Abstract
Dry eye in humans displays increased prevalence in the aged and in women. Here, we investigated the ocular surfaces and lacrimal glands of aged mice of both sexes. We surveyed three different ages [young, middle-aged (6 to 9 months), and elderly] by investigating severity markers of dry eye disease (DED). We observed an age-dependent dry eye phenotype as early as 6 to 9 months: increased corneal surface irregularity, increased corneal barrier disruption, conjunctival CD4+ T-cell infiltration, and loss of mucin-filled goblet cells. Expression of interferon-γ, IL-17 mRNA transcripts was increased in the conjunctiva and IL-17A, matrix metallopeptidase 9, and chemokine ligand 20 in the corneas of elderly mice. Elderly male mice develop more of a skewed response of type 1 T helper cell, whereas female mice have a bias toward type 17 T helper cell in the conjunctiva. In the lacrimal gland, an increase in CD4+ and CD8+ T cells and B cells and a decrease in activated dendritic cells were observed. Adoptive transfer of CD4+ T cells isolated from elderly mice transferred DED into young immunodeficient recipients, which was more pronounced from male donors. Our findings show the development of DED in aging mice. Pathogenic CD4+ T cells that develop with aging are capable of transferring DED from older mice to naive immunodeficient recipients. Taken together, our results indicate that age-related autoimmunity contributes to development of DED with aging.
Alterations in cellular immunity are a known aspect of aging.1 With increasing age, the thymus begins to involute, leading to a decrease in the number of naive T lymphocytes in the circulation. A compensatory autoproliferation of existing, mature T cells may lead to the expansion of CD4+, CD28− T cells. These cells are resistant to apoptosis and have been shown to be associated with a variety of autoimmune disorders such as rheumatoid arthritis, multiple sclerosis, and diabetes mellitus.2 Aging similarly affects the humoral immune system. A significant decrease in naive B cells is found in old age, with a concomitant increase in the life span of mature B cells. This increase of long-lasting B cells increases the possibility for autoreactivity against a neoantigen.3 It has also been proposed that dysfunctions in B-cell suppression and an age-related predilection for cellular response of type 2 T helper (Th2) cells may allow for and promote the reactivation of self-reactive memory B cells.4
Age-related changes in the innate and adaptive immune system are hallmarked by increased levels of proinflammatory cytokines at baseline and in response to stimuli because of dysfunction of antigen-presenting cells (APCs).5,6 Dendritic cells (DCs) are professional APCs that are largely responsible for initiating this antigen response, regardless if it is harmful or not.7 They can either activate the immune response or induce tolerance. The proper functioning of this family of APCs is crucial to the health of the ocular surface. Phagocytotic and pinocytotic abilities are significantly decreased in DCs from aged subjects,8 making antigen capture and presentation impaired. In contrast DCs in aged subjects release more tumor necrosis factor-α and IL-6 with Toll-like receptor stimulation,9 promoting a more proinflammatory environment. They have also been found to release greater amounts of IL-23 than those of younger subjects, promoting the development of Th17 cells that are associated with autoimmunity.10 Such age-related changes in DC function create a milieu ripe for developing autoimmunity on the ocular surface.
Dry eye disease (DED) has been shown to increase in prevalence with age.11 It has been reported that 6% of patients aged 40 years and as many as 15% to 33% of elderly patients older than 65 years are affected by the disease.11–13 The morbidity that this disease can cause the elderly population is tremendous. DED can ultimately decrease contrast sensitivity and increase higher order visual aberrations.14 Visual impairments have been associated with falls and hip fractures in the elderly,15 and complications from these falls are the leading cause of death from injury in men and women older than 65 years.16 As vision deteriorates, one's ability to perform activities of daily living becomes exceedingly more difficult. In the elderly, limitations in physical abilities confound such difficulties, making them less capable of independent living.
A number of common age-associated changes in the eye contribute to development of DED. These include a decrease in aqueous tear production17; decreases in corneal epithelial nerve density18 and thus sensitivity,19 conjunctivochalasis, ectropion, and lid laxity20; and decreases in supportive sex hormones.21,22 The role of immune dysfunction in the pathogenesis of the DED observed in the elderly has not been sufficiently investigated.
Elderly women are more commonly afflicted with DED than men. This is supported by the clinical findings of lower tear production in women than men through the sixth decade of life,23 and the observation that DED is more common in women who experience premature menopause due to primary ovarian failure.24 However, the basis for the sex predilection for DED has not yet been established. As with aging, a commonality between menopause and DED is the immune system, which sex hormones are known to have a significant influence over. Current studies show conflicting data as to which hormones promote inflammation and thus autoimmunity.25–27
Age-related alterations in the ocular surface immune system alone may increase the potential for the development of DED. In this study we investigated the immunocytologic profile of age-related DED. To accomplish this, we compared groups of male and female mice at three different ages: young, mid-life, and elderly. We hypothesized that dry eye-associated pathological changes of the cornea and conjunctiva will develop with age in mice and that there be accompanying age-related accumulation of CD4+, CD8+, and activated DCs in the ocular surface and lacrimal gland (LG) tissues.
Materials and Methods
Animals
C57BL/6 mice of both sexes, at three different age groups were evaluated: young (8 weeks old; The Jackson Laboratory, Bar Harbor, ME), middle-aged retired breeder (6 to 9 months olds; The Jackson Laboratory), and elderly (24 months old; National Institute on Aging, Bethesda, MD). Recombination activating gene-1 (RAG1) knockout (KO) mice were purchased from The Jackson Laboratory for establishing breeding colonies in our facility and were used at 8 weeks of age.
Our animals were kept in a standard vivarium facility that is managed by Baylor Center for Comparative Medicine. Mice were exposed to a 12-hour light cycle while in the dry eye room and in the vivarium, because both facilities have timers that control the light/dark cycle.
Mice under desiccating stress (DS) were kept in a separate room in our laboratory, whereby humidity control is achieved by a special heating, ventilating, and air-conditioning unit on the ceiling, and further desiccation is provided by two portable dehumidifiers.
C57BL/6 mice (n = 262) were used in this study, 131 of each sex as follows: 46 young C57BL/6 mice (no treatment), 41 middle-aged mice, 39 mice elderly mice, and 5 young C57BL/6 mice subjected to DS.
C57BL/6 mice (n = 46) of both sexes at 8 weeks of age were used for these studies as follows: histological sections (n = 5; eyes and LG), flow cytometry (n = 12, LG), corneal permeability (n = 12 animals), gene analysis (n = 12, cornea and conjunctiva), and adoptive transfer donors (n = 5, isolation of CD4+ T cells). Evaluation of corneal smoothness was performed immediately after the euthanasia, on the same mice that were used for flow cytometry (n = 12). Tear measurements and tear collection for epidermal growth factor (EGF) assays were performed in live mice on two consecutive days in mice later used for corneal staining or for flow cytometry (n = 12).
C57BL/6 mice (n = 41) of both sexes at 6 to 9 weeks of age were evaluated. The same read-outs evaluated in the young C57BL/6 mice were used, except for adoptive transfer. C57BL/6 mice (n = 39) of both sexes at 24 months of age were evaluated in the similar manner as young C57BL/6 mice except for corneal permeability.
Young C57BL/6 mice (n = 5) of both sexes were subjected to DS for 5 days as positive controls for the adoptive transfer experiments. RAG1 KO mice (n = 30) were used as recipients of adoptively transferred CD4+ T lymphocytes from mice of both sexes (n = 5 per age group/sex) 8 weeks old, 24 months old, and subjected to DS for 5 days.
This research protocol was approved by the Baylor College of Medicine Center for Comparative Medicine, and it conformed to the standards of the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
Evaluation of Corneal Smoothness
Corneal smoothness was assessed in 12 mice of the young, middle-aged, and elderly groups of both sexes as previously published.28 Both eyes were imaged, and results were averaged with all 24 samples. Results are presented as means ± SD.
Tear Volume Measurements
Phenol red threads tests were used to measure tear volume, as previously described.29 Briefly, a phenol red impregnated thread (Zone-Quick; Showa Yakuhin Kako Co., Tokyo, Japan) was held in the lateral canthus of each eye for 20 seconds. A color change from yellow to red occurs in the thread when tears are absorbed. The distance was converted to volume according to a previously published standard.29
Corneal Permeability
Corneal epithelial permeability to Oregon-green-dextran (OGD; 70,000 molecular weight; Invitrogen, Eugene, OR) was assessed in 12 C57BL/6 mice of both sexes and all ages (young, middle-age, and elderly), as previously described.28 The severity of corneal OGD staining was graded in digital images by two masked observers, using NIS Elements version 3.0 (Nikon, Melville, NY). Both corneas were rinsed with PBS and photographed with a high dynamic and resolution digital camera (Coolsnap HQ2; Photometrics, Tucson, AZ) attached to a stereoscopic zoom microscope (SMZ 1500; Nikon), under fluorescence excitation at 470 nm. Results are presented as means ± SD of gray levels.
Tear Washings and EGF Enzyme-Linked Immunosorbent Assay
Tear fluid washings were collected from 12 animals/group/age (young, middle-aged, elderly) in two independent experiments, using a previously reported method.30 One sample consisted of tear washings from both eyes of 1 mouse pooled (2 μL) in PBS + 0.1% bovine serum albumin (8 μL) and stored at −80°C until the assay was performed. Results are presented as means ± SD (pg/mL).
Histology, PAS Staining, and IHC
Five right extraorbital LGs and right eyes from each sex/age, at 8 weeks, 6 to 9 months, and 24 months, were surgically excised, fixed in 10% formalin, and embedded in paraffin, and 8-μm sections were cut. Sections were stained with H&E for evaluating morphology and with PAS reagent for measuring goblet cell (GC) density, and images were acquired and processed as previously published.31
For immunohistochemistry, five left extraorbital LGs and left eyes from each sex/age at 8 weeks, 6 to 9 months, and 24 months were excised, embedded in optimal cutting temperature compound (VWR, Suwanee, GA), and flash frozen in liquid nitrogen. Sagittal 8-μm sections were cut with a cryostat (HM 500; Micron, Waldorf, Germany) and placed on glass slides that were stored at −80°C.
Immunohistochemistry was performed to detect cells in the conjunctiva and LGs stained positively for CD4 (clone H129.9; 10 μg/mL), CD8α (clone 53e6.7; 3.125 μg/mL) (both from BD Bioscience, San Diego, CA) as previously published.28 CD4/CD8 ratio was calculated by dividing the average number of CD4+ T cells by the average number CD8+ T cells in the conjunctival epithelium, as previously published.32
RNA Isolation and Real-Time PCR
Total RNA collected from the conjunctiva and cornea was extracted with a Qiagen MicroPlus RNA isolation Kit (Qiagen, Valencia, CA) and processed, and cDNA was synthesized as previously published.28 Twelve samples per group/age were used, and one sample consisted of pooled conjunctiva of right and left eyes of the same animal.
Real-time PCR was performed with specific MGB probes (Taqman; Applied Biosystems, Inc., Foster City, CA) and PCR master mix (Taqman Gene Expression Master Mix), in a commercial thermocycling system (StepOnePlus Real-Time PCR System; Applied Biosystems), according to the manufacturer's recommendations. Murine MGB probes were GAPDH (Mm99999915), MMP-9 (Mm00442991), IL17A (Mm00439618), IFNG; (Mm00801778), CCL20 (Mm00444228), and IL13 (Mm00434204). The GAPDH gene was used as an endogenous reference for each reaction. The results of real-time PCR were analyzed by the comparative CT method whereby target change equaled 2−ΔΔCT. The results were normalized by the CT value of GAPDH. The mean CT of relative mRNA level in the 8-week-old female group was used as the calibrator.
Flow Cytometric Analysis
Right and left extraorbital LGs from one mouse per age/sex were excised and pooled into a single sample from mice at 8 weeks, 6 to 9 months, and 24 months of age (n = 12 animals/group divided into three independent experiments with four samples per group/age/experiment). Single-cell suspensions of LGs containing 1 × 106 cells were prepared as previously described.33 Briefly, single-cell suspensions of collagenase-digested LGs were stained with anti-CD16/32, followed by cell surface staining as follows: tube 1, anti–CD4-fluorescein isothiocyanate (FITC; GK1.5; BD Pharmingen, San Diego, CA), anti–CD8α-phosphatidylethanolamine (PE; clone 53–6.7; BD Pharmingen), anti–APC-B220 (clone RA3-6B2; BD Pharmingen); tube 2, anti–CD11c-FITC (clone HL3; BD Pharmingen), anti–CD11b-APC (Clone M1/70; BD Pharmingen), and anti–major histocompatibility complex (MHC) II (I-AI-E-PE, clone 2G9; BD Pharmingen); and tube 3, anti–natural killer (NK)-PE (pan-NK marker, CD49b, clone DX5; BD Pharmingen) and anti–γδ T-cell receptor–FITC (clone GL3; BD Pharmingen). Single-cell preparations of splenocytes obtained from young mice were stained with the same antibodies and served as positive controls. The gating strategy used in this study was as follows: lymphocytes and monocytes were individually identified on the basis of forward scatter and side scatter properties, subsequently gated on the basis of forward scatter height versus forward scatter area (singlets 1), then gated on side scatter height versus side scatter area (singlets 2). Propidium iodide exclusion was used to discriminate live cells. Because of paucity of some markers in the LGs, a minimum of 50,000 to 300,000 events were collected. A BD LSRII Benchtop cytometer was used for flow cytometry, and data were analyzed with BD Diva software version 6.7 (BD Pharmingen) and FlowJo software version 7.6.5 (Tree Star Inc., Ashland, OR).
Murine DS Model
DS was induced by subcutaneous injection of scopolamine hydrobromide (0.5 mg/0.2 mL; Sigma-Aldrich, St. Louis, MO), four times daily (8 AM, noon, 2 PM, and 5 PM), for 5 consecutive days in 8-week-old C57BL/6 mice of both sexes (n = 5), as previously published.28 Mice were placed in a cage with a perforated plastic screen on one side to allow airflow from a fan placed 6 inches in front of it for 16 hours per day. Room humidity was maintained at 30% to 35%.
Isolation of Murine CD4+ T Cells and Adoptive Transfer
Superior cervical lymph nodes and spleens from donor mice of both sexes from nonstressed mice at 8 weeks and 24 months and after DS for 5 days (at 8 weeks) were meshed gently between two frosted end glass slides, as previously described.28 Adoptive transfer recipients (RAG1 KO mice, sex specific, at 8 weeks of age) received 5 × 106 untouched CD4+ cells intraperitoneally and were sacrificed 72 hours after the initial adoptive transfer.
Repeatability of Data and Statistical Analysis
All experiments were repeated at least two times. After completion of all experiments, data were averaged, and graphs were generated. Sample size calculation was performed with StateMate Software version 2.0 (GraphPad Inc., San Diego, CA), based on preliminary data.
Two-way analysis of variance with Tukey's post hoc testing was used for overall statistical comparisons. P < 0.05 was considered statistically significant. These tests were performed with GraphPad Prism 5.0 software (GraphPad Inc., San Diego, CA).
Results
Mice Develop Ocular Surface Disease with Age
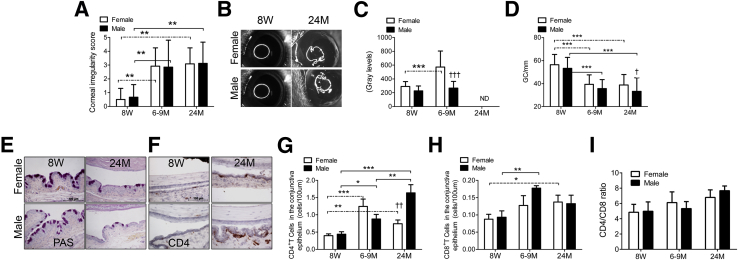
Hallmarks of DED include increased corneal surface irregularity, disrupted corneal barrier function, and conjunctival GC loss.28,32,34 Corneal irregularity was measured by grading the distortion of a circular light reflected off the corneal surface. Videokeratoscopic surface irregularity indices that were based on a similar principle were found to correlate with the severity of corneal epithelial disease in patients with dry eye,34 and corneal surface irregularity significantly increased in mice with experimental dry eye.28
As seen in Figure 1, A and B, corneal irregularity increased with age in both male and female mice. This increase was observed at the 6 to 9 months of age and did not further increase at age 24 months. No difference was observed between sexes at any age (Figure 1, A and B, and Table 1).
Figure 1.
Ocular surface phenotype in young (8 weeks), middle-aged (6 to 9 months), and elderly (24 months) C57BL/6 mice. A: Corneal irregularity score. Bar graphs show means ± SD of three independent experiments with four animals per experiment (8 eyes per experiment, yielding a final sample of 24 eyes per age/sex). B: Representative corneal rings used to generate the graph in A. C: Corneal Oregon-Green dextran fluorescence intensity score. Bar graphs show means ± SD of three independent experiments with four animals per experiment (8 eyes per experiment, yielding a final sample of 24 eyes per age/sex). D: Number of PAS+ conjunctival goblet cells counted in paraffin-embedded sections expressed as number per millimeter. Bar graphs show means ± SD of two independent experiments with two to three animals per age and sex, yielding a final sample of five right eyes for each group). Both eyes/animals were independently evaluated, and results were averaged. E: Representative images of conjunctiva sections stained with PAS used to generate the bar graph in D. F: Representative images of conjunctiva frozen sections immunostained for CD4 (in red) used to generate the bar graph in G. G: CD4+ T cells infiltrating the conjunctival epithelium. H: CD8+ T cells infiltrating the conjunctival epithelium. Both CD4 and CD8 evaluation, bar graphs show means ± SD of two independent experiments with two to three animals per age and sex, yielding a final sample of five left eyes for each group). I: CD4/CD8 ratio in conjunctiva epithelium. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 for within strain comparison; ††P < 0.01, and †††P < 0.001 for sex comparison. Original magnification: ×40 (E and G). Scale bars: 100 μm (D and G). ND, not determined; 8W, 8 weeks; 6-9M, 6 to 9 months; 24M, 24 months. Dashed lines indicate female-to-female comparison; solid lines indicate male-to-male comparisons.
Table 1.
Summary of Findings in Ocular Surface and Lacrimal Gland with Aging
| Parameters | Age comparison with young mice (8 weeks) | Middle-aged (6–9 months) mice | Old (24 months) mice | Significant sex effect/time point |
|---|---|---|---|---|
| Ocular surface parameters | Corneal irregularity | ↑∗ | ↑∗ | F = M |
| Corneal permeability | ↑∗ | ND | F >> M | |
| GC density | ↓∗ | ↓∗ | M << F (24 months) | |
| CD4+ T-cell infiltration in conjunctiva | ↑∗ | ↑∗† | M > F | |
| Gene expression analysis in cornea and conjunctiva | MMP-9 mRNA in cornea | ↑∗ | ↑∗† | F > M (24 months) |
| IL-17A mRNA in cornea | ↑∗ | ↑∗† | F > M (6–9 months and 24 months) | |
| CCL20 mRNA in cornea | ↑∗ | ↑∗† | F > M (24 months) | |
| IFN-γ mRNA in conjunctiva | ∅ | ↑∗† | M > F (24 months) | |
| IL-17A mRNA in conjunctiva | ↑∗ | ↑∗† | F > M | |
| IL-13/IFN-γ ratio in conjunctiva | ↓∗ | ↓∗ | ↓ M, ∅ F | |
| IL-17/IFN-γ ratio in conjunctiva | ↑∗ | ↑∗† | F >> M | |
| LG evaluation | Tear volume | ↑∗ | ↑∗† | F = M |
| Tear EGF concentration | ↑∗ | ↑∗ | M > F (24 months) | |
| CD4+ T-cell infiltration | ↑∗ | ↑∗† | M > F (24 months) | |
| CD8+ T-cell infiltration | ↑∗ | ↑∗† | M > F (24 months) | |
| B-cell infiltration | ↑∗ | ↑∗† | F > M (24 months) | |
| CD11c+MHC II+ | ∅ | ↓∗† | F = M (24 months) | |
| CD11b+MHC II+ | ∅ | ↓∗† | F = M (24 months) |
Mice were compared with young mice (8 weeks old).
F, female; M, male; CCL20, chemokine ligand 20; EGF, epidermal growth factor; GC, goblet cell; IFN-γ, interferon γ; LG, lacrimal gland; MHC, major histocompatibility complex; MMP-9, matrix metallopeptidase 9; ND, not determined; ∅, no change; ↑, increase; ↓, decrease.
P ≤ 0.05 compared to young mice.
P ≤ 0.05 compared to 6- to 9-month-old mice.
Corneal barrier function studies showed that middle-aged female mice have significantly increased permeability to the fluorescent 70-kDa molecule OGD compared with young female and middle-aged male mice (Figure 1C). We were unable to use this technique in the elderly group because of the ubiquitous presence of cataracts in these mice, which prevent accurate measurement of corneal fluorescence as a result of increased background fluorescence from the cataratous lens (data not shown).
Filled GCs were identified on paraffin-embedded conjunctival sections stained with PAS. A significant decrease in GC density in conjunctiva (cells/mm) was observed at the 6- to 9-month age and persisted at 24 months (Figure 1, D and E). In contrast to corneal irregularity, male mice exhibited significantly greater loss of mucin-filled GCs at 24 months than female mice. No differences were observed between sexes in younger mice.
DED is accompanied by an increase in CD4+ T cells and an increase in the CD4/CD8 ratio in the conjunctival epithelium.32 To evaluate the density of CD4+ and CD8+ T cells that infiltrate the conjunctival epithelium, we evaluated conjunctiva cryosections immunostained for each T-cell subset. Aging was associated with an increase in the number of CD4+ cells in the conjunctival epithelium in both the middle-aged and elderly groups (Figure 1, F and G), consistent with our hypothesis that older mice develop dry eye. A difference between sexes was observed at 24 months, with male mice having a greater number of infiltrating CD4+ T cells than female mice. The number of CD8+ cells in the conjunctival epithelium increased at the 6- to 9- month age in male mice and at 24 months in female mice (Figure 1H) and an increased CD4/CD8 ratio was observed with aging (Figure 1I). No significant difference was found between sexes at any age.
Increased Ocular Surface Inflammation with Aging
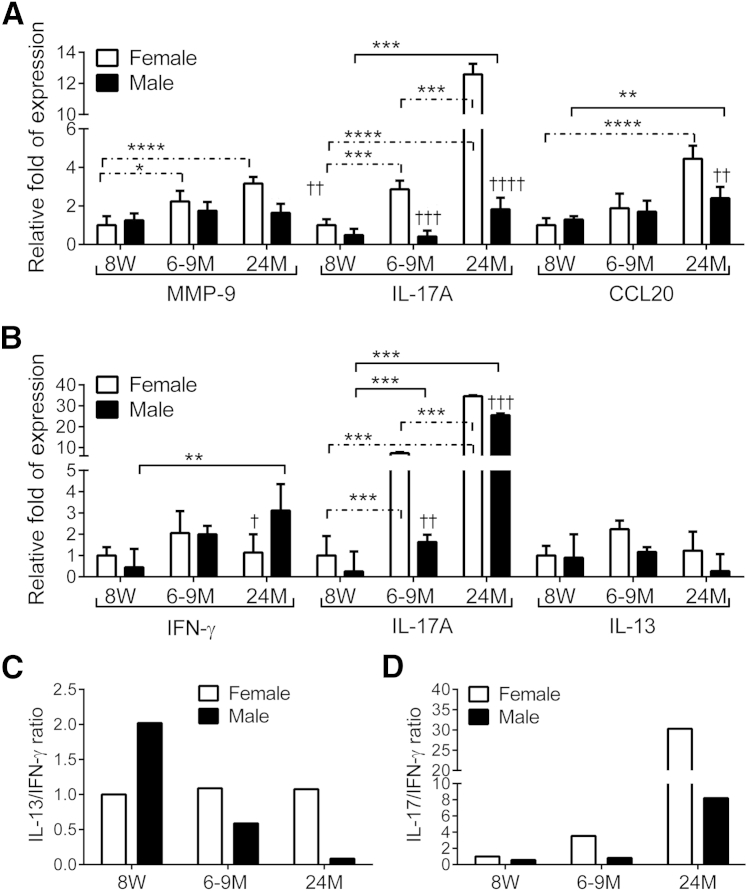
In our inducible DS model, we have found increased levels of transcripts that encode encoding matrix metalloproteinase 9 (MMP-9) and T-cell–related cytokines, notably IL-17A and IFN-γ, in the conjunctiva.31 To investigate if the age-related dry eye phenotype was accompanied by increased levels of the same inflammatory mediators we performed real-time PCR for MMP-9, T-cell related cytokines, and chemokines in corneal epithelium and in whole thickness biopsies of conjunctiva (containing epithelium, stroma, and resident inflammatory cells) of mice of all ages and both sexes. Our results are summarized in Figure 2, A and B.
Figure 2.
Aging induces expression of T-cell–related cytokines in cornea and conjunctiva. A: Relative fold of expression of MMP-9, IL-17A, and CCL20 mRNA in cornea. Bar graphs show means ± SD of one representative experiment with six samples per age and sex (experiment was repeated twice with similar results). One sample consisted of pooled right and left corneas of the same animal. B: Relative fold expression changes in IFN-γ, IL-17A, and IL-13 mRNA in conjunctiva. Bar graphs show means ± SD of one representative experiment with six samples per age and sex (experiment was repeated twice with similar results). One sample consisted of pooled right and left corneas of the same animal. C: IL-17/IFN-γ ratio in conjunctiva. D: IL-13/IFN-γ ratio in conjunctiva. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, and ∗∗∗∗P < 0.0001 for within strain comparison; ††P < 0.01, †††P < 0.001, and ††††P < 0.0001 for sex comparison. CCL20, chemokine ligand 20; IFN-γ, interferon γ; MMP-9, matrix metallopeptidase 9; 8W, 8 weeks; 6–9M, 6 to 9 months; 24M, 24 months. Dashed lines indicate female-to-female comparison; solid lines indicate male-to-male comparisons.
An increase was found in MMP-9, IL-17A, and CCL20 mRNA in the cornea with aging in female mice, reaching statistical significance at 6 to 9 and 24 months for MMP-9 and IL-17A and at 24 months for CCL20 compared with baseline. Levels of MMP-9, IL-17A, and CCL20 were also higher in female mice than in male mice (Figure 2A). We observed that a significant age-related increase was found in IL-17A mRNA transcripts in both sexes at 6 to 9 months and 24 months in conjunctiva (Figure 2B), but female mice had significantly higher levels than male mice. IFN-γ mRNA transcripts were significantly increased in elderly male mice, compared with young mice of the same sex and also with female mice of the same age (Figure 2B), whereas no significant change in IL-13 with aging was observed.
Another way to interpret the changes in T-cell cytokines with aging is to display the data as ratios of signature cytokines. We have previously shown that the Th2 cytokine IL-13 is a homeostatic factor for conjunctival GCs,35 whereas the Th1 cytokine IFN-γ promotes epithelial metaplasia, apoptosis, and GC loss.32,36 It has been noted in other systems that these Th cytokines antagonize each other in vivo and in vitro.37–41 We used the mean values of gene expression of Th17, Th1, and Th2 signature cytokines, IL-17A, IFN-γ, and IL-13, respectively, to generate Figure 2, C and D. Interestingly, the IL-13/IFN-γ ratio (Figure 2C) and the IL-17A/IFN-γ ratio (Figure 2D) at the 6- to 9-month and 24-month time points were lower in male mice than in similarly aged female mice. These findings suggest that male mice develop a Th1-skewed response, whereas female mice develop more of a Th17-skewed response with aging.
LG Function and Inflammation
Tear volume has been used as a measure of LG function, but conflicting results have been found in rodents.42–44 Tear volume was measured with the phenol red cotton-thread technique.45 We observed that tear volume increased from 8 weeks to 6 to 9 months and from 6 to 9 months to 24 months in female mice (Figure 3A). Male mice also exhibited a significant increase in tear volume with age, present at 6 to 9 months and sustained at 24 months. No difference was found between sexes at any of the three age groups.
Figure 3.
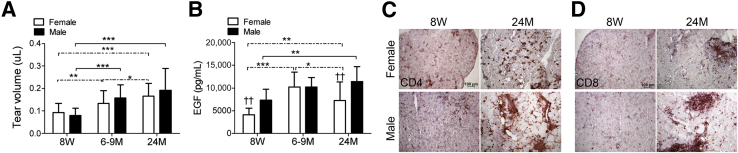
Lacrimal gland evaluation in young (8 weeks), middle-aged (6 to 9 months), and old (24 months) C57BL/6 mice. A and B: Tear volume (A; means ± SD) and tear EGF concentrations (B) were measured by enzyme linked immunosorbent assay. Tear washings from both right and left eyes from one mouse per age and sex were collected and pooled into a single tube, yielding a final sample of 12 individual samples per group and age divided into three independent experiments with four samples per experiment). Bar graphs show the means ± SD. C and D: Representative images of lacrimal gland frozen sections immunostained for CD4 (in red, C) and CD8 (in red, D). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 for within strain comparison; ††P < 0.01 for sex comparison. Original magnification: ×40 (C and D). EGF, epidermal growth factor; 8W, 8 weeks; 6-9M, 6 to 9 months; 24M, 24 months. Dashed lines indicate female-to-female comparison; solid lines indicate male-to-male comparisons.
EGF is a multifunctional cytokine that regulates proliferation, differentiation, and survival of epithelial cells. EGF is secreted by LGs in humans,46,47 is measurable in tears of mice,30,48,49 and tear EGF concentration is significantly lower in murine models of Sjögren syndrome.48 In human patients, decreased EGF concentration was noted in patients with aqueous tear-deficient dry eye compared with control subjects50 and inversely correlated the severity of corneal and conjunctival disease measured by dye staining.46
Because we had previously shown that EGF concentration in murine tears inversely correlated with total inflammatory cell infiltrate, as well as CD4 and CD8 infiltration in LGs of autoimmune prone CD25 KO mice,48 we assayed EGF in tears of all mice of both sexes at all three time points. Figure 3B summarizes our findings. Mirroring the tear volume results, tear EGF concentration significantly increased in the middle-age and elderly groups. We observed that young and elderly female mice have significantly lower tear EGF concentration than similarly aged male mice, and a significant increase in EGF concentration in both sexes at the 6- to 9-month and 24-month time points was seen.
Lymphocytic infiltration, mainly periductally, has been noted in the aged human LG.51–53 In contrast to marked lymphocytic infiltration of the LG that develops with aging in certain autoimmune strains of mice that have been used as models of Sjögren syndrome, the T-cell infiltration in aged C57BL/6 mice is relatively mild.33,49,54,55 CD4+ and CD8+ T cells in LG cryosections were identified by immunohistochemical staining. Overall, minimal infiltration was observed in the young LG, whereas increased T-cell infiltration of periductal and acini was noted with aging (Figure 3, C and D). However, preservation of LG parenchyma was found with normal-appearing filled acini in >90% of the gland (data not shown).
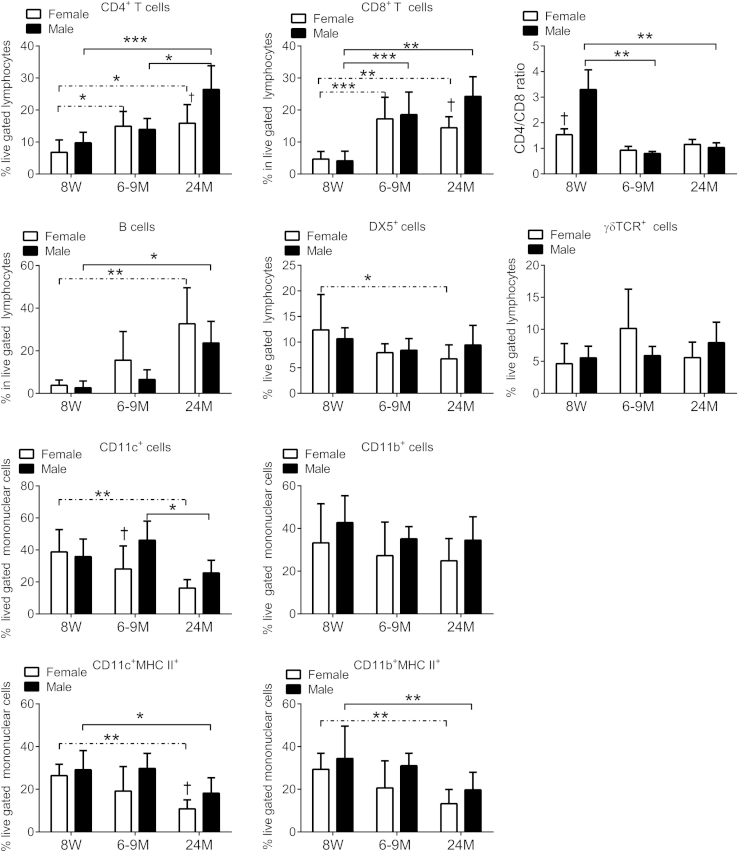
Flow cytometric analysis of freshly isolated cells from LGs of all age groups and both sexes was used to quantify the immune cell subsets that infiltrated the gland, including CD4, CD8, and γδ T cells; B cells, monocytes, DCs, and NK cells. Our flow cytometric analysis is presented in Figure 4. We found that aging increases the CD4+ and CD8+ T-cell populations which are more pronounced in female mice at 6 to 9 months of age and in male mice at 24 months. In contrast to the conjunctiva (Figure 1I), the CD4/CD8 ratio in the LG decreases with age (Figure 4). We also noted an increase in B cells with aging, with significantly higher numbers in old female mice. In contrast, a significant decrease was found in NK+ cells in female mice at 24 months and no change in the frequency of γδ T+ cells with aging.
Figure 4.
Flow cytometric analysis of LG in young (8 weeks), middle-aged (6 to 9 months), and old (24 months) C57BL/6 mice. Right and left extraorbital LGs from one mouse per group and sex were excised and pooled into a single tube, yielding a final sample of 12 individual LG samples per group and age divided into three independent experiments with four samples per experiment). Bar graphs show means ± SD. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 for within strain comparison; †P < 0.05 for sex comparison. LG, lacrimal gland; MHC, major histocompatibility complex; TCR, T-cell receptor; 8W, 8 weeks; 6-9M, 6 to 9 months; 24M, 24 months. Dashed lines indicate female-to-female comparison; solid lines indicate male-to-male comparisons.
We observed that aging significantly decreased the frequency of CD11c+ cells in female mice at 24 months, with no change in the number of CD11b+ cells. However, when evaluating CD11c+ MHC II+ and CD11b+ MHC II+ cells we observed that both populations decreased at 24 months. Interestingly, at 24 months, CD11c+ MHC II+ cell, activated DCs were more numerous in male mice.
Taken together, our results show that the autoimmune infiltration in aged LGs was composed of CD4+, CD8+, and B cells and this parallels a decrease in CD11c+ MHC II+, CD11b+ MHC II+, and NK+ cells.
CD4+ T Cells from Aged Mice Induce Autoimmune Lacrimal Keratoconjunctivitis
Dry eye is a multifactorial disease. Increasing evidence indicates that effector CD4+ T cells contribute to the LG immunopathology in Sjögren syndrome and perhaps with aging. Inflammation in the LGs, cornea, and conjunctiva, which results in increased T-cell infiltration and conjunctival GC loss, has been induced by transferring CD4+ T cells from mice subjected to DS to T-cell–deficient mice that have not been exposed to DS.56
We observed a dry eye phenotype as mice aged (increased corneal irregularity, increased corneal barrier disruption, GC loss, and increased CD4+ T-cell infiltration in the conjunctiva and LGs), despite the lack of LG secretory function, suggesting that ocular surface changes appear to be a result of age-related increase in inflammation. We hypothesized that aging leads to accumulation of autoreactive, pathogenic CD4+ T cells. To test this hypothesis, we performed adoptive transfer of freshly isolated CD4+ T cells from young (8 weeks) and old (24 months) mice of both sexes, using immunodeficient RAG1 KO mice as recipients. Our standard adoptive transfer protocol, which uses young C57BL/6 mice subjected to DS for 5 days, was used as a positive control.
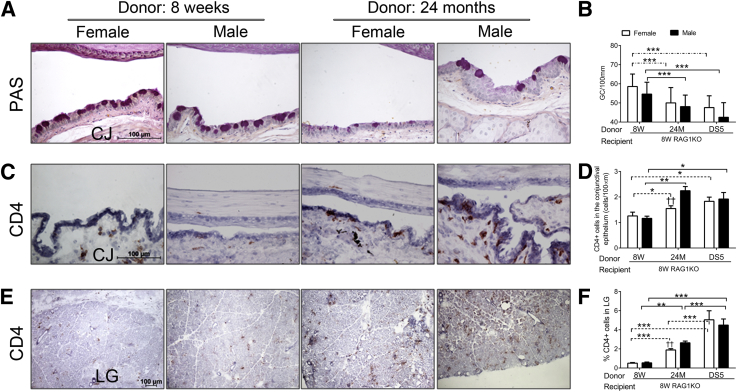
Our results from the adoptive transfer experiments are summarized in Figure 5 and Table 2. Young RAG1 KO mice that received CD4+ T cells from elderly mice of both sexes showed a decrease in conjunctival GC density (Figure 5, A and B), mirroring the reduction in GC density seen in the aged donor mice (Figure 1D). The magnitude of GC loss after adoptive transfer of CD4+ T cells from aged mice was comparable with that seen after transfer of CD4+ T cells from young C57BL/6 donors subjected to DS (Figure 5B) which were used as positive controls. No sex difference in GC density was observed in RAG1 KO recipients of elderly CD4+ T cells.
Figure 5.
Results of adoptive of CD4+ T cells from young (8 weeks), middle-aged (6 to 9 months), and old (24 months) C57BL/6 mice to 8-week-old RAG1KO recipients. Mice subjected to DS for 5 days were used as positive controls. A: Representative images of conjunctiva sections stained with PAS used to generate the bar graph in B. B: Number of PAS+ conjunctival goblet cells counted in paraffin-embedded sections expressed as number per millimeter. Bar graphs show the means ± SD of two independent experiments with two to three animals per age and sex, yielding a final sample of five right eyes per age and sex). Both eyes/animals were independently evaluated, and results were averaged. C: Representative images of conjunctiva frozen sections immunostained for CD4 (in red) used to generate the bar graph in D. D: CD4+ T cells infiltrating the conjunctival epithelium. Bar graphs show the means ± SD of two independent experiments with two to three animals per age and sex, yielding a final sample of five right eyes for each group). Both eyes/animals were independently evaluated, and results were averaged. E: Representative images of LG frozen sections immunostained for CD4 (in red) used to generate the bar graph in F. F: CD4+ T cells in the LG. Bar graphs show means ± SD of two independent experiments with two to three animals per age and sex, yielding a final sample of five individual left LGs per group and age). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 for within strain comparison. ††P < 0.01 for sex comparison. Original magnification: ×40 (A, C, and E). CJ, conjunctiva; DS, desiccating stress; DS5, desiccating stress for 5 days; LG, lacrimal gland; RAG1KO, recombination activating gene-1 knockout; 8W, 8 weeks; 6-9M, 6 to 9 months; 24M, 24 months. Dashed lines indicate female-to-female comparison; solid lines indicate male-to-male comparisons.
Table 2.
Summary of Changes Observed in Young RAG1 KO after Receiving CD4+ T Cells Isolated from Young (8 weeks) and Old (24 months) Mice of Both Sexes
| Donor age compared with young donor | Old mice | Significant sex effect/time point |
|---|---|---|
| GC density | ↓∗ | F = M |
| CD4+ T-cell infiltration in conjunctiva | ↑∗ | M >> F |
| CD4+ T-cell infiltration in LG | ↑∗ | M >> F |
F, female; M, male; GC, goblet cell; LG, lacrimal gland; ND, not determined; RAG1 KO, recombination activating gene-1 knockout; ↑, increase; ↓, decrease.
P ≤ 0.05 compared to young (8 weeks old) mice.
With respect to the number of CD4+ T cells that infiltrated the conjunctiva, a statistically significant increase was found in RAG1 KO recipients of the elderly group for both sexes with greater infiltration seen from male donors (Figure 5, C and D). The extent of conjunctival cell infiltration was similar to that seen in the positive control donor group (DS for 5 days). The age and sex effect observed in the adoptive transfer (Figure 5, B and D) was similar to what was observed in the donor mice (Figure 1, D and G).
Cryosections of LGs stained for CD4+ T cells in RAG1 KO recipients were evaluated. Recipients of CD4+ T cells from elderly donors showed significantly higher numbers of infiltrating cells than those receiving cells from young donors (Figure 5, E and F). Similar to the conjunctiva, greater CD4+ T-cell infiltration was seen in recipients of elderly male donors than female donors. However, unlike the conjunctiva, the increase in CD4+ T cells observed in LGs of RAG1 KO recipients of cells from older mice was not quite to the degree observed in the positive control group that received CD4+ T cells from C57BL/6 mice that were subjected to DS.
Discussion
The strong correlation between age and the prevalence of DED has been well established.11 Although alterations in the immune response associated with aging have been identified,57 there is a paucity of information whether these changes affect the mucosal-associated lymphoid tissue in the ocular surface and LG and contribute to development of clinical DED.
Evidence is increasing that the ocular surface epithelial disease that develops in dry eye results from inflammation that is initiated by changes in tear composition, such as increased tear osmolarity that results from decreased LG secretion or increased evaporation due to lipid tear deficiency.58 In human dry eye, CD4+ T cells have been found to infiltrate the conjunctiva and produce IFN-γ and IL-17.31 The antigen that incites this immune reaction is yet to be determined, but experiments in mouse models indicate that the ocular surface DCs activated by dry eye and migrating to the regional lymph nodes are essential for this autoimmune reaction.59 Because increased age is the strongest risk factor for dry eye in humans, we hypothesized that changes in the immune environment of the ocular surface with aging predispose to the development of surface epithelial changes. To test this hypothesis, we evaluated the effects of aging on immune/inflammatory cell populations in the LG and ocular surface of the C57BL/6 mouse strain that we have found is susceptible to developing autoimmune lacrimal keratoconjunctivitis in response to short-term DS.31,32
Our results showed that aging induces signs of DED in mice, characterized by an increased corneal irregularity, corneal barrier disruption, and decreased density of filled conjunctival GCs. These findings were accompanied by an increase in CD4+ T-cell infiltration in the conjunctiva and increased expression of IFN-γ and IL-17A transcripts in conjunctiva. In the LG, we observed an increase in both CD4+ T cells and CD8+ T cells, B cells, and a decrease in activated APCs with no change in LG function measured by EGF concentration in tears. The contribution of pathogenic CD4+ T cells to the development of age-related dry eye was confirmed by the results of adoptive transfer experiments in which CD4+ T cells from older donor mice produced greater loss of mucin-filled GCs and CD4 infiltration in both the ocular surface and LG of young, naive immunodeficient RAG1 KO recipient mice (Tables 1 and 2).
One unique aspect of our study is the detailed characterization of the ocular surface with aging. We observed in aged mice the same dry eye phenotype induced by an environmental challenge with low humidity and pharmacological blockade of LG secretion in young C57BL/6 mice: increased corneal irregularity, increased corneal barrier disruption, increased conjunctival GCs, and increased CD4+ T-cell infiltration in the conjunctiva, which was accompanied by increased IL-17A and MMP-9 transcripts in the cornea and IL-17A and IFN-γ transcripts in the conjunctiva.28,31,32,60 As with human dry eye, many of these changes were present at middle age.61–63
We had previously reported that the Th1 cytokine IFN-γ stimulates cornified envelope precursor production and promotes GC loss in the conjunctival epithelium32 and that neutralization of IL-17A ameliorates corneal barrier disruption in an experimental model of dry eye.31 Interestingly, increased expression of IFN-γ mRNA transcripts and lower GC density was found in conjunctiva of older male mice, whereas increased expression of IL-17A mRNA transcripts and increased OGD intensity score as a marker of corneal barrier disruption was significantly higher in older female mice. Our studies agree with Williams et al64 who found an increase of CD4+ T cells in the conjunctiva of aged subjects and increased IFN-γ production compared with young subjects. These results suggest that the female sex prevalence for dry eye could be a consequence of an immunological bias toward a Th17 response. IL-17A has been shown to cause barrier disruption in mice and increased expression of MMPs,31 which both lead to increased desquamation and break-down of tight junctions of the corneal epithelium. Because epithelial corneal disease is responsible for the irritation and blurred vision symptoms reported by most patients with dry eye, neutralization of IL-17A may prove to be an effective new therapeutic strategy for dry eye. Preclinical studies in mice have shown success of treating corneal disease by interfering with Th17 pathway.31,65,66
We observed that tear volume paradoxically increased with aging, specifically in male animals. Changes in tear parameters (such as tear volume, tear meniscus height, and tear flow) are often found in elderly subjects.17,67–70 In Sprague-Dawley rats, tear volume increased with aging in male rats but not female rats.42 Increase,44 decrease,43 or no change71 in tear volume has been noted in mice, depending on the genetic background. Tear volume is also a composite of fluid secretion by the LG and conjunctival and corneal epithelia. In addition, an increase in leakage of conjunctival blood vessels, such as can occur with ocular surface inflammation, can increase tear volume. In favor of this observation, we noted that in the severely autoimmune strain CD25 KO, which has 60% destruction of LG as early as 8 weeks of age, tear volume increases with age (data not shown). Marko et al44 described an increase in tear volume in the Spdef null mouse, which lacks GCs and has inflammatory cell infiltration of the conjunctiva.
Consistent with previous studies that noted mild-to-moderate lymphocytic infiltration of rodent LGs,53,55 we noted an increase in LG inflammation with age. Our tear volume findings suggest that this level of inflammation is not sufficient to decrease secretory function. More severe lymphocytic inflammation or higher levels of inflammatory cytokines may be needed to affect LG function as seen in LGs of autoimmune mouse strains, such as nonobese diabetic, IqL/Jic, CD25 KO, and C57BL/6.NOD-Aec1Aec2 mice.33,33,48,72–76 Our findings suggest that age-related increases in inflammatory mediators make a greater contribution to development of ocular surface disease than inflammation in the LG.
A review of the literature of aged humans and aged LGs indicates that there is increased focal infiltration by T and B cells and an accumulation of mast cells.53,55,77 Our results showed an increase of CD4+ T cells, CD8+ T cells, and B cells with age, with no change in DX5+ NK cells and γδ T-cell receptor+ cells, and a decrease in certain DC populations (CD11c+, CD11c+MHCII+, and CD11b+MHCII+). Sex-specific changes were also observed: old male mice had a higher percentage of CD4+, CD8+, and CD11c+MHC II+ cells than age-matched female mice. We have previously demonstrated a significant increase in CD11c+ and CD11c+MHC II+ cells in the draining nodes59,78 and a decrease in the conjunctiva (manuscript under review) in our acute model of DS. The decrease in both CD11c+ and CD11c+MHC II+ cells within the aged LG suggests potential immunodysregulation that remains to be defined.
To determine whether CD4+ T cells in elderly mice contribute to the observed GC loss, we adoptively transferred CD4+ T cells from young or aged C57BL/6 donor mice to young naive immunodeficient RAG1 KO recipients. Compared with the young donors, a significantly greater number of CD4+ T cells from aged donors homed to the ocular surface and LGs of the recipients, and this T-cell infiltration was accompanied by conjunctival GC loss. These findings suggest that the CD4+ T cells in aged mice are more pathogenic and are capable of inducing a dry eye phenotype. There is a body of work to support that this is a valid model to demonstrate autoreactive T cells. Adoptive transfer of CD4+ T cells to immunodeficient recipients, such as RAG-1 KO mice has been used to characterize autoreactive T cells in a number of different experimentally induced autoinflammatory models, including inflammatory bowel disease, experimental autoimmune encephalomyelitis, and experimental autoimmune uveitis.79–81 We have previously used this method to show that autoreactive CD4+ T cells generated in mice subjected to 5 days of DS were capable of causing DED (GC loss and CD4+ T-cell infiltration of the conjunctiva and LG) when adoptively transferred to naive immunodeficient recipients.28,31,78 The present study used the same model to compare the disease-causing potential of CD4+ T cells isolated from young unstressed donors, young mice with environmentally induced dry eye, or unstressed old mice. We observed minimal disease in the RAG recipients of young unstressed donors. If autoimmunity would develop from these cells, then we would have observed it in those recipients. In contrast, only CD4+ T cells from old unstressed donors and young mice subjected to desiccation produced DED in the adoptive transfer recipients. We have previously reported that transfer of pathogenic CD4+ T cells from donors subjected to desiccation into immunocompetent recipients was not capable of causing disease, unless we depleted regulatory T cells with anti-CD25 antibody.56 We did not investigate the cause for the increased pathogenicity of CD4+ T cells with aging; however, we speculate that it might be due to increased exposure to the inciting antigen, reduced regulatory cell activity, accumulation of pathogenic CD4+ T cells, or reduction of tolerogenic DCs. The adoptive transfer experiments of aged CD4+ T cells into young mice offers the possibility of manipulating various immune components and gaining greater insight approaches for future studies in aging.
Taken together, our present findings indicate that dry eye may be an ideal condition to investigate the mechanisms by which age-related alterations in immunoregulation leads to development of autoimmunity.
Footnotes
Supported by NIH grant EY11915 (S.C.P.) and core grant for Vision ResearchEY-002520-36 (Department of Ophthalmology); Fight for Sight grant-in-aid (C.S.d.P.); Hartford Foundation (C.S.d.P.); Fight for Sight with Women's Eye Health.org Summer Student Fellowship (A.J.M.); Research to Prevent Blindness, Oshman Foundation; William Stamps Farish Fund; Hamill Foundation; and by the Cytometry and Cell Sorting Core at Baylor College of Medicine which is funded by the NIH National Institute of Allergy and Infectious Diseases grants P30AI036211, NCIP30CA125123, and NCRRS10RR024574.
The sponsor agencies had no involvement in the study design; data collection, analysis, and interpretation of data; writing of the report; or the decision to submit the paper for publication.
Disclosures: None declared.
References
- 1.De Benedictis G., Carrieri G., Varcasia O., BonaFe M., Franceschi C. Inherited variability of the mitochondrial genome and successful aging in humans. Ann N Y Acad Sci. 2000;908:208–218. doi: 10.1111/j.1749-6632.2000.tb06648.x. [DOI] [PubMed] [Google Scholar]
- 2.Goronzy J.J., Weyand C.M. Aging, autoimmunity and arthritis: T-cell senescence and contraction of T-cell repertoire diversity - catalysts of autoimmunity and chronic inflammation. Arthritis Res Ther. 2003;5:225–234. doi: 10.1186/ar974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson S.A., Cambier J.C. Ageing, autoimmunity and arthritis: senescence of the B cell compartment - implications for humoral immunity. Arthritis Res Ther. 2004;6:131–139. doi: 10.1186/ar1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stacy S., Krolick K.A., Infante A.J., Kraig E. Immunological memory and late onset autoimmunity. Mech Ageing Dev. 2002;123:975–985. doi: 10.1016/s0047-6374(02)00035-0. [DOI] [PubMed] [Google Scholar]
- 5.Bruunsgaard H., Pedersen M., Pedersen B.K. Aging and proinflammatory cytokines. Curr Opin Hematol. 2001;8:131–136. doi: 10.1097/00062752-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Fagiolo U., Cossarizza A., Scala E., Fanales-Belasio E., Ortolani C., Cozzi E., Monti D., Franceschi C., Paganelli R. Increased cytokine production in mononuclear cells of healthy elderly people. Eur J Immunol. 1993;23:2375–2378. doi: 10.1002/eji.1830230950. [DOI] [PubMed] [Google Scholar]
- 7.Grolleau-Julius A., Garg M.R., Mo R., Stoolman L.L., Yung R.L. Effect of aging on bone marrow-derived murine CD11c+CD4-CD8alpha- dendritic cell function. J Gerontol A Biol Sci Med Sci. 2006;61:1039–1047. doi: 10.1093/gerona/61.10.1039. [DOI] [PubMed] [Google Scholar]
- 8.Agrawal A., Agrawal S., Cao J.N., Su H., Osann K., Gupta S. Altered innate immune functioning of dendritic cells in elderly humans: a role of phosphoinositide 3-kinase-signaling pathway. J Immunol. 2007;178:6912–6922. doi: 10.4049/jimmunol.178.11.6912. [DOI] [PubMed] [Google Scholar]
- 9.Agrawal A., Agrawal S., Tay J., Gupta S. Biology of dendritic cells in aging. J Clin Immunol. 2008;28:14–20. doi: 10.1007/s10875-007-9127-6. [DOI] [PubMed] [Google Scholar]
- 10.Myer R.G., El M.R., High K.P. Prostaglandin E2-dependent IL-23 production in aged murine dendritic cells. Exp Gerontol. 2010;45:834–841. doi: 10.1016/j.exger.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moss S.E., Klein R., Klein B.E. Incidence of dry eye in an older population. Arch Ophthalmol. 2004;122:369–373. doi: 10.1001/archopht.122.3.369. [DOI] [PubMed] [Google Scholar]
- 12.Bandeen-Roche K., Munoz B., Tielsch J.M., West S.K., Schein O.D. Self-reported assessment of dry eye in a population-based setting. Invest Ophthalmol Vis Sci. 1997;38:2469–2475. [PubMed] [Google Scholar]
- 13.Lin P.Y., Tsai S.Y., Cheng C.Y., Liu J.H., Chou P., Hsu W.M. Prevalence of dry eye among an elderly Chinese population in Taiwan: the Shihpai Eye Study. Ophthalmology. 2003;110:1096–1101. doi: 10.1016/S0161-6420(03)00262-8. [DOI] [PubMed] [Google Scholar]
- 14.Chotikavanich S., de Paiva C.S., Li D.-Q., Chen J.J., Bian F., Farley W.J., Pflugfelder S.C. Production and activity of matrix metalloproteinase-9 on the ocular surface increase in dysfunctional tear syndrome. Invest Ophthalmol Vis Sci. 2009;50:3203–3209. doi: 10.1167/iovs.08-2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ivers R.Q., Cumming R.G., Mitchell P., Attebo K. Visual impairment and falls in older adults: the Blue Mountains Eye Study. J Am Geriatr Soc. 1998;46:58–64. doi: 10.1111/j.1532-5415.1998.tb01014.x. [DOI] [PubMed] [Google Scholar]
- 16.Sattin R.W. Falls among older persons: a public health perspective. Annu Rev Public Health. 1992;13:489–508. doi: 10.1146/annurev.pu.13.050192.002421. [DOI] [PubMed] [Google Scholar]
- 17.Furukawa R.E., Polse K.A. Changes in tear flow accompanying aging. Am J Optom Physiol Opt. 1978;55:69–74. doi: 10.1097/00006324-197802000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Benitez del Castillo J.M., Wasfy M.A., Fernandez C., Garcia-Sanchez J. An in vivo confocal masked study on corneal epithelium and subbasal nerves in patients with dry eye. Invest Ophthalmol Vis Sci. 2004;45:3030–3035. doi: 10.1167/iovs.04-0251. [DOI] [PubMed] [Google Scholar]
- 19.Bourcier T., Acosta M.C., Borderie V., Borras F., Gallar J., Bury T., Laroche L., Belmonte C. Decreased corneal sensitivity in patients with dry eye. Invest Ophthalmol Vis Sci. 2005;46:2341–2345. doi: 10.1167/iovs.04-1426. [DOI] [PubMed] [Google Scholar]
- 20.Bashour M., Harvey J. Causes of involutional ectropion and entropion–age-related tarsal changes are the key. Ophthal Plast Reconstr Surg. 2000;16:131–141. doi: 10.1097/00002341-200003000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Versura P., Campos E.C. Menopause and dry eye. A possible relationship. Gynecol Endocrinol. 2005;20:289–298. doi: 10.1080/09513590400027257. [DOI] [PubMed] [Google Scholar]
- 22.Forsblad-d'Elia H., Carlsten H., Labrie F., Konttinen Y.T., Ohlsson C. Low serum levels of sex steroids are associated with disease characteristics in primary Sjogren's syndrome; supplementation with dehydroepiandrosterone restores the concentrations. J Clin Endocrinol Metab. 2009;94:2044–2051. doi: 10.1210/jc.2009-0106. [DOI] [PubMed] [Google Scholar]
- 23.McCarty C.A., Bansal A.K., Livingston P.M., Stanislavsky Y.L., Taylor H.R. The epidemiology of dry eye in Melbourne, Australia. Ophthalmology. 1998;105:1114–1119. doi: 10.1016/S0161-6420(98)96016-X. [DOI] [PubMed] [Google Scholar]
- 24.Smith J.A., Vitale S., Reed G.F., Grieshaber S.A., Goodman L.A., Vanderhoof V.H., Calis K.A., Nelson L.M. Dry eye signs and symptoms in women with premature ovarian failure. Arch Ophthalmol. 2004;122:151–156. doi: 10.1001/archopht.122.2.151. [DOI] [PubMed] [Google Scholar]
- 25.McMurray R.W. Estrogen, prolactin, and autoimmunity: actions and interactions. Int Immunopharmacol. 2001;1:995–1008. doi: 10.1016/s1567-5769(01)00045-5. [DOI] [PubMed] [Google Scholar]
- 26.Islander U., Erlandsson M.C., Hasseus B., Jonsson C.A., Ohlsson C., Gustafsson J.A., Dahlgren U., Carlsten H. Influence of oestrogen receptor alpha and beta on the immune system in aged female mice. Immunology. 2003;110:149–157. doi: 10.1046/j.1365-2567.2003.01704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baeza I., De Castro N.M., Gimenez-Llort L., De la Fuente M. Ovariectomy, a model of menopause in rodents, causes a premature aging of the nervous and immune systems. J Neuroimmunol. 2010;219:90–99. doi: 10.1016/j.jneuroim.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 28.de Paiva C.S., Volpe E.A., Gandhi N.B., Zhang X., Zheng X., Pitcher J.D., III, Farley W.J., Stern M.E., Niederkorn J.Y., Li D.Q., Flavell R.A., Pflugfelder S.C. Disruption of TGF-beta signaling improves ocular surface epithelial disease in experimental autoimmune keratoconjunctivitis sicca. PLoS One. 2011;6:e29017. doi: 10.1371/journal.pone.0029017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stewart P., Chen Z., Farley W., Olmos L., Pflugfelder S.C. Effect of experimental dry eye on tear sodium concentration in the mouse. Eye Contact Lens. 2005;31:175–178. doi: 10.1097/01.icl.0000161705.19602.c9. [DOI] [PubMed] [Google Scholar]
- 30.Pitcher J., III, de Paiva C.S., Pelegrino F., McClellan A., Raince J., Pangelinan S., Rahimy E., Farley W., Stern M., Li D., Pflugfelder S. Pharmacological cholinergic blockade stimulates inflammatory cytokine production and lymphocytic infiltration in the mouse lacrimal gland. Invest Ophthalmol Vis Sci. 2011;52:3221–3227. doi: 10.1167/iovs.09-4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Paiva C.S., Chotikavanich S., Pangelinan S.B., Pitcher J.I., Fang B., Zheng X., Ma P., Farley W.J., Siemasko K.S., Niederkorn J.Y., Stern M.E., Li D.-Q., Pflugfelder S.C. IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunol. 2009;2:243–253. doi: 10.1038/mi.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Paiva C.S., Villarreal A.L., Corrales R.M., Rahman H.T., Chang V.Y., Farley W.J., Stern M.E., Niederkorn J.Y., Li D.Q., Pflugfelder S.C. Dry eye-induced conjunctival epithelial squamous metaplasia is modulated by interferon-{gamma} Invest Ophthalmol Vis Sci. 2007;48:2553–2560. doi: 10.1167/iovs.07-0069. [DOI] [PubMed] [Google Scholar]
- 33.Rahimy E., Pitcher J.D., III, Pangelinan S.B., Chen W., Farley W.J., Niederkorn J.Y., Stern M.E., Li D.Q., Pflugfelder S.C., de Paiva C.S. Spontaneous autoimmune dacryoadenitis in aged CD25KO mice. Am J Pathol. 2010;177:744–753. doi: 10.2353/ajpath.2010.091116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Paiva C.S., Lindsey J.L., Pflugfelder S.C. Assessing the severity of keratitis sicca with videokeratoscopic indices. Ophthalmology. 2003;110:1102–1109. doi: 10.1016/s0161-6420(03)00245-8. [DOI] [PubMed] [Google Scholar]
- 35.de Paiva C.S., Raince J.K., McClellan A.J., Shanmugam K.P., Pangelinan S.B., Volpe E.A., Corrales R.M., Farley W.J., Corry D.B., Li D.Q., Pflugfelder S.C. Homeostatic control of conjunctival mucosal goblet cells by NKT-derived IL-13. Mucosal Immunol. 2011;4:397–408. doi: 10.1038/mi.2010.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang X., Chen W., de Paiva C.S., Corrales R.M., Volpe E.A., McClellan A.J., Farley W.J., Li D.Q., Pflugfelder S.C. Interferon-gamma exacerbates dry eye-induced apoptosis in conjunctiva through dual apoptotic pathways. Invest Ophthalmol Vis Sci. 2011;52:6279–6285. doi: 10.1167/iovs.10-7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Porter P., Susarla S.C., Polikepahad S., Qian Y., Hampton J., Kiss A., Vaidya S., Sur S., Ongeri V., Yang T., Delclos G.L., Abramson S., Kheradmand F., Corry D.B. Link between allergic asthma and airway mucosal infection suggested by proteinase-secreting household fungi. Mucosal Immunol. 2009;2:504–517. doi: 10.1038/mi.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ford J.G., Rennick D., Donaldson D.D., Venkayya R., McArthur C., Hansell E., Kurup V.P., Warnock M., Grunig G. Il-13 and IFN-gamma: interactions in lung inflammation. J Immunol. 2001;167:1769–1777. doi: 10.4049/jimmunol.167.3.1769. [DOI] [PubMed] [Google Scholar]
- 39.Irifune K., Yokoyama A., Sakai K., Watanabe A., Katayama H., Ohnishi H., Hamada H., Nakajima M., Kohno N., Higaki J. Adoptive transfer of T-helper cell type 1 clones attenuates an asthmatic phenotype in mice. Eur Respir J. 2005;25:653–659. doi: 10.1183/09031936.05.00021304. [DOI] [PubMed] [Google Scholar]
- 40.Brookes S.M., Cohen S.B., Price E.J., Webb L.M., Feldmann M., Maini R.N., Venables P.J. T cell clones from a Sjogren's syndrome salivary gland biopsy produce high levels of IL-10. Clin Exp Immunol. 1996;103:268–272. doi: 10.1046/j.1365-2249.1996.d01-623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mitsias D.I., Tzioufas A.G., Veiopoulou C., Zintzaras E., Tassios I.K., Kogopoulou O., Moutsopoulos H.M., Thyphronitis G. The Th1/Th2 cytokine balance changes with the progress of the immunopathological lesion of Sjogren's syndrome. Clin Exp Immunol. 2002;128:562–568. doi: 10.1046/j.1365-2249.2002.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sullivan D.A., Hann L.E., Yee L., Allansmith M.R. Age- and gender-related influence on the lacrimal gland and tears. Acta Ophthalmol (Copenh) 1990;68:188–194. doi: 10.1111/j.1755-3768.1990.tb01902.x. [DOI] [PubMed] [Google Scholar]
- 43.Kojima T., Wakamatsu T.H., Dogru M., Ogawa Y., Igarashi A., Ibrahim O.M., Inaba T., Shimizu T., Noda S., Obata H., Nakamura S., Wakamatsu A., Shirasawa T., Shimazaki J., Negishi K., Tsubota K. Age-related dysfunction of the lacrimal gland and oxidative stress: evidence from the Cu,Zn-superoxide dismutase-1 (Sod1) knockout mice. Am J Pathol. 2012;180:1879–1896. doi: 10.1016/j.ajpath.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 44.Marko C.K., Menon B.B., Chen G., Whitsett J.A., Clevers H., Gipson I.K. Spdef null mice lack conjunctival goblet cells and provide a model of dry eye. Am J Pathol. 2013;183:35–48. doi: 10.1016/j.ajpath.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Villareal A.L., Farley W., Pflugfelder S.C. Effect of topical ophthalmic epinastine and olopatadine on tear volume in mice. Eye Contact Lens. 2006;32:272–276. doi: 10.1097/01.icl.0000224360.10319.b1. [DOI] [PubMed] [Google Scholar]
- 46.Lam H., Blieden L., de Paiva C.S., Farley W.J., Stern M.E., Pflugfelder S.C. Tear cytokine profiles in dysfunctional tear syndrome. Am J Ophthalmol. 2009;147:198–205. doi: 10.1016/j.ajo.2008.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoshino K., Monroy D., Pflugfelder S.C. Cholinergic stimulation of lactoferrin and epidermal growth factor secretion by the human lacrimal gland. Cornea. 1996;15:617–621. [PubMed] [Google Scholar]
- 48.Pelegrino F.S., Volpe E.A., Gandhi N.B., Li D.Q., Pflugfelder S.C., de Paiva C.S. Deletion of interferon-gamma delays onset and severity of dacryoadenitis in CD25KO mice. Arthritis Res Ther. 2012;14:R234. doi: 10.1186/ar4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Paiva C.S., Hwang C.S., Pitcher J.D., III, Pangelinan S.B., Rahimy E., Chen W., Yoon K.C., Farley W.J., Niederkorn J.Y., Stern M.E., Li D.Q., Pflugfelder S.C. Age-related T-cell cytokine profile parallels corneal disease severity in Sjogren's syndrome-like keratoconjunctivitis sicca in CD25KO mice. Rheumatology (Oxford) 2010;49:246–258. doi: 10.1093/rheumatology/kep357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pflugfelder S.C., Jones D., Ji Z., Afonso A., Monroy D. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjogren's syndrome keratoconjunctivitis sicca. Curr Eye Res. 1999;19:201–211. doi: 10.1076/ceyr.19.3.201.5309. [DOI] [PubMed] [Google Scholar]
- 51.Nasu M., Matsubara O., Yamamoto H. Post-mortem prevalence of lymphocytic infiltration of the lacrymal gland: a comparative study in autoimmune and non-autoimmune diseases. J Pathol. 1984;143:11–15. doi: 10.1002/path.1711430104. [DOI] [PubMed] [Google Scholar]
- 52.Obata H., Yamamoto S., Horiuchi H., Machinami R. Histopathologic study of human lacrimal gland. Statistical analysis with special reference to aging. Ophthalmology. 1995;102:678–686. doi: 10.1016/s0161-6420(95)30971-2. [DOI] [PubMed] [Google Scholar]
- 53.Rios J.D., Horikawa Y., Chen L.L., Kublin C.L., Hodges R.R., Dartt D.A., Zoukhri D. Age-dependent alterations in mouse exorbital lacrimal gland structure, innervation and secretory response. Exp Eye Res. 2005;80:477–491. doi: 10.1016/j.exer.2004.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zoukhri D., Kublin C.L. Impaired neurotransmission in lacrimal and salivary glands of a murine model of Sjogren's syndrome. Adv Exp Med Biol. 2002;506:1023–1028. doi: 10.1007/978-1-4615-0717-8_144. [DOI] [PubMed] [Google Scholar]
- 55.Rocha E.M., Alves M., Rios J.D., Dartt D.A. The aging lacrimal gland: changes in structure and function. Ocul Surf. 2008;6:162–174. doi: 10.1016/s1542-0124(12)70177-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Niederkorn J.Y., Stern M.E., Pflugfelder S.C., de Paiva C.S., Corrales R.M., Gao J., Siemasko K. Desiccating stress induces T cell-mediated Sjogren's Syndrome-like lacrimal keratoconjunctivitis. J Immunol. 2006;176:3950–3957. doi: 10.4049/jimmunol.176.7.3950. [DOI] [PubMed] [Google Scholar]
- 57.Pflugfelder S.C., Huang A.J.W., Schuchovski P.T., Pereira I.C., Tseng S.C.G. Conjunctival cytological features of primary Sjogren syndrome. Ophthalmology. 1990;97:985–991. doi: 10.1016/s0161-6420(90)32478-8. [DOI] [PubMed] [Google Scholar]
- 58.Stern M.E., Schaumburg C.S., Dana R., Calonge M., Niederkorn J.Y., Pflugfelder S.C. Autoimmunity at the ocular surface: pathogenesis and regulation. Mucosal Immunol. 2010;3:425–442. doi: 10.1038/mi.2010.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schaumburg C.S., Siemasko K.F., de Paiva C.S., Wheeler L.A., Niederkorn J.Y., Pflugfelder S.C., Stern M.E. Ocular surface APCs are necessary for autoreactive T cell-mediated experimental autoimmune lacrimal keratoconjunctivitis. J Immunol. 2011;187:3653–3662. doi: 10.4049/jimmunol.1101442. [DOI] [PubMed] [Google Scholar]
- 60.de Paiva C.S., Corrales R.M., Villarreal A.L., Farley W., Li D.Q., Stern M.E., Pflugfelder S.C. Apical corneal barrier disruption in experimental murine dry eye is abrogated by methylprednisolone and doxycycline. Invest Ophthalmol Vis Sci. 2006;47:2847–2856. doi: 10.1167/iovs.05-1281. [DOI] [PubMed] [Google Scholar]
- 61.Schein O.D., Munoz B., Tielsch J.M., Bandeen-Roche K., West S. Prevalence of dry eye among the elderly. Am J Ophthalmol. 1997;124:723–728. doi: 10.1016/s0002-9394(14)71688-5. [DOI] [PubMed] [Google Scholar]
- 62.Schaumberg D.A., Sullivan D.A., Buring J.E., Dana M.R. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003;136:318–326. doi: 10.1016/s0002-9394(03)00218-6. [DOI] [PubMed] [Google Scholar]
- 63.Schaumberg D.A., Dana R., Buring J.E., Sullivan D.A. Prevalence of dry eye disease among US men: estimates from the Physicians' Health Studies. Arch Ophthalmol. 2009;127:763–768. doi: 10.1001/archophthalmol.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Williams G.P., Denniston A.K., Oswal K.S., Tomlins P.J., Barry R.J., Rauz S., Curnow S.J. The dominant human conjunctival epithelial CD8alphabeta+ T cell population is maintained with age but the number of CD4+ T cells increases. Age (Dordr) 2012;34:1517–1528. doi: 10.1007/s11357-011-9316-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chauhan S.K., El A.J., Ecoiffier T., Goyal S., Zhang Q., Saban D.R., Dana R. Autoimmunity in dry eye is due to resistance of Th17 to Treg suppression. J Immunol. 2009;182:1247–1252. doi: 10.4049/jimmunol.182.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dohlman T.H., Chauhan S.K., Kodati S., Hua J., Chen Y., Omoto M., Sadrai Z., Dana R. The CCR6/CCL20 axis mediates Th17 cell migration to the ocular surface in dry eye disease. Invest Ophthalmol Vis Sci. 2013;54:4081–4091. doi: 10.1167/iovs.12-11216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cui L., Shen M., Wang J., Jiang J., Li M., Chen D., Chen Z., Tao A., Lu F. Age-related changes in tear menisci imaged by optical coherence tomography. Optom Vis Sci. 2011;88:1214–1219. doi: 10.1097/OPX.0b013e3182271297. [DOI] [PubMed] [Google Scholar]
- 68.Patel S., Wallace I. Tear meniscus height, lower punctum lacrimale, and the tear lipid layer in normal aging. Optom Vis Sci. 2006;83:731–739. doi: 10.1097/01.opx.0000236810.17338.cf. [DOI] [PubMed] [Google Scholar]
- 69.Mathers W.D., Lane J.A., Zimmerman M.B. Tear film changes associated with normal aging. Cornea. 1996;15:229–234. doi: 10.1097/00003226-199605000-00001. [DOI] [PubMed] [Google Scholar]
- 70.Hamano T., Mitsunaga S., Kotani S., Hamano T., Hamano K., Hamano H., Sakamoto R., Tamura H. Tear volume in relation to contact lens wear and age. CLAO J. 1990;16:57–61. [PubMed] [Google Scholar]
- 71.Modulo C.M., Machado Filho E.B., Malki L.T., Dias A.C., de Souza J.C., Oliveira H.C., Jorge I.C., Santos Gomes I.B., Meyrelles S.S., Rocha E.M. The role of dyslipidemia on ocular surface, lacrimal and meibomian gland structure and function. Curr Eye Res. 2012;37:300–308. doi: 10.3109/02713683.2011.631720. [DOI] [PubMed] [Google Scholar]
- 72.Esch T.R., Jonsson M.V., Levanos V.A., Poveromo J.D., Sorkin B.C. Leukocytes infiltrating the submandibular glands of NOD mice express E-cadherin. J Autoimmun. 2000;15:387–393. doi: 10.1006/jaut.2000.0451. [DOI] [PubMed] [Google Scholar]
- 73.Saitoh-Inagawa W., Hiroi T., Yanagita M., Iijima H., Uchio E., Ohno S., Aoki K., Kiyono H. Unique characteristics of lacrimal glands as a part of mucosal immune network: high frequency of IgA-committed B-1 cells and NK1.1+ alphabeta T cells. Invest Ophthalmol Vis Sci. 2000;41:138–144. [PubMed] [Google Scholar]
- 74.Cha S., Brayer J., Gao J., Brown V., Killedar S., Yasunari U., Peck A.B. A dual role for interferon-gamma in the pathogenesis of Sjogren's syndrome-like autoimmune exocrinopathy in the nonobese diabetic mouse. Scand J Immunol. 2004;60:552–565. doi: 10.1111/j.0300-9475.2004.01508.x. [DOI] [PubMed] [Google Scholar]
- 75.Sharma R., Zheng L., Guo X., Fu S.M., Ju S.T., Jarjour W.N. Novel animal models for Sjogren's syndrome: expression and transfer of salivary gland dysfunction from regulatory T cell-deficient mice. J Autoimmun. 2006;27:289–296. doi: 10.1016/j.jaut.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nguyen C.Q., Peck A.B. Unraveling the pathophysiology of Sjogren syndrome-associated dry eye disease. Ocul Surf. 2009;7:11–27. doi: 10.1016/s1542-0124(12)70289-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Williams R.M., Singh J., Sharkey K.A. Innervation and mast cells of the rat lacrimal gland: the effects of age. Adv Exp Med Biol. 1994;350:67–74. doi: 10.1007/978-1-4615-2417-5_12. [DOI] [PubMed] [Google Scholar]
- 78.Zhang X., Volpe E.A., Gandhi N.B., Schaumburg C.S., Siemasko K.F., Pangelinan S.B., Kelly S.D., Hayday A.C., Li D.Q., Stern M.E., Niederkorn J.Y., Pflugfelder S.C., de Paiva C.S. NK cells promote Th-17 mediated corneal barrier disruption in dry eye. PLoS One. 2012;7:e36822. doi: 10.1371/journal.pone.0036822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fahlen L., Read S., Gorelik L., Hurst S.D., Coffman R.L., Flavell R.A., Powrie F. T cells that cannot respond to TGF-beta escape control by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2005;201:737–746. doi: 10.1084/jem.20040685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Veldhoen M., Hocking R.J., Flavell R.A., Stockinger B. Signals mediated by transforming growth factor-beta initiate autoimmune encephalomyelitis, but chronic inflammation is needed to sustain disease. Nat Immunol. 2006;7:1151–1156. doi: 10.1038/ni1391. [DOI] [PubMed] [Google Scholar]
- 81.Luger D., Silver P.B., Tang J., Cua D., Chen Z., Iwakura Y., Bowman E.P., Sgambellone N.M., Chan C.C., Caspi R.R. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J Exp Med. 2008;205:799–810. doi: 10.1084/jem.20071258. [DOI] [PMC free article] [PubMed] [Google Scholar]