz J, Buchdunger E, Hofmann F, Garcia-Echeverria C, Krystal GW. The insulin-like growth factor-I (IGF-I) receptor kinase inhibitor NVP-ADW742, in combination with STI571, delineates a spectrum of dependence of small cell lung cancer on IGF-I and stem cell factor signaling. Mol Cancer Ther. 2004;3:527–35. [PubMed] [Google Scholar]
- 409.Zhou H, Rao J, Lin J, Yin B, Sheng H, Lin F. et al. The insulin-like growth factor-I receptor kinase inhibitor NVP-ADW742 sensitizes medulloblastoma to the effects of chemotherapy. Oncol Rep. 2011;25:1565–71. doi: 10.3892/or.2011.1233. doi:10.3892/or.2011.1233. [DOI] [PubMed] [Google Scholar]
- 410.Beauchamp MC, Knafo A, Yasmeen A, Carboni JM, Gottardis MM, Pollak MN. et al. BMS-536924 sensitizes human epithelial ovarian cancer cells to the PARP inhibitor, 3-aminobenzamide. Gynecol Oncol. 2009;115:193–8. doi: 10.1016/j.ygyno.2009.07.009. doi:10.1016/j.ygyno.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 411.Dool CJ, Mashhedi H, Zakikhani M, David S, Zhao Y, Birman E. et al. IGF1/insulin receptor kinase inhibition by BMS-536924 is better tolerated than alloxan-induced hypoinsulinemia and more effective than metformin in the treatment of experimental insulin-responsive breast cancer. Endocr Relat Cancer. 2011;18:699–709. doi: 10.1530/ERC-11-0136. doi:10.1530/erc-11-0136. [DOI] [PubMed] [Google Scholar]
- 412.Hou X, Huang F, Carboni JM, Flatten K, Asmann YW, Ten Eyck C. et al. Drug efflux by breast cancer resistance protein is a mechanism of resistance to the benzimidazole insulin-like growth factor receptor/insulin receptor inhibitor, BMS-536924. Mol Cancer Ther. 2011;10:117–25. doi: 10.1158/1535-7163.MCT-10-0438. doi:10.1158/1535-7163.mct-10-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Huang F, Greer A, Hurlburt W, Han X, Hafezi R, Wittenberg GM. et al. The mechanisms of differential sensitivity to an insulin-like growth factor-1 receptor inhibitor (BMS-536924) and rationale for combining with EGFR/HER2 inhibitors. Cancer Res. 2009;69:161–70. doi: 10.1158/0008-5472.CAN-08-0835. doi:10.1158/0008-5472.can-08-0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Wahner Hendrickson AE, Haluska P, Schneider PA, Loegering DA, Peterson KL, Attar R. et al. Expression of insulin receptor isoform A and insulin-like growth factor-1 receptor in human acute myelogenous leukemia: effect of the dual-receptor inhibitor BMS-536924 in vitro. Cancer Res. 2009;69:7635–43. doi: 10.1158/0008-5472.CAN-09-0511. doi:10.1158/0008-5472.can-09-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Gilbert JA, Adhikari LJ, Lloyd RV, Rubin J, Haluska P, Carboni JM. et al. Molecular markers for novel therapies in neuroendocrine (carcinoid) tumors. Endocr Relat Cancer. 2010;17:623–36. doi: 10.1677/ERC-09-0318. doi:10.1677/erc-09-0318. [DOI] [PubMed] [Google Scholar]
- 416.Svejda B, Kidd M, Timberlake A, Harry K, Kazberouk A, Schimmack S. et al. Serotonin and the 5-HT7 receptor: The link between hepatocytes, IGF-1 and small intestinal neuroendocrine tumors. Cancer Sci. 2013 doi: 10.1111/cas.12174. doi:10.1111/cas.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Gilbert JA, Lloyd RV, Ames MM. Lack of mutations in EGFR in gastroenteropancreatic neuroendocrine tumors. N Engl J Med. 2005;353:209–10. doi: 10.1056/NEJM200507143530219. doi:10.1056/nejm200507143530219. [DOI] [PubMed] [Google Scholar]
- 418.Bojko A, Reichert K, Adamczyk A, Ligeza J, Ligeza J, Klein A. The effect of tyrphostins AG494 and AG1478 on the autocrine growth regulation of A549 and DU145 cells. Folia Histochem Cytobiol. 2012;50:186–95. doi: 10.5603/fhc.2012.0028. [DOI] [PubMed] [Google Scholar]
- 419.Yan Y, Ai Z, Wang J, Xu Y, Teng Y. Influence of epidermal growth factor receptor inhibitor AG1478 on epithelial-mesenchymal transition in endometrial carcinoma cells. Int J Gynecol Cancer. 2012;22:1457–62. doi: 10.1097/IGC.0b013e31826ed2be. doi:10.1097/IGC.0b013e31826ed2be. [DOI] [PubMed] [Google Scholar]
- 420.Jin X, Jin X, Sohn YW, Yin J, Kim SH, Joshi K. et al. Blockade of EGFR signaling promotes glioma stem-like cell invasiveness by abolishing ID3-mediated inhibition of p27(KIP1) and MMP3 expression. Cancer Lett. 2013;328:235–42. doi: 10.1016/j.canlet.2012.09.005. doi:10.1016/j.canlet.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 421.Eriksson B, Janson ET, Bax ND, Mignon M, Morant R, Opolon P. et al. The use of new somatostatin analogues, lanreotide and octastatin, in neuroendocrine gastro-intestinal tumours. Digestion. 1996;57(Suppl 1):77–80. doi: 10.1159/000201402. [DOI] [PubMed] [Google Scholar]
- 422.Cap J, Marekova M, Cerman J, Malirova E, Suba P, Netuka D. et al. Inhibition of hormone secretion in GH-secreting pituitary adenomas by receptor-subtype specific somatostatin analogues in vitro. Gen Physiol Biophys. 2003;22:201–12. [PubMed] [Google Scholar]
- 423.Duran-Prado M, Saveanu A, Luque RM, Gahete MD, Gracia-Navarro F, Jaquet P. et al. A potential inhibitory role for the new truncated variant of somatostatin receptor 5, sst5TMD4, in pituitary adenomas poorly responsive to somatostatin analogs. J Clin Endocrinol Metab. 2010;95:2497–502. doi: 10.1210/jc.2009-2247. doi:10.1210/jc.2009-2247. [DOI] [PubMed] [Google Scholar]
- 424.Saveanu A, Gunz G, Guillen S, Dufour H, Culler MD, Jaquet P. Somatostatin and dopamine-somatostatin multiple ligands directed towards somatostatin and dopamine receptors in pituitary adenomas. Neuroendocrinology. 2006;83:258–63. doi: 10.1159/000095536. doi:10.1159/000095536. [DOI] [PubMed] [Google Scholar]
- 425.Zatelli MC, Tagliati F, Taylor JE, Rossi R, Culler MD, degli Uberti EC. Somatostatin receptor subtypes 2 and 5 differentially affect proliferation in vitro of the human medullary thyroid carcinoma cell line tt. J Clin Endocrinol Metab. 2001;86:2161–9. doi: 10.1210/jcem.86.5.7489. [DOI] [PubMed] [Google Scholar]
- 426.Bocci G, Culler MD, Fioravanti A, Orlandi P, Fasciani A, Colucci R. et al. In vitro antiangiogenic activity of selective somatostatin subtype-1 receptor agonists. Eur J Clin Invest. 2007;37:700–8. doi: 10.1111/j.1365-2362.2007.01848.x. doi:10.1111/j.1365-2362.2007.01848.x. [DOI] [PubMed] [Google Scholar]
- 427.Fusco A, Gunz G, Jaquet P, Dufour H, Germanetti AL, Culler MD. et al. Somatostatinergic ligands in dopamine-sensitive and -resistant prolactinomas. Eur J Endocrinol. 2008;158:595–603. doi: 10.1530/EJE-07-0806. doi:10.1530/eje-07-0806. [DOI] [PubMed] [Google Scholar]
- 428.Gruszka A, Kunert-Radek J, Radek A, Pisarek H, Taylor J, Dong JZ. et al. The effect of selective sst1, sst2, sst5 somatostatin receptors agonists, a somatostatin/dopamine (SST/DA) chimera and bromocriptine on the "clinically non-functioning" pituitary adenomas in vitro. Life Sci. 2006;78:689–93. doi: 10.1016/j.lfs.2005.05.061. doi:10.1016/j.lfs.2005.05.061. [DOI] [PubMed] [Google Scholar]
- 429.Ruscica M, Arvigo M, Gatto F, Dozio E, Feltrin D, Culler MD. et al. Regulation of prostate cancer cell proliferation by somatostatin receptor activation. Mol Cell Endocrinol. 2010;315:254–62. doi: 10.1016/j.mce.2009.11.006. doi:10.1016/j.mce.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 430.Ludvigsen E, Stridsberg M, Taylor JE, Culler MD, Oberg K, Janson ET. Subtype selective interactions of somatostatin and somatostatin analogs with sst1, sst2, and sst5 in BON-1 cells. Med Oncol. 2004;21:285–95. doi: 10.1385/MO:21:3:285. doi:10.1385/mo:21:3:285. [DOI] [PubMed] [Google Scholar]
- 431.Barbieri F, Pattarozzi A, Gatti M, Aiello C, Quintero A, Lunardi G. et al. Differential efficacy of SSTR1, -2, and -5 agonists in the inhibition of C6 glioma growth in nude mice. Am J Physiol Endocrinol Metab. 2009;297:E1078–88. doi: 10.1152/ajpendo.00292.2009. doi:10.1152/ajpendo.00292.2009. [DOI] [PubMed] [Google Scholar]
- 432.Barbieri F, Pattarozzi A, Gatti M, Porcile C, Bajetto A, Ferrari A. et al. Somatostatin receptors 1, 2, and 5 cooperate in the somatostatin inhibition of C6 glioma cell proliferation in vitro via a phosphotyrosine phosphatase-eta-dependent inhibition of extracellularly regulated kinase-1/2. Endocrinology. 2008;149:4736–46. doi: 10.1210/en.2007-1762. doi:10.1210/en.2007-1762. [DOI] [PubMed] [Google Scholar]
- 433.Gruszka A, Culler MD, Melmed S. Somatostatin analogs and chimeric somatostatin-dopamine molecules differentially regulate human growth hormone and prolactin gene expression and secretion in vitro. Mol Cell Endocrinol. 2012;362:104–9. doi: 10.1016/j.mce.2012.05.020. doi:10.1016/j.mce.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 434.Zatelli MC, Tagliati F, Piccin D, Taylor JE, Culler MD, Bondanelli M. et al. Somatostatin receptor subtype 1-selective activation reduces cell growth and calcitonin secretion in a human medullary thyroid carcinoma cell line. Biochem Biophys Res Commun. 2002;297:828–34. doi: 10.1016/s0006-291x(02)02307-0. [DOI] [PubMed] [Google Scholar]
- 435.Tallent M, Liapakis G, O'Carroll AM, Lolait SJ, Dichter M, Reisine T. Somatostatin receptor subtypes SSTR2 and SSTR5 couple negatively to an L-type Ca2+ current in the pituitary cell line AtT-20. Neuroscience. 1996;71:1073–81. doi: 10.1016/0306-4522(95)00510-2. [DOI] [PubMed] [Google Scholar]
- 436.Prevost G, Veber N, Viollet C, Roubert V, Roubert P, Benard J. et al. Somatostatin-14 mainly binds the somatostatin receptor subtype 2 in human neuroblastoma tumors. Neuroendocrinology. 1996;63:188–97. doi: 10.1159/000126957. [DOI] [PubMed] [Google Scholar]
- 437.Cescato R, Loesch KA, Waser B, Macke HR, Rivier JE, Reubi JC. et al. Agonist-biased signaling at the sst2A receptor: the multi-somatostatin analogs KE108 and SOM230 activate and antagonize distinct signaling pathways. Mol Endocrinol. 2010;24:240–9. doi: 10.1210/me.2009-0321. doi:10.1210/me.2009-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Cescato R, Schulz S, Waser B, Eltschinger V, Rivier JE, Wester HJ. et al. Internalization of sst2, sst3, and sst5 receptors: effects of somatostatin agonists and antagonists. J Nucl Med. 2006;47:502–11. [PubMed] [Google Scholar]
- 439.Mentlein R, Eichler O, Forstreuter F, Held-Feindt J. Somatostatin inhibits the production of vascular endothelial growth factor in human glioma cells. Int J Cancer. 2001;92:545–50. doi: 10.1002/ijc.1223. [DOI] [PubMed] [Google Scholar]
- 440.Stark A, Mentlein R. Somatostatin inhibits glucagon-like peptide-1-induced insulin secretion and proliferation of RINm5F insulinoma cells. Regul Pept. 2002;108:97–102. doi: 10.1016/s0167-0115(02)00152-0. [DOI] [PubMed] [Google Scholar]
- 441.Yang SK, Parkington HC, Blake AD, Keating DJ, Chen C. Somatostatin increases voltage-gated K+ currents in GH3 cells through activation of multiple somatostatin receptors. Endocrinology. 2005;146:4975–84. doi: 10.1210/en.2005-0696. doi:10.1210/en.2005-0696. [DOI] [PubMed] [Google Scholar]
- 442.Arvigo M, Gatto F, Ruscica M, Ameri P, Dozio E, Albertelli M. et al. Somatostatin and dopamine receptor interaction in prostate and lung cancer cell lines. J Endocrinol. 2010;207:309–17. doi: 10.1677/JOE-10-0342. doi:10.1677/joe-10-0342. [DOI] [PubMed] [Google Scholar]
- 443.Florio T, Barbieri F, Spaziante R, Zona G, Hofland LJ, van Koetsveld PM. et al. Efficacy of a dopamine-somatostatin chimeric molecule, BIM-23A760, in the control of cell growth from primary cultures of human non-functioning pituitary adenomas: a multi-center study. Endocr Relat Cancer. 2008;15:583–96. doi: 10.1677/ERC-07-0271. doi:10.1677/erc-07-0271. [DOI] [PubMed] [Google Scholar]
- 444.Ferone D, Arvigo M, Semino C, Jaquet P, Saveanu A, Taylor JE. et al. Somatostatin and dopamine receptor expression in lung carcinoma cells and effects of chimeric somatostatin-dopamine molecules on cell proliferation. Am J Physiol Endocrinol Metab. 2005;289:E1044–50. doi: 10.1152/ajpendo.00209.2005. doi:10.1152/ajpendo.00209.2005. [DOI] [PubMed] [Google Scholar]
- 445.Gatto F, Barbieri F, Gatti M, Wurth R, Schulz S, Ravetti JL. et al. Balance between somatostatin and D2 receptor expression drives TSH-secreting adenoma response to somatostatin analogues and dopastatins. Clin Endocrinol (Oxf) 2012;76:407–14. doi: 10.1111/j.1365-2265.2011.04200.x. doi:10.1111/j.1365-2265.2011.04200.x. [DOI] [PubMed] [Google Scholar]
- 446.Ren SG, Kim S, Taylor J, Dong J, Moreau JP, Culler MD. et al. Suppression of rat and human growth hormone and prolactin secretion by a novel somatostatin/dopaminergic chimeric ligand. J Clin Endocrinol Metab. 2003;88:5414–21. doi: 10.1210/jc.2003-030302. [DOI] [PubMed] [Google Scholar]
- 447.Saveanu A, Lavaque E, Gunz G, Barlier A, Kim S, Taylor JE. et al. Demonstration of enhanced potency of a chimeric somatostatin-dopamine molecule, BIM-23A387, in suppressing growth hormone and prolactin secretion from human pituitary somatotroph adenoma cells. J Clin Endocrinol Metab. 2002;87:5545–52. doi: 10.1210/jc.2002-020934. [DOI] [PubMed] [Google Scholar]
- 448.Gruszka A, Ren SG, Dong J, Culler MD, Melmed S. Regulation of growth hormone and prolactin gene expression and secretion by chimeric somatostatin-dopamine molecules. Endocrinology. 2007;148:6107–14. doi: 10.1210/en.2007-0378. doi:10.1210/en.2007-0378. [DOI] [PubMed] [Google Scholar]
- 449.Chen L, Han L, Shi Z, Zhang K, Liu Y, Zheng Y. et al. LY294002 enhances cytotoxicity of temozolomide in glioma by down-regulation of the PI3K/Akt pathway. Mol Med Rep. 2012;5:575–9. doi: 10.3892/mmr.2011.674. doi:10.3892/mmr.2011.674. [DOI] [PubMed] [Google Scholar]
- 450.Gong C, Liao H, Wang J, Lin Y, Qi J, Qin L. et al. LY294002 induces G0/G1 cell cycle arrest and apoptosis of cancer stem-like cells from human osteosarcoma via down-regulation of PI3K activity. Asian Pac J Cancer Prev. 2012;13:3103–7. doi: 10.7314/apjcp.2012.13.7.3103. [DOI] [PubMed] [Google Scholar]
- 451.Guo RX, Zhang RF, Wang XY, Shi HR, Qiao YH. [Effects of PD98059 and LY294002 on subcutaneous xenograft of human endometrial carcinoma in nude mice] Zhonghua Fu Chan Ke Za Zhi. 2011;46:446–52. [PubMed] [Google Scholar]
- 452.Li C, Liu VW, Chan DW, Yao KM, Ngan HY. LY294002 and metformin cooperatively enhance the inhibition of growth and the induction of apoptosis of ovarian cancer cells. Int J Gynecol Cancer. 2012;22:15–22. doi: 10.1097/IGC.0b013e3182322834. doi:10.1097/IGC.0b013e3182322834. [DOI] [PubMed] [Google Scholar]
- 453.Liu J, Fu XQ, Zhou W, Yu HG, Yu JP, Luo HS. LY294002 potentiates the anti-cancer effect of oxaliplatin for gastric cancer via death receptor pathway. World J Gastroenterol. 2011;17:181–90. doi: 10.3748/wjg.v17.i2.181. doi:10.3748/wjg.v17.i2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Sun JL, Zhu BS, Gong W, Zhang P, Yu LY, Zhao K. et al. [Effects of combined therapy of LY294002 and SN50 on nude mice model with gastric cancer] Zhonghua Wei Chang Wai Ke Za Zhi. 2011;14:364–7. [PubMed] [Google Scholar]
- 455.Wempe SL, Gamarra-Luques CD, Telleria CM. Synergistic lethality of mifepristone and LY294002 in ovarian cancer cells. Cancer Growth Metastasis. 2013;6:1–13. doi: 10.4137/CGM.S11124. doi:10.4137/cgm.s11124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Wu D, Tao J, Xu B, Qing W, Li P, Lu Q. et al. Phosphatidylinositol 3-kinase inhibitor LY294002 suppresses proliferation and sensitizes doxorubicin chemotherapy in bladder cancer cells. Urol Int. 2011;87:105–13. doi: 10.1159/000322849. doi:10.1159/000322849. [DOI] [PubMed] [Google Scholar]
- 457.Yoshioka T, Yogosawa S, Yamada T, Kitawaki J, Sakai T. Combination of a novel HDAC inhibitor OBP-801/YM753 and a PI3K inhibitor LY294002 synergistically induces apoptosis in human endometrial carcinoma cells due to increase of Bim with accumulation of ROS. Gynecol Oncol. 2013;129:425–32. doi: 10.1016/j.ygyno.2013.02.008. doi:10.1016/j.ygyno.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 458.Al-Rawi MA, Rmali K, Mansel RE, Jiang WG. Interleukin 7 induces the growth of breast cancer cells through a wortmannin-sensitive pathway. Br J Surg. 2004;91:61–8. doi: 10.1002/bjs.4449. doi:10.1002/bjs.4449. [DOI] [PubMed] [Google Scholar]
- 459.Boehle AS, Kurdow R, Boenicke L, Schniewind B, Faendrich F, Dohrmann P. et al. Wortmannin inhibits growth of human non-small-cell lung cancer in vitro and in vivo. Langenbecks Arch Surg. 2002;387:234–9. doi: 10.1007/s00423-002-0314-x. doi:10.1007/s00423-002-0314-x. [DOI] [PubMed] [Google Scholar]
- 460.Li J, Li F, Wang H, Wang X, Jiang Y, Li D. Wortmannin reduces metastasis and angiogenesis of human breast cancer cells via nuclear factor-kappaB-dependent matrix metalloproteinase-9 and interleukin-8 pathways. J Int Med Res. 2012;40:867–76. doi: 10.1177/147323001204000305. [DOI] [PubMed] [Google Scholar]
- 461.Teranishi F, Takahashi N, Gao N, Akamo Y, Takeyama H, Manabe T. et al. Phosphoinositide 3-kinase inhibitor (wortmannin) inhibits pancreatic cancer cell motility and migration induced by hyaluronan in vitro and peritoneal metastasis in vivo. Cancer Sci. 2009;100:770–7. doi: 10.1111/j.1349-7006.2009.01084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Yun J, Lv YG, Yao Q, Wang L, Li YP, Yi J. Wortmannin inhibits proliferation and induces apoptosis of MCF-7 breast cancer cells. Eur J Gynaecol Oncol. 2012;33:367–9. [PubMed] [Google Scholar]
- 463.Zhang F, Zhang T, Jiang T, Zhang R, Teng ZH, Li C. et al. Wortmannin potentiates roscovitine-induced growth inhibition in human solid tumor cells by repressing PI3K/Akt pathway. Cancer Lett. 2009;286:232–9. doi: 10.1016/j.canlet.2009.05.039. doi:10.1016/j.canlet.2009.05.039. [DOI] [PubMed] [Google Scholar]
- 464.Bagci-Onder T, Wakimoto H, Anderegg M, Cameron C, Shah K. A dual PI3K/mTOR inhibitor, PI-103, cooperates with stem cell-delivered TRAIL in experimental glioma models. Cancer Res. 2011;71:154–63. doi: 10.1158/0008-5472.CAN-10-1601. doi:10.1158/0008-5472.can-10-1601. [DOI] [PubMed] [Google Scholar]
- 465.Gedaly R, Angulo P, Hundley J, Daily MF, Chen C, Koch A. et al. PI-103 and sorafenib inhibit hepatocellular carcinoma cell proliferation by blocking Ras/Raf/MAPK and PI3K/AKT/mTOR pathways. Anticancer Res. 2010;30:4951–8. [PMC free article] [PubMed] [Google Scholar]
- 466.Kojima K, Shimanuki M, Shikami M, Samudio IJ, Ruvolo V, Corn P. et al. The dual PI3 kinase/mTOR inhibitor PI-103 prevents p53 induction by Mdm2 inhibition but enhances p53-mediated mitochondrial apoptosis in p53 wild-type AML. Leukemia. 2008;22:1728–36. doi: 10.1038/leu.2008.158. doi:10.1038/leu.2008.158. [DOI] [PubMed] [Google Scholar]
- 467.Lopez-Fauqued M, Gil R, Grueso J, Hernandez-Losa J, Pujol A, Moline T. et al. The dual PI3K/mTOR inhibitor PI-103 promotes immunosuppression, in vivo tumor growth and increases survival of sorafenib-treated melanoma cells. Int J Cancer. 2010;126:1549–61. doi: 10.1002/ijc.24926. doi:10.1002/ijc.24926. [DOI] [PubMed] [Google Scholar]
- 468.Park S, Chapuis N, Bardet V, Tamburini J, Gallay N, Willems L. et al. PI-103, a dual inhibitor of Class IA phosphatidylinositide 3-kinase and mTOR, has antileukemic activity in AML. Leukemia. 2008;22:1698–706. doi: 10.1038/leu.2008.144. doi:10.1038/leu.2008.144. [DOI] [PubMed] [Google Scholar]
- 469.Zou ZQ, Zhang XH, Wang F, Shen QJ, Xu J, Zhang LN. et al. A novel dual PI3Kalpha/mTOR inhibitor PI-103 with high antitumor activity in non-small cell lung cancer cells. Int J Mol Med. 2009;24:97–101. doi: 10.3892/ijmm_00000212. [DOI] [PubMed] [Google Scholar]
- 470.Zhao Y, Duan S, Zeng X, Liu C, Davies NM, Li B. et al. Prodrug strategy for PSMA-targeted delivery of TGX-221 to prostate cancer cells. Mol Pharm. 2012;9:1705–16. doi: 10.1021/mp3000309. doi:10.1021/mp3000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Meuillet EJ, Zuohe S, Lemos R, Ihle N, Kingston J, Watkins R. et al. Molecular pharmacology and antitumor activity of PHT-427, a novel Akt/phosphatidylinositide-dependent protein kinase 1 pleckstrin homology domain inhibitor. Mol Cancer Ther. 2010;9:706–17. doi: 10.1158/1535-7163.MCT-09-0985. doi:10.1158/1535-7163.mct-09-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Reid JM, Walden CA, Qin R, Ziegler KL, Haslam JL, Rajewski RA. et al. Phase 0 clinical chemoprevention trial of the Akt inhibitor SR13668. Cancer Prev Res (Phila) 2011;4:347–53. doi: 10.1158/1940-6207.CAPR-10-0313. doi:10.1158/1940-6207.capr-10-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Mandal M, Kim S, Younes MN, Jasser SA, El-Naggar AK, Mills GB. et al. The Akt inhibitor KP372-1 suppresses Akt activity and cell proliferation and induces apoptosis in thyroid cancer cells. Br J Cancer. 2005;92:1899–905. doi: 10.1038/sj.bjc.6602595. doi:10.1038/sj.bjc.6602595. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 474.Mandal M, Younes M, Swan EA, Jasser SA, Doan D, Yigitbasi O. et al. The Akt inhibitor KP372-1 inhibits proliferation and induces apoptosis and anoikis in squamous cell carcinoma of the head and neck. Oral Oncol. 2006;42:430–9. doi: 10.1016/j.oraloncology.2005.09.011. doi:10.1016/j.oraloncology.2005.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Zeng Z, Samudio IJ, Zhang W, Estrov Z, Pelicano H, Harris D. et al. Simultaneous inhibition of PDK1/AKT and Fms-like tyrosine kinase 3 signaling by a small-molecule KP372-1 induces mitochondrial dysfunction and apoptosis in acute myelogenous leukemia. Cancer Res. 2006;66:3737–46. doi: 10.1158/0008-5472.CAN-05-1278. doi:10.1158/0008-5472.can-05-1278. [DOI] [PubMed] [Google Scholar]
- 476.de Frias M, Iglesias-Serret D, Cosialls AM, Coll-Mulet L, Santidrian AF, Gonzalez-Girones DM. et al. Akt inhibitors induce apoptosis in chronic lymphocytic leukemia cells. Haematologica. 2009;94:1698–707. doi: 10.3324/haematol.2008.004028. doi:10.3324/haematol.2008.004028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Gallia GL, Tyler BM, Hann CL, Siu IM, Giranda VL, Vescovi AL. et al. Inhibition of Akt inhibits growth of glioblastoma and glioblastoma stem-like cells. Mol Cancer Ther. 2009;8:386–93. doi: 10.1158/1535-7163.MCT-08-0680. doi:10.1158/1535-7163.mct-08-0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Han EK, Leverson JD, McGonigal T, Shah OJ, Woods KW, Hunter T. et al. Akt inhibitor A-443654 induces rapid Akt Ser-473 phosphorylation independent of mTORC1 inhibition. Oncogene. 2007;26:5655–61. doi: 10.1038/sj.onc.1210343. doi:10.1038/sj.onc.1210343. [DOI] [PubMed] [Google Scholar]
- 479.Liu X, Shi Y, Woods KW, Hessler P, Kroeger P, Wilsbacher J. et al. Akt inhibitor a-443654 interferes with mitotic progression by regulating aurora a kinase expression. Neoplasia. 2008;10:828–37. doi: 10.1593/neo.08408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Somnay Y, Simon K, Harrison AD, Kunnimalaiyaan S, Chen H, Kunnimalaiyaan M. Neuroendocrine phenotype alteration and growth suppression through apoptosis by MK-2206, an allosteric inhibitor of AKT, in carcinoid cell lines in vitro. Anticancer Drugs. 2013;24:66–72. doi: 10.1097/CAD.0b013e3283584f75. doi:10.1097/CAD.0b013e3283584f75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Feldman RI, Wu JM, Polokoff MA, Kochanny MJ, Dinter H, Zhu D. et al. Novel small molecule inhibitors of 3-phosphoinositide-dependent kinase-1. J Biol Chem. 2005;280:19867–74. doi: 10.1074/jbc.M501367200. doi:10.1074/jbc.M501367200. [DOI] [PubMed] [Google Scholar]
- 482.Duran I, Kortmansky J, Singh D, Hirte H, Kocha W, Goss G. et al. A phase II clinical and pharmacodynamic study of temsirolimus in advanced neuroendocrine carcinomas. Br J Cancer. 2006;95:1148–54. doi: 10.1038/sj.bjc.6603419. doi:10.1038/sj.bjc.6603419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Eum KH, Ahn SK, Kang H, Lee M. Differential inhibitory effects of two Raf-targeting drugs, sorafenib and PLX4720, on the growth of multidrug-resistant cells. Mol Cell Biochem. 2013;372:65–74. doi: 10.1007/s11010-012-1446-0. doi:10.1007/s11010-012-1446-0. [DOI] [PubMed] [Google Scholar]
- 484.Jiang CC, Lai F, Thorne RF, Yang F, Liu H, Hersey P. et al. MEK-independent survival of B-RAFV600E melanoma cells selected for resistance to apoptosis induced by the RAF inhibitor PLX4720. Clin Cancer Res. 2011;17:721–30. doi: 10.1158/1078-0432.CCR-10-2225. doi:10.1158/1078-0432.ccr-10-2225. [DOI] [PubMed] [Google Scholar]
- 485.Nehs MA, Nagarkatti S, Nucera C, Hodin RA, Parangi S. Thyroidectomy with neoadjuvant PLX4720 extends survival and decreases tumor burden in an orthotopic mouse model of anaplastic thyroid cancer. Surgery. 2010;148:1154–62. doi: 10.1016/j.surg.2010.09.001. discussion 62. doi:10.1016/j.surg.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Nucera C, Nehs MA, Nagarkatti SS, Sadow PM, Mekel M, Fischer AH. et al. Targeting BRAFV600E with PLX4720 displays potent antimigratory and anti-invasive activity in preclinical models of human thyroid cancer. Oncologist. 2011;16:296–309. doi: 10.1634/theoncologist.2010-0317. doi:10.1634/theoncologist.2010-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Oikonomou E, Koc M, Sourkova V, Andera L, Pintzas A. Selective BRAFV600E inhibitor PLX4720, requires TRAIL assistance to overcome oncogenic PIK3CA resistance. PLoS One. 2011;6:e21632.. doi: 10.1371/journal.pone.0021632. doi:10.1371/journal.pone.0021632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Shao Y, Aplin AE. BH3-only protein silencing contributes to acquired resistance to PLX4720 in human melanoma. Cell Death Differ. 2012;19:2029–39. doi: 10.1038/cdd.2012.94. doi:10.1038/cdd.2012.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Gibney GT, Zager JS. Clinical development of dabrafenib in BRAF mutant melanoma and other malignancies. Expert Opin Drug Metab Toxicol. 2013;9:893–9. doi: 10.1517/17425255.2013.794220. doi:10.1517/17425255.2013.794220. [DOI] [PubMed] [Google Scholar]
- 490.Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M. et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358–65. doi: 10.1016/S0140-6736(12)60868-X. doi:10.1016/s0140-6736(12)60868-x. [DOI] [PubMed] [Google Scholar]
- 491.Long GV, Trefzer U, Davies MA, Kefford RF, Ascierto PA, Chapman PB. et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:1087–95. doi: 10.1016/S1470-2045(12)70431-X. doi:10.1016/s1470-2045(12)70431-x. [DOI] [PubMed] [Google Scholar]
- 492.Lee NV, Lira ME, Pavlicek A, Ye J, Buckman D, Bagrodia S. et al. A novel SND1-BRAF fusion confers resistance to c-Met inhibitor PF-04217903 in GTL16 cells though MAPK activation. PLoS One. 2012;7:e39653.. doi: 10.1371/journal.pone.0039653. doi:10.1371/journal.pone.0039653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Torti VR, Wojciechowicz D, Hu W, John-Baptiste A, Evering W, Troche G. et al. Epithelial tissue hyperplasia induced by the RAF inhibitor PF-04880594 is attenuated by a clinically well-tolerated dose of the mek inhibitor PD-0325901. Mol Cancer Ther. 2012;11:2274–83. doi: 10.1158/1535-7163.MCT-11-0984. doi:10.1158/1535-7163.mct-11-0984. [DOI] [PubMed] [Google Scholar]
- 494.Montagut C, Sharma SV, Shioda T, McDermott U, Ulman M, Ulkus LE. et al. Elevated CRAF as a potential mechanism of acquired resistance to BRAF inhibition in melanoma. Cancer Res. 2008;68:4853–61. doi: 10.1158/0008-5472.CAN-07-6787. doi:10.1158/0008-5472.can-07-6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Whittaker SR, Theurillat JP, Van Allen E, Wagle N, Hsiao J, Cowley GS. et al. A genome-scale RNA interference screen implicates NF1 loss in resistance to RAF inhibition. Cancer Discov. 2013;3:350–62. doi: 10.1158/2159-8290.CD-12-0470. doi:10.1158/2159-8290.cd-12-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Baines P, Fisher J, Truran L, Davies E, Hallett M, Hoy T. et al. The MEK inhibitor, PD98059, reduces survival but does not block acute myeloid leukemia blast maturation in vitro. Eur J Haematol. 2000;64:211–8. doi: 10.1034/j.1600-0609.2000.90139.x. [DOI] [PubMed] [Google Scholar]
- 497.Kanda S, Kanetake H, Miyata Y. Long-term exposure of human renal carcinoma cells to PD98059 induces epithelial-mesenchymal transition-like phenotype and enhanced motility. Mol Cell Biochem. 2008;309:69–76. doi: 10.1007/s11010-007-9644-x. doi:10.1007/s11010-007-9644-x. [DOI] [PubMed] [Google Scholar]
- 498.Mandic A, Viktorsson K, Heiden T, Hansson J, Shoshan MC. The MEK1 inhibitor PD98059 sensitizes C8161 melanoma cells to cisplatin-induced apoptosis. Melanoma Res. 2001;11:11–9. doi: 10.1097/00008390-200102000-00002. [DOI] [PubMed] [Google Scholar]
- 499.Xu LL, Mei JH, Chen JX, Xu S, Qin HY, Wang SS. [Study the role of PD98059 in ovarian carcinoma cell line HO-8910] Zhonghua Bing Li Xue Za Zhi. 2008;37:625–6. [PubMed] [Google Scholar]
- 500.Yao J, Qian C, Shu T, Zhang X, Zhao Z, Liang Y. Combination treatment of PD98059 and DAPT in gastric cancer through induction of apoptosis and downregulation of WNT/beta-catenin. Cancer Biol Ther. 2013;14(9):833–839. doi: 10.4161/cbt.25332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Zhang YJ, Fang JY, Sun DF, Zhao SL, Shen GF, Zheng Q. et al. [Synergistic effect of rapamycin (RPM) and PD98059 on cell cycle and mTOR signal transduction in human colorectal cancer cells] Zhonghua Zhong Liu Za Zhi. 2007;29:889–93. [PubMed] [Google Scholar]
- 502.Flores LG 2nd, Yeh HH, Soghomonyan S, Young D, Bankson J, Hu Q. et al. Monitoring therapy with MEK inhibitor U0126 in a novel Wilms tumor model in Wt1 knockout Igf2 transgenic mice using 18F-FDG PET with dual-contrast enhanced CT and MRI: early metabolic response without inhibition of tumor growth. Mol Imaging Biol. 2013;15:175–85. doi: 10.1007/s11307-012-0588-5. doi:10.1007/s11307-012-0588-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Freeman MR, Kim J, Lisanti MP, Di Vizio D. A metabolic perturbation by U0126 identifies a role for glutamine in resveratrol-induced cell death. Cancer Biol Ther. 2011;12:966–77. doi: 10.4161/cbt.12.11.18136. doi:10.4161/cbt.12.11.18136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Ge X, Fu YM, Meadows GG. U0126, a mitogen-activated protein kinase kinase inhibitor, inhibits the invasion of human A375 melanoma cells. Cancer Lett. 2002;179:133–40. doi: 10.1016/s0304-3835(02)00004-6. [DOI] [PubMed] [Google Scholar]
- 505.Georgakis GV, Li Y, Rassidakis GZ, Medeiros LJ, Younes A. The HSP90 inhibitor 17-AAG synergizes with doxorubicin and U0126 in anaplastic large cell lymphoma irrespective of ALK expression. Exp Hematol. 2006;34:1670–9. doi: 10.1016/j.exphem.2006.07.002. doi:10.1016/j.exphem.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 506.Hong SK, Kim JH, Lin MF, Park JI. The Raf/MEK/extracellular signal-regulated kinase 1/2 pathway can mediate growth inhibitory and differentiation signaling via androgen receptor downregulation in prostate cancer cells. Exp Cell Res. 2011;317:2671–82. doi: 10.1016/j.yexcr.2011.08.008. doi:10.1016/j.yexcr.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Horiuchi H, Kawamata H, Fujimori T, Kuroda Y. A MEK inhibitor (U0126) prolongs survival in nude mice bearing human gallbladder cancer cells with K-ras mutation: analysis in a novel orthotopic inoculation model. Int J Oncol. 2003;23:957–63. [PubMed] [Google Scholar]
- 508.Horiuchi H, Kawamata H, Furihata T, Omotehara F, Hori H, Shinagawa Y. et al. A MEK inhibitor (U0126) markedly inhibits direct liver invasion of orthotopically inoculated human gallbladder cancer cells in nude mice. J Exp Clin Cancer Res. 2004;23:599–606. [PubMed] [Google Scholar]
- 509.Ito M, Zhao N, Zeng Z, Chang CC, Zu Y. Synergistic growth inhibition of anaplastic large cell lymphoma cells by combining cellular ALK gene silencing and a low dose of the kinase inhibitor U0126. Cancer Gene Ther. 2010;17:633–44. doi: 10.1038/cgt.2010.20. doi:10.1038/cgt.2010.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.James JA, Smith MA, Court EL, Yip C, Ching Y, Willson C. et al. An investigation of the effects of the MEK inhibitor U0126 on apoptosis in acute leukemia. Hematol J. 2003;4:427–32. doi: 10.1038/sj.thj.6200327. doi:10.1038/sj.thj.6200327. [DOI] [PubMed] [Google Scholar]
- 511.Kerr AH, James JA, Smith MA, Willson C, Court EL, Smith JG. An investigation of the MEK/ERK inhibitor U0126 in acute myeloid leukemia. Ann N Y Acad Sci. 2003;1010:86–9. doi: 10.1196/annals.1299.013. [DOI] [PubMed] [Google Scholar]
- 512.Lind CR, Gray CW, Pearson AG, Cameron RE, O'Carroll SJ, Narayan PJ. et al. The mitogen-activated/extracellular signal-regulated kinase kinase 1/2 inhibitor U0126 induces glial fibrillary acidic protein expression and reduces the proliferation and migration of C6 glioma cells. Neuroscience. 2006;141:1925–33. doi: 10.1016/j.neuroscience.2006.05.038. doi:10.1016/j.neuroscience.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 513.Marampon F, Gravina GL, Di Rocco A, Bonfili P, Di Staso M, Fardella C. et al. MEK/ERK inhibitor U0126 increases the radiosensitivity of rhabdomyosarcoma cells in vitro and in vivo by downregulating growth and DNA repair signals. Mol Cancer Ther. 2011;10:159–68. doi: 10.1158/1535-7163.MCT-10-0631. doi:10.1158/1535-7163.mct-10-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Yip-Schneider MT, Schmidt CM. MEK inhibition of pancreatic carcinoma cells by U0126 and its effect in combination with sulindac. Pancreas. 2003;27:337–44. doi: 10.1097/00006676-200311000-00012. [DOI] [PubMed] [Google Scholar]
- 515.Zou ZQ, Zhang LN, Wang F, Bellenger J, Shen YZ, Zhang XH. The novel dual PI3K/mTOR inhibitor GDC-0941 synergizes with the MEK inhibitor U0126 in non-small cell lung cancer cells. Mol Med Rep. 2012;5:503–8. doi: 10.3892/mmr.2011.682. doi:10.3892/mmr.2011.682. [DOI] [PubMed] [Google Scholar]
- 516.Iida S, Miki Y, Ono K, Akahira J, Nakamura Y, Suzuki T. et al. Synergistic anti-tumor effects of RAD001 with MEK inhibitors in neuroendocrine tumors: a potential mechanism of therapeutic limitation of mTOR inhibitor. Mol Cell Endocrinol. 2012;350:99–106. doi: 10.1016/j.mce.2011.11.024. doi:10.1016/j.mce.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 517.Kunnimalaiyaan M, Ndiaye M, Chen H. Neuroendocrine tumor cell growth inhibition by ZM336372 through alterations in multiple signaling pathways. Surgery. 2007;142:959–64. doi: 10.1016/j.surg.2007.09.020. discussion -64. doi:10.1016/j.surg.2007.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Hail N Jr, Chen P, Bushman LR. Teriflunomide (leflunomide) promotes cytostatic, antioxidant, and apoptotic effects in transformed prostate epithelial cells: evidence supporting a role for teriflunomide in prostate cancer chemoprevention. Neoplasia. 2010;12:464–75. doi: 10.1593/neo.10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Hail N Jr, Chen P, Rower J, Bushman LR. Teriflunomide encourages cytostatic and apoptotic effects in premalignant and malignant cutaneous keratinocytes. Apoptosis. 2010;15:1234–46. doi: 10.1007/s10495-010-0518-4. doi:10.1007/s10495-010-0518-4. [DOI] [PubMed] [Google Scholar]
- 520.Adler JT, Cook M, Luo Y, Pitt SC, Ju J, Li W. et al. Tautomycetin and tautomycin suppress the growth of medullary thyroid cancer cells via inhibition of glycogen synthase kinase-3beta. Mol Cancer Ther. 2009;8:914–20. doi: 10.1158/1535-7163.MCT-08-0712. doi:10.1158/1535-7163.mct-08-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Magae J, Watanabe C, Osada H, Cheng XC, Isono K. Induction of morphological change of human myeloid leukemia and activation of protein kinase C by a novel antibiotic, tautomycin. J Antibiot (Tokyo) 1988;41:932–7. doi: 10.7164/antibiotics.41.932. [DOI] [PubMed] [Google Scholar]
- 522.Suganuma M, Okabe S, Sueoka E, Nishiwaki R, Komori A, Uda N. et al. Tautomycin: an inhibitor of protein phosphatases 1 and 2A but not a tumor promoter on mouse skin and in rat glandular stomach. J Cancer Res Clin Oncol. 1995;121:621–7. doi: 10.1007/BF01197780. [DOI] [PubMed] [Google Scholar]
- 523.Kappes A, Vaccaro A, Kunnimalaiyaan M, Chen H. ZM336372, a Raf-1 activator, inhibits growth of pheochromocytoma cells. J Surg Res. 2006;133:42–5. doi: 10.1016/j.jss.2006.02.002. doi:10.1016/j.jss.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 524.Tatake RJ, O'Neill MM, Kennedy CA, Wayne AL, Jakes S, Wu D. et al. Identification of pharmacological inhibitors of the MEK5/ERK5 pathway. Biochem Biophys Res Commun. 2008;377:120–5. doi: 10.1016/j.bbrc.2008.09.087. doi:10.1016/j.bbrc.2008.09.087. [DOI] [PubMed] [Google Scholar]
- 525.Razumovskaya E, Sun J, Ronnstrand L. Inhibition of MEK5 by BIX02188 induces apoptosis in cells expressing the oncogenic mutant FLT3-ITD. Biochem Biophys Res Commun. 2011;412:307–12. doi: 10.1016/j.bbrc.2011.07.089. doi:10.1016/j.bbrc.2011.07.089. [DOI] [PubMed] [Google Scholar]
- 526.Yang Q, Lee JD. Targeting the BMK1 MAP kinase pathway in cancer therapy. Clin Cancer Res. 2011;17:3527–32. doi: 10.1158/1078-0432.CCR-10-2504. doi:10.1158/1078-0432.ccr-10-2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Weekes CD, Von Hoff DD, Adjei AA, Leffingwell DP, Eckhardt SG, Gore L. et al. Multicenter phase I trial of the mitogen-activated protein kinase 1/2 inhibitor BAY 86-9766 in patients with advanced cancer. Clin Cancer Res. 2013;19:1232–43. doi: 10.1158/1078-0432.CCR-12-3529. doi:10.1158/1078-0432.ccr-12-3529. [DOI] [PubMed] [Google Scholar]
- 528.Morris EJ, Jha S, Restaino CR, Dayananth P, Zhu H, Cooper A, Discovery of a Novel ERK Inhibitor with Activity in Models of Acquired Resistance to BRAF and MEK Inhibitors. Cancer Discov. 2013. doi:10.1158/2159-8290.cd-13-0070. [DOI] [PubMed]
- 529.Li H, Wang X, Chen T, Qu J. p38 inhibitor SB203580 sensitizes the resveratrol-induced apoptosis in human lung adenocarcinoma (A549) cells. J Biochem Mol Toxicol. 2012;26:251–7. doi: 10.1002/jbt.21413. doi:10.1002/jbt.21413. [DOI] [PubMed] [Google Scholar]
- 530.Zhao YM, Su B, Yang XJ, Shi JY, Tang L, Zhang J. et al. [Small molecule inhibitor SB203580 enhances the antitumor effect of gefitinib in PC-9 and A549 lung cancer cell lines] Zhonghua Zhong Liu Za Zhi. 2013;35:103–8. doi: 10.3760/cma.j.issn.0253-3766.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 531.He D, Zhao XQ, Chen XG, Fang Y, Singh S, Talele TT. et al. BIRB796, the inhibitor of p38 mitogen-activated protein kinase, enhances the efficacy of chemotherapeutic agents in ABCB1 overexpression cells. PLoS One. 2013;8:e54181.. doi: 10.1371/journal.pone.0054181. doi:10.1371/journal.pone.0054181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Yasui H, Hideshima T, Ikeda H, Jin J, Ocio EM, Kiziltepe T. et al. BIRB 796 enhances cytotoxicity triggered by bortezomib, heat shock protein (Hsp) 90 inhibitor, and dexamethasone via inhibition of p38 mitogen-activated protein kinase/Hsp27 pathway in multiple myeloma cell lines and inhibits paracrine tumour growth. Br J Haematol. 2007;136:414–23. doi: 10.1111/j.1365-2141.2006.06443.x. doi:10.1111/j.1365-2141.2006.06443.x. [DOI] [PubMed] [Google Scholar]
- 533.Grossi V, Liuzzi M, Murzilli S, Martelli N, Napoli A, Ingravallo G. et al. Sorafenib inhibits p38alpha activity in colorectal cancer cells and synergizes with the DFG-in inhibitor SB202190 to increase apoptotic response. Cancer Biol Ther. 2012;13:1471–81. doi: 10.4161/cbt.22254. doi:10.4161/cbt.22254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Hirosawa M, Nakahara M, Otosaka R, Imoto A, Okazaki T, Takahashi S. The p38 pathway inhibitor SB202190 activates MEK/MAPK to stimulate the growth of leukemia cells. Leuk Res. 2009;33:693–9. doi: 10.1016/j.leukres.2008.09.028. doi:10.1016/j.leukres.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 535.Hideshima T, Akiyama M, Hayashi T, Richardson P, Schlossman R, Chauhan D. et al. Targeting p38 MAPK inhibits multiple myeloma cell growth in the bone marrow milieu. Blood. 2003;101:703–5. doi: 10.1182/blood-2002-06-1874. doi:10.1182/blood-2002-06-1874. [DOI] [PubMed] [Google Scholar]
- 536.Jemaa M, Vitale I, Kepp O, Berardinelli F, Galluzzi L, Senovilla L. et al. Selective killing of p53-deficient cancer cells by SP600125. EMBO Mol Med. 2012;4:500–14. doi: 10.1002/emmm.201200228. doi:10.1002/emmm.201200228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Jin HO, Seo SK, Woo SH, Kim ES, Lee HC, Yoo DH. et al. SP600125 negatively regulates the mammalian target of rapamycin via ATF4-induced Redd1 expression. FEBS Lett. 2009;583:123–7. doi: 10.1016/j.febslet.2008.11.035. doi:10.1016/j.febslet.2008.11.035. [DOI] [PubMed] [Google Scholar]
- 538.Kim JA, Lee J, Margolis RL, Fotedar R. SP600125 suppresses Cdk1 and induces endoreplication directly from G2 phase, independent of JNK inhibition. Oncogene. 2010;29:1702–16. doi: 10.1038/onc.2009.464. doi:10.1038/onc.2009.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Kim JH, Kim TH, Kang HS, Ro J, Kim HS, Yoon S. SP600125, an inhibitor of Jnk pathway, reduces viability of relatively resistant cancer cells to doxorubicin. Biochem Biophys Res Commun. 2009;387:450–5. doi: 10.1016/j.bbrc.2009.07.036. doi:10.1016/j.bbrc.2009.07.036. [DOI] [PubMed] [Google Scholar]
- 540.Li JY, Huang JY, Xing B, Ren KW, Li M, Wei D. et al. SP600125, a JNK inhibitor, suppresses growth of JNK-inactive glioblastoma cells through cell-cycle G2/M phase arrest. Pharmazie. 2012;67:942–6. [PubMed] [Google Scholar]
- 541.Moon DO, Choi YH, Kim GY. Role of p21 in SP600125-induced cell cycle arrest, endoreduplication, and apoptosis. Cell Mol Life Sci. 2011;68:3249–60. doi: 10.1007/s00018-011-0626-5. doi:10.1007/s00018-011-0626-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Moon DO, Kim MO, Choi YH, Kim ND, Chang JH, Kim GY. Bcl-2 overexpression attenuates SP600125-induced apoptosis in human leukemia U937 cells. Cancer Lett. 2008;264:316–25. doi: 10.1016/j.canlet.2008.02.011. doi:10.1016/j.canlet.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 543.Renlund N, Pieretti-Vanmarcke R, O'Neill FH, Zhang L, Donahoe PK, Teixeira J. c-Jun N-terminal kinase inhibitor II (SP600125) activates Mullerian inhibiting substance type II receptor-mediated signal transduction. Endocrinology. 2008;149:108–15. doi: 10.1210/en.2007-0529. doi:10.1210/en.2007-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Wang M, Atayar C, Rosati S, Bosga-Bouwer A, Kluin P, Visser L. JNK is constitutively active in mantle cell lymphoma: cell cycle deregulation and polyploidy by JNK inhibitor SP600125. J Pathol. 2009;218:95–103. doi: 10.1002/path.2521. doi:10.1002/path.2521. [DOI] [PubMed] [Google Scholar]
- 545.Zhang T, Inesta-Vaquera F, Niepel M, Zhang J, Ficarro SB, Machleidt T. et al. Discovery of potent and selective covalent inhibitors of JNK. Chem Biol. 2012;19:140–54. doi: 10.1016/j.chembiol.2011.11.010. doi:10.1016/j.chembiol.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppl. 1-7.